BaTiO3 Nanocarriers: Advancing Targeted Therapies with Smart Drug Release
Abstract
1. Introduction
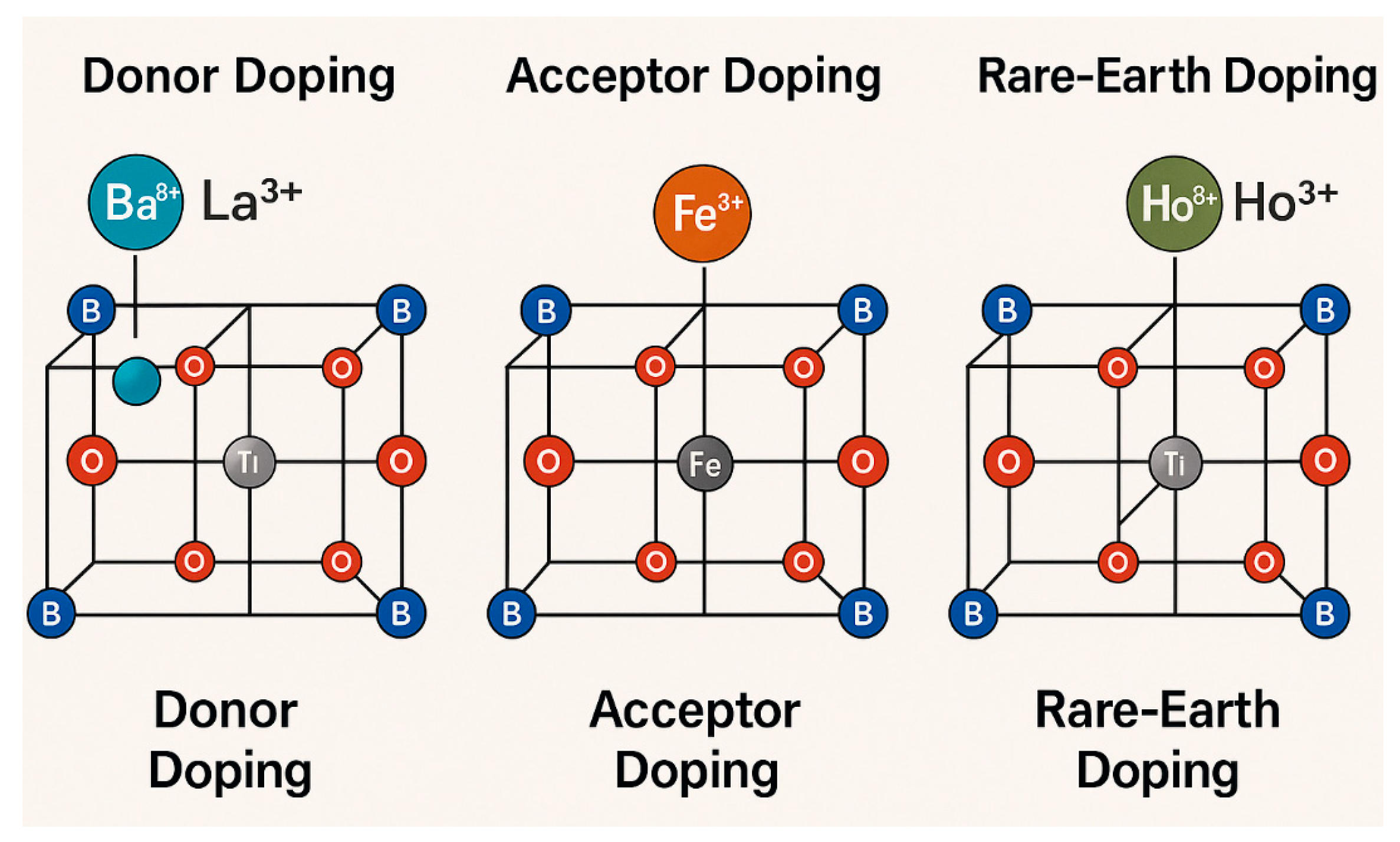
2. Structural Properties and Synthesis Methods of BaTiO3
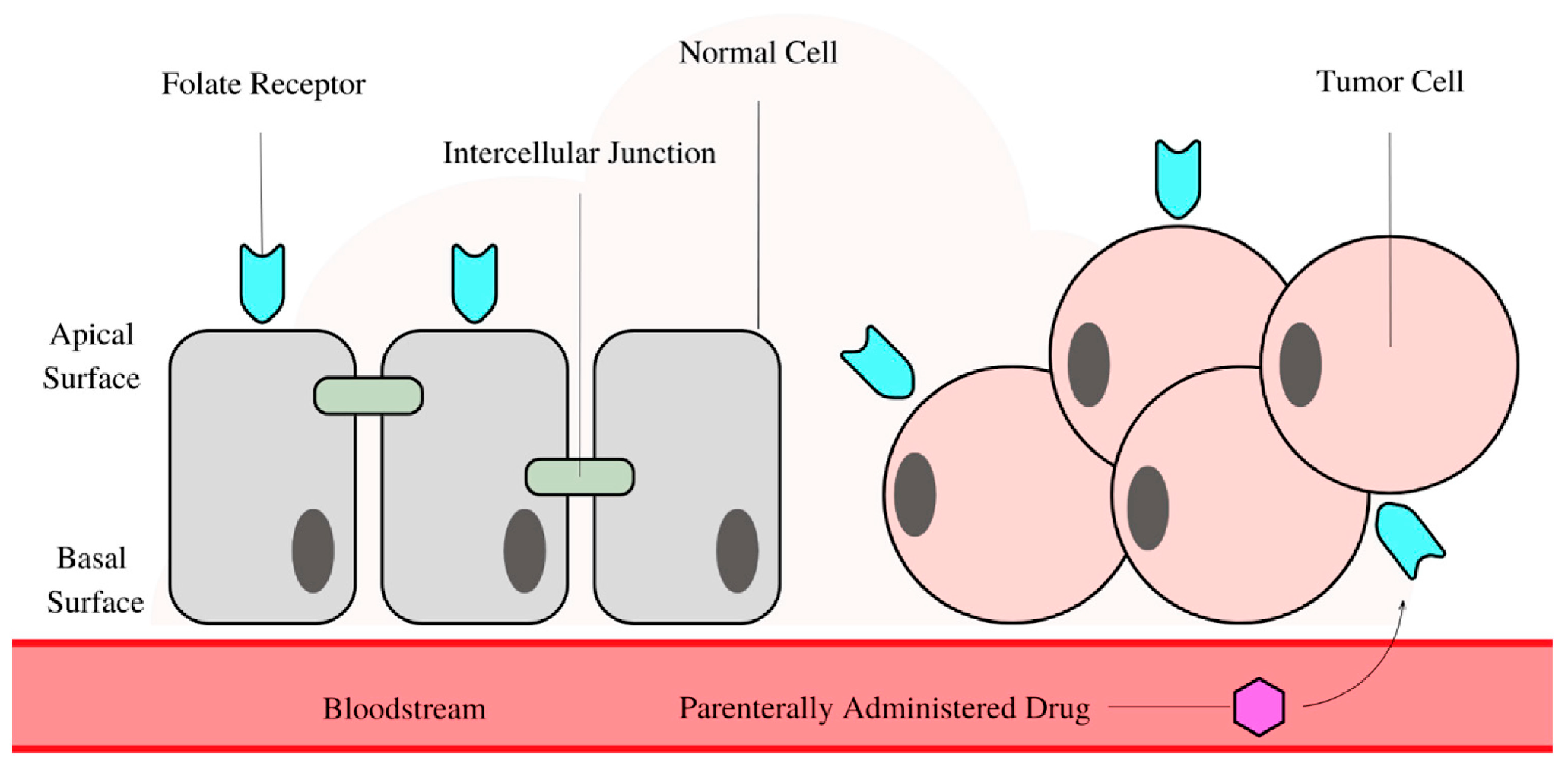
3. The Need for Targeted Drug Delivery and Biomedical Relevance of BaTiO3 in the Context of Smart Drug Delivery
3.1. Antibacterial Applications of BaTiO3 (BTNPs)
3.2. Tissue Regeneration and Wound Healing
3.3. Use of Piezoelectric Properties for Controlled Drug Release
3.4. Catalytic Approaches in Smart Drug Release
3.5. Photothermal and Photodynamic Therapy Using BaTiO3
3.6. Enhanced Cellular Uptake Through Surface Charge Modulation
3.7. Synergistic Drug Delivery for Cancer Cell Targeting
3.8. Integration into the Multifunctional Platforms
4. Critical Comparison of BaTiO3 Nanocarriers with Conventional Drug Delivery Platforms
5. The Advantages of BaTiO3 and Comparison with Other Nanocarriers
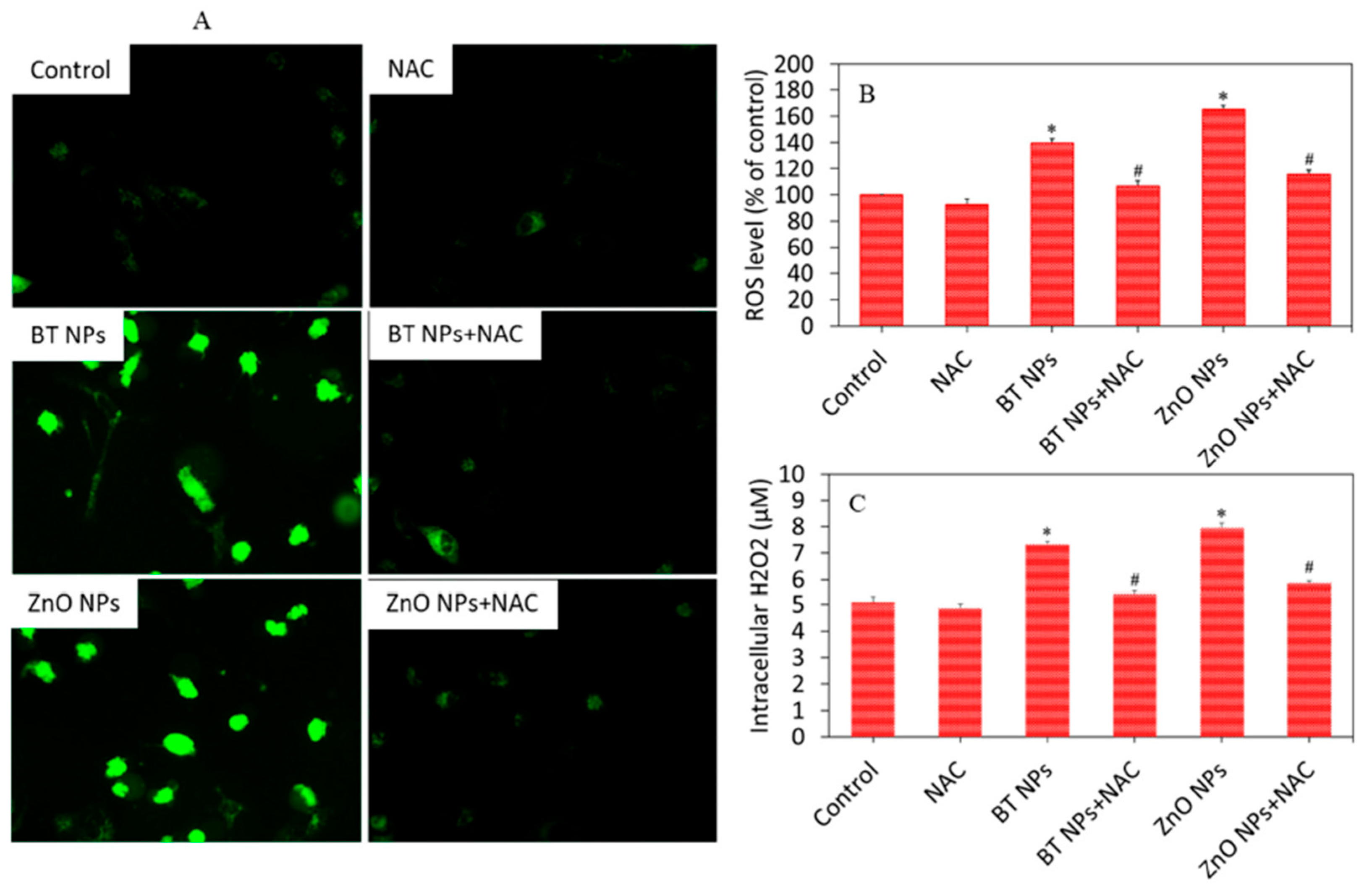
5.1. Biocompatibility and Low Cytotoxicity
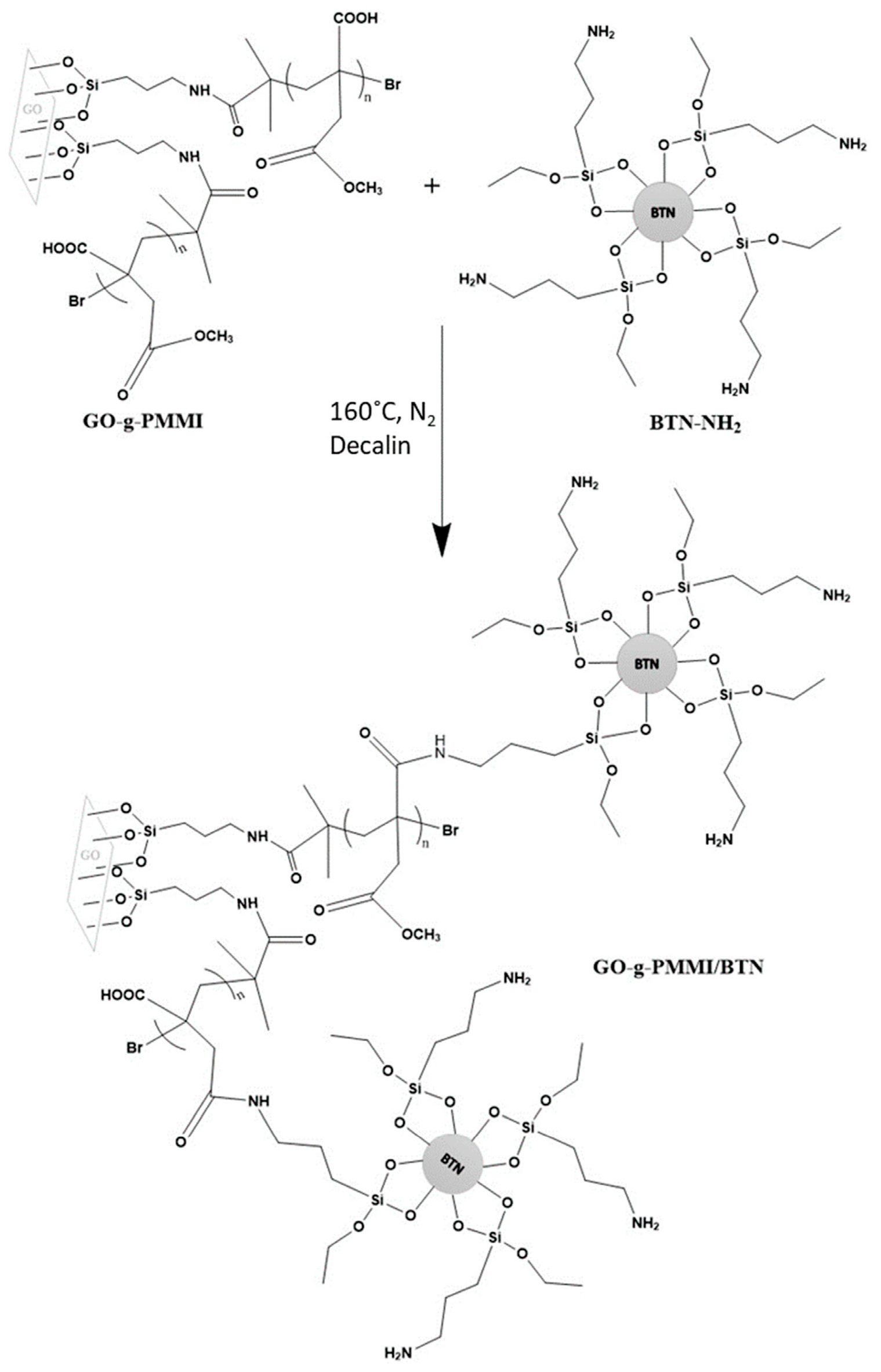
5.2. Drug Loading Capacity, Modifiability, and Multifunctionality
5.3. Research Challenges and Future Directions
5.4. Biosafety, Biodistribution, and Clinical Translation Potential of BTNPs
5.5. Clinical Translation and Regulatory Challenges of BaTiO3 Nanocarriers
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BTNPs | Barium titanate (BaTiO3) nanoparticles |
| SDDS | Smart drug delivery systems |
| SDT | Sonodynamic therapy |
| LIPUS | Low intensity pulsed ultrasound |
| PTT | Photothermal therapy |
| PDT | Photodynamic therapy |
| BTO-Ov | Oxygen-vacancy-engineered barium titanate |
| NIR | Near-infrared |
| ROS | Reactive oxygen species |
| EPR | Enhanced permeability and retention |
| SHG | Second-harmonic generation |
| CLSM | Confocal laser scanning microscopy |
| BBB | Blood–brain barrier |
| GBM | Glioblastoma multiforme |
| TNBC | Triple-negative breast cancer |
| TfR | Transferrin receptor |
| FA | Folic acid |
| GSH | Glutathione |
| CSNP | Core–shell nanoparticle |
| GBT | Graphene–barium titanate nanocomposite |
| GO–BaTiO3 | Graphene oxide–barium titanate nanocomposite |
| PMMA/PEO | Poly(methyl methacrylate)/Poly(ethylene oxide) |
| PEO/SF | Poly(ethylene oxide)/Silk fibroin |
| PHB | Polyhydroxybutyrate |
| PEI | Polyethyleneimine |
| DSPE-PEG | A lipid-polymer conjugate composed of 1,2-distearoyl-sn-glycero- 3-phosphoethanolamine (DSPE) and polyethylene glycol (PEG) |
| RAFT | Reversible Addition-Fragmentation chain Transfer polymerization |
| MAPK/JNK | Mitogen-activated protein kinase/Jun N-terminal kinase signaling pathway |
| OXPHOS | Oxidative phosphorylation |
| MLCCs | Multilayer ceramic capacitors |
| ML | Machine learning |
| FDA | U.S. Food and Drug Administration |
| EMA | European Medicines Agency |
| GMP | Good Manufacturing Practices |
References
- Merz, W.J. The electric and optical behavior of BaTiO3 single-domain crystals. Phys. Rev. 1949, 76, 1221. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R.; Jaffe, H. Piezoelectric Ceramics; Academic Press: London, UK, 1971. [Google Scholar]
- Lines, M.E.; Glass, A.M. Principles and Applications of Ferroelectrics and Related Materials; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Setter, N.; Waser, R. Electroceramic Materials. Acta Mater. 2000, 48, 151–178. [Google Scholar] [CrossRef]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef]
- Sood, A.; Singhmar, R.; Sahoo, S.; Lee, D.; Kim, C.M.; Kumar, A.; Han, S.S. Physicochemical, electrochemical, and biological characterization of field-assisted gold nanocluster-coated barium titanate nanoparticles for biomedical applications. J. Mater. Chem. B 2024, 12, 525–539. [Google Scholar] [CrossRef]
- Jordan, T.; O’Brien, M.A.; Spatarelu, C.-P.; Luke, G.P. Antibody-conjugated barium titanate nanoparticles for cell-specific targeting. ACS Appl. Nano Mater. 2020, 3, 2636–2646. [Google Scholar] [CrossRef]
- Linke, L.K.; Dehm, K.E.; Gubanov, K.; Fink, R.H.; Szyja, B.M.; Crisp, R.W. Colloidal organometallic synthesis of solution- processable barium titanate nanoparticles for nanoelectronic applications. Nanoscale 2025, 17, 7917–7925. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liang, X. Piezoelectric nanomaterials activated by ultrasound in disease treatment. Pharmaceutics 2023, 15, 1338. [Google Scholar] [CrossRef] [PubMed]
- Sikiru, S.; Soleimani, H.; Rostami, A.; Khodapanah, L. The architecture of BaTiO3 nanoparticles synthesis via temperature-responsive for improved oil recovery: A molecular dynamics simulation and core-flooding experimental study. Crystals 2025, 15, 8. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and cytotoxicity of gold nanoparticles: Recent advances in methodologies and regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Fakhar-e-Alam, M.; Saddique, S.; Hossain, N.; Shahzad, A.; Ullah, I.; Sohail, A.; Junaid, M.; Khan, I.; Saadullah, M. Synthesis, characterization, and application of BaTiO3 nanoparticles for anti-cancer activity. J. Clust. Sci. 2023, 34, 1745–1755. [Google Scholar] [CrossRef]
- Domrzalski, J.N.; Stevens, T.E.; Van Ginhoven, R.M.; Fritzsching, K.J.; Walder, B.J.; Johnson, E.M.; Lewis, R.E.; Vreeland, E.C.; Pearce, C.J.; Vargas, D.A. Surface functionalized barium titanate nanoparticles: A combined experimental and computational study. ECS J. Solid State Sci. Technol. 2022, 11, 063006. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Shi, J. Piezocatalytic tumor therapy by ultrasound-triggered and BaTiO3-mediated piezoelectricity. Adv. Mater. 2020, 32, 2001976. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, W.; Lai, Z.; Qiu, G.; Bu, Z.; Zhu, X.; Wang, Q.; Yu, Y.; Liu, J. Nanotechnology-enabled sonodynamic therapy against malignant tumors. Nanoscale Adv. 2024, 6, 1974–1991. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Ding, Y.; Zhang, Z.; Huang, T.; Zhang, Y.; Wan, X.; Wang, Z.L.; Li, L. Piezotronic effect-augmented Cu2−xO–BaTiO3 sonosensitizers for multifunctional cancer dynamic therapy. ACS Nano 2022, 16, 9304–9316. [Google Scholar] [CrossRef]
- Wang, Y.; Barhoumi, A.; Tong, R.; Wang, W.; Ji, T.; Deng, X.; Li, L.; Lyon, S.A.; Reznor, G.; Zurakowski, D.; et al. BaTiO3-core Au-shell nanoparticles for photothermal therapy and bimodal imaging. Acta Biomater. 2018, 72, 287–294. [Google Scholar] [CrossRef]
- Ball, J.P.; Mound, B.A.; Nino, J.C.; Allen, J.B. Biocompatible evaluation of barium titanate foamed ceramic structures for orthopedic applications. J. Biomed. Mater. Res. A 2014, 102, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Kapoor, D.U.; Sharma, J.B.; Gandhi, S.M.; Prajapati, B.G.; Thanawuth, K.; Limmatvapirat, S.; Sriamornsak, P. AI-driven design and optimization of nanoparticle-based drug delivery systems. Sci. Eng. Health Stud. 2024, 18, 24010003. [Google Scholar] [CrossRef]
- Bhange, M.; Telange, D. Convergence of nanotechnology and artificial intelligence in the fight against liver cancer: A comprehensive review. Discov. Oncol. 2025, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.M.; Dawber, M.; Lichtensteiger, C.; Ahn, C.H.; Triscone, J.M. Modern Physics of Ferroelectrics: Essential Background. In Physics of Ferroelectrics. Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2007; Volume 105. [Google Scholar] [CrossRef]
- Hlinka, J.; Márton, P. Phenomenological model of a 90° domain wall in BaTiO3-type ferroelectrics. Phys. Rev. B 2006, 74, 104104. [Google Scholar] [CrossRef]
- Uchino, K. (Ed.) Advanced Piezoelectric Materials: Science and Technology; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Setter, N.; Cross, L.E. The role of B-site cation disorder in diffuse phase transition behavior of perovskite ferroelectrics. J. Mater. Sci. 1980, 51, 4356–4360. [Google Scholar] [CrossRef]
- An, K.; Yu, Z.; Bai, H.; Liu, D.; Qiao, L.; Lv, X.; Shao, L.; Feng, J.; Cao, Y.; Li, L.; et al. Oxygen vacancy redistribution and ferroelectric polarization relaxation on epitaxial perovskite films during an electrocatalytic process. J. Mater. Chem. A 2024, 12, 9672–9680. [Google Scholar] [CrossRef]
- Marjanović, M.; Dimitrijević, D.; Paunović, V.; Prijić, Z. Microstructural and dielectrical characterization of Ho doped BaTiO3 ceramics. Serbian J. Electr. Eng. 2014, 11, 247–256. [Google Scholar] [CrossRef]
- Bae, H.; Lee, K.T. Effect of tetragonal to cubic phase transition on the upconversion luminescence properties of A/B site erbium-doped perovskite BaTiO3. RSC Adv. 2019, 9, 2451–2457. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Hao, J. Structure and electrical properties of BaTiO3 prepared by sol–gel process. J. Alloys Compd. 2009, 482, 137–140. [Google Scholar] [CrossRef]
- Swaminathan, V.; Pramana, S.S.; White, T.J.; Chen, L.; Chukka, R.; Ramanujan, R.V. Microwave synthesis of noncentrosymmetric BaTiO3 truncated nanocubes for charge storage applications. ACS Appl. Mater. Interfaces 2010, 2, 3037–3042. [Google Scholar] [CrossRef]
- Javed, S.; Alshehri, S.; Shoaib, A.; Ahsan, W.; Sultan, M.H.; Alqahtani, S.S.; Kazi, M.; Shakeel, F. Chronicles of nanoerythrosomes: An erythrocyte-based biomimetic smart drug delivery system as a therapeutic and diagnostic tool in cancer therapy. Pharmaceutics 2021, 13, 368. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Gozali, D.; Shamsuddin, S.; Muchtaridi, M. Chitosan-based nano-smart drug delivery system in breast cancer therapy. Pharmaceutics 2023, 15, 879. [Google Scholar] [CrossRef]
- Dubey, A.K.; Thrivikraman, G.; Basu, B. Absence of systemic toxicity in mouse model towards BaTiO3 nanoparticulate based eluate treatment. J. Mater. Sci. Mater. Med. 2015, 26, 103. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.K.A.; Fouad, H.; Abdal-hay, A.; Abd El-salam, N.M.; Abdelrazek Khalil, K. Fabrication and characterization of piezoelectric PEO/SF/BaTiO3 scaffolds for cardiac tissue engineering. J. Compos. Sci. 2023, 7, 200. [Google Scholar] [CrossRef]
- Kareem, Y.M.; Hamad, T.I. Assessment of the antibacterial effect of Barium Titanate nanoparticles against Staphylococcus epidermidis adhesion after addition to maxillofacial silicone. F1000Research 2023, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, Z.; Xu, Y.; Ning, X.; Wang, D.; Cao, J.; Feng, Y. Procedural promotion of wound healing by graphene-barium titanate nanosystem with white light irradiation. Int. J. Nanomed. 2023, 18, 4507–4520. [Google Scholar] [CrossRef]
- Jaafar, H.K.; Hashim, A.; Rabee, B.H. Synthesis of PMMA/PEO/SiC/BaTiO3 Nanostructures for antibacterial and radiation- shielding applications. Nanosyst. Phys. Chem. Math. 2024, 22, 447–456. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; Bai, Y.; Liu, Y.; Wang, Y.; Liu, O.; Yan, F.; Tang, Z.; Zhang, X.; Deng, X. Electroactive BaTiO3 nanoparticle-functionalized fibrous scaffolds enhance osteogenic differentiation of mesenchymal stem cells. Int. J. Nanomed. 2017, 12, 4007–4018. [Google Scholar] [CrossRef]
- Strangis, G.; Labardi, M.; Gallone, G.; Milazzo, M.; Capaccioli, S.; Forli, F.; Cinelli, P.; Berrettini, S.; Seggiani, M.; Danti, S.; et al. 3D printed piezoelectric BaTiO3/polyhydroxybutyrate nanocomposite scaffolds for bone tissue engineering. Bioengineering 2024, 11, 193. [Google Scholar] [CrossRef]
- Marino, A.; Almici, E.; Migliorin, S.; Tapeinos, C.; Battaglini, M.; Cappello, V.; Marchetti, M.; de Vito, G.; Cicchi, R.; Pavone, F.S.; et al. Piezoelectric barium titanate nanostimulators for the treatment of glioblastoma multiforme. J. Colloid Interface Sci. 2019, 538, 449–461. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Q.; Wu, R.; Sun, S.; Zhang, J.; Chen, J.; Gong, M.; Chen, C.; Liang, X. Ultrasound-triggered piezocatalysis for selectively controlled NO gas and chemodrug release to enhance drug penetration in pancreatic cancer. ACS Nano 2023, 17, 3557–3573. [Google Scholar] [CrossRef]
- Wang, P.; Tang, Q.; Zhang, L.; Xu, M.; Sun, L.; Sun, S.; Zhang, J.; Wang, S.; Liang, X. Ultrasmall barium titanate nanoparticles for highly efficient hypoxic tumor therapy via ultrasound triggered piezocatalysis and water splitting. ACS Nano 2021, 15, 11326–11340. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, S.; Wang, P.; Sun, L.; Wang, Y.; Zhang, L.; Xu, M.; Chen, J.; Wu, R.; Zhang, J.; et al. Genetically engineering cell membrane-coated BTO nanoparticles for MMP2-activated piezocatalysis-immunotherapy. Adv. Mater. 2023, 35, 2300964. [Google Scholar] [CrossRef]
- Gong, C.; Shi, S.; Dong, P.; Kan, B.; Gou, M.; Wang, X.; Li, X.; Luo, F.; Zhao, X.; Wei, Y.; et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int. J. Pharm. 2009, 365, 89–99. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, H.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multi-functional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Q.; Wang, Y.; Xu, M.; Sun, S.; Zhang, J.; Wu, R.; Yue, X.; Li, X.; Chen, Q.; et al. Ultrasound-induced piezocatalysis triggered NO generation for enhanced hypoxic tumor therapy. ACS Appl. Mater. Interfaces 2023, 15, 15220–15234. [Google Scholar] [CrossRef] [PubMed]
- Cafarelli, A.; Marino, A.; Vannozzi, L.; Puigmartí-Luis, J.; Pané, S.; Ciofani, G.; Ricotti, L. Piezoelectric nanomaterials activated by ultrasound: The pathway from discovery to future clinical adoption. ACS Nano 2021, 15, 11066–11086. [Google Scholar] [CrossRef] [PubMed]
- Sarwan, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. Hybrid thermo-responsive polymer systems and their biomedical applications. Front. Mater. 2020, 7, 73. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Wu, Z.; Feng, L.; Yu, S.; Zhang, H.; Gao, J.; Mai, Y.W.; Jia, Y. Enhancement of pyroelectric catalysis of ferroelectric BaTiO3 crystal: The action mechanism of electric poling. Ceram. Int. 2020, 46, 16763–16769. [Google Scholar] [CrossRef]
- Thomas, R.G.; Surendran, S.P.; Jeong, Y.Y. Tumor microenvironment-stimuli responsive nanoparticles for anticancer therapy. Front. Mol. Biosci. 2020, 7, 610533. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.; Zhang, Z.; Zhao, Y.; Yang, S.; Zhang, Y.; Yao, S.; Wang, S.; Huang, T.; Zhang, Y.; et al. Oxygen vacancy- engineered BaTiO3 nanoparticles for synergistic cancer photothermal, photodynamic, and catalytic therapy. Nano Res. 2022, 15, 7304–7312. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Yuan, H.; Sun, X. ZnO quantum dots decorated BaTiO3 for cancer sonodynamic therapy. Ultrason. Sonochem. 2024, 110, 107036. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zeng, Z.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Pan, S. Barium titanate/ZnAl-layered double hydroxide catalysts for piezoelectrically enhanced photocatalytic degradation of coexisting pollutants and antibiotic resistance genes. J. Environ. Chem. Eng. 2024, 12, 114227. [Google Scholar] [CrossRef]
- Cai, W.; Ma, X.; Chen, J.; Shi, R.; Wang, Y.; Yang, Y.; Jing, D.; Yuan, H.; Du, J.; Que, M. Synergy of oxygen vacancy and piezoelectricity effect promotes the CO2 photoreduction by BaTiO3. Appl. Surf. Sci. 2023, 619, 156773. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Qian, J.; He, S. Polyelectrolyte coated BaTiO3 nanoparticles for second harmonic generation imaging-guided photodynamic therapy with improved stability and enhanced cellular uptake. RSC Adv. 2016, 6, 40615–40625. [Google Scholar] [CrossRef]
- Angelats-Silva, L.M.; Pérez-Azahuanche, F.; Roldan-Lopez, J.A.; Emelianov, N.A.; Céspedes-Vásquez, R.B.; Valverde-Alva, M.A. Influence of the surface modification of BaTiO3 nanoparticles by hydrolyzed chitosan obtained from shrimp exoskeletons on the optical response intensity of the second harmonic. MRS Adv. 2022, 7, 260–264. [Google Scholar] [CrossRef]
- Kralj, S.; Rojnik, M.; Romih, R.; Jagodič, M.; Kos, J.; Makovec, D. Effect of surface charge on the cellular uptake of fluorescent magnetic nanoparticles. J. Nanopart. Res. 2012, 14, 1151. [Google Scholar] [CrossRef]
- Sood, A.; Desseigne, M.; Dev, A.; Maurizi, L.; Kumar, A.; Millot, N.; Han, S.S. A comprehensive review on barium titanate nanoparticles as a persuasive piezoelectric material for biomedical applications: Prospects and challenges. Small 2023, 19, 2206401. [Google Scholar] [CrossRef]
- Safari Sharafshadeh, M.; Tafvizi, F.; Khodarahmi, P.; Ehtesham, S. Folic acid-functionalized PEGylated niosomes co-encapsulated cisplatin and doxoribicin exhibit enhanced anticancer efficacy. Cancer Nano 2024, 15, 14. [Google Scholar] [CrossRef]
- Murugan, C.; Lee, H.; Park, S. Tumor-targeted molybdenum disulfide@barium titanate core–shell nanomedicine for dual photothermal and chemotherapy of triple-negative breast cancer cells. J. Mater. Chem. B 2023, 11, 1044–1056. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Woo Joo, S.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, J.; He, Z. Deep penetration of nanoparticulate drug delivery systems into tumors: Challenges and solutions. Curr. Med. Chem. 2013, 20, 2881–2891. [Google Scholar] [CrossRef]
- Cuenca-Bracamonte, Q.; Yazdani-Pedram, M.; Aguilar-Bolados, H. Electrical properties of polyetherimide-based nanocomposites filled with reduced graphene oxide and graphene oxide-barium titanate-based hybrid nanoparticles. Polymers 2022, 14, 4266. [Google Scholar] [CrossRef]
- Wu, H.; Dong, H.; Tang, Z.; Chen, Y.; Liu, Y.; Wang, M.; Wei, X.; Wang, N.; Bao, S.; Yu, D.; et al. Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophages and bone repair via MAPK/JNK inhibition and OXPHOS activation. Biomaterials 2023, 293, 121990. [Google Scholar] [CrossRef]
- Wang, X.; Bai, R. Advances in smart delivery of magnetic field-targeted drugs in cardiovascular diseases. Drug Deliv. 2023, 30, 2256495. [Google Scholar] [CrossRef]
- Hossain, S.; Hossain, S. Magnetic and optical characterization of cobalt ferrite–barium titanate core–shell for biomedical applications. IEEE Trans. Magn. 2022, 58, 2501208. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2025, 4, 145–160. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Wu, C.-W.; Vivero-Escoto, J.L.; Lin, V.S.-Y. Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Bargero, A.; Ciofani, G. Piezoelectric nanomaterials: Latest applications in biomedicine and challenges in clinical translation. Nanomedicine 2024, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A.; Alshamsan, A. Barium titanate (BaTiO3) nanoparticles exert cytotoxicity through oxidative stress in human lung carcinoma (A549) cells. Nanomaterials 2020, 10, 2309. [Google Scholar] [CrossRef]
- Alfareed, T.M.; Slimani, Y.; Almessiere, M.A.; Nawaz, M.; Khan, F.A.; Baykal, A.; Al-Suhaimi, E.A. Biocompatibility and colorectal anti-cancer activity study of nanosized BaTiO3 coated spinel ferrites. Sci. Rep. 2022, 12, 14127. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Chang, C. The immune effects of naturally occurring and synthetic nanoparticles. J. Autoimmun. 2010, 34, J234–J246. [Google Scholar] [CrossRef]
- Ahmed, M.; Kurungottu, P.; Swetha, K.; Atla, S.; Ashok, N.; Nagamalleswari, E.; Bonam, S.R.; Sahu, B.D.; Kurapati, R. Role of NLRP3 inflammasome in nanoparticle adjuvant-mediated immune response. Biomater. Sci. 2025, 13, 2164–2178. [Google Scholar] [CrossRef]
- Yan, Z.; Tran, H.; Ma, D.; Xie, J. Emerging piezoelectric metamaterials for biomedical applications. Mater. Interfaces 2024, 1, 13–34. [Google Scholar] [CrossRef]
- Cui, K.; Li, T.; Ma, Y.; Zhang, C.; Zhang, K.; Qi, C.; Cai, K. Ultrasound-responsive drug delivery system based on piezoelectric catalytic mechanisms. J. Funct. Biomater. 2025, 16, 304. [Google Scholar] [CrossRef]
- Qiao, Y.; Islam, M.S.; Wang, L.; Yan, Y.; Zhang, J.; Benicewicz, B.C.; Ploehn, H.J.; Tang, C. Thiophene polymer-grafted barium titanate nanoparticles toward nanodielectric composites. Chem. Mater. 2014, 26, 5319–5326. [Google Scholar] [CrossRef]
- Pilch, J.; Potęga, A.; Kowalczyk, A.; Kasprzak, A.; Kowalik, P.; Bujak, P.; Paluszkiewicz, E.; Augustin, E.; Nowicka, A.M. pH-responsive drug delivery nanoplatforms as smart carriers of unsymmetrical bisacridines for targeted cancer therapy. Pharmaceutics 2023, 15, 201. [Google Scholar] [CrossRef]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, Z.; Zhong, G.; Shi, L.; Xie, J.; Hao, J. Transducer materials mediated deep brain stimulation in neurological disorders. Adv. Funct. Mater. 2025, 8, 2506050. [Google Scholar] [CrossRef]
- Ibrahim, M.A.I.; Othman, R.; Chee, C.F.; Ahmad Fisol, F. Evaluation of folate-functionalized nanoparticle drug delivery systems —Effectiveness and concerns. Biomedicines 2023, 11, 2080. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, C.; You, Y.; Wang, Y.; Wei, R.; Liu, X. Fabrication of BaTiO3-loaded graphene nanosheets-based polyarylene ether nitrile nanocomposites with enhanced dielectric and crystallization properties. Nanomaterials 2019, 9, 1667. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Goel, S.; Chianella, I.; Yazdani Nezhad, H. Graphene nanoplatelets/Barium titanate polymer nanocomposite fibril: A remanufactured multifunctional material with unprecedented electrical, thermomechanical, and electromagnetic properties. Adv. Sustain. Syst. 2023, 7, 2300177. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/media/157812/download (accessed on 30 July 2025).
- European Medicines Agency (EMA). Multidisciplinary: Nanomedicines—Scientific Guidelines; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Bosetti, R.; Jones, S.L. Cost-effectiveness of nanomedicine: Estimating the real size of nano-costs. Nanomedicine 2019, 14, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-N.; Lee, D.-S.; Ju Park, H.; Kim, J.-S. Barium titanate nanoparticles sensitize treatment-resistant breast cancer cells to the antitumor action of tumor-treating fields. Sci. Rep. 2020, 10, 2560. [Google Scholar] [CrossRef]
- Candito, M.; Simoni, E.; Gentilin, E.; Martini, A.; Marioni, G.; Danti, S.; Astolfi, L. Neuron Compatibility and antioxidant activity of barium titanate and lithium niobate nanoparticles. Int. J. Mol. Sci. 2022, 23, 1761. [Google Scholar] [CrossRef]
- International Council for Harmonisation (ICH); U.S. Food and Drug Administration (FDA). ICH M3(R2). Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals; EMA: Amsterdam, The Netherlands, 11 June 2009. [Google Scholar]
- National Cancer Institute’s Nanotechnology Characterization Laboratory (NCL): Protocols & Assay Cascade (Immunocompatibility, Complement/Cytokines); National Cancer Institute—Nanotechnology Characterization Laboratory (Cancer.gov): Frederick, MD, USA, 2005.
- Dobrovolskaia, M.A. Lessons learned from immunological characterization of nanomaterials at the Nanotechnology Characterization Laboratory. Front. Immunol. 2022, 13, 984252. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: Pyrogen and Endotoxins Testing: Questions and Answers. Silver Spring, MD, USA, 2012 (issued 27 June 2012). Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pyrogen-and-endotoxins-testing-questions-and-answers (accessed on 14 September 2025).
- United States Pharmacopeial Convention (USP). General Chapter <71> Sterility Tests. In United States Pharmacopeia and National Formulary (USP–NF) [Online]; United States Pharmacopeial Convention: Rockville, MD, USA; Available online: https://www.usp.org/harmonization-standards/pdg/general-methods/sterility-test (accessed on 14 September 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćurčić, M.; Hadžić, B.; Gilić, M.; Lazarević, Z.; Ilić, A. BaTiO3 Nanocarriers: Advancing Targeted Therapies with Smart Drug Release. Pharmaceutics 2025, 17, 1203. https://doi.org/10.3390/pharmaceutics17091203
Ćurčić M, Hadžić B, Gilić M, Lazarević Z, Ilić A. BaTiO3 Nanocarriers: Advancing Targeted Therapies with Smart Drug Release. Pharmaceutics. 2025; 17(9):1203. https://doi.org/10.3390/pharmaceutics17091203
Chicago/Turabian StyleĆurčić, Milica, Branka Hadžić, Martina Gilić, Zorica Lazarević, and Andjelija Ilić. 2025. "BaTiO3 Nanocarriers: Advancing Targeted Therapies with Smart Drug Release" Pharmaceutics 17, no. 9: 1203. https://doi.org/10.3390/pharmaceutics17091203
APA StyleĆurčić, M., Hadžić, B., Gilić, M., Lazarević, Z., & Ilić, A. (2025). BaTiO3 Nanocarriers: Advancing Targeted Therapies with Smart Drug Release. Pharmaceutics, 17(9), 1203. https://doi.org/10.3390/pharmaceutics17091203







