Development and Evaluation of a Modified Fixed-Dose Combination Antihypertensive Tablet Containing S-Amlodipine Besylate: A Bioequivalence and Stability Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Compatibility Study Method
2.2.2. Preparation of Single-Layer Fixed-Dose Combination Products
2.2.3. Preparation of Bilayer, Fixed-Dose Combination Products
Olmesartan Medoxomil (First Layer), Hydrochlorothiazide and S-Amlodipine Besylate (Second Layer)
Olmesartan Medoxomil and Hydrochlorothiazide (First Layer), S-Amlodipine Besylate (Second Layer)
2.2.4. Product Quality Evaluation
2.2.5. Stability Method
Stability Test Under Stressed Conditions
Stability Test of Bilayer Tablet Under Accelerated Conditions
2.2.6. In Vitro Test
In Vitro Evaluation of Single-Layer Tablet
In Vitro Evaluation of Bi-Layer Tablet
2.2.7. In Vivo Pharmacokinetic Study
In Vivo Pharmacokinetic Study of Single-Layer Tablet
In Vivo Pharmacokinetic Study of Bi-Layer Tablet
3. Results
3.1. Compatibility Study
3.2. Stability
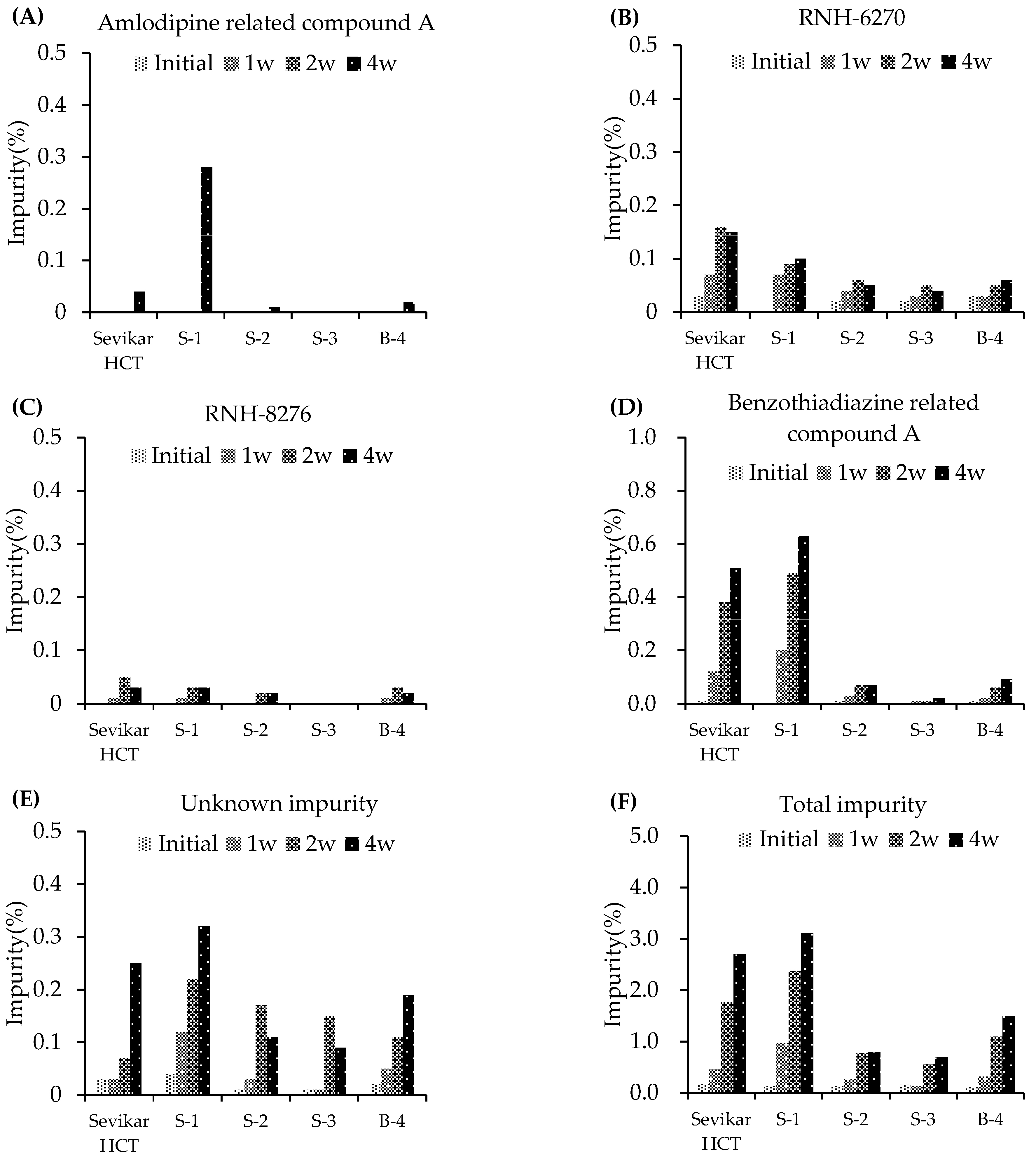
3.2.1. Stability Testing Under Stress Conditions
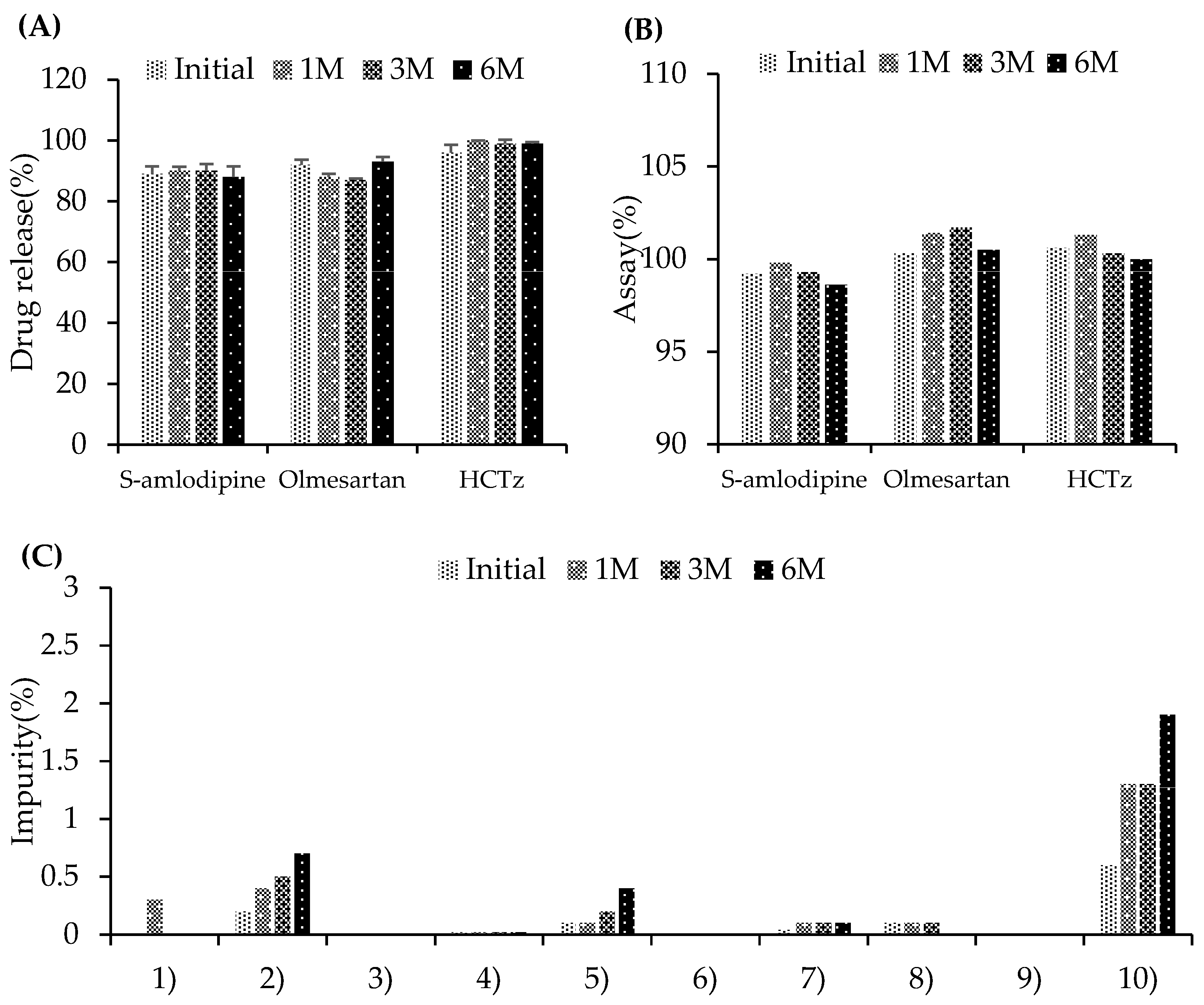
3.2.2. Stability Testing Under Accelerated Conditions
3.3. In Vitro Test
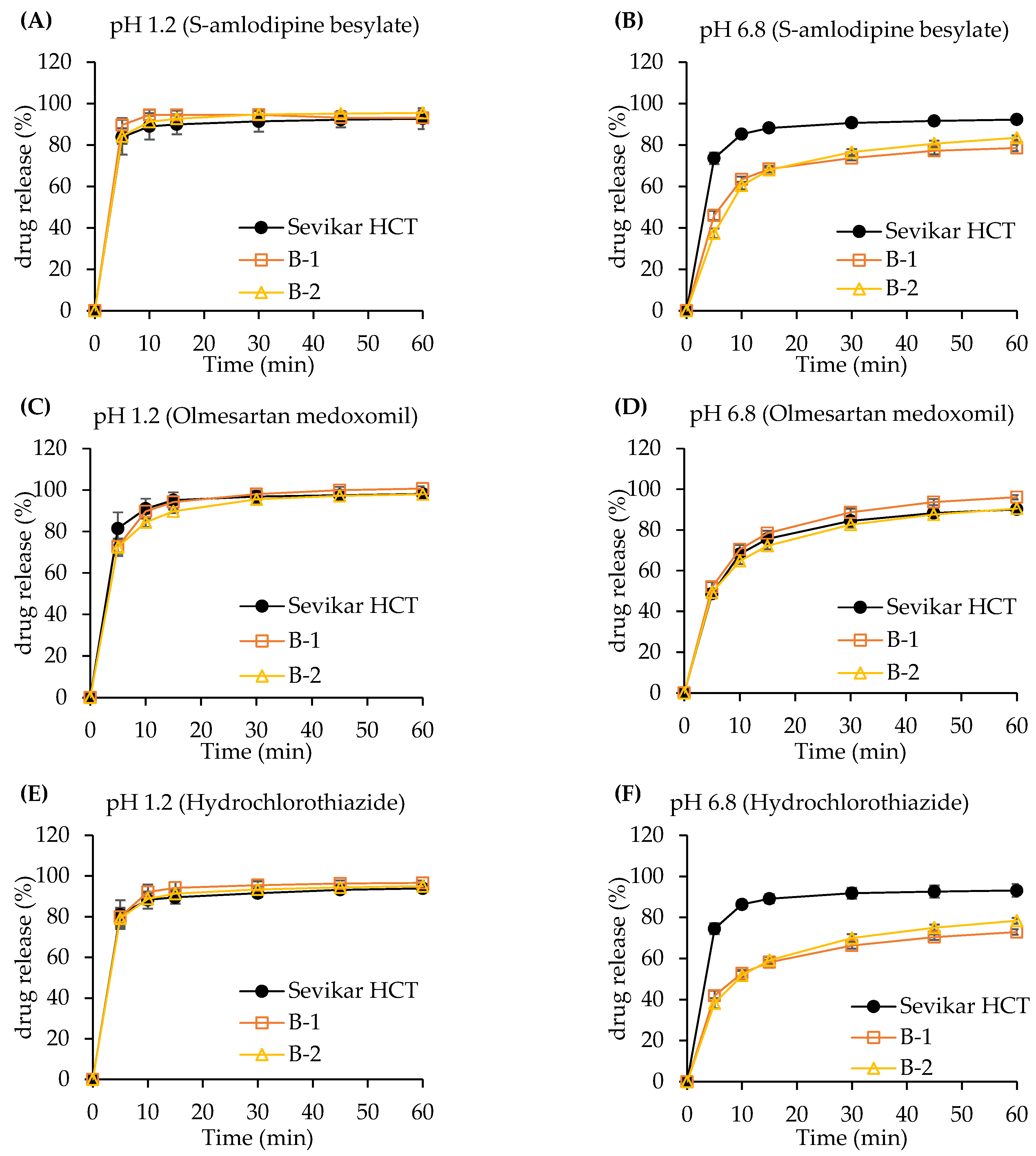
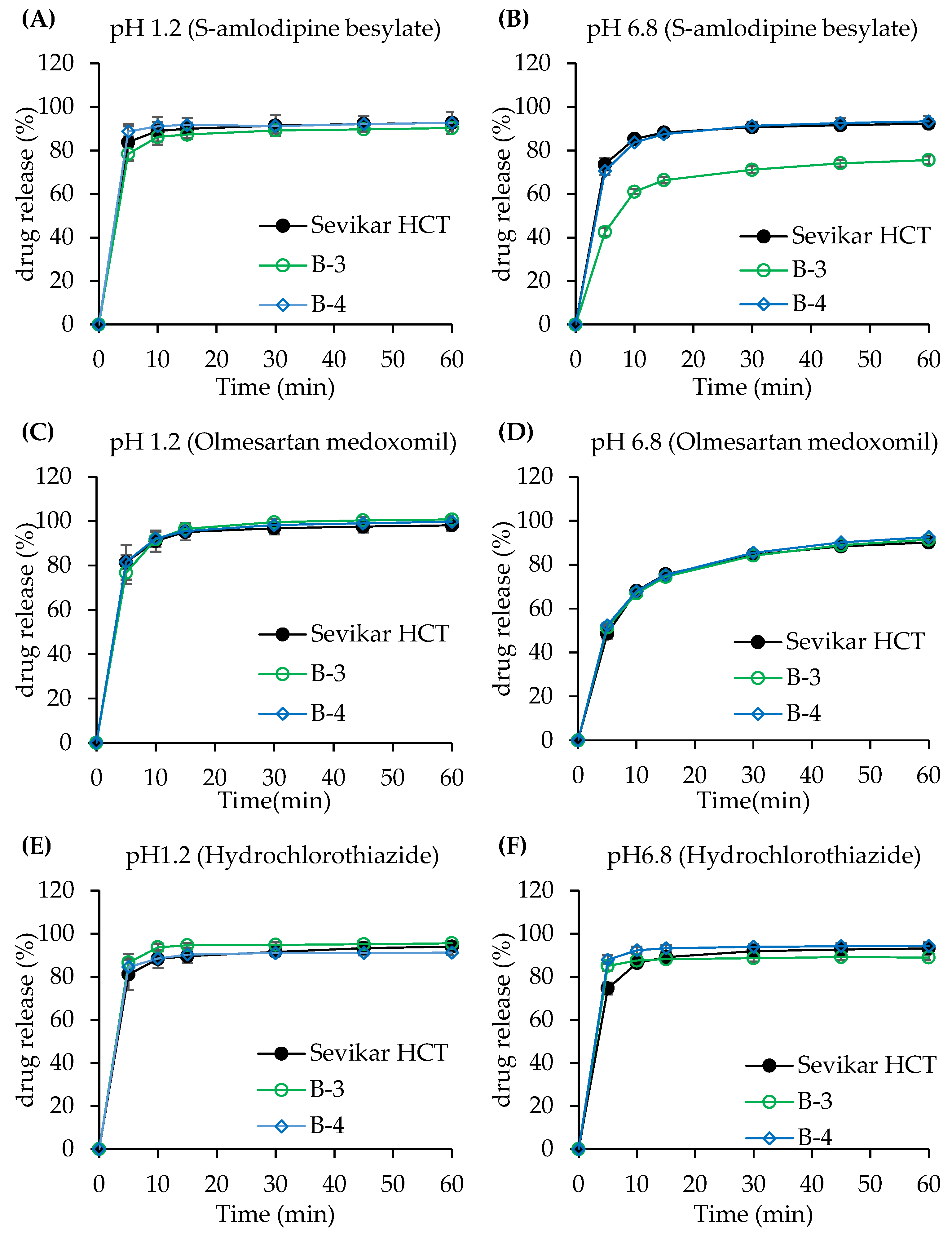
3.3.1. Dissolution Test of Single-Layer Tablet
3.3.2. Dissolution Test of Bi-Layer Tablet
3.4. Pharmacokinetics
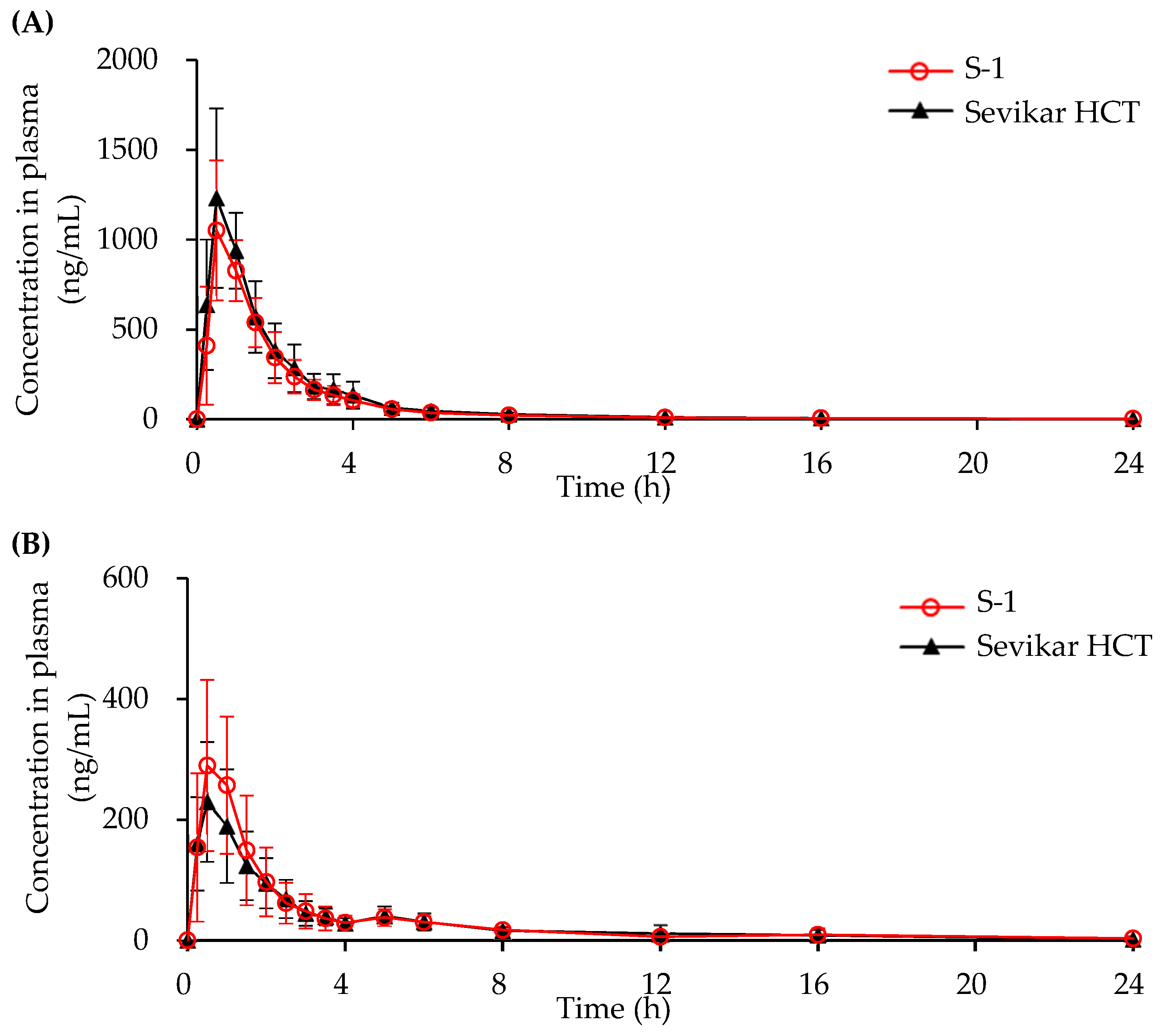
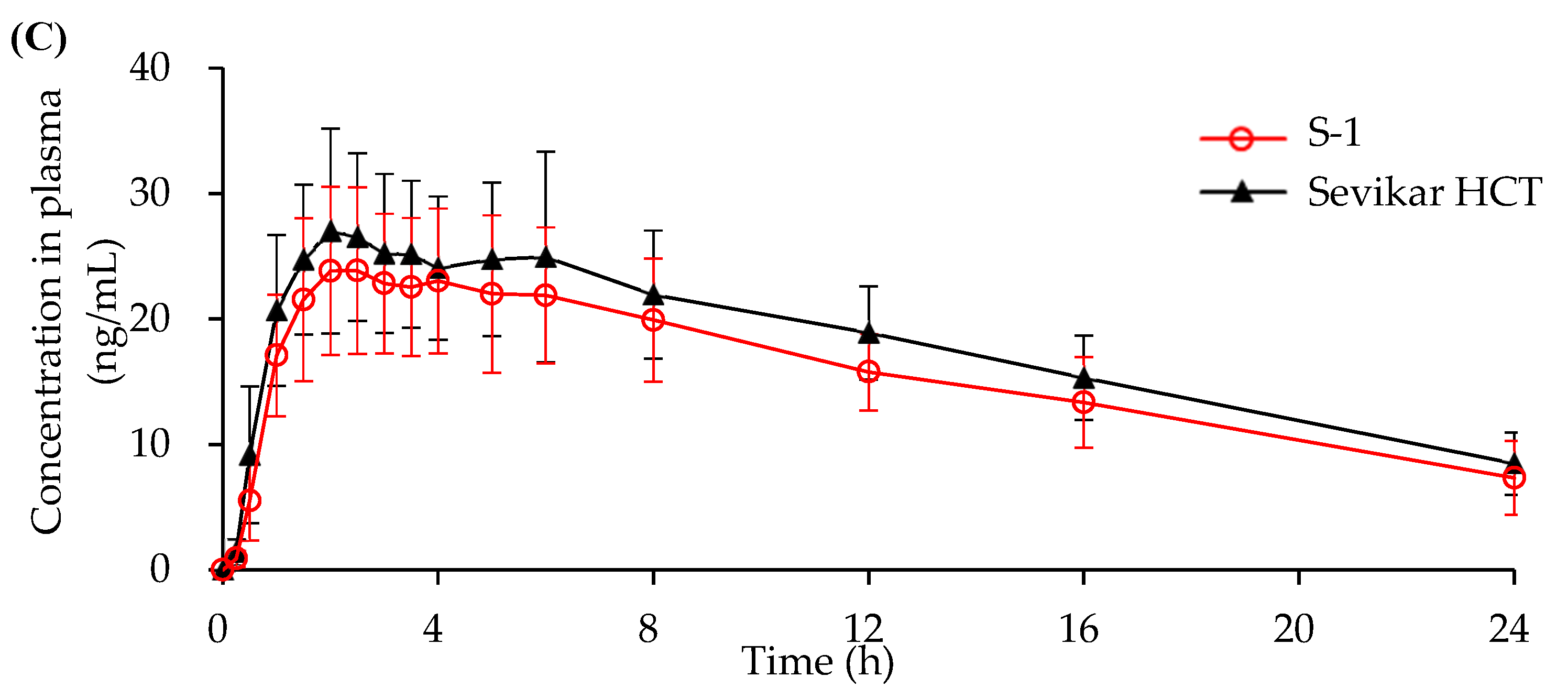
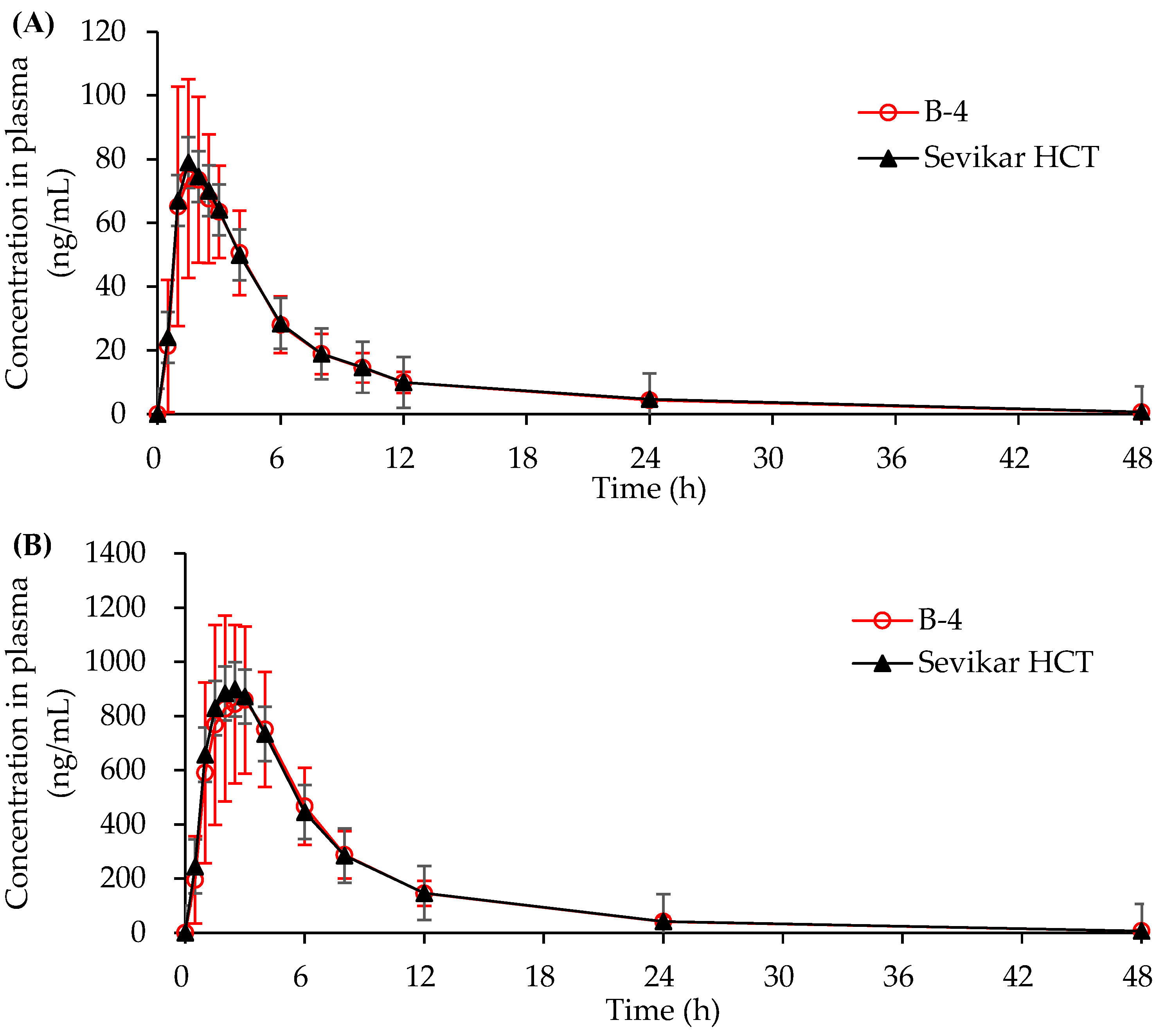
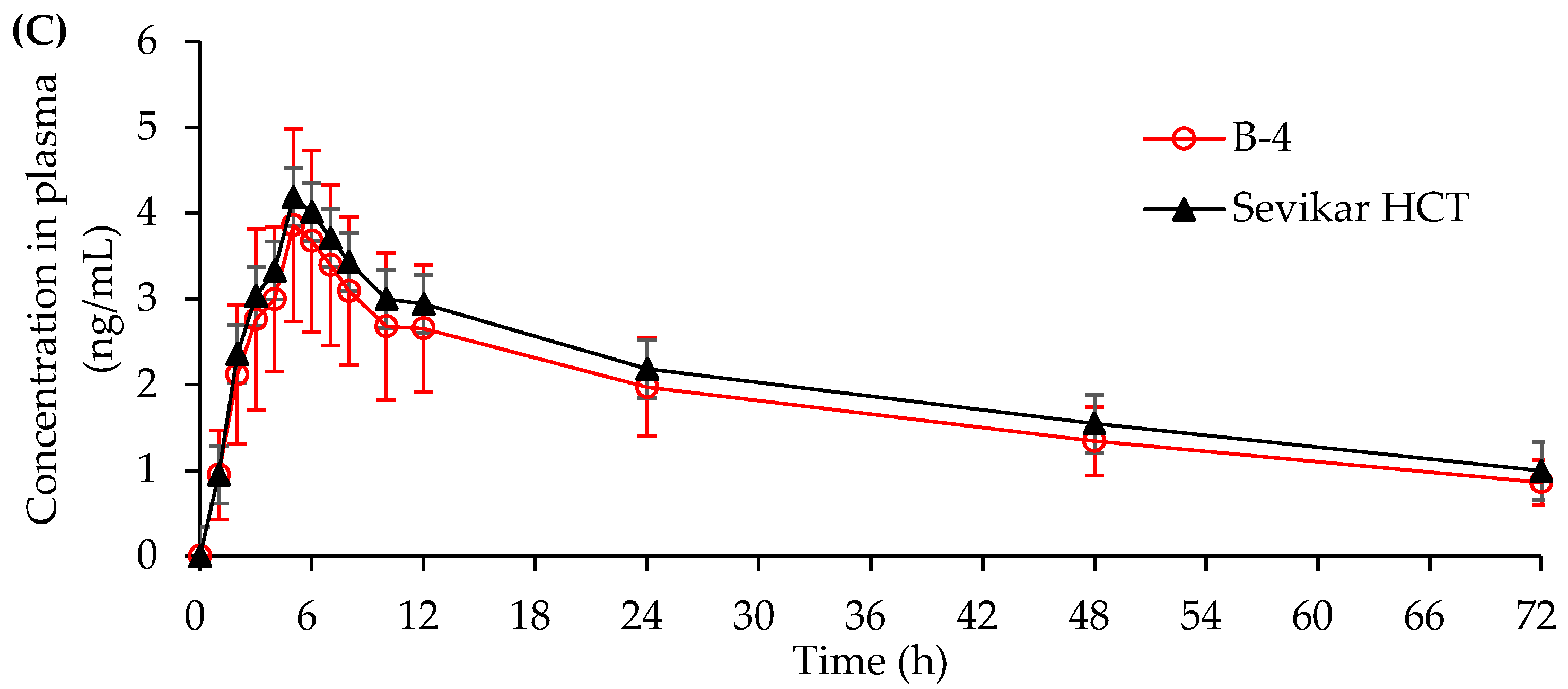
3.4.1. In Vivo Beagle Pharmacokinetic Study
3.4.2. In Vivo Human Pharmacokinetic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Test Items | Specifications | Test Results | |
|---|---|---|---|
| Content uniformity | Olmesartan medoxomil | NMT 15% | 3.2% |
| S-amlodipine besylate | NMT 15% | 9.8% | |
| Hydrochlorothiazide | NMT 15% | 0.7% | |
| Assay | Olmesartan medoxomil | 90.0 ~ 110.0% | 102.8% |
| S-amlodipine besylate | 90.0 ~ 110.0% | 98.2% | |
| Hydrochlorothiazide | 90.0 ~ 110.0% | 102.7% | |
| Dissolution test | Olmesartan medoxomil | ≥75% (45 min) | 85% |
| S-amlodipine besylate | ≥70% (45 min) | 83% | |
| Hydrochlorothiazide | ≥80% (30 min) | 93% | |
| Impurity test | Amlodipine related compound A | ≤0.5% | 0.2% |
| RNH-6270 | ≤2.5% | 0.2% | |
| RNH-8276 | ≤0.5% | 0.03% | |
| RNH-6373 | ≤0.5% | ND | |
| Benzothiadiazine related compound A | ≤1.0% | 0.04% | |
| Unknown impurity | ≤0.2% | 0.1% | |
| Total impurity | ≤5.0% | 0.9% | |
| Amlodipine Related Compound A | Unknown Impurity | Total Impurity | ||
|---|---|---|---|---|
| S-amlodipine besylate | Initial | ND | ND | ND |
| 1w | <LOQ | <LOQ | <LOQ | |
| 2w | <LOQ | <LOQ | <LOQ | |
| 4w | 0.06 | 0.16 | 0.30 | |
| S-amlodipine besylate + Silicified microcrystalline Cellulose | Initial | ND | ND | ND |
| 1w | ND | <LOQ | <LOQ | |
| 2w | ND | <LOQ | <LOQ | |
| 4w | <LOQ | 0.21 | 0.31 | |
| S-amlodipine besylate + Manitol | Initial | ND | ND | ND |
| 1w | <LOQ | <LOQ | <LOQ | |
| 2w | 0.09 | 0.25 | 0.45 | |
| 4w | 0.23 | 0.77 | 1.53 | |
| S-amlodipine besylate + Lactose | Initial | ND | ND | ND |
| 1w | <LOQ | <LOQ | <LOQ | |
| 2w | <LOQ | <LOQ | <LOQ | |
| 4w | <LOQ | 0.24 | 0.34 | |
| S-amlodipine besylate + Pregelatinized starch | Initial | ND | ND | ND |
| 1w | <LOQ | <LOQ | <LOQ | |
| 2w | <LOQ | <LOQ | <LOQ | |
| 4w | 0.05 | 0.22 | 0.39 | |
| S-amlodipine besylate + Low-substituted Hydroxypropyl cellulose | Initial | ND | ND | ND |
| 1w | 0.06 | <LOQ | 0.06 | |
| 2w | 0.07 | 0.15 | 0.28 | |
| 4w | 0.09 | 0.24 | 0.43 | |
| S-amlodipine besylate + Croscarmellose sodium | Initial | ND | ND | ND |
| 1w | <LOQ | 0.07 | 0.07 | |
| 2w | 0.05 | 0.20 | 0.42 | |
| 4w | 0.21 | 0.26 | 0.81 | |
| S-amlodipine besylate + Crospovidone | Initial | ND | ND | ND |
| 1w | <LOQ | <LOQ | <LOQ | |
| 2w | <LOQ | 0.15 | 0.26 | |
| 4w | 0.15 | 0.24 | 0.39 | |
| S-amlodipine besylate + Sodium starch glycolate | Initial | ND | ND | ND |
| 1w | <LOQ | <LOQ | <LOQ | |
| 2w | < LOQ | 0.19 | 0.36 | |
| 4w | 0.09 | 0.23 | 0.65 | |
| S-amlodipine besylate + Povidone | Initial | ND | ND | ND |
| 1w | 0.06 | <LOQ | 0.06 | |
| 2w | <LOQ | 015 | 0.28 | |
| 4w | 0.09 | 0.24 | 0.65 | |
| S-amlodipine besylate + Silicon dioxide | Initial | ND | ND | ND |
| 1w | 0.07 | 0.06 | 0.06 | |
| 2w | 0.09 | 0.19 | 0.27 | |
| 4w | 0.21 | 0.23 | 0.49 | |
| S-amlodipine besylate + Magnesium stearate | Initial | ND | ND | ND |
| 1w | <LOQ | <LOQ | <LOQ | |
| 2w | <LOQ | <LOQ | <LOQ | |
| 4w | <LOQ | 0.23 | 0.30 |
| RNH-6270 | RNH-6373 | RNH-8276 | Unknown Impurity | Total Impurity | ||
|---|---|---|---|---|---|---|
| Olmesartan medoxomil | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 2w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 4w | ND | ND | <LOQ | 0.05 | 0.05 | |
| Olmesartan medoxomil + Silicified microcrystalline Cellulose | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 2w | ND | ND | <LOQ | 0.06 | 0.06 | |
| 4w | ND | ND | <LOQ | 0.06 | 0.11 | |
| Olmesartan medoxomil + Manitol | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 2w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 4w | ND | ND | <LOQ | 0.05 | 0.05 | |
| Olmesartan medoxomil + Lactose | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 2w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 4w | ND | ND | <LOQ | 0.05 | 0.10 | |
| Olmesartan medoxomil + Pregelatinized starch | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 2w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 4w | ND | ND | <LOQ | 0.05 | 0.10 | |
| Olmesartan medoxomil + Low-substituted Hydroxypropyl cellulose | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 2w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 4w | ND | ND | <LOQ | 0.05 | 0.10 | |
| Olmesartan medoxomil + Croscarmellose sodium | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 2w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 4w | ND | ND | <LOQ | 0.10 | 0.23 | |
| Olmesartan medoxomil + Crospovidone | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 2w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 4w | ND | ND | <LOQ | 0.05 | 0.10 | |
| Olmesartan medoxomil + Sodium starch glycolate | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 2w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 4w | ND | ND | <LOQ | 0.05 | 0.05 | |
| Olmesartan medoxomil + Povidone | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | <LOQ | <LOQ | |
| 2w | ND | ND | <LOQ | 0.06 | 0.06 | |
| 4w | ND | ND | <LOQ | 0.06 | 0.10 | |
| Olmesartan medoxomil + Silicon dioxide | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 2w | ND | ND | <LOQ | 0.07 | 0.14 | |
| 4w | ND | ND | <LOQ | 0.10 | 0.23 | |
| Olmesartan medoxomil + Magnesium stearate | Initial | ND | ND | ND | <LOQ | <LOQ |
| 1w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 2w | ND | ND | <LOQ | 0.05 | 0.05 | |
| 4w | ND | ND | <LOQ | 0.05 | 0.11 |
| Benzothiadiazine Related Compound A | Unknown Impurity | Total Impurity | ||
|---|---|---|---|---|
| Hydrochlorothiazide | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.08 | 0.08 | |
| 2w | <LOQ | 0.08 | 0.08 | |
| 4w | <LOQ | 0.08 | 0.08 | |
| Hydrochlorothiazide + Silicified microcrystalline Cellulose | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.10 | 0.10 | |
| 2w | <LOQ | 0.10 | 0.10 | |
| 4w | <LOQ | 0.10 | 0.10 | |
| Hydrochlorothiazide + Manitol | Initial | <LOQ | 0.09 | 0.09 |
| 1w | <LOQ | 0.08 | 0.08 | |
| 2w | <LOQ | 0.07 | 0.07 | |
| 4w | <LOQ | 0.08 | 0.08 | |
| Hydrochlorothiazide + Lactose | Initial | <LOQ | 0.09 | 0.09 |
| 1w | <LOQ | 0.08 | 0.08 | |
| 2w | <LOQ | 0.08 | 0.08 | |
| 4w | <LOQ | 0.07 | 0.07 | |
| Hydrochlorothiazide + Pregelatinized starch | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.09 | 0.09 | |
| 2w | <LOQ | 0.08 | 0.08 | |
| 4w | <LOQ | 0.07 | 0.07 | |
| Hydrochlorothiazide + Low-substituted Hydroxypropyl cellulose | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.07 | 0.07 | |
| 2w | <LOQ | 0.08 | 0.08 | |
| 4w | <LOQ | 0.07 | 0.07 | |
| Hydrochlorothiazide + Croscarmellose sodium | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.08 | 0.08 | |
| 2w | <LOQ | 0.08 | 0.08 | |
| 4w | <LOQ | 0.07 | 0.07 | |
| Hydrochlorothiazide + Crospovidone | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.09 | 0.09 | |
| 2w | <LOQ | 0.06 | 0.06 | |
| 4w | <LOQ | 0.07 | 0.07 | |
| Hydrochlorothiazide + Sodium starch glycolate | Initial | <LOQ | 0.09 | 0.09 |
| 1w | <LOQ | 0.08 | 0.08 | |
| 2w | <LOQ | 0.07 | 0.07 | |
| 4w | <LOQ | 0.07 | 0.07 | |
| Hydrochlorothiazide + Povidone | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.13 | 0.13 | |
| 2w | <LOQ | 0.07 | 0.07 | |
| 4w | <LOQ | 0.07 | 0.07 | |
| Hydrochlorothiazide + Silicon dioxide | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.09 | 0.09 | |
| 2w | <LOQ | 0.08 | 0.08 | |
| 4w | <LOQ | 0.08 | 0.08 | |
| Hydrochlorothiazide + Magnesium stearate | Initial | <LOQ | 0.10 | 0.10 |
| 1w | <LOQ | 0.09 | 0.09 | |
| 2w | <LOQ | 0.06 | 0.06 | |
| 4w | <LOQ | 0.05 | 0.05 |
References
- Brunner, H.R. Clinical efficacy and tolerability of olmesartan. Clin. Ther. 2004, 26, A28–A32. [Google Scholar] [CrossRef]
- Ma, S.-F.; Anraku, M.; Iwao, Y.; Yamasaki, K.; Kragh-Hansen, U.; Yamaotsu, N.; Hirono, S.; Ikeda, T.; Otagiri, M. Hydrolysis of angiotensin II receptor blocker prodrug olmesartan medoxomil by human serum albumin and identification of its catalytic active sites. Drug Metab. Dispos. 2005, 33, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M. Angiotensin II type 1 receptor blockers. Circulation 2001, 103, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Neutel, J.M.; Elliott, W.J.; Izzo, J.L., Jr.; Chen, C.L.; Masonson, H.N. Antihypertensive efficacy of olmesartan medoxomil, a new angiotensin II receptor antagonist, as assessed by ambulatory blood pressure measurements. J. Clin. Hypertens. 2002, 4, 325–331. [Google Scholar] [CrossRef][Green Version]
- Scott, L.J.; McCormack, P.L. Olmesartan medoxomil: A review of its use in the management of hypertension. Drugs 2008, 68, 1239–1272. [Google Scholar] [CrossRef]
- Ernst, M.E.; Fravel, M.A. Thiazide and the thiazide-like diuretics: Review of hydrochlorothiazide, chlorthalidone, and indapamide. Am. J. Hypertens. 2022, 35, 573–586. [Google Scholar] [CrossRef]
- Mondaca-Ruff, D.; Araos, P.; Yanez, C.E.; Novoa, U.F.; Mora, I.G.; Ocaranza, M.P.; Jalil, J.E. Hydrochlorothiazide reduces cardiac hypertrophy, fibrosis and rho-kinase activation in DOCA-salt induced hypertension. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 724–735. [Google Scholar] [CrossRef]
- Wener, J.; Friedman, R.; Mayman, A.; Schucher, R. Hydrochlorothiazide (hydrodiuril) in the management of cardiac oedema. Can. Med. Assoc. J. 1959, 81, 221. [Google Scholar]
- Herman, L.L.; Weber, P.; Bashir, K. Hydrochlorothiazide. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bargagli, M.; Anderegg, M.A.; Fuster, D.G. Effects of thiazides and new findings on kidney stones and dysglycemic side effects. Acta Physiol. 2024, 240, e14155. [Google Scholar] [CrossRef]
- Musini, V.M.; Nazer, M.; Bassett, K.; Wright, J.M. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst. Rev. 2014, 2014, CD003824. [Google Scholar] [CrossRef]
- Morales-Olivas, F. Diuretics use in the management of hypertension. Hipertens. Y Riesgo Vasc. 2024, 41, 186–193. [Google Scholar] [CrossRef]
- Mason, R.P. Mechanisms of plaque stabilization for the dihydropyridine calcium channel blocker amlodipine: Review of the evidence. Atherosclerosis 2002, 165, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Puscas, I.; Gilau, L.; Coltau, M.; Pasca, R.; Domuta, G.; Baican, M.; Hecht, A. Calcium channel blockers reduce blood pressure in part by inhibiting vascular smooth muscle carbonic anhydrase I. Cardiovasc. Drugs Ther. 2000, 14, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Glasser, S.P.; West, T.W. Clinical safety and efficacy of once-a-day amlodipine for chronic stable angina pectoris. Am. J. Cardiol. 1988, 62, 518–522. [Google Scholar] [PubMed]
- Chahine, R.A.; Feldman, R.L.; Giles, T.D.; Nicod, P.; Raizner, A.E.; Weiss, R.J.; Vanov, S.K.; Study, A. Randomized placebo-controlled trial of amlodipine in vasospastic angina. J. Am. Coll. Cardiol. 1993, 21, 1365–1370. [Google Scholar]
- Abernethy, D.R. The pharmacokinetic profile of amlodipine. Am. Heart J. 1989, 118, 1100–1103. [Google Scholar] [CrossRef]
- Meluzín, J.; Štejfa, M.; Novák, M.; Zeman, K.; Špinarová, L.; Julínek, J.; Toman, J.; Šimek, P. Amlodipine in patients with stable angina pectoris treated with nitrates and beta-blockers. The influence on exercise tolerance, systolic and diastolic functions of the left ventricle. Int. J. Cardiol. 1992, 37, 101–109. [Google Scholar] [CrossRef]
- Lukša, J.; Josič, D.; Kremser, M.; Kopitar, Z.; Milutinovič, S. Pharmacokinetic behaviour of R-(+)-and S-(−)-amlodipine after single enantiomer administration. J. Chromatogr. B Biomed. Sci. Appl. 1997, 703, 185–193. [Google Scholar] [CrossRef]
- Kim, S.A.; Park, S.; Chung, N.; Lim, D.-S.; Yang, J.-Y.; Oh, B.-H.; Tahk, S.-J.; Ahn, T.-H. Efficacy and safety profiles of a new S (—)-amlodipine nicotinate formulation versus racemic amlodipine besylate in adult Korean patients with mild to moderate hypertension: An 8-week, multicenter, randomized, double-blind, double-dummy, parallel-group, phase III, noninferiority clinical trial. Clin. Ther. 2008, 30, 845–857. [Google Scholar]
- Liu, F.; Qiu, M.; Zhai, S.-D. Tolerability and effectiveness of (S)-amlodipine compared with racemic amlodipine in hypertension: A systematic review and meta-analysis. Curr. Ther. Res. 2010, 71, 1–29. [Google Scholar] [CrossRef]
- Dalal, J.; Mohan, J.; Iyengar, S.; Hiremath, J.; Sathyamurthy, I.; Bansal, S.; Kahali, D.; Dasbiswas, A. S-Amlodipine: An Isomer with Difference—Time to Shift from Racemic Amlodipine. Int. J. Hypertens. 2018, 2018, 8681792. [Google Scholar] [CrossRef]
- Hancu, G.; Modroiu, A. Chiral switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef]
- Guerrero-García, C.; Rubio-Guerra, A.F. Combination therapy in the treatment of hypertension. Drugs Context 2018, 7, 212531. [Google Scholar] [CrossRef]
- DiPette, D.J.; Skeete, J.; Ridley, E.; Campbell, N.R.; Lopez-Jaramillo, P.; Kishore, S.P.; Jaffe, M.G.; Coca, A.; Townsend, R.R.; Ordunez, P. Fixed-dose combination pharmacologic therapy to improve hypertension control worldwide: Clinical perspective and policy implications. J. Clin. Hypertens. 2018, 21, 4. [Google Scholar] [CrossRef]
- Kishore, S.P.; Salam, A.; Rodgers, A.; Jaffe, M.G.; Frieden, T. Fixed-dose combinations for hypertension. Lancet 2018, 392, 819–820. [Google Scholar] [CrossRef]
- Verma, A.A.; Khuu, W.; Tadrous, M.; Gomes, T.; Mamdani, M.M. Fixed-dose combination antihypertensive medications, adherence, and clinical outcomes: A population-based retrospective cohort study. PLoS Med. 2018, 15, e1002584. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Olmesartan medoxomil/amlodipine/hydrochlorothiazide: Fixed-dose combination in hypertension. Drugs 2011, 71, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Bramlage, P.; Fronk, E.-M.; Wolf, W.-P.; Smolnik, R.; Sutton, G.; Schmieder, R.E. Safety and effectiveness of a fixed-dose combination of olmesartan, amlodipine, and hydrochlorothiazide in clinical practice. Vasc. Health Risk Manag. 2014, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marques da Silva, P.; Haag, U.; Guest, J.F.; Brazier, J.E.; Soro, M. Health-related quality of life impact of a triple combination of olmesartan medoxomil, amlodipine besylate and hydrochlorotiazide in subjects with hypertension. Health Qual. Life Outcomes 2015, 13, 24. [Google Scholar] [CrossRef]
- Shende, P.K.; Shrawne, C.; Gaud, R. Multi-layer Tablet: Current scenario and recent advances. Int. J. Drug Deliv. 2012, 4, 418. [Google Scholar]
- Wang, X.; Cui, F.; Yonezawa, Y.; Sunada, H. Preparation and evaluation of combination tablet containing incompatible active ingredients. Chem. Pharm. Bull. 2003, 51, 772–778. [Google Scholar] [CrossRef][Green Version]
- Webster, R.; Patel, A.; Selak, V.; Billot, L.; Bots, M.L.; Brown, A.; Bullen, C.; Cass, A.; Crengle, S.; Elley, C.R. Effectiveness of fixed dose combination medication (‘polypills’) compared with usual care in patients with cardiovascular disease or at high risk: A prospective, individual patient data meta-analysis of 3140 patients in six countries. Int. J. Cardiol. 2016, 205, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Galappatthy, P.; Waniganayake, Y.C.; Sabeer, M.I.; Wijethunga, T.J.; Galappatthy, G.K.; Ekanayaka, R.A. Leg edema with (S)-amlodipine vs conventional amlodipine given in triple therapy for hypertension: A randomized double blind controlled clinical trial. BMC Cardiovasc. Disord. 2016, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Bauer, M.; Boussac, N.; Khan-Malek, R.; Munden, P.; Sardaro, M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J. Pharm. Biomed. Anal. 1998, 17, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Ellison, C.D.; Conner, D.P.; Lesko, L.J.; Hussain, A.S. Influence of drug release properties of conventional solid dosage forms on the systemic exposure of highly soluble drugs. AAPS PharmSci. 2001, 3, 24. [Google Scholar] [CrossRef]
- Thakkar, H.P.; Patel, B.V.; Thakkar, S.P. Development and characterization of nanosuspensions of olmesartan medoxomil for bioavailability enhancement. J. Pharm. Bioallied Sci. 2011, 3, 426–434. [Google Scholar] [CrossRef]
- Cardot, J.; Beyssac, E.; Alric, M. In vitro-in vivo correlation: Importance of dissolution in IVIVC. Dissolution Technol. 2007, 14, 15. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Akimoto, M.; Nagahata, N.; Furuya, A.; Fukushima, K.; Higuchi, S.; Suwa, T. Gastric pH profiles of beagle dogs and their use as an alternative to human testing. Eur. J. Pharm. Biopharm. 2000, 49, 99–102. [Google Scholar] [CrossRef]
- Chai, R.; Gao, H.; Ma, Z.; Guo, M.; Fu, Q.; Liu, H.; He, Z. In vitro and in vivo evaluation of olmesartan medoxomil microcrystals and nanocrystals: Preparation, characterization, and pharmacokinetic comparison in beagle dogs. Curr. Drug Deliv. 2019, 16, 500–510. [Google Scholar] [CrossRef]
- Beermann, B.; Groschinsky-Grind, M. Pharmacokinetics of hydrochlorothiazide in man. Eur. J. Clin. Pharmacol. 1977, 12, 297–303. [Google Scholar] [CrossRef]
- Khaled, K.A.; Asiri, Y.A.; El-Sayed, Y.M. In vivo evaluation of hydrochlorothiazide liquisolid tablets in beagle dogs. Int. J. Pharm. 2001, 222, 1–6. [Google Scholar] [CrossRef]
- Stopher, D.; Beresford, A.; Macrae, P.; Humphrey, M. The metabolism and pharmacokinetics of amlodipine in humans and animals. J. Cardiovasc. Pharmacol. 1988, 12, S55–S59. [Google Scholar] [CrossRef]

| Ingredient | Formulation Code | ||
|---|---|---|---|
| S-1 | S-2 | S-3 | |
| Olmesartan medoxomil | 40.00 | 40.00 | 40.00 |
| Hydrochlorothiazide | 12.50 | 12.50 | 12.50 |
| S-amlodipine besylate dihydrate | 7.38 | - | - |
| Amlodipine besylate | - | 6.94 | - |
| Pregelatinized starch | 105.00 | 105.00 | 105.00 |
| Silicified microcrystalline cellulose | 118.82 | 112.41 | 112.41 |
| Croscarmellose sodium | 15.00 | 15.00 | 15.00 |
| Magnesium stearate | 1.20 | 1.20 | 1.20 |
| Opadty II pink(85F25437) | 10.00 | 10.00 | 10.00 |
| Total weight | 309.90 | 303.05 | 295.11 |
| Ingredient | Formulation Code | |
|---|---|---|
| B-1 | B-2 | |
| Olmesartan medoxomil | 40.00 | 40.00 |
| Silicified microcrystalline cellulose | 59.50 | 59.50 |
| Pregelatinized starch | 52.50 | 52.50 |
| Croscarmellose sodium | 15.00 | 15.00 |
| Magnesium stearate | 3.00 | 3.00 |
| First weight | 170.00 | 170.00 |
| Hydrochlorothiazide | 12.50 | 12.50 |
| S-amlodipine besylate dihydrate | 7.38 | 7.38 |
| Silicified microcrystalline cellulose | 39.10 | 39.10 |
| Pregelatinized starch | 33.02 | 28.27 |
| Croscarmellose sodium | - | 4.75 |
| Magnesium stearate | 3.00 | 3.00 |
| Second weight | 95.00 | 95.00 |
| Total weight | 265.00 | 265.00 |
| Ingredient | Formulation Code | ||
|---|---|---|---|
| B-3 | B-4 | ||
| Olmesartan granule | Olmesartan medoxomil | 40.00 | 40.00 |
| Silicified microcrystalline cellulose | 59.50 | 59.50 | |
| Pregelatinized starch | 52.50 | 52.50 | |
| Croscarmellose sodium | 15.00 | 15.00 | |
| Hydrochlorothiazide powder | Hydrochlorothiazide | 12.50 | 12.50 |
| Silicified microcrystalline cellulose | 12.25 | 12.25 | |
| Pregelatinized starch | 8.15 | 8.15 | |
| Silicon dioxide | 2.10 | 2.10 | |
| Magnesium stearate | 3.00 | 3.00 | |
| First weight | 205.00 | 205.00 | |
| S-amlodipine powder | S-amlodipine besylate dihydrate | 7.38 | 7.38 |
| Silicified microcrystalline cellulose | 39.67 | 63.42 | |
| Pregelatinized starch | 20.00 | 53.76 | |
| Low-substituted hydroxypropyl cellulose | 25.00 | 15.00 | |
| Crospovidone | - | 7.50 | |
| Silicon dioxide | 0.95 | 0.94 | |
| Magnesium stearate | 2.00 | 2.00 | |
| Second weight | 95.00 | 150.00 | |
| Total weight | 300.00 | 355.00 | |
| Formulation | Slope k (%/week) | R2 |
|---|---|---|
| Sevikar HCT® | 0.669 | 0.949 |
| S-1 | 0.757 | 0.926 |
| S-2 | 0.177 | 0.780 |
| S-3 | 0.152 | 0.941 |
| B-4 | 0.366 | 0.924 |
| Parameters | Hydrochlorothiazide | Olmesartan Medoxomil | S-Amlodipine Besylate | |||
|---|---|---|---|---|---|---|
| Test | Reference | Test | Reference | Test | Reference | |
| AUC0-t (ng/mL) | 1902.0 ±378.8 | 2227.2 ±617.7 | 695.3 ±216.5 | 639.6 ±210.1 | 369.1 ±85.7 | 422.3 ±87.6 |
| Cmax (ng/mL) | 1131.5 ±318.5 | 1320.3 ±363.0 | 367.5 ±108.3 | 266.3 ±78.7 | 26.5 ±6.5 | 29.7 ±8.1 |
| Tmax (h) | 0.67 ±0.24 | 0.67 ±0.24 | 0.67 ±0.29 | 0.55 ±0.30 | 3.60 ±1.72 | 3.0 ±1.2 |
| T1/2 (h) | 4.83 ±2.81 | 4.17 ±1.27 | 4.21 ±1.90 | 4.12 ±4.11 | 10.91 ±3.07 | 10.71 ±2.63 |
| Parameters | Hydrochlorothiazide | Olmesartan Medoxomil | S-Amlodipine Besylate | |||
|---|---|---|---|---|---|---|
| T/R Ratio | 90% CI | T/R Ratio | 90% CI | T/R Ratio | 90% CI | |
| AUC0-t | 0.85 | 0.81, 0.92 | 1.08 | 0.96, 1.21 | 0.87 | 0.84, 0.90 |
| Cmax | 0.86 | 0.75, 0.99 | 1.38 | 1.13, 1.64 | 0.89 | 0.85, 0.95 |
| Parameters | Hydrochlorothiazide | Olmesartan Medoxomil | S-Amlodipine Besylate | |||
|---|---|---|---|---|---|---|
| Test | Reference | Test | Reference | Test | Reference | |
| AUC0-t (ng/mL) | 524.29 ±150.48 | 525.16 ±129.75 | 6825.01 ±1929.15 | 6913.78 ±2105.56 | 125.49 ±34.10 | 140.81 ±41.32 |
| Cmax (ng/mL) | 87.59 ±26.69 | 89.42 ±23.74 | 963.13 ±294.25 | 987.24 ±300.80 | 3.99 ±1.08 | 4.32 ±1.20 |
| Tmax (h) | 1.5 | 1.5 | 2.5 | 2.0 | 5.0 | 5.0 |
| T1/2 (h) | 9.30 ±1.50 | 9.42 ±1.30 | 7.94 ±1.72 | 8.01 ±1.74 | 39.92 ±8.58 | 40.31 ±7.20 |
| Parameters | Hydrochlorothiazide | Olmesartan Medoxomil | S-Amlodipine Besylate | |||
|---|---|---|---|---|---|---|
| T/R Ratio | 90% CI | T/R Ratio | 90% CI | T/R Ratio | 90% CI | |
| AUC0-t | 0.99 | 0.95, 1.04 | 1.00 | 0.94, 1.05 | 0.90 | 0.86, 0.93 |
| Cmax | 0.97 | 0.90, 1.05 | 0.98 | 0.92, 1.05 | 0.92 | 0.89, 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.W.; Jeong, J.-H.; Park, C.-W. Development and Evaluation of a Modified Fixed-Dose Combination Antihypertensive Tablet Containing S-Amlodipine Besylate: A Bioequivalence and Stability Study. Pharmaceutics 2025, 17, 1235. https://doi.org/10.3390/pharmaceutics17091235
Moon HW, Jeong J-H, Park C-W. Development and Evaluation of a Modified Fixed-Dose Combination Antihypertensive Tablet Containing S-Amlodipine Besylate: A Bioequivalence and Stability Study. Pharmaceutics. 2025; 17(9):1235. https://doi.org/10.3390/pharmaceutics17091235
Chicago/Turabian StyleMoon, Hyeon Woo, Jin-Hyuk Jeong, and Chun-Woong Park. 2025. "Development and Evaluation of a Modified Fixed-Dose Combination Antihypertensive Tablet Containing S-Amlodipine Besylate: A Bioequivalence and Stability Study" Pharmaceutics 17, no. 9: 1235. https://doi.org/10.3390/pharmaceutics17091235
APA StyleMoon, H. W., Jeong, J.-H., & Park, C.-W. (2025). Development and Evaluation of a Modified Fixed-Dose Combination Antihypertensive Tablet Containing S-Amlodipine Besylate: A Bioequivalence and Stability Study. Pharmaceutics, 17(9), 1235. https://doi.org/10.3390/pharmaceutics17091235






