Box-Behnken Design Assisted Optimization and Characterization of Chitosan Film for Simultaneous Topical Delivery of Ascorbic Acid and Metronidazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Response Surface Methodology (RSM)
2.3. Film Preparation
2.4. Mechanical Test
2.5. Surface pH Measurement
2.6. Viscosity Measurement
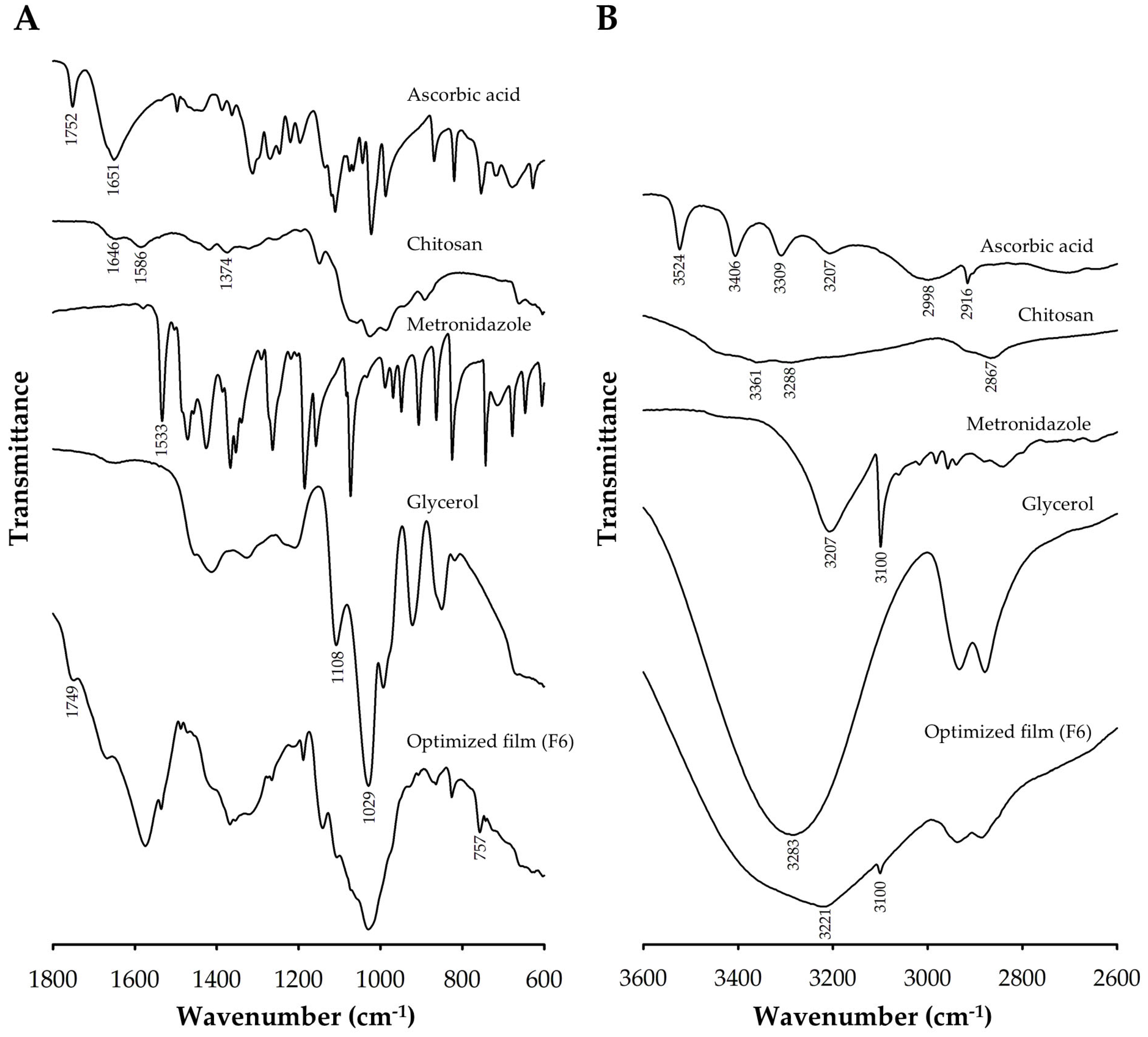
2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
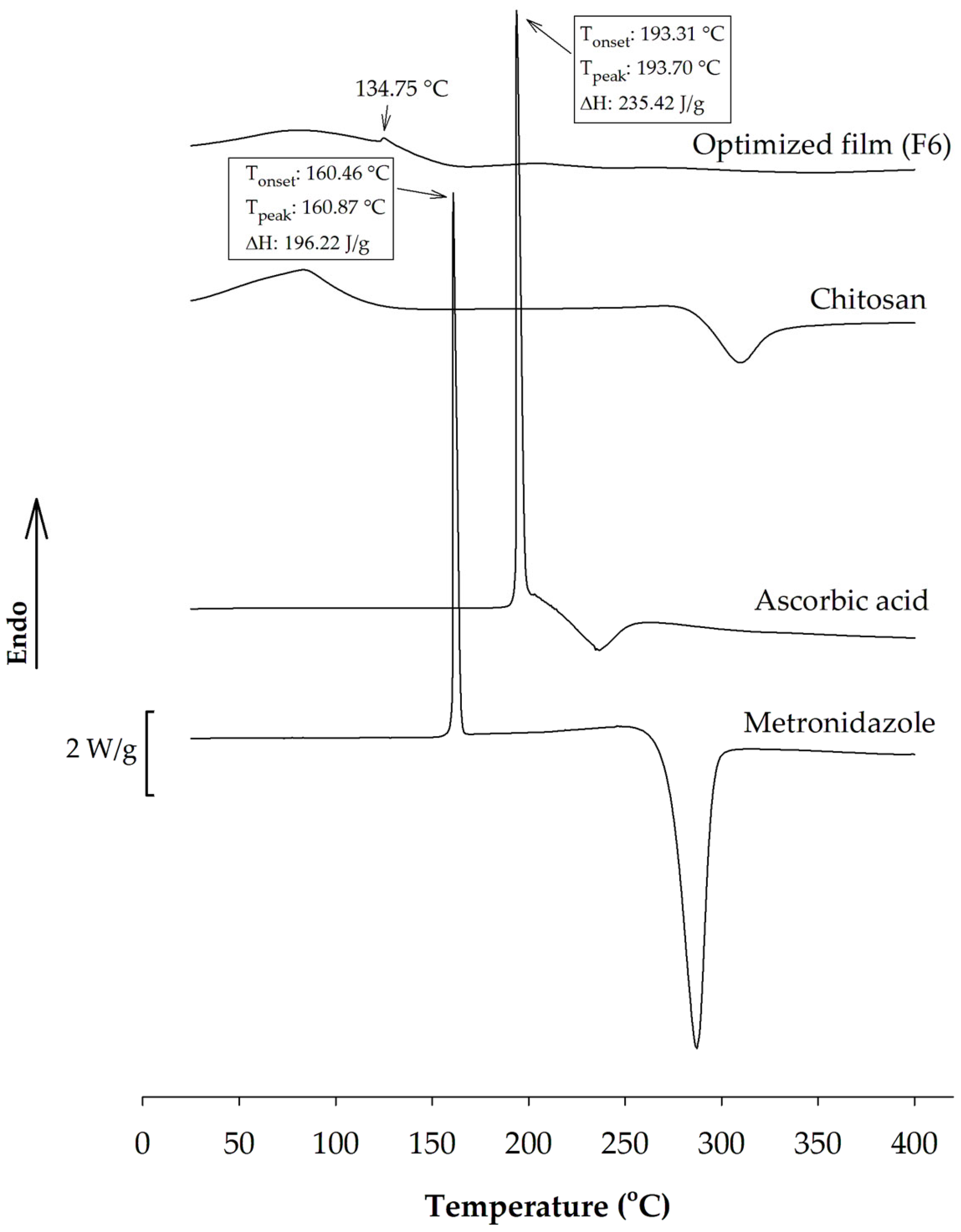
2.8. Differential Scanning Calorimetry (DSC)
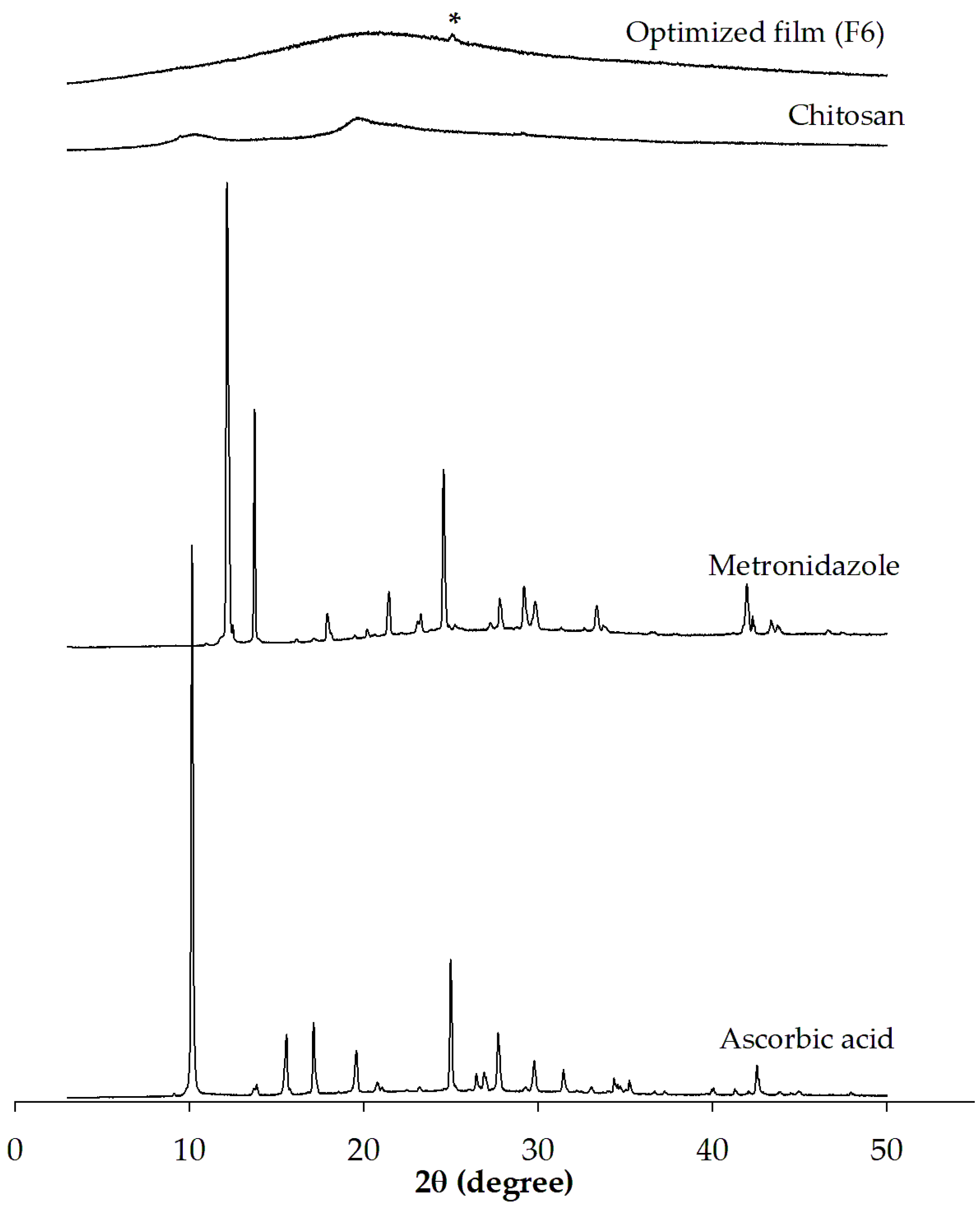
2.9. Powder X-Ray Diffraction (PXRD) Analysis
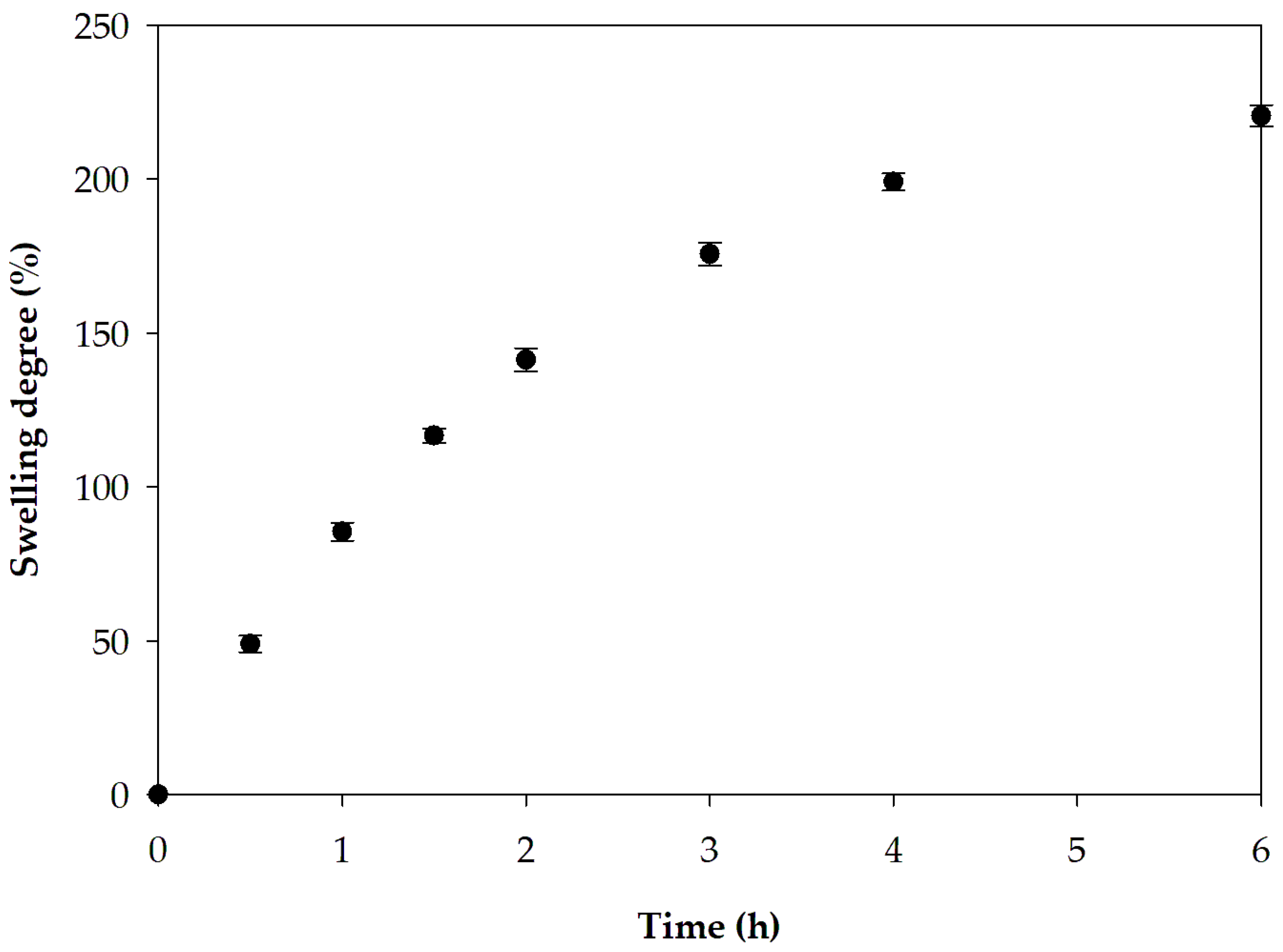
2.10. Swelling Study
2.11. Drug Content Study
2.12. In Vitro Release Study
3. Results and Discussion
3.1. RSM Analysis and Optimization
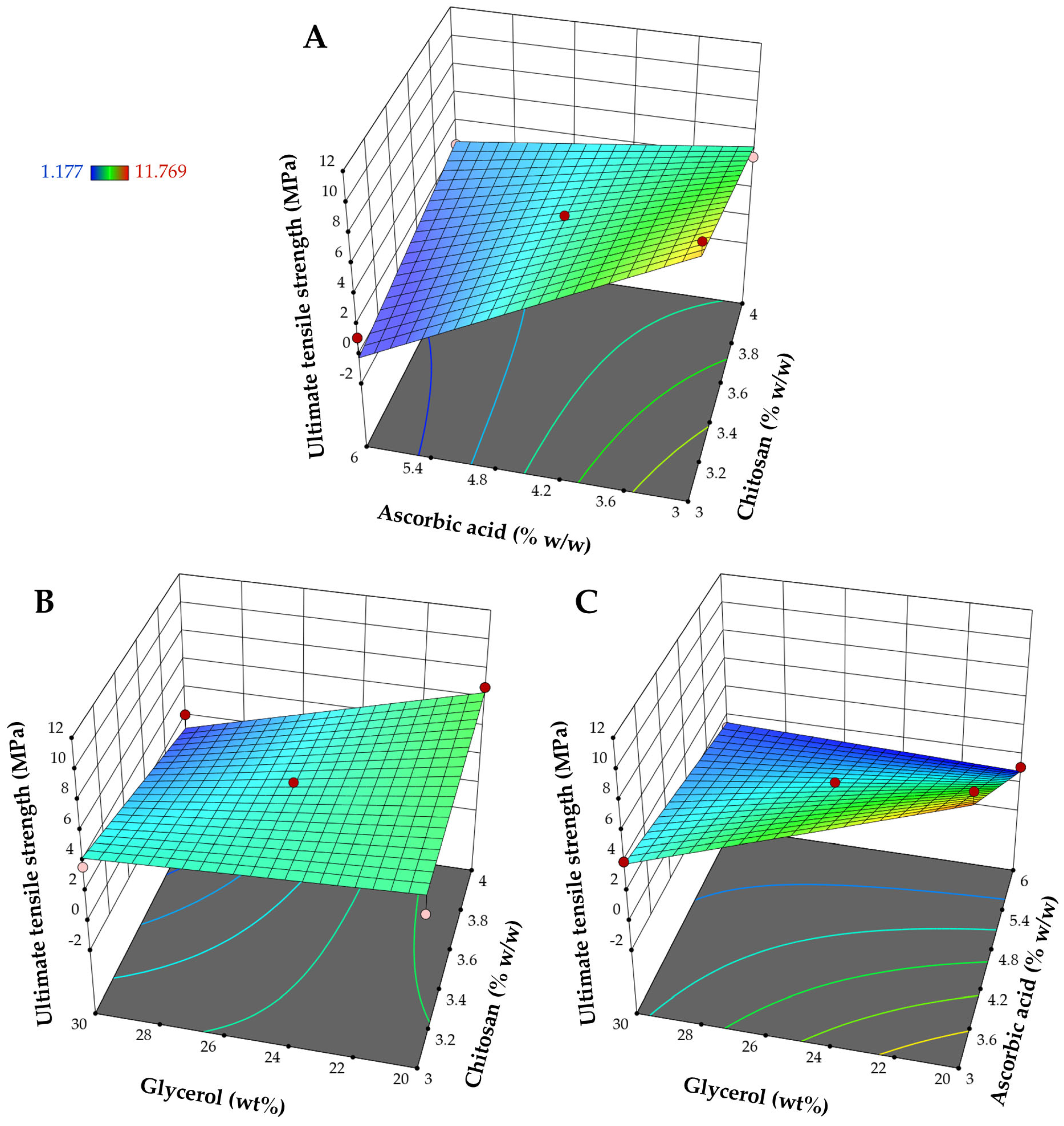
3.1.1. Influence on Ultimate Tensile Strength (Y1)
3.1.2. Influence on Elongation at Break (Y2)
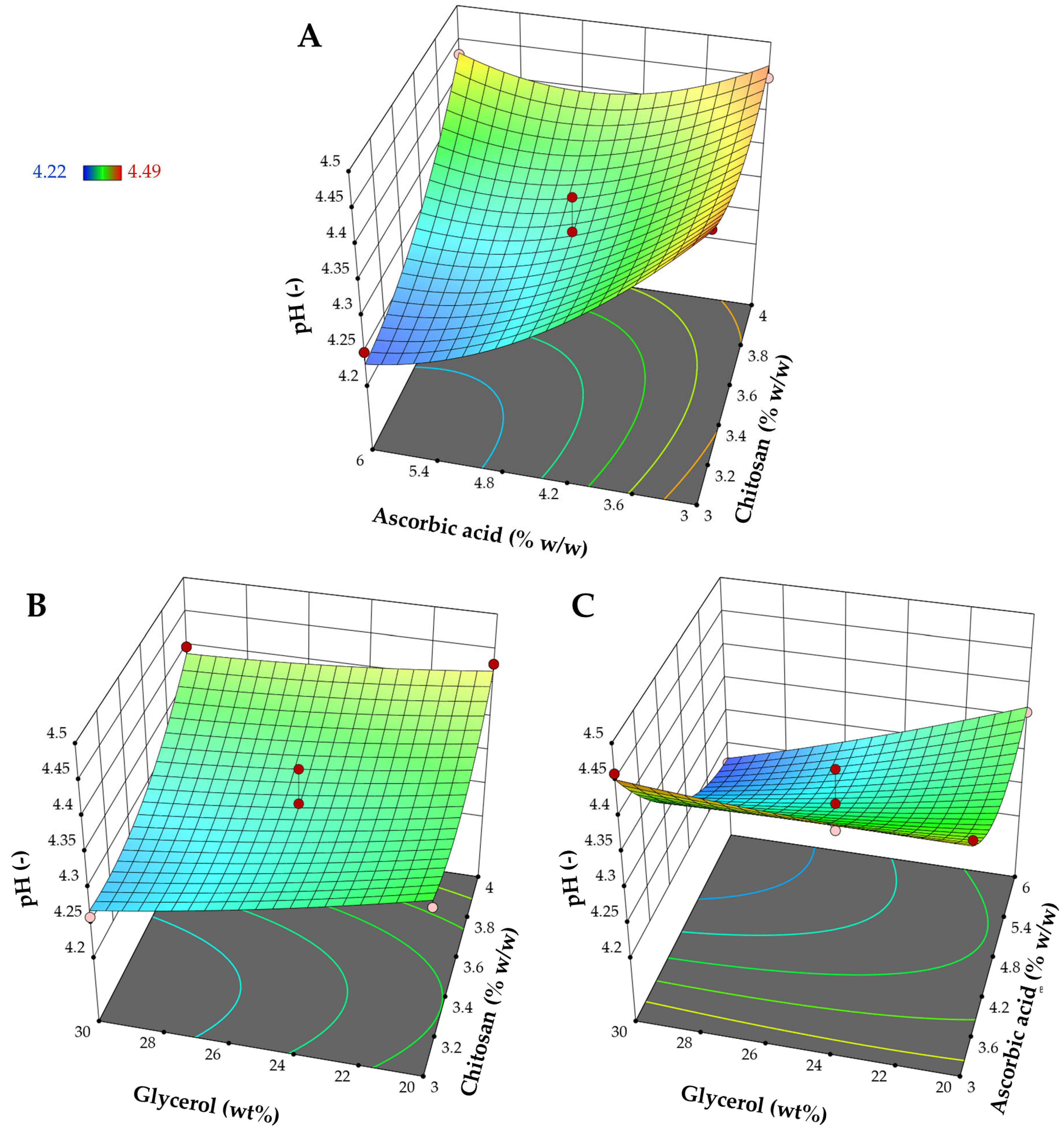
3.1.3. Influence on pH (Y3)
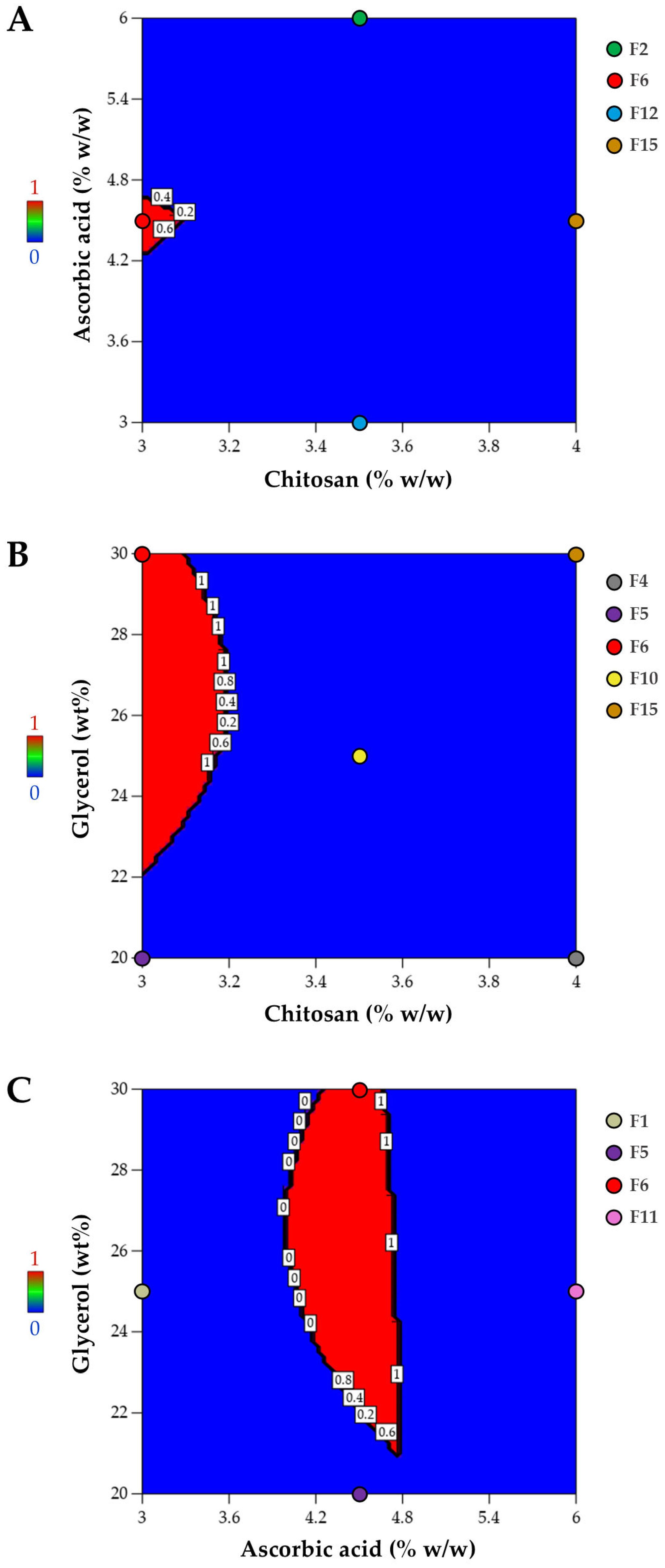
3.1.4. Optimized Composition for Film Preparation
3.2. Characterization of Film with Optimized Composition
3.2.1. FTIR Analysis
3.2.2. DSC Analysis
3.2.3. PXRD Analysis
3.2.4. Swelling Capacity
3.2.5. Drug Content
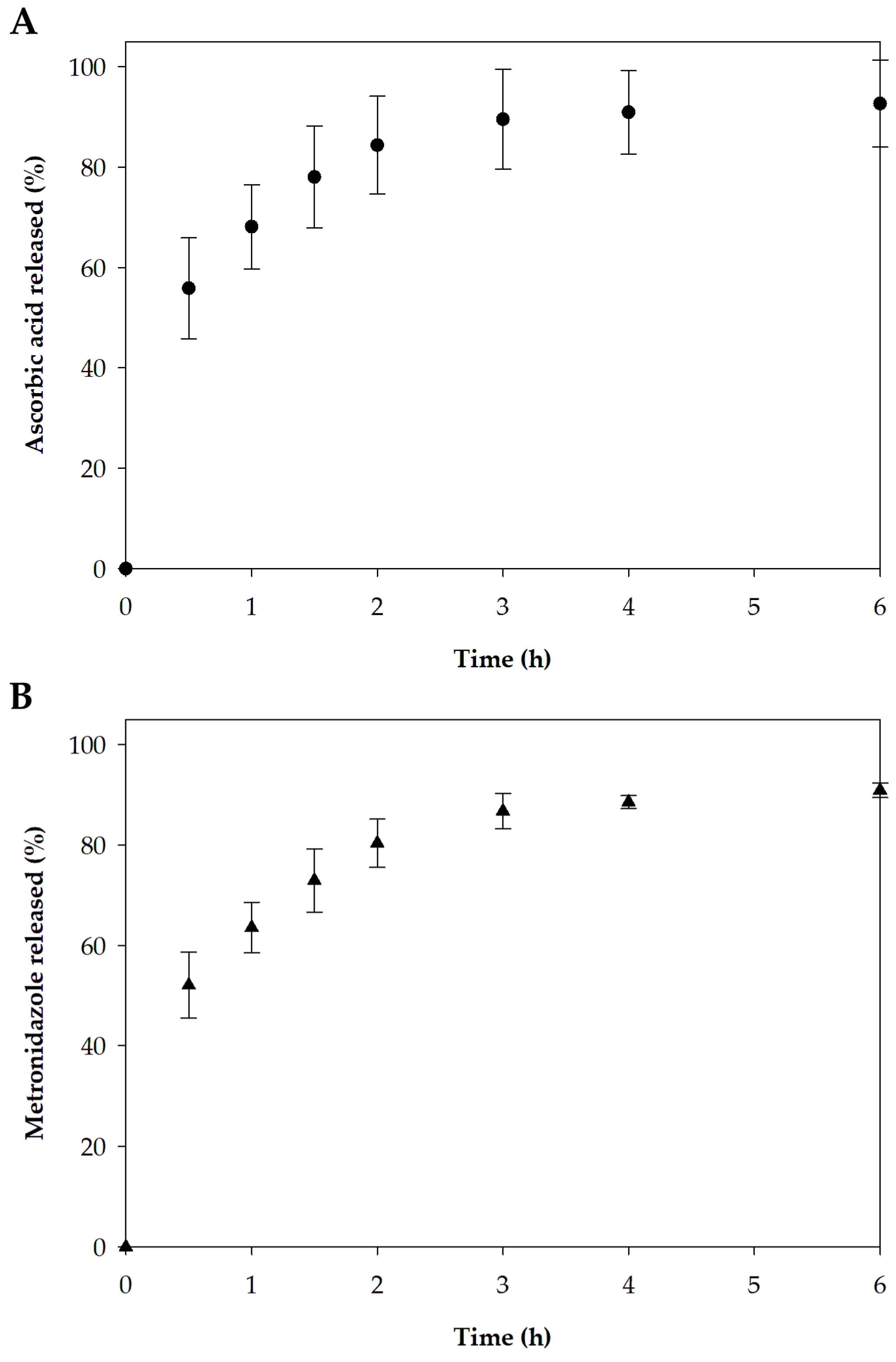
3.2.6. In Vitro Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celebioglu, A.; Uyar, T. Green synthesis of polycyclodextrin/drug inclusion complex nanofibrous hydrogels: pH-dependent release of acyclovir. ACS Appl. Bio Mater. 2023, 6, 3798–3809. [Google Scholar] [CrossRef]
- Voss, G.T.; Gularte, M.S.; Vogt, A.G.; Giongo, J.L.; Vaucher, R.A.; Echenique, J.V.Z.; Soares, M.P.; Luchese, C.; Wilhelm, E.A.; Fajardo, A.R. Polysaccharide-based film loaded with vitamin C and propolis: A promising device to accelerate diabetic wound healing. Int. J. Pharm. 2018, 552, 340–351. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-derived polysaccharides and their therapeutic potential in wound healing application—A review. Int. J. Biol. Macromol. 2023, 253, 127331. [Google Scholar] [CrossRef] [PubMed]
- Al-Achi, A.; Gupta, M.R.; Stagner, W.C. Dispersed system product design. In Integrated Pharmaceutics: Applied Preformulation, Product Design, and Regulatory Science; John Wiley and Sons: Hoboken, NJ, USA, 2022; pp. 298–335. [Google Scholar]
- Parhi, R.; Sahoo, S.K.; Das, A. Applications of polysaccharides in topical and transdermal drug delivery—A recent update of literature. Braz. J. Pharm. Sci. 2023, 58, e20802. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Jaber, N.; Al-Remawi, M.; Al Odwan, G.; Qinna, N. Chitosan-biotin topical film: Preparation and evaluation of burn wound healing activity. Pharm. Dev. Technol. 2022, 27, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Maneewattanapinyo, P.; Pichayakorn, W.; Monton, C.; Dangmanee, N.; Wunnakup, T.; Suksaeree, J. Effect of ionic liquid on silver-nanoparticle-complexed Ganoderma applanatum and its topical film formulation. Pharmaceutics 2023, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Meedecha, P.; Srisang, N.; Eawsakul, K.; Ongtanasup, T.; Tambunlertchai, S.; Sokjabok, S.; Chungcharoen, T.; Srisang, S.; Limmun, W. Preparation and evaluation of blend polymer films for wound dressing using vancomycin-loaded polycaprolactone and carboxymethyl cellulose via crosslinking methods: Effect of mechanical strength, antibacterial activity, and cytotoxicity. J. Mech. Behav. Biomed. Mater. 2024, 151, 106339. [Google Scholar] [CrossRef]
- Yu, Y.; Su, Z.; Peng, Y.; Zhong, Y.; Wang, L.; Xin, M.; Li, M. Recent advances in modifications, biotechnology, and biomedical applications of chitosan-based materials: A review. Int. J. Biol. Macromol. 2025, 289, 138772. [Google Scholar] [CrossRef]
- Sorgun, S.; Küçük, H.; Birgin, K. Some distance-based topological indices of certain polysaccharides. J. Mol. Struct. 2022, 1250, 131716. [Google Scholar] [CrossRef]
- Zhang, R.; Chang, S.J.; Jing, Y.; Wang, L.; Chen, C.J.; Liu, J.T. Application of chitosan with different molecular weights in cartilage tissue engineering. Carbohydr. Polym. 2023, 314, 120890. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhang, Y.; Wang, S.; Zhao, J.; Wang, T. Glutathione depletion-based pH-responsive injectable hydrogels for synergistic treatment of colon tumor. Int. J. Biol. Macromol. 2025, 297, 139557. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Jia, L.; Yang, R.; Tao, H.; Cui, H.; Lin, L.; Khojah, E.; Bushnaq, T.; Shi, C. Fabrication of guar gum/chitosan edible films reinforced with orange essential oil nanoemulsion for cheese preservation. Int. J. Biol. Macromol. 2025, 285, 138285. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films: Effect of the type of solvent acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Janik, W.; Ledniowska, K.; Nowotarski, M.; Kudła, S.; Knapczyk-Korczak, J.; Stachewicz, U.; Nowakowska-Bogdan, E.; Sabura, E.; Nosal-Kovalenko, H.; Turczyn, R.; et al. Chitosan-based films with alternative eco-friendly plasticizers: Preparation, physicochemical properties and stability. Carbohydr. Polym. 2023, 301, 120277. [Google Scholar] [CrossRef] [PubMed]
- Hon, S.L. Vitamin C (ascorbic acid). In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 805–807. [Google Scholar]
- Tan, W.; Dong, F.; Zhang, J.; Zhao, X.; Li, Q.; Guo, Z. Physical and antioxidant properties of edible chitosan ascorbate films. J. Agric. Food Chem. 2019, 67, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Liping, L.; Kexin, L.; Huipu, D.; Jia, L.; Jie, Z. Study on preparation of a chitosan/vitamin C complex and its properties in cosmetics. Nat. Prod. Commun. 2020, 15, 1934578X20946876. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.B.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Utomo, E.; Wardoyo, L.A.H.; Larrañeta, E.; Donnelly, R.F. Metronidazole nanosuspension loaded dissolving microarray patches: An engineered composite pharmaceutical system for the treatment of skin and soft tissue infection. Biomater. Adv. 2022, 140, 213073. [Google Scholar] [CrossRef]
- Sampaio, C.; Biondo-Simões, M.; Trindade, L.; Olandowski, M.; Matias, J. Metronidazole concentration in the bloodstream following its topical application, at different concentration levels, on experimental skin wounds during healing by secondary intention. Acta Cir. Bras. 2019, 34, e20190010000004. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Phan, N.H.T.; Trinh, C.D.; Tran, T.V.; Pham, B.T.T.; Quynh, B.T.P.; Phung, T.K. Glycerol-plasticized chitosan film for the preservation of orange. J. Food Saf. 2022, 42, e12943. [Google Scholar] [CrossRef]
- Kruželák, J.; Hložeková, K.; Kvasničáková, A.; Džuganová, M.; Chodák, I.; Hudec, I. Application of plasticizer glycerol in lignosulfonate-filled rubber compounds based on SBR and NBR. Materials 2023, 16, 635. [Google Scholar] [CrossRef]
- Fernandes, A.S.; de Souza Ferreira, S.B.; Bruschi, M.L. Design as strategy for evaluation of the mechanical properties of binary mixtures composed of poly(methyl vinyl ether-alt-maleic anhydride) and Pluronic F127 for biomedical applications. J. Mech. Behav. Biomed. Mater. 2023, 138, 105608. [Google Scholar] [CrossRef] [PubMed]
- Kraisit, P.; Hirun, N.; Limpamanoch, P.; Sawaengsuk, Y.; Janchoochai, N.; Manasaksirikul, O.; Limmatvapirat, S. Effect of Cremophor RH40, hydroxypropyl methylcellulose, and mixing speed on physicochemical properties of films containing nanostructured lipid carriers loaded with furosemide using the Box–Behnken design. Polymers 2024, 16, 1605. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Cui, M.; Kang, K.; Park, B.; Son, Y.; Khim, E.; Jang, M.; Khim, J. Application of Box-Behnken design with response surface methodology for modeling and optimizing ultrasonic oxidation of arsenite with H2O2. Open Chem. 2014, 12, 164–172. [Google Scholar] [CrossRef]
- Beg, S.; Akhter, S. Box–Behnken designs and their applications in pharmaceutical product development. In Design of Experiments for Pharmaceutical Product Development: Volume I: Basics and Fundamental Principles; Beg, S., Ed.; Springer: Singapore, 2021; pp. 77–85. [Google Scholar]
- Mahajan, H.S.; Deshmukh, S.R. Development and evaluation of gel-forming ocular films based on xyloglucan. Carbohydr. Polym. 2015, 122, 243–247. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of surfactants and drug load on physico-mechanical and dissolution properties of nanocrystalline tadalafil-loaded oral films. Eur. J. Pharm. Sci. 2017, 109, 372–380. [Google Scholar] [CrossRef]
- Agwa, M.M.; Sabra, S.; Atwa, N.A.; Dahdooh, H.A.; Lithy, R.M.; Elmotasem, H. Potential of frankincense essential oil-loaded whey protein nanoparticles embedded in frankincense resin as a wound healing film based on green technology. J. Drug Deliv. Sci. Technol. 2022, 71, 103291. [Google Scholar] [CrossRef]
- Manshor, N.M.; Jai, J.; Hamzah, F.; Yusof, N.M. Rheological properties of cassava starch film forming solution with kaffir lime oil. Int. J. Recent Technol. Eng. 2019, 8, 6781–6786. [Google Scholar] [CrossRef]
- Voss, G.T.; Gularte, M.S.; de Oliveira, R.L.; Luchese, C.; Fajardo, A.R.; Wilhelm, E.A. Biopolymeric films as delivery vehicles for controlled release of hydrocortisone: Promising devices to treat chronic skin diseases. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111074. [Google Scholar] [CrossRef]
- Silva, C.N.S.; Cruz, M.V.; Fernandes, K.F.; Batista, K.A. Production of anti-inflammatory films based on cashew gum polysaccharide and polyvinyl alcohol for wound dressing applications. 3Biotech 2023, 13, 299. [Google Scholar] [CrossRef]
- Ghorbani, M.; Hassani, N.; Raeisi, M. Development of gelatin thin film reinforced by modified gellan gum and naringenin-loaded zein nanoparticle as a wound dressing. Macromol. Res. 2022, 30, 397–405. [Google Scholar] [CrossRef]
- Suksaeree, J.; Maneewattanapinyo, P.; Panrat, K.; Pichayakorn, W.; Monton, C. Solvent-cast polymeric films from pectin and Eudragit® NE 30D for transdermal drug delivery systems. J. Polym. Environ. 2021, 29, 3174–3184. [Google Scholar] [CrossRef]
- Mady, O.; Hussien, S.; Abdelkader, D.H.; elZahaby, E. Metoclopramide loaded buccal films for potential treatment of migraine symptoms: In vitro and in vivo study. Pharm. Dev. Technol. 2023, 28, 650–659. [Google Scholar] [CrossRef]
- Hirun, N.; Tantishaiyakul, V.; Sangfai, T.; Rugmai, S.; Soontaranon, S. Nano-structure, phase transition and morphology of gallic acid and xyloglucan hydrogel. Polym. Bull. 2016, 73, 2211–2226. [Google Scholar] [CrossRef]
- Sampaopan, Y.; Suksaeree, J. Formulation development and pharmaceutical evaluation of Lysiphyllum strychnifolium topical patches for their anti-inflammatory potential. AAPS PharmSciTech 2022, 23, 116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Getti, G.; Boateng, J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv. Transl. Res. 2018, 8, 1751–1768. [Google Scholar] [CrossRef]
- Kumar, R. Modified mix design and statistical modelling of lightweight concrete with high volume micro fines waste additive via the Box-Behnken design approach. Cem. Concr. Compos. 2020, 113, 103706. [Google Scholar] [CrossRef]
- Abla, K.K.; Mneimneh, A.T.; Allam, A.N.; Mehanna, M.M. Application of Box-Behnken design in the preparation, optimization, and in-vivo pharmacokinetic evaluation of oral tadalafil-loaded niosomal film. Pharmaceutics 2023, 15, 173. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Tananuwong, K.; Krochta, J.M. Whey protein film with oxygen scavenging function by incorporation of ascorbic acid. J. Food Sci. 2011, 76, E561–E568. [Google Scholar] [CrossRef]
- Kowalczyk, D. Biopolymer/candelilla wax emulsion films as carriers of ascorbic acid—A comparative study. Food Hydrocoll. 2016, 52, 543–553. [Google Scholar] [CrossRef]
- Da Matta, M.D., Jr.; Sarmento, S.B.S.; de Oliveira, L.M.; Zocchi, S.S. Mechanical properties of pea starch films associated with xanthan gum and glycerol. Starke 2011, 63, 274–282. [Google Scholar] [CrossRef]
- Edikresnha, D.; Suciati, T.; Munir, M.M.; Khairurrijal, K. Polyvinylpyrrolidone/cellulose acetate electrospun composite nanofibres loaded by glycerine and garlic extract with in vitro antibacterial activity and release behaviour test. RSC Adv. 2019, 9, 26351–26363. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, S.; Gao, S.; Muchakayala, R.; Liu, R.; Ma, Q. Mg-ion conducting gel polymer electrolyte membranes containing biodegradable chitosan: Preparation, structural, electrical and electrochemical properties. Polym. Test. 2017, 62, 278–286. [Google Scholar] [CrossRef]
- Son, T.W.; Kim, B.G.; Park, Y.M.; Lim, H.S.; Kwon, O.K. Effects of mixing ratio on the mechanical and thermal properties of polyelectrolyte complex film. Macromol. Res. 2006, 14, 267–271. [Google Scholar] [CrossRef]
- Do Amaral Sobral, P.J.; Gebremariam, G.; Drudi, F.; De Aguiar Saldanha Pinheiro, A.C.; Romani, S.; Rocculi, P.; Dalla Rosa, M. Rheological and viscoelastic properties of chitosan solutions prepared with different chitosan or acetic acid concentrations. Foods 2022, 11, 2692. [Google Scholar] [CrossRef]
- Brown, C.D.; Kreilgaard, L.; Nakakura, M.; Caram-Lelham, N.; Pettit, D.K.; Gombotz, W.R.; Hoffman, A.S. Release of PEGylated granulocyte-macrophage colony-stimulating factor from chitosan/glycerol films. J. Control. Release 2001, 72, 35–46. [Google Scholar] [CrossRef]
- Adekogbe, I.; Ghanem, A. Fabrication and characterization of DTBP-crosslinked chitosan scaffolds for skin tissue engineering. Biomaterials 2005, 26, 7241–7250. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Z.; Liu, J.; Dong, P.; Tian, F.; Li, F.; Meng, X. Electrospun kaolin-loaded chitosan/PEO nanofibers for rapid hemostasis and accelerated wound healing. Int. J. Biol. Macromol. 2022, 217, 998–1011. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.A.; Sim, H.J.; Lima, M.D.; Baughman, R.H.; Kim, S.J. Temperature-responsive tensile actuator based on multi-walled carbon nanotube yarn. Nanomicro Lett. 2016, 8, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of acidic pH on wound healing in vivo: A novel perspective for wound treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef]
- Patil, N.; Wairkar, S. Chitosan and α-cellulose-based mupirocin topical film-forming spray: Optimization, in vitro characterization, antimicrobial studies and wound healing activity. Int. J. Biol. Macromol. 2024, 254, 127622. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and characterization of novel eggshell membrane-chitosan blend films for potential wound-care dressing: From waste to medicinal products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Carra, J.B.; Wessel, K.B.B.; Pereira, G.N.; Oliveira, M.C.; Pattini, P.M.T.; Masquetti, B.L.; Amador, I.R.; Bruschi, M.L.; Casagrande, R.; Georgetti, S.R.; et al. Bioadhesive polymeric films containing rhamnolipids, an innovative antimicrobial topical formulation. AAPS PharmSciTech 2024, 25, 177. [Google Scholar] [CrossRef]
- Mori, D.; Punit, R.; Ramesh, P.; Kiran, D.; Chavda, J. Preparation and optimization of multi-functional directly compressible excipient: An integrated approach of principal component analysis and design of experiments. Drug Dev. Ind. Pharm. 2020, 46, 2010–2021. [Google Scholar] [CrossRef]
- Krampe, R.; Visser, J.C.; Frijlink, H.W.; Breitkreutz, J.; Woerdenbag, H.J.; Preis, M. Oromucosal film preparations: Points to consider for patient centricity and manufacturing processes. Expert. Opin. Drug Deliv. 2016, 13, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Dahl, D.K.; Whitesell, A.N.; Sharma-Huynh, P.; Maturavongsadit, P.; Janusziewicz, R.; Fox, R.J.; Loznev, H.T.; Button, B.; Schorzman, A.N.; Zamboni, W.; et al. A mucoadhesive biodissolvable thin film for localized and rapid delivery of lidocaine for the treatment of vestibulodynia. Int. J. Pharm. 2022, 612, 121288. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in MRI. Appl. Nanosci. 2015, 5, 435–441. [Google Scholar] [CrossRef]
- Yohannan Panicker, C.; Tresa Varghese, H.; Philip, D. FT-IR, FT-Raman and SERS spectra of Vitamin C. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 802–804. [Google Scholar] [CrossRef]
- Liu, K.; Lin, X.; Chen, L.; Huang, L.; Cao, S. Dual-functional chitosan–methylisothiazolinone/microfibrillated cellulose biocomposites for enhancing antibacterial and mechanical properties of agar films. Cellulose 2014, 21, 519–528. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; de la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Scuota, S.; Bazzucchi, V.; Mura, P. Development and microbiological evaluation of chitosan and chitosan-alginate microspheres for vaginal administration of metronidazole. Int. J. Pharm. 2021, 598, 120375. [Google Scholar] [CrossRef]
- Utomo, E.; Domínguez-Robles, J.; Anjani, Q.K.; Picco, C.J.; Korelidou, A.; Magee, E.; Donnelly, R.F.; Larrañeta, E. Development of 3D-printed vaginal devices containing metronidazole for alternative bacterial vaginosis treatment. Int. J. Pharm. X 2023, 5, 100142. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, S.M.; Hammoudeh, A.Y.; Mahmoud, A. Identification and quantification of diethylene glycol contamination in glycerine raw material. Spectrosc. Lett. 2019, 52, 60–65. [Google Scholar] [CrossRef]
- Guo, Y.; Qu, Y.; Yu, J.; Song, L.; Chen, S.; Qin, Z.; Gong, J.; Zhan, H.; Gao, Y.; Zhang, J. A chitosan-vitamin C based injectable hydrogel improves cell survival under oxidative stress. Int. J. Biol. Macromol. 2022, 202, 102–111. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; El-Sawy, N.M.; Hegazy, E.S.A. Antioxidative properties of irradiated chitosan/vitamin C complex and their use as food additive for lipid storage. J. Appl. Polym. Sci. 2015, 132, 42105. [Google Scholar] [CrossRef]
- Halim, A.L.A.; Kamari, A. Active biopolymer film based on carboxymethyl cellulose and ascorbic acid for food preservation. AIP Conf. Proc. 2017, 1847, 040002. [Google Scholar]
- Kristó, K.; Módra, S.; Hornok, V.; Süvegh, K.; Ludasi, K.; Aigner, Z.; Kelemen, A.; Sovány, T.; Pintye-Hódi, K.; Regdon, G. Investigation of surface properties and free volumes of chitosan-based buccal mucoadhesive drug delivery films containing ascorbic acid. Pharmaceutics 2022, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Ioan, B.; Marieta, M.-P.; Irina, K.; Sorin, I.F. Spectroscopic investigation of the interaction between β-cyclodextrin and ascorbic acid. J. Phys. Conf. Ser. 2009, 182, 012004. [Google Scholar]
- Song, J.E.; Jun, S.H.; Ryoo, J.Y.; Kang, N.G. Formulation of ascorbic acid and betaine-based therapeutic deep eutectic system for enhanced transdermal delivery of ascorbic acid. Pharmaceutics 2024, 16, 687. [Google Scholar] [CrossRef]
- Szentmihályi, K.; Klébert, S.; May, Z.; Bódis, E.; Mohai, M.; Trif, L.; Feczkó, T.; Károly, Z. Immobilization of metronidazole on mesoporous silica materials. Pharmaceutics 2022, 14, 2332. [Google Scholar] [CrossRef]
- Xiaolin, T.; Dafeng, T.; Zhongyan, W.; Fengkui, M. Synthesis and evaluation of chitosan-vitamin C complexes. J. Appl. Polym. Sci. 2009, 114, 2986–2991. [Google Scholar] [CrossRef]
- Aresta, A.; Calvano, C.D.; Trapani, A.; Cellamare, S.; Zambonin, C.G.; De Giglio, E. Development and analytical characterization of vitamin(s)-loaded chitosan nanoparticles for potential food packaging applications. J. Nanopart. Res. 2013, 15, 1592. [Google Scholar] [CrossRef]
- Farshforoush, P.; Ghanbarzadeh, S.; Goganian, A.M.; Hamishehkar, H. Novel metronidazole-loaded hydrogel as a gastroretentive drug delivery system. Iran. Polym. J. 2017, 26, 895–901. [Google Scholar] [CrossRef]
- Gómez-Burgaz, M.; Torrado, G.; Torrado, S. Characterization and superficial transformations on mini-matrices made of interpolymer complexes of chitosan and carboxymethylcellulose during in vitro clarithromycin release. Eur. J. Pharm. Biopharm. 2009, 73, 130–139. [Google Scholar] [CrossRef]
- Khan, N.; Singh, A.K.; Saneja, A. Preparation, characterization, and antioxidant activity of L-ascorbic acid/HP-β-cyclodextrin inclusion complex-incorporated electrospun nanofibers. Foods 2023, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Scolari, I.R.; Páez, P.L.; Sánchez-Borzone, M.E.; Granero, G.E. Promising chitosan-coated alginate-Tween 80 nanoparticles as rifampicin coadministered ascorbic acid delivery carrier against Mycobacterium tuberculosis. AAPS PharmSciTech 2019, 20, 67. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Grabska-Zielińska, S.; Skopińska-Wiśniewska, J.; Jakubowska, E. Examining the impact of squaric acid as a crosslinking agent on the properties of chitosan-based films. Int. J. Mol. Sci. 2021, 22, 3329. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Uzair, B.; Menaa, F.; Braga, V.A. Fabrication, physical characterizations, and in vitro, in vivo evaluation of ginger extract-loaded gelatin/poly(vinyl alcohol) hydrogel films against burn wound healing in animal model. AAPS PharmSciTech 2020, 21, 323. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Bogdan, C.; Spînu, N.; Suciu, M.; Pop, A.; Țoc, A.; Tomuță, I. Design, optimization and pharmaceutical characterization of wound healing film dressings with chloramphenicol and ibuprofen. Drug Dev. Ind. Pharm. 2024, 50, 446–459. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.I.; Dattilo, M.; Patitucci, F.; Malivindi, R.; Pezzi, V.; Tzanov, T.; Puoci, F. Synthesis and evaluation of wound healing properties of hydro-diab hydrogel loaded with green-synthetized AGNPS: In vitro and in ex vivo studies. Drug Deliv. Transl. Res. 2022, 12, 1881–1894. [Google Scholar] [CrossRef]
- Khaloo Kermani, P.; Zargar Kharazi, A. A promising antibacterial wound dressing made of electrospun poly (glycerol sebacate) (PGS)/gelatin with local delivery of ascorbic acid and pantothenic acid. J. Polym. Environ. 2023, 31, 2504–2518. [Google Scholar] [CrossRef]
- Lewicka, K.; Smola-Dmochowska, A.; Dobrzyński, P.; Śmigiel-Gac, N.; Jelonek, K.; Musiał-Kulik, M.; Rychter, P. Microspheres based on blends of chitosan derivatives with carrageenan as vitamin carriers in cosmeceuticals. Polymers 2024, 16, 1815. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Yoshii, A.C.; Rocha e Silva, H.; Stringhetti Ferreira Cury, B.; Chorilli, M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: Preparation and characterization. Pharm. Dev. Technol. 2015, 20, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.A.; Parmar, S.J.; Easwaran, S. Metronidazole-loaded nanostructured lipid carriers to improve skin deposition and retention in the treatment of rosacea. Drug Dev. Ind. Pharm. 2019, 45, 1039–1051. [Google Scholar] [CrossRef]
- Adamczyk, O.; Deptuch, A.; Tarnawski, T.R.; Zieliński, P.M.; Drzewicz, A.; Juszyńska-Gałązka, E. Electrospun fiber mats with metronidazole: Design, evaluation, and release kinetics. J. Phys. Chem. B 2025. [Google Scholar] [CrossRef]


| Factors | Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| Chitosan (X1) | 3 | 3.5 | 4 |
| Ascorbic acid (X2) | 3 | 4.5 | 6 |
| Glycerol (X3) | 20 | 25 | 30 |
| Formulations | Coded Levels of Independent Factors | ||
|---|---|---|---|
| X1 | X2 | X3 | |
| F1 | −1 | −1 | 0 |
| F2 | 0 | +1 | +1 |
| F3 | 0 | 0 | 0 |
| F4 | +1 | 0 | −1 |
| F5 | −1 | 0 | −1 |
| F6 | −1 | 0 | +1 |
| F7 | 0 | 0 | 0 |
| F8 | 0 | 0 | 0 |
| F9 | 0 | +1 | −1 |
| F10 | 0 | 0 | 0 |
| F11 | −1 | +1 | 0 |
| F12 | 0 | −1 | +1 |
| F13 | 0 | −1 | −1 |
| F14 | 0 | 0 | 0 |
| F15 | +1 | 0 | +1 |
| F16 | +1 | +1 | 0 |
| F17 | +1 | −1 | 0 |
| Responses | Models | Sequential p-Value | R2 | Adjusted R2 | Predicted R2 | Lack-of-Fit p-Value | Remarks |
|---|---|---|---|---|---|---|---|
| Ultimate tensile strength (Y1) | Linear | 0.0011 | 0.6997 | 0.6303 | 0.3831 | 0.0086 | |
| 2FI | 0.0009 | 0.9377 | 0.9003 | 0.7292 | 0.0864 | Suggested | |
| Quadratic | 0.7616 | 0.9467 | 0.8782 | 0.2656 | 0.0419 | ||
| Elongation at break (Y2) | Linear | <0.0001 | 0.8598 | 0.8275 | 0.7258 | 0.1135 | |
| 2FI | 0.6260 | 0.8814 | 0.8102 | 0.4703 | 0.0834 | ||
| Quadratic | 0.0131 | 0.9722 | 0.9365 | 0.7762 | 0.4495 | Suggested | |
| pH (Y3) | Linear | 0.0069 | 0.5945 | 0.5009 | 0.2846 | 0.1155 | |
| 2FI | 0.1135 | 0.7707 | 0.6331 | 0.3765 | 0.1730 | ||
| Quadratic | 0.0151 | 0.9440 | 0.8719 | 0.7485 | 0.7997 | Suggested |
| Source | Ultimate Tensile Strength (Y1) | Elongation at Break (Y2) | pH (Y3) | |||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Model | <0.0001 | 0.0001 | 0.0013 | |||
| Intercept | 4.34 | - | 69.85 | - | 4.31 | - |
| X1 | −0.5228 | 0.1487 | −24.13 | 0.0001 | 0.0450 | 0.0036 |
| X2 | −3.12 | <0.0001 | 39.55 | <0.0001 | −0.0725 | 0.0002 |
| X3 | −1.59 | 0.0008 | 9.63 | 0.0200 | −0.0300 | 0.0244 |
| X1X2 | 1.91 | 0.0023 | −9.91 | 0.0658 | 0.0550 | 0.0076 |
| X1X3 | −1.08 | 0.0447 | −3.66 | 0.4468 | 0.0150 | 0.3459 |
| X2X3 | 1.92 | 0.0023 | 0.8643 | 0.8546 | −0.0400 | 0.0309 |
| X12 | - | - | 10.43 | 0.0508 | 0.0405 | 0.0266 |
| X22 | - | - | −0.7586 | 0.8689 | 0.0505 | 0.0101 |
| X32 | - | - | −18.94 | 0.0037 | 0.0055 | 0.7151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.; Kraisit, P.; Santhan, S.; Hirun, N. Box-Behnken Design Assisted Optimization and Characterization of Chitosan Film for Simultaneous Topical Delivery of Ascorbic Acid and Metronidazole. Pharmaceutics 2025, 17, 562. https://doi.org/10.3390/pharmaceutics17050562
Khan B, Kraisit P, Santhan S, Hirun N. Box-Behnken Design Assisted Optimization and Characterization of Chitosan Film for Simultaneous Topical Delivery of Ascorbic Acid and Metronidazole. Pharmaceutics. 2025; 17(5):562. https://doi.org/10.3390/pharmaceutics17050562
Chicago/Turabian StyleKhan, Bilawal, Pakorn Kraisit, Supaporn Santhan, and Namon Hirun. 2025. "Box-Behnken Design Assisted Optimization and Characterization of Chitosan Film for Simultaneous Topical Delivery of Ascorbic Acid and Metronidazole" Pharmaceutics 17, no. 5: 562. https://doi.org/10.3390/pharmaceutics17050562
APA StyleKhan, B., Kraisit, P., Santhan, S., & Hirun, N. (2025). Box-Behnken Design Assisted Optimization and Characterization of Chitosan Film for Simultaneous Topical Delivery of Ascorbic Acid and Metronidazole. Pharmaceutics, 17(5), 562. https://doi.org/10.3390/pharmaceutics17050562






