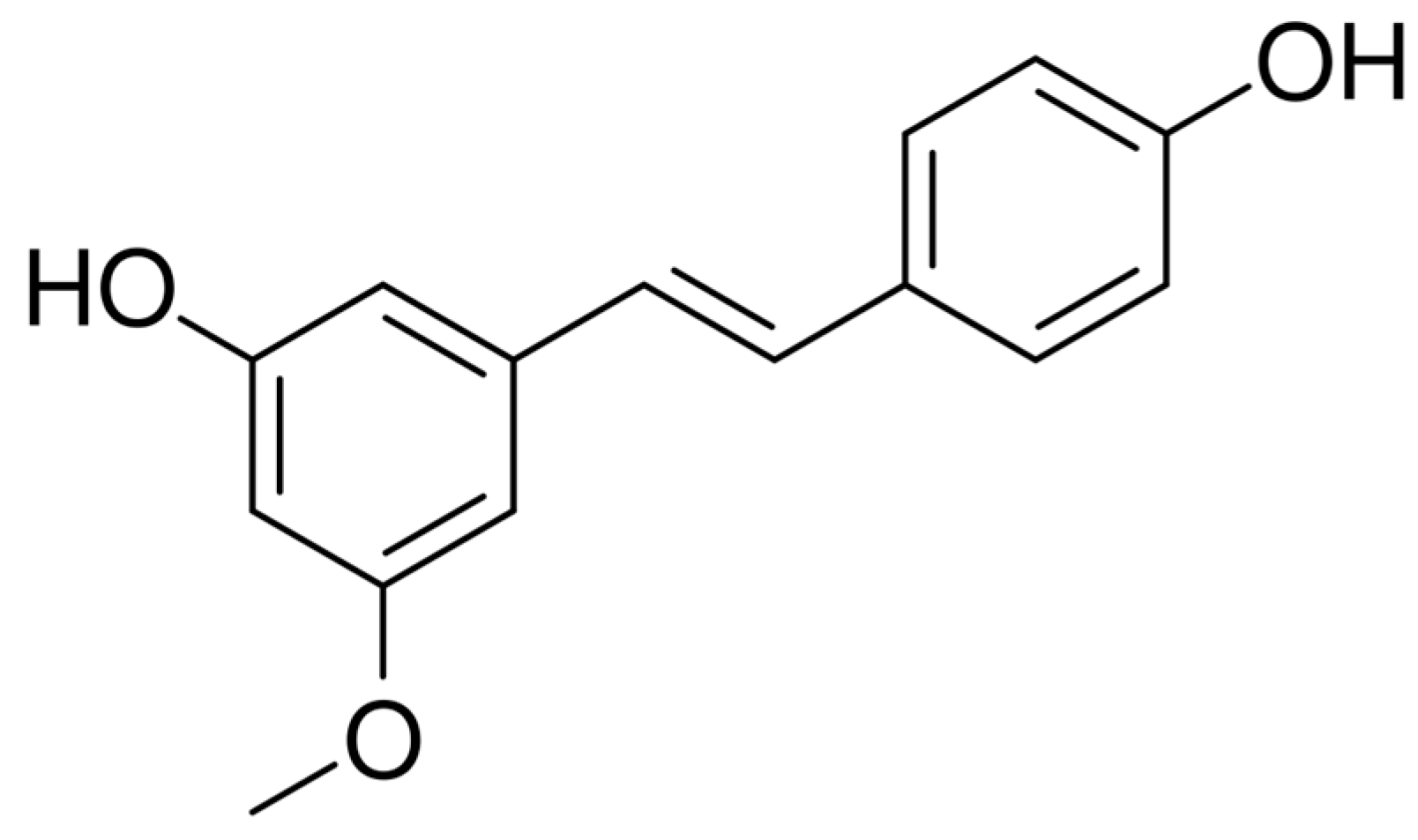
Pinostilbene as a Potential Cytotoxic Agent in Cancer Cell Lines: Improvement of Solubility and Stability by Cyclodextrin Encapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cell Line and Culture Conditions
2.2.2. Cytotoxicity Test
2.2.3. Determination of Stoichiometry and Encapsulation Constants
2.2.4. Molecular Docking
2.2.5. Fluorimetric Studies and Determination of pH Influence
2.2.6. Determination of Aqueous Solubility
2.2.7. Determination of Stability in Aqueous Solution
2.2.8. Pinostilbene Complexation in HP-β-CD and Characterization
2.2.9. In Vitro Release Studies
2.2.10. Data Analysis
3. Results and Discussion
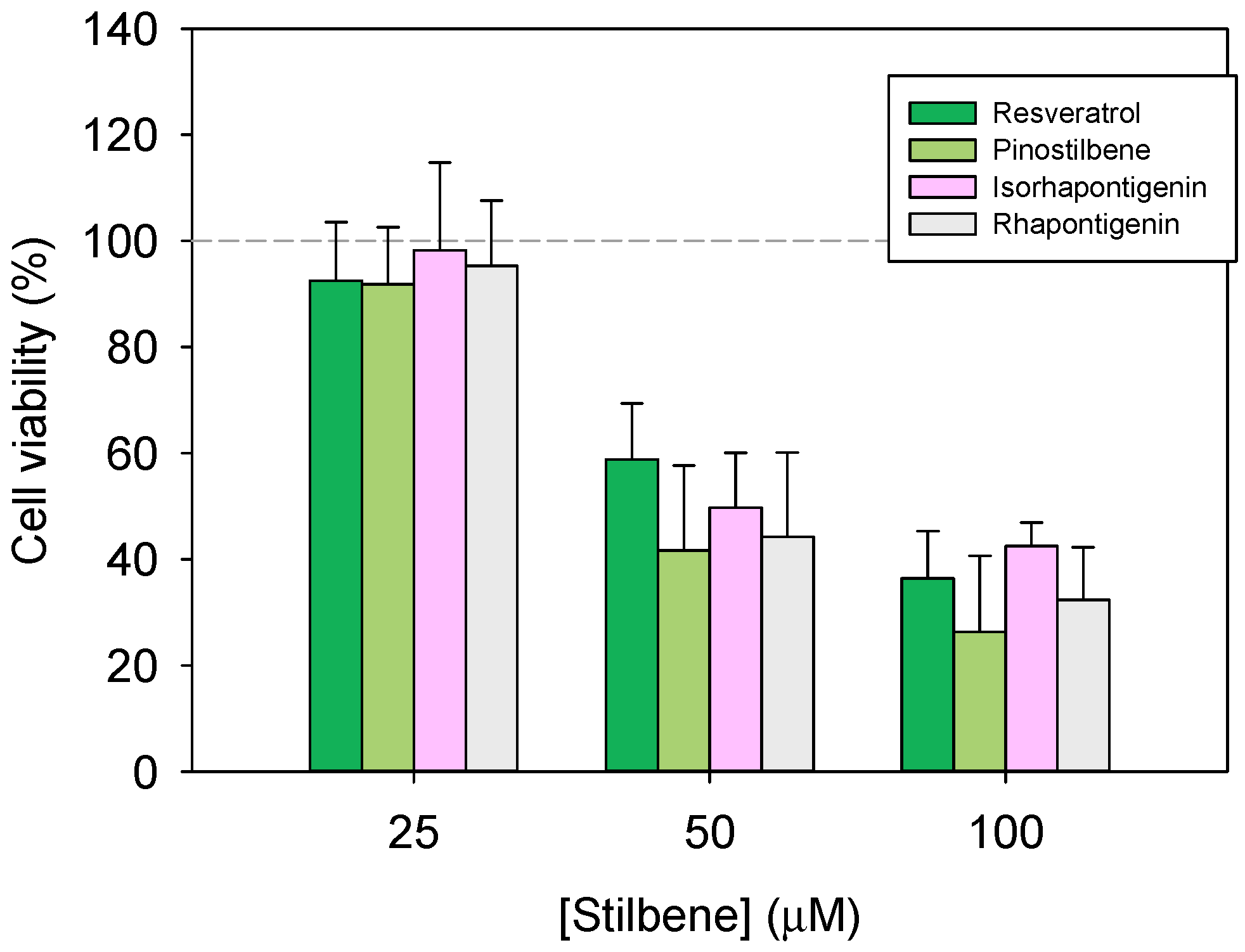
3.1. Inhibition of Caco-2 Proliferation After Pinostilbene Treatment
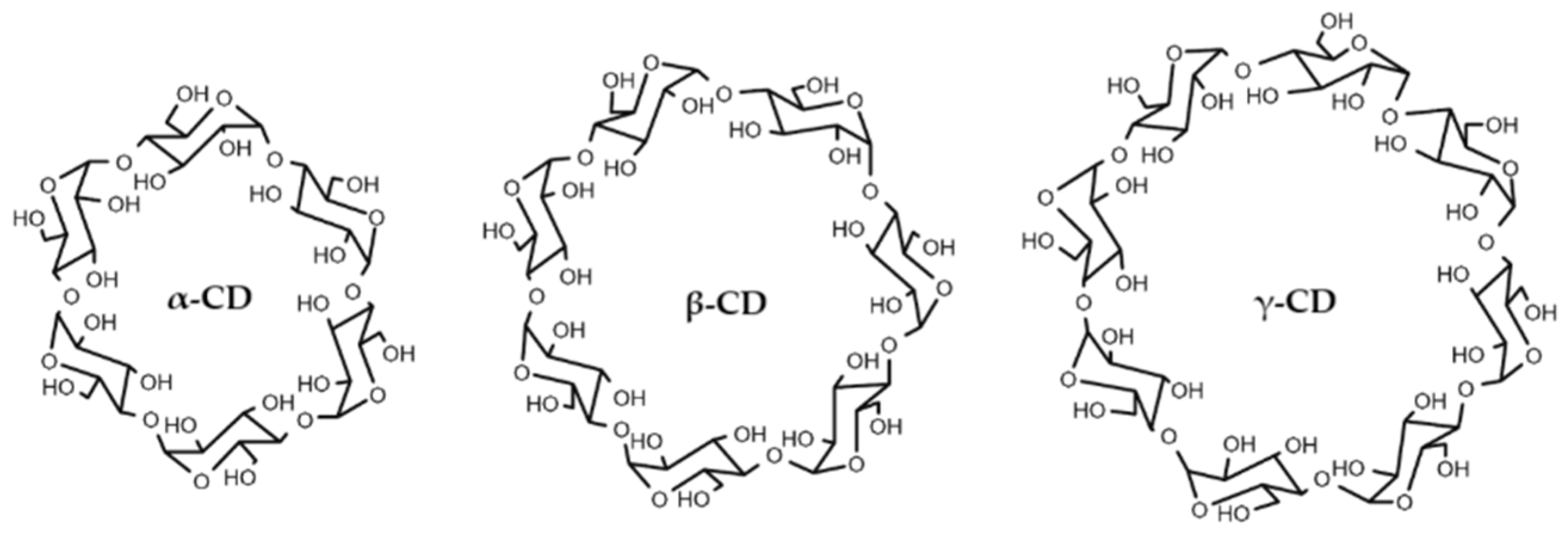
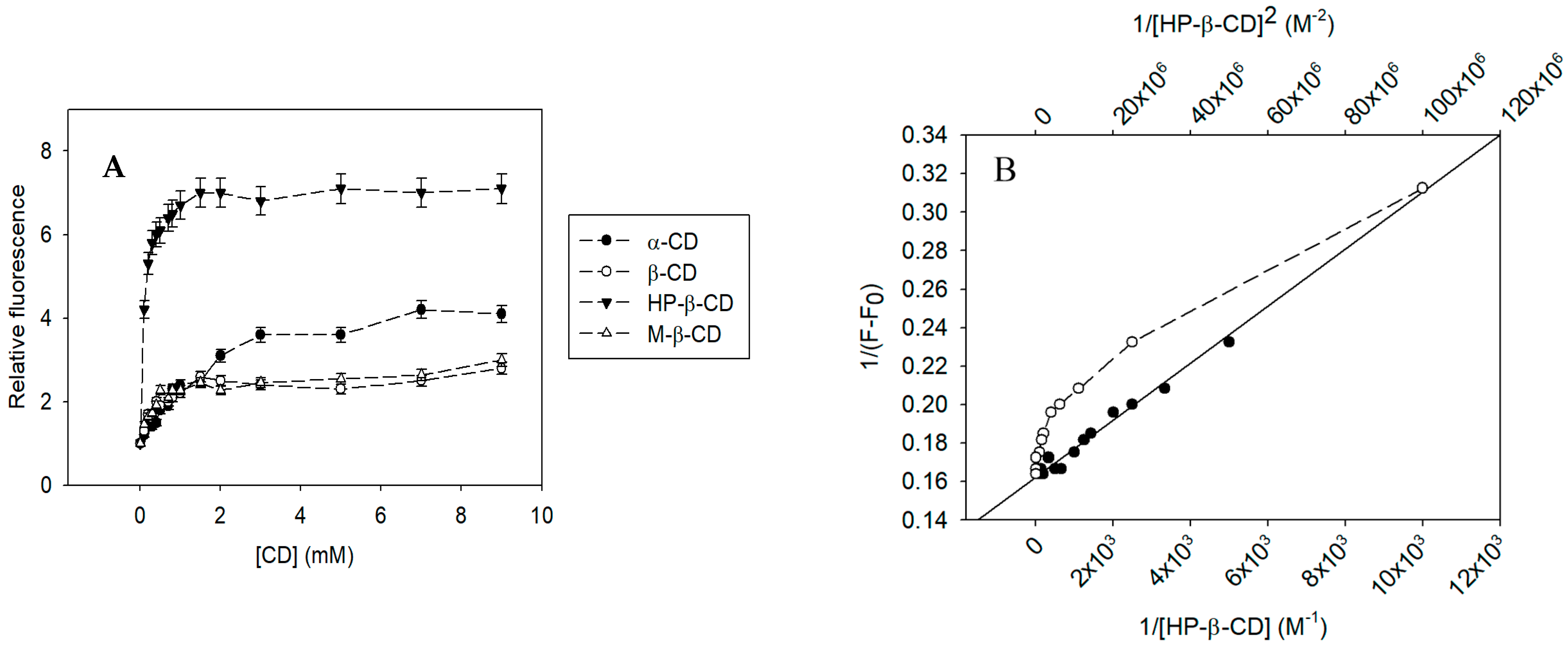
3.2. Physicochemical Study of the Inclusion Complexes with Pinostilbene
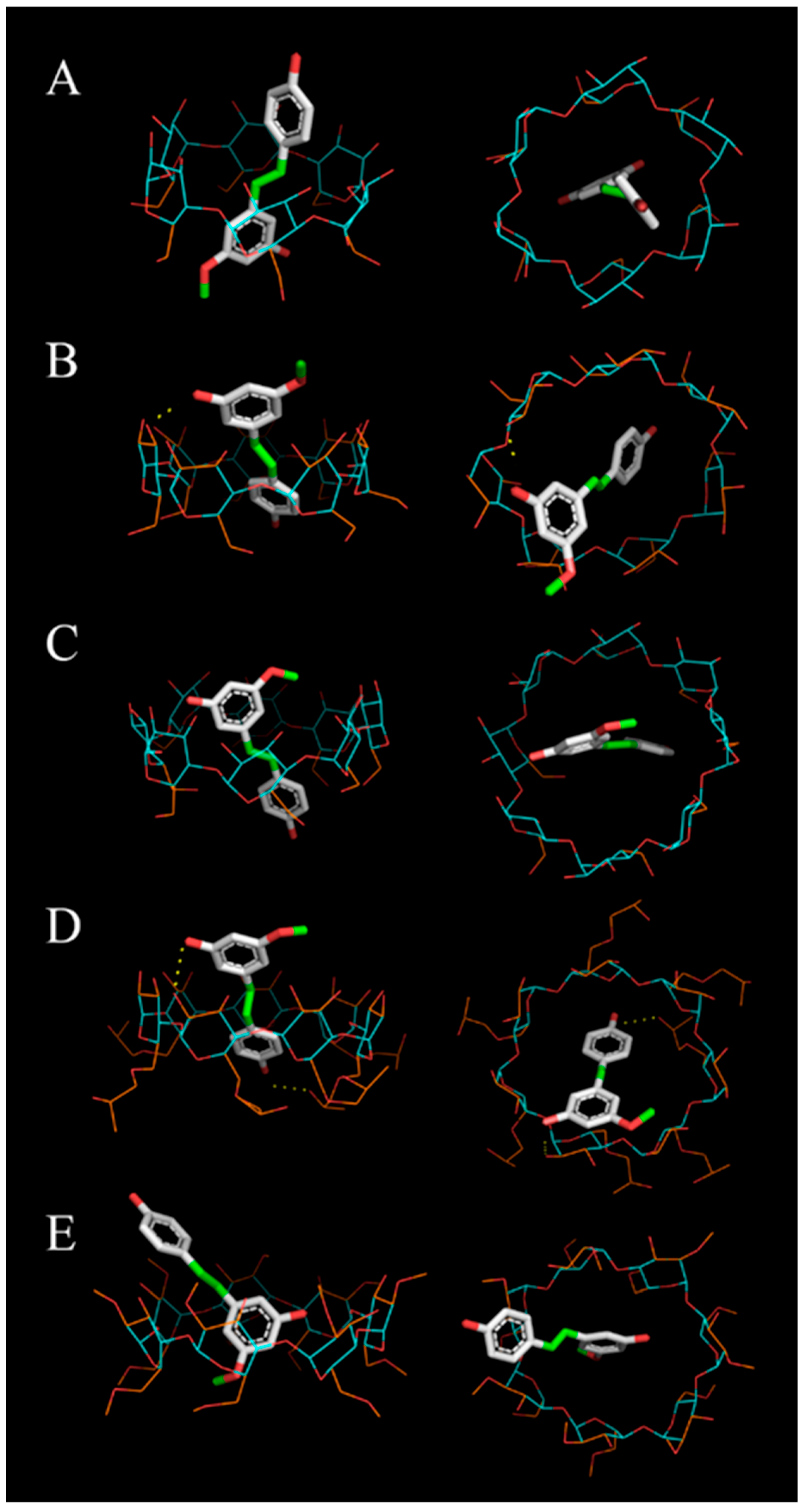
3.3. Molecular Docking of Pinostilbene in CDs
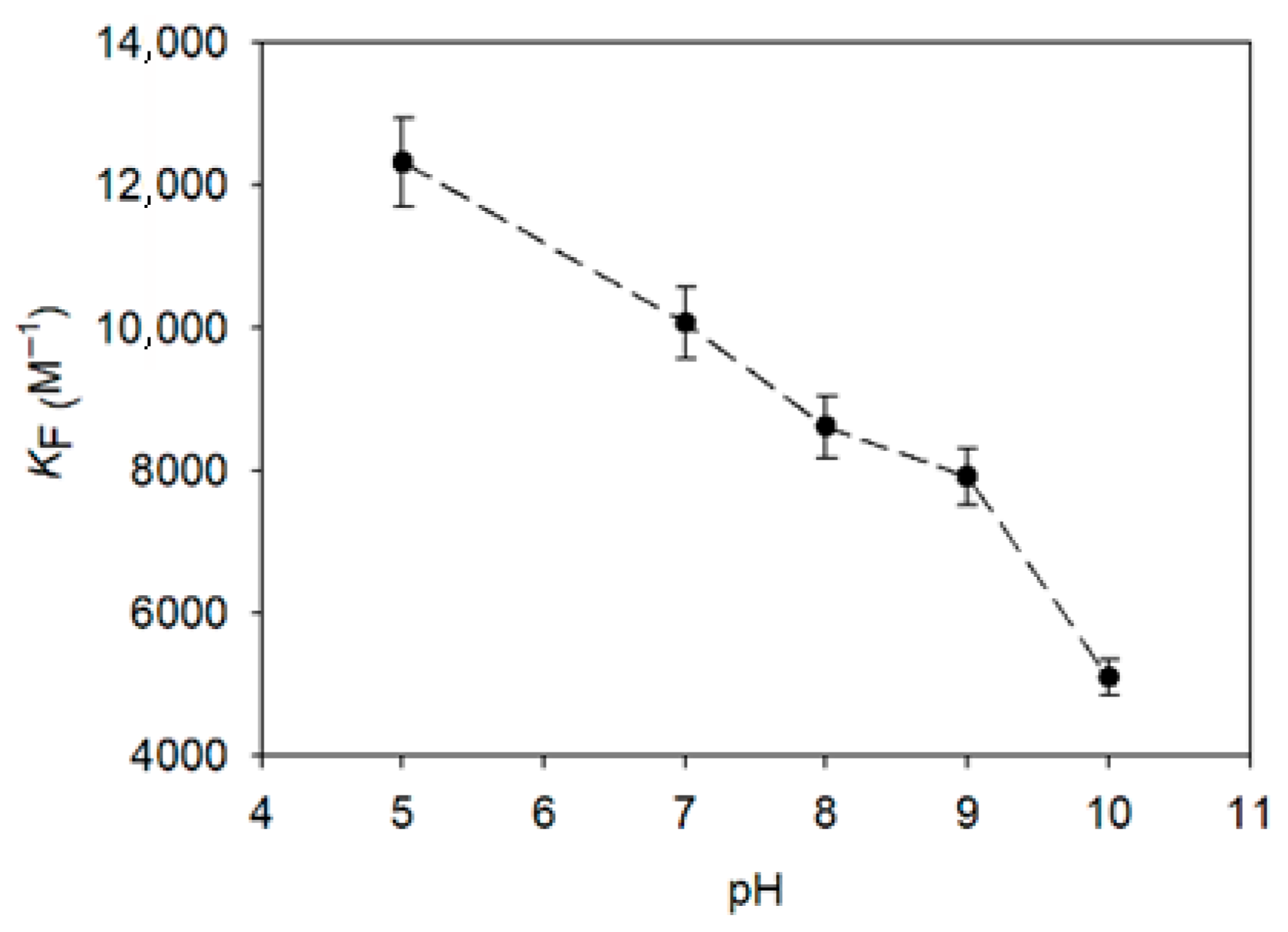
3.4. Influence of pH on Pinostilbene Encapsulation
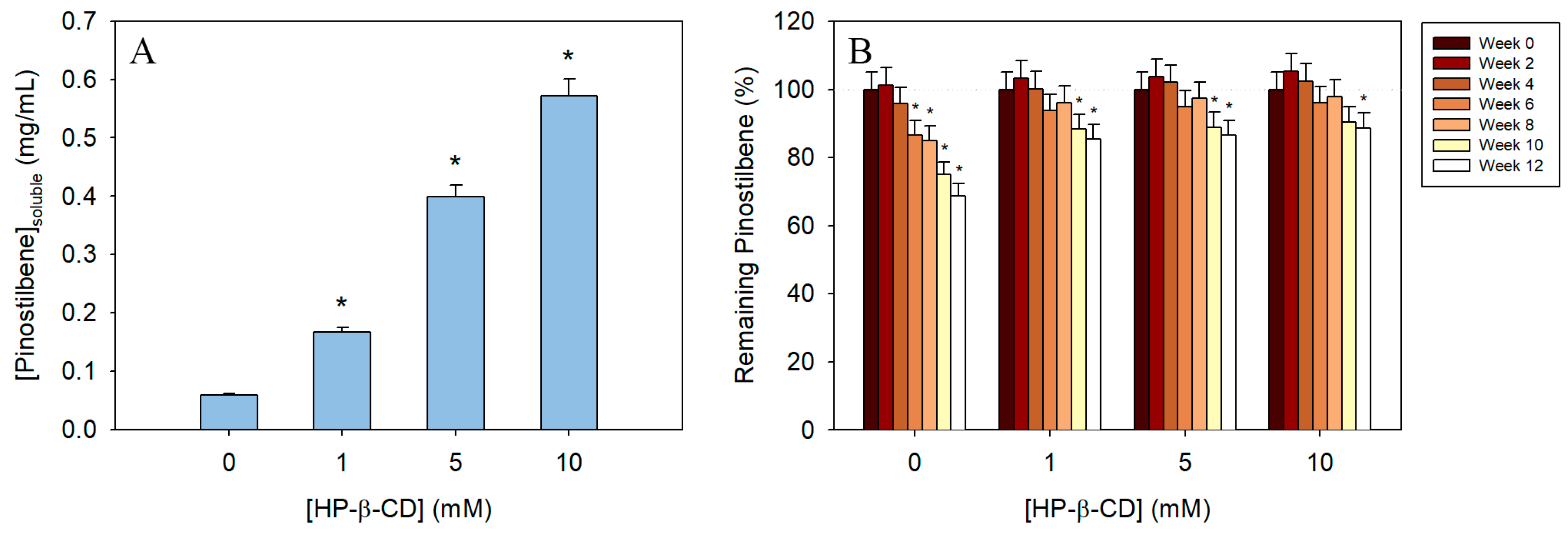
3.5. Solubility of Free and Encapsulated Pinostilbene
3.6. Time-Course Evaluation of the Stability of the Inclusion Complexes
3.7. DSC Analysis
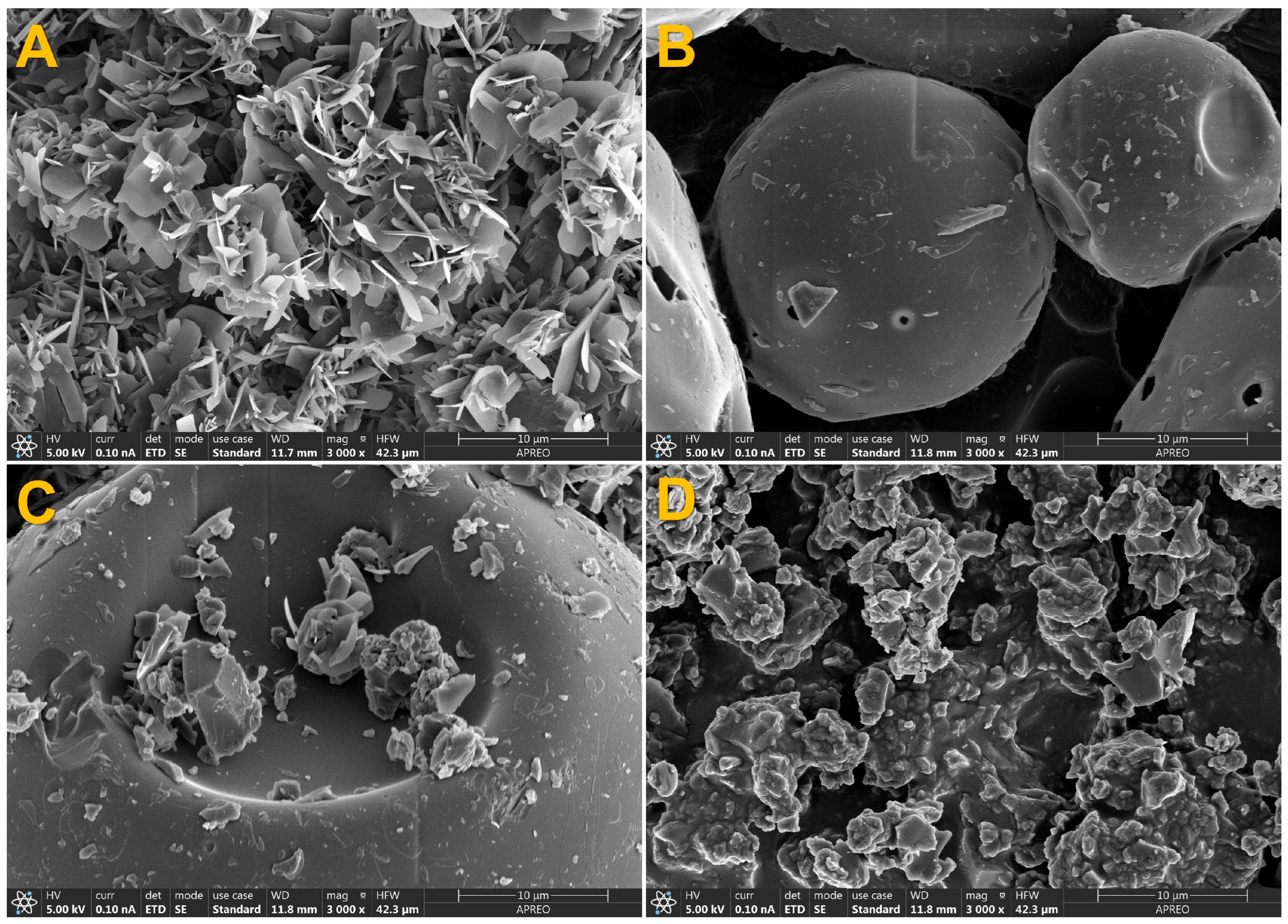
3.8. SEM Analysis
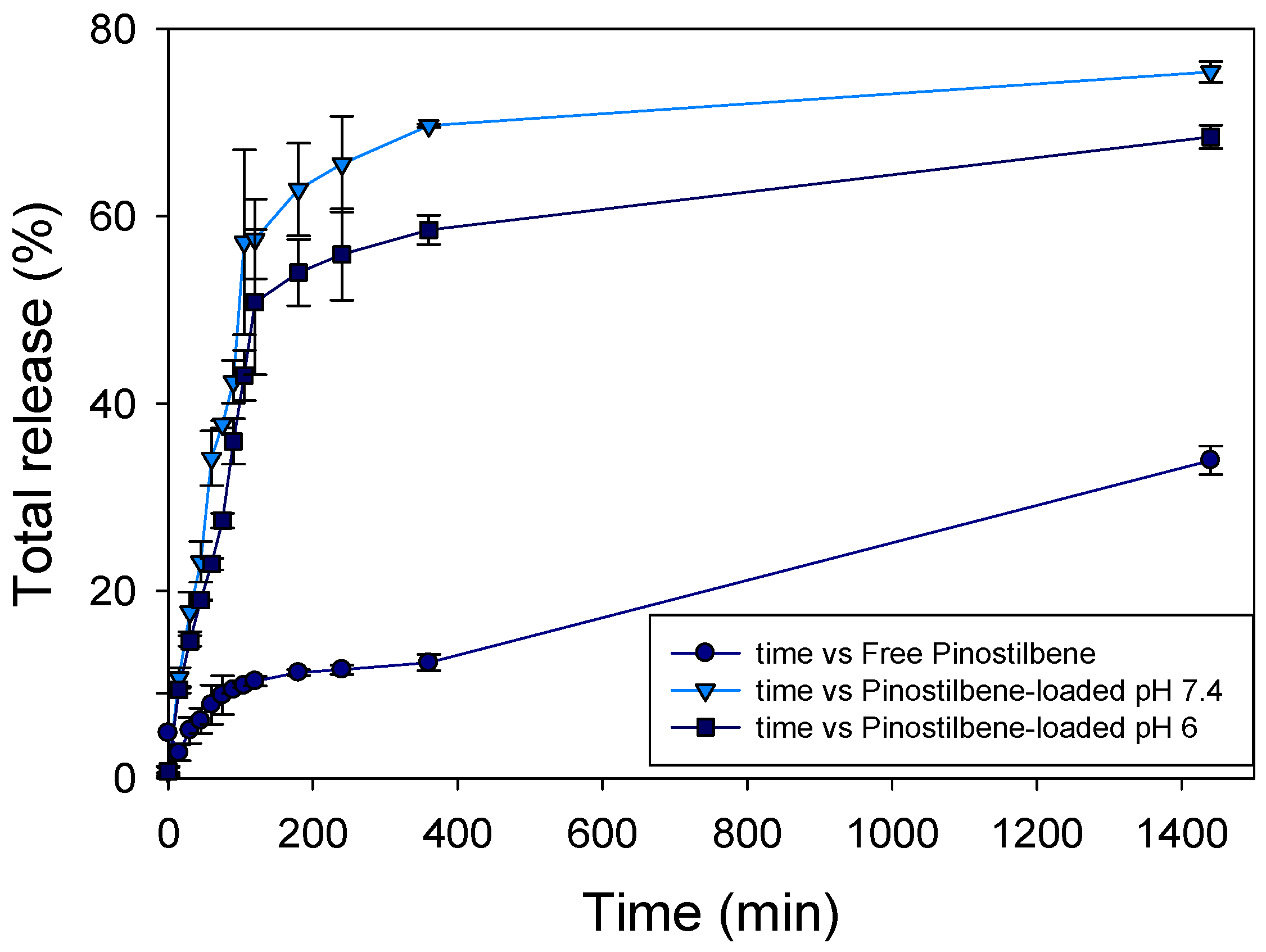
3.9. Releasing-Profile Comparison
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Gea-González, A.; Hernández-García, S.; Henarejos-Escudero, P.; Martínez-Rodríguez, P.; García-Carmona, F.; Gandía-Herrero, F. Polyphenols from traditional Chinese medicine and Mediterranean diet are effective against Aβ toxicity in vitro and in vivo in Caenorhabditis elegans. Food Funct. 2022, 13, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Han, S.H.; Jo, K.W.; Cho, Y.; Kim, K.-T. Pinostilbene inhibits full-length and splice variant of androgen receptor in prostate cancer. Sci. Rep. 2023, 13, 16663. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.P.; Chánique, A.M.; Martínez-Márquez, A.; Bru-Martínez, R.; Kourist, R.; Parra, L.P.; Schüller, A. Rational Design of Resveratrol O-methyltransferase for the Production of Pinostilbene. Int. J. Mol. Sci. 2021, 22, 4345. [Google Scholar] [CrossRef]
- Jeong, Y.J.; An, C.H.; Woo, S.G.; Jeong, H.J.; Kim, Y.-M.; Park, S.-J.; Yoon, B.D.; Kim, C.Y. Production of pinostilbene compounds by the expression of resveratrol O-methyltransferase genes in Escherichia coli. Enzyme Microb. Technol. 2014, 54, 8–14. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Fujitaka, Y.; Doi, S.; Hamada, H.; Kuboki, A.; Kiriake, Y.; Iwaki, T.; Saikawa, T.; et al. Synthesis of Glycosides of Resveratrol, Pinostilbene, and Piceatannol by Bioconversion with Phytolacca americana. Nat. Prod. Commun. 2019, 2019, 1–3. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef]
- Zakova, T.; Rondevaldova, J.; Bernardos, A.; Landa, P.; Kokoska, L. The relationship between structure and in vitro antistaphylococcal effect of plant-derived stilbenes. Acta Microbiol. Immunol. Hung. 2018, 65, 467–476. [Google Scholar] [CrossRef]
- Leláková, V.; Šmejkal, K.; Jakubczyk, K.; Veselý, O.; Landa, P.; Václavík, J.; Bobáľ, P.; Pížová, H.; Temml, V.; Steinacher, T.; et al. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem. 2019, 285, 431–440. [Google Scholar] [CrossRef]
- Chao, J.; Li, H.; Cheng, K.-W.; Yu, M.-S.; Chang, R.C.-C.; Wang, M. Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. J. Nutr. Biochem. 2010, 21, 482–489. [Google Scholar] [CrossRef]
- Chung, Y.C.; Hyun, C.-G. Inhibitory effects of pinostilbene on adipogenesis in 3T3-L1 adipocytes: A study of possible mechanisms. Int. J. Mol. Sci. 2021, 22, 13446. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Chin, M.-C.; Lin, C.-C.; His, Y.-T.; Lo, Y.-S.; Chuang, Y.-C.; Chen, M.-K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell. Physiol. Biochem. 2018, 50, 911–923. [Google Scholar] [CrossRef]
- Staskiewicz, A.; Wong, E.; Tucker, M.; Farhin, R.; Park, J.; Saade, R.; Alkhazali, T.; Dang, T.; Wang, X. Cytotoxic and Apoptotic Effects of Pinostilbene and Bortezomib Combination Treatment on Human Multiple Myeloma Cells. Int. J. Mol. Sci. 2023, 24, 12590. [Google Scholar] [CrossRef]
- Jarosova, V.; Vesely, O.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of stilbenoids by human faecal microbiota. Molecules 2019, 24, 1155. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Figueiras, A.; Gallardo, E.; Nerín, C.; Domingues, F.C. Strategies to improve the solubility and stability of stilbene antioxidants: A comparative study between cyclodextrins and bile acids. Food Chem. 2014, 145, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.d.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.d.; Neves de Lima, Á.A. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; González-Ramón, A.; García-Carmona, F.; López-Nicolás, J.M. Recent advances in the treatment of Niemann pick disease type C: A mini-review. Int. J. Pharm. 2020, 584, 119440. [Google Scholar] [CrossRef]
- Matencio Durán, A.; Trotta, F.; Khazaei Monfared, Y.; Caldera, F.; López Nicolás, J.M. Cyclodextrin Derivatives for the Treatment of Gout and Hyperuricemia. WO2024133757A1, 27 June 2024. [Google Scholar]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Molecular encapsulation and bioactivity of gnetol, a resveratrol analogue, for use in foods. J. Sci. Food Agric. 2022, 102, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Orcajada, S.; Vidal-Sánchez, F.J.; Conesa, I.; Matencio, A.; López-Nicolás, J.M. Improvement of the Physicochemical Limitations of Rhapontigenin, a Cytotoxic Analogue of Resveratrol against Colon Cancer. Biomolecules 2023, 13, 1270. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Orcajada, S.; Vidal-Sánchez, F.J.; Conesa, I.; Escribano-Naharro, F.; Matencio, A.; López-Nicolás, J.M. Antiproliferative Effects in Colorectal Cancer and Stabilisation in Cyclodextrins of the Phytoalexin Isorhapontigenin. Biomedicines 2023, 11, 3023. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Navarro-Orcajada, S.; Conesa, I.; Munoz-Sanchez, I.; Laveda-Cano, L.; Cano-Yelo, D.; Garcia-Carmona, F.; López-Nicolás, J.M. Evaluation of juice and milk “food models” fortified with oxyresveratrol and β-Cyclodextrin. Food Hydrocoll. 2020, 98, 105250. [Google Scholar] [CrossRef]
- Caco-2 [Caco2]—HTB-37|ATCC. Available online: https://www.atcc.org/products/htb-37 (accessed on 27 August 2025).
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- PubChem Pinostilbene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5473050 (accessed on 27 August 2025).
- Ciclodextrinas (Jmol). Available online: https://water.lsbu.ac.uk/water/cycloh.html (accessed on 31 August 2025).
- van Aalten, D.M.; Bywater, R.; Findlay, J.B.; Hendlich, M.; Hooft, R.W.; Vriend, G. PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J. Comput. Aided Mol. Des. 1996, 10, 255–262. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Uberti, F.; Trotta, F.; Pagliaro, P.; Bisericaru, D.M.; Cavalli, R.; Ferrari, S.; Penna, C.; Matencio, A. Developing New Cyclodextrin-Based Nanosponges Complexes to Improve Vitamin D Absorption in an In Vitro Study. Int. J. Mol. Sci. 2023, 24, 5322. [Google Scholar] [CrossRef]
- Matencio, A.; Bisericaru, D.M.; Conesa, I.; Er-Rahmani, S.; Pedrazzo, A.R.; López-Nicolás, J.M.; Trotta, F. Study of progesterone complexation in cyclodextrins and cyclodextrin-based nanosponges as an example of solvent-free complexation. J. Drug Deliv. Sci. Technol. 2024, 98, 105893. [Google Scholar] [CrossRef]
- Bisericaru, D.M.; Pérez-Jiménez, M.; Romero-Muñoz, M.; Caldera, F.; Bordignon, S.; Trotta, F.; Matencio, A. Optimizing 6-benzylaminopurine for micropropagation: A cyclodextrin monomers and polymers approach. Carbohydr. Polym. 2025, 362, 123658. [Google Scholar] [CrossRef]
- England, C.G.; Miller, M.C.; Kuttan, A.; Trent, J.O.; Frieboes, H.B. Release kinetics of paclitaxel and cisplatin from two and three layered gold nanoparticles. Eur. J. Pharm. Biopharm. 2015, 92, 120–129. [Google Scholar] [CrossRef]
- Paul, S.; Mizuno, C.S.; Lee, H.J.; Zheng, X.; Chajkowisk, S.; Rimoldi, J.M.; Conney, A.; Suh, N.; Rimando, A.M. In vitro and in vivo studies on stilbene analogs as potential treatment agents for colon cancer. Eur. J. Med. Chem. 2010, 45, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Moreno, J.J. Resveratrol Analogs with Antioxidant Activity Inhibit Intestinal Epithelial Cancer Caco-2 Cell Growth by Modulating Arachidonic Acid Cascade. J. Agric. Food Chem. 2019, 67, 819–828. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; García-Carmona, F. Rapid, simple and sensitive determination of the apparent formation constants of trans-resveratrol complexes with natural cyclodextrins in aqueous medium using HPLC. Food Chem. 2008, 109, 868–875. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; Méndez-Cazorla, L.; García-Carmona, F. Physicochemical Study of the Complexation of Pterostilbene by Natural and Modified Cyclodextrins. J. Agric. Food Chem. 2009, 57, 5294–5300. [Google Scholar] [CrossRef]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Encapsulation of piceatannol, a naturally occurring hydroxylated analogue of resveratrol, by natural and modified cyclodextrins. Food Funct. 2016, 7, 2367–2373. [Google Scholar] [CrossRef]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. The inclusion complex of oxyresveratrol in modified cyclodextrins: A thermodynamic, structural, physicochemical, fluorescent and computational study. Food Chem. 2017, 232, 177–184. [Google Scholar] [CrossRef]
- Bethune, S.J.; Schultheiss, N.; Henck, J.-O. Improving the Poor Aqueous Solubility of Nutraceutical Compound Pterostilbene through Cocrystal Formation. Cryst. Growth Des. 2011, 11, 2817–2823. [Google Scholar] [CrossRef]
- Kershaw, J.; Kim, K.-H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef]
- Suzuki, Y.; Muangnoi, C.; Thaweesest, W.; Teerawonganan, P.; Bhuket, P.R.N.; Titapiwatanakun, V.; Yoshimura-Fujii, M.; Sritularak, B.; Likhitwitayawuid, K.; Rojsitthisak, P. Exploring novel cocrystalline forms of oxyresveratrol to enhance aqueous solubility and permeability across a cell monolayer. Biol. Pharm. Bull. 2019, 42, 1004–1012. [Google Scholar] [CrossRef]
- Qiu, Y.; Lai, J.; Zhang, Y.; Fang, S.; Guo, Z.; Liang, X. Improved Bioavailability of Stilbenes from Cajanus cajan (L.) Millsp. Leaves Achieved by Hydroxypropyl-β-Cyclodextrin Inclusion: Preparation, Characterization and Pharmacokinetic Assessment. Molecules 2025, 30, 2526. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Hernández-García, S.; García-Carmona, F.; López-Nicolás, J.M. An integral study of cyclodextrins as solubility enhancers of α-methylstilbene, a resveratrol analogue. Food Funct. 2017, 8, 270–277. [Google Scholar] [CrossRef] [PubMed]

| CD | KF (M−1) | R2 | |
|---|---|---|---|
| 1:1 Model | 1:2 Model | ||
| α-CD | 633.62 ± 31.68 | 0.972 | 0.821 |
| β-CD | 3503.62 ± 175.18 | 0.949 | 0.734 |
| γ-CD | - | - | - |
| HP-β-CD | 10,074.45 ± 503.72 | 0.976 | 0.868 |
| M-β-CD | 4870.24 ± 243.51 | 0.955 | 0.782 |
| Conditions | K | R2 |
|---|---|---|
| Free pinostilbene | 0.581 | 0.891 |
| Pinostilbene—loaded pH 7.4 | 4.546 | 0.984 |
| Pinostilbene—loaded pH 6 | 3.716 | 0.986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conesa, I.; Navarro-Orcajada, S.; Vidal-Sánchez, F.J.; Torralba-Antón, E.; Carrión-Espinosa, M.; Matencio, A.; López-Nicolás, J.M. Pinostilbene as a Potential Cytotoxic Agent in Cancer Cell Lines: Improvement of Solubility and Stability by Cyclodextrin Encapsulation. Pharmaceutics 2025, 17, 1219. https://doi.org/10.3390/pharmaceutics17091219
Conesa I, Navarro-Orcajada S, Vidal-Sánchez FJ, Torralba-Antón E, Carrión-Espinosa M, Matencio A, López-Nicolás JM. Pinostilbene as a Potential Cytotoxic Agent in Cancer Cell Lines: Improvement of Solubility and Stability by Cyclodextrin Encapsulation. Pharmaceutics. 2025; 17(9):1219. https://doi.org/10.3390/pharmaceutics17091219
Chicago/Turabian StyleConesa, Irene, Silvia Navarro-Orcajada, Francisco José Vidal-Sánchez, Elena Torralba-Antón, Marta Carrión-Espinosa, Adrián Matencio, and José Manuel López-Nicolás. 2025. "Pinostilbene as a Potential Cytotoxic Agent in Cancer Cell Lines: Improvement of Solubility and Stability by Cyclodextrin Encapsulation" Pharmaceutics 17, no. 9: 1219. https://doi.org/10.3390/pharmaceutics17091219
APA StyleConesa, I., Navarro-Orcajada, S., Vidal-Sánchez, F. J., Torralba-Antón, E., Carrión-Espinosa, M., Matencio, A., & López-Nicolás, J. M. (2025). Pinostilbene as a Potential Cytotoxic Agent in Cancer Cell Lines: Improvement of Solubility and Stability by Cyclodextrin Encapsulation. Pharmaceutics, 17(9), 1219. https://doi.org/10.3390/pharmaceutics17091219







