Pancreatic Cancer-Targeting Cascade Nanoamplifier Enables Self-Replenishing H2O2 Generation and Autophagy Disruption in Chemodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
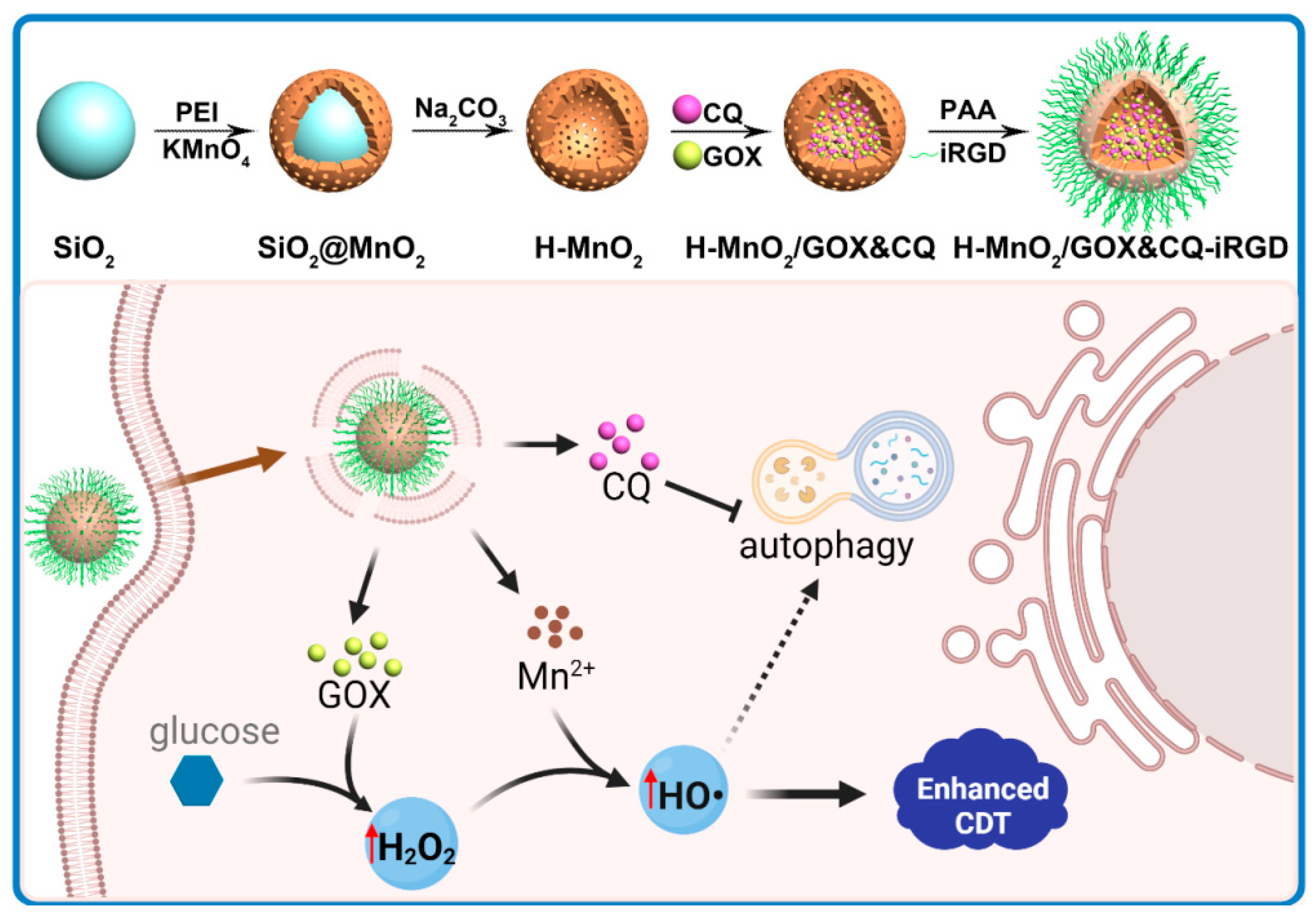
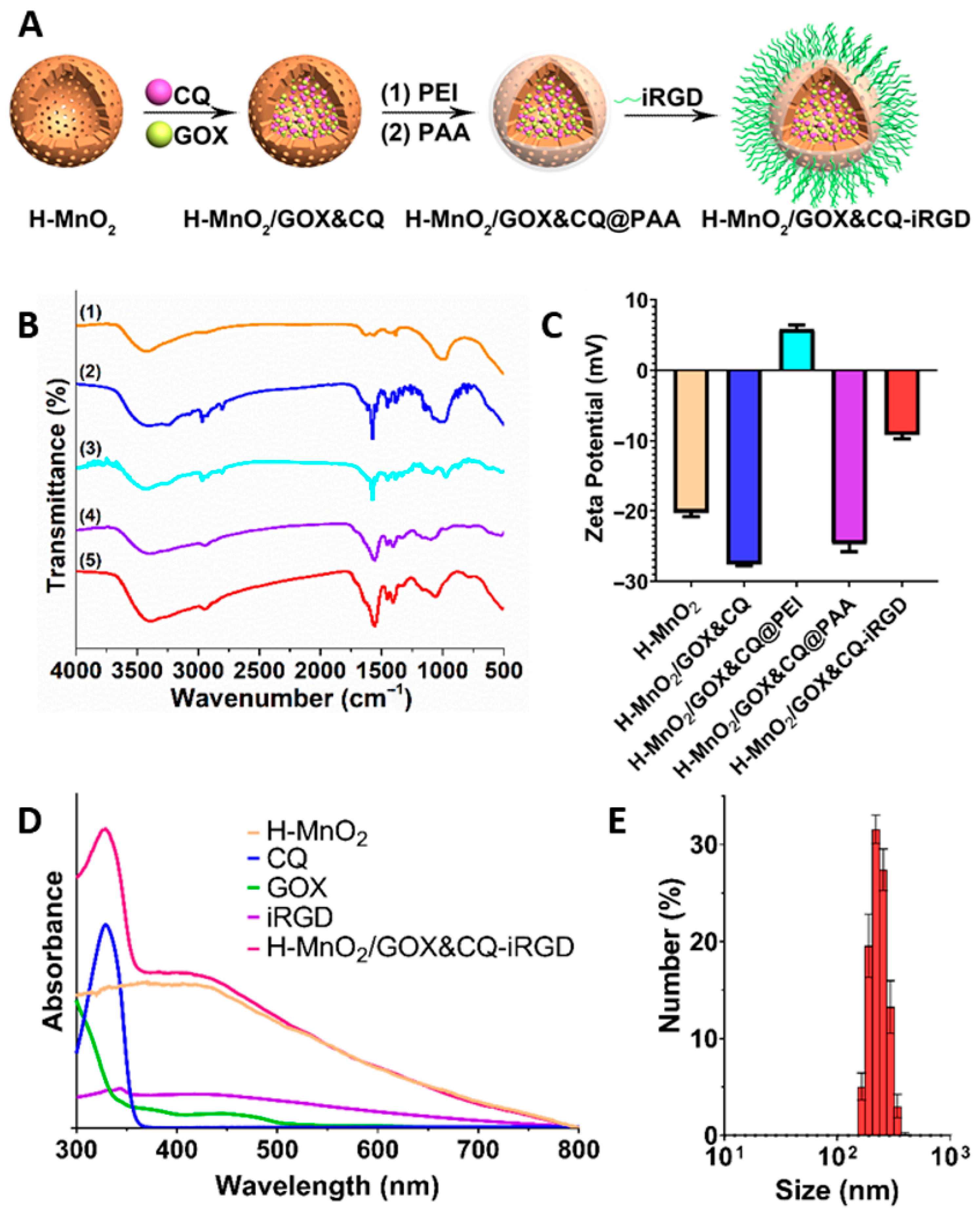
2.2.1. Construction and Characterization of H-MnO2/GOX&CQ-iRGD
2.2.2. Detection of O2, H2O2, and ·OH Generation and the Release of Mn2+, CQ, and GOX from H-MnO2/GOX&CQ-iRGD Nanoparticles
2.2.3. Therapeutic Study of H-MnO2/GOX&CQ-iRGD on Human Pancreatic Cancer Cells
2.2.4. Imaging and Therapeutic Evaluation of H-MnO2/GOX&CQ-iRGD in Pancreatic Tumor-Bearing Mice
2.3. Statistical Analysis
3. Results
3.1. Construction and Characterization of the Cascade Nanoamplifier
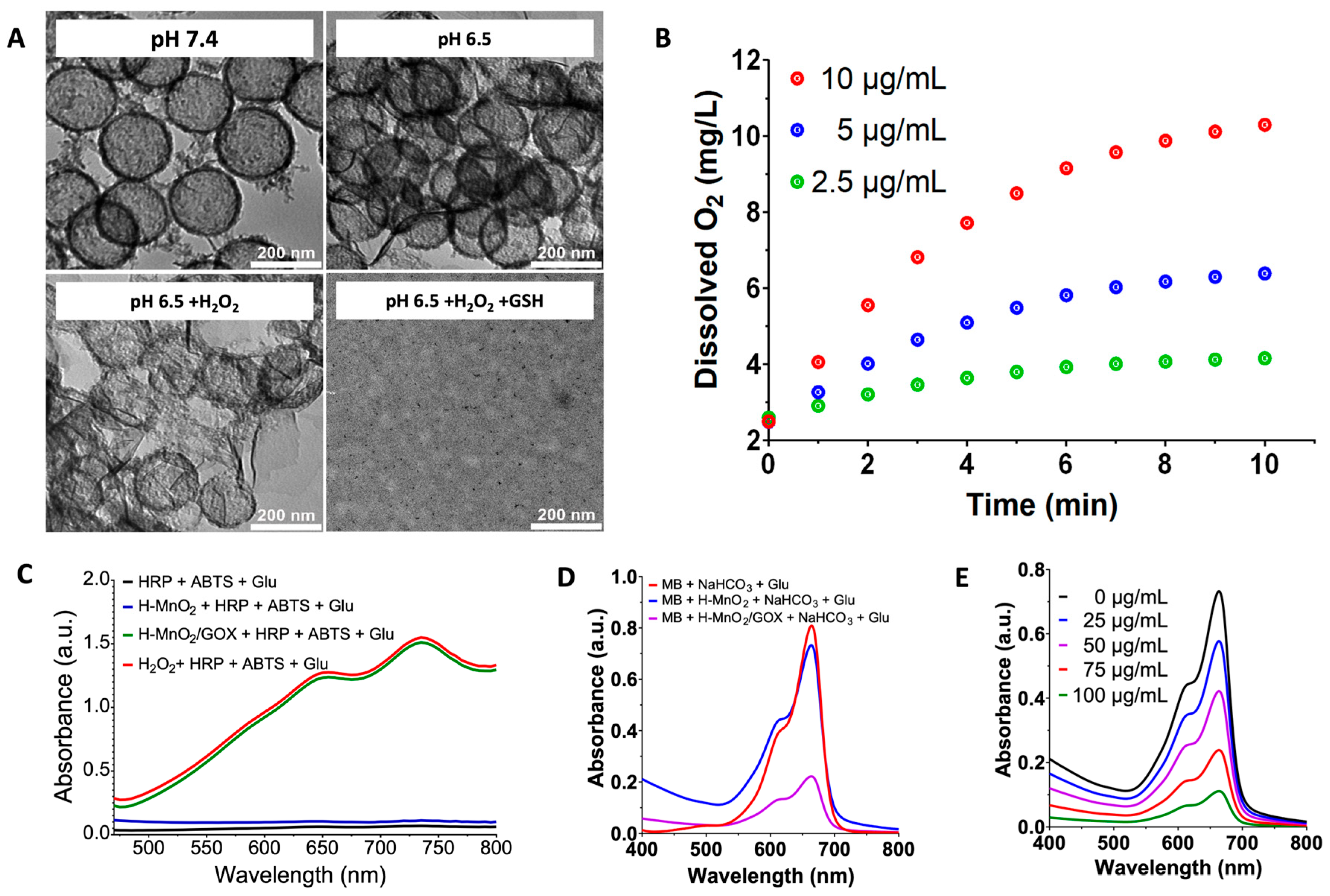
3.2. TME-Induced Shell Collapse and Enhanced Fenton Reaction of the Cascade Nanoamplifier (Solution Level)
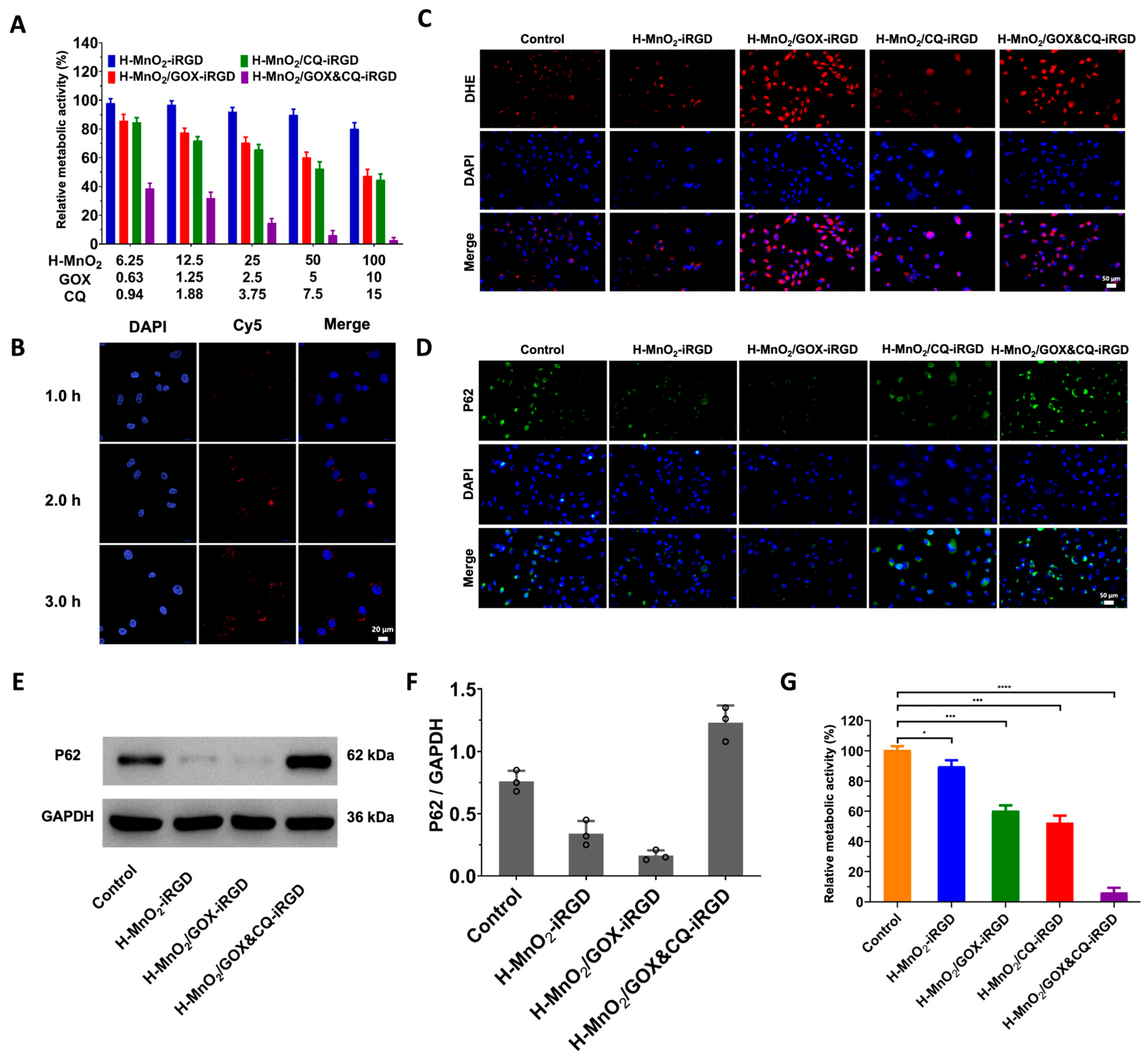
3.3. Pancreatic Cancer Cell-Specific Cascade Nanoamplifier Enhances CDT (Cellular Level)
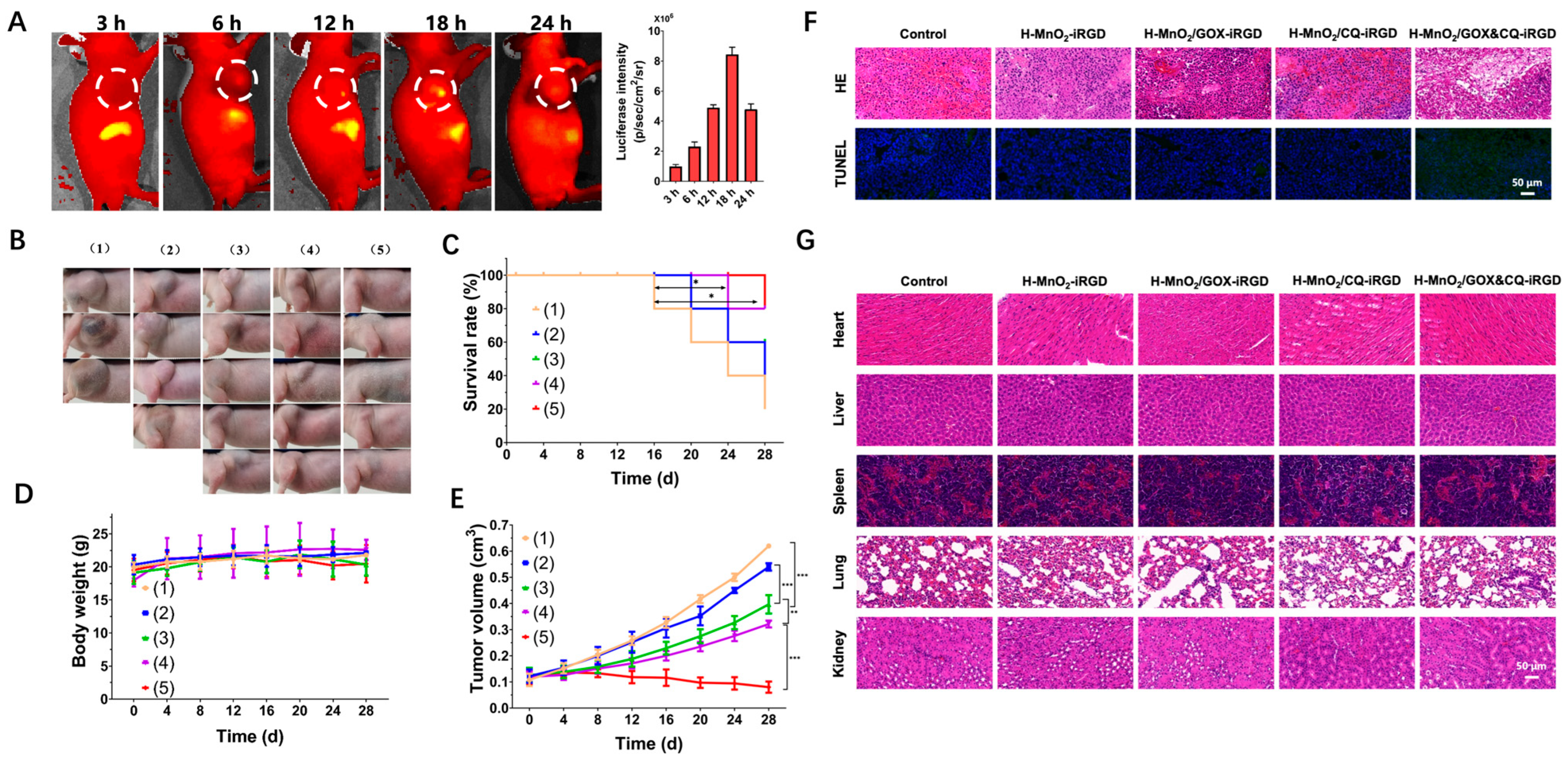
3.4. Cascade Nanoamplifier-Enhanced CDT for Pancreatic Cancer in Animal Models (In Vivo Level)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GOX | glucose oxidase |
| CQ | chloroquine |
| TME | tumor microenvironment |
| CDT | chemodynamic therapy |
| GSH | glutathione |
| PEI | polyethyleneimine |
| ROS | reactive oxygen species |
| BPEI | branched polyethyleneimine |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [PubMed]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomedicine meets Fenton chemistry. Chem. Rev. 2021, 121, 1981–2019. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Jia, Q.; Li, Y. Tumor Microenvironment Activated Vanadium−Doped Carbon Dots for Fluorescence Imaging and Chemodynamic Therapy. Crystals 2023, 13, 652. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, C.; Guo, Z.; Tan, G.; Niu, D.; Li, Y.; Zhu, W.H. Spatio-Temporally Reporting Dose-Dependent Chemotherapy via Uniting Dual-Modal MRI/NIR Imaging. Angew. Chem. Int. Ed. 2020, 59, 21143–21150. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, P.; Liu, X.; Li, Y.; Wu, W.; Gao, X.; Liu, B. An activity-based photosensitizer to reverse hypoxia and oxidative resistance for tumor photodynamic eradication. Adv. Mater. 2022, 34, 2206659. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, G.; Guo, Z.; Lu, J.; Ye, J.; Xu, W.; Gao, X.; Yue, K.; Wei, Y.; Zhao, L. Ultra-sensitive iron-doped Palladium nanocrystals with enhanced hydroxyl radical generation for chemo-/chemodynamic Nanotherapy. Adv. Funct. Mater. 2022, 32, 2107518. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, H.; Xu, C.; Tong, Z.; Zhang, S.; Bai, Y.; Chen, Y.; Xu, Q.; Zhou, L.; Ding, H.; et al. A nanomedicine fabricated from gold nanoparticles-decorated metal–organic framework for cascade chemo/chemodynamic cancer therapy. Adv. Sci. 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Cheng, B.; Li, D.; Li, C.; Zhuang, Z.; Wang, P.; Liu, G. The Application of Biomedicine in Chemodynamic Therapy: From Material Design to Improved Strategies. Bioengineering 2023, 10, 925. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Hua, W.; Gu, W.; Zhuang, H.; Li, H.; Jiang, X.; Mao, Y.; Liu, Y.; Jin, D.; et al. Heterostructures with built-in electric fields for long-lasting chemodynamic therapy. Angew. Chem. Int. Ed. 2023, 62, e202300356. [Google Scholar] [CrossRef]
- Zhao, P.; Li, H.; Bu, W. A forward vision for chemodynamic therapy: Issues and opportunities. Angew. Chem. Int. Ed. 2023, 62, e202210415. [Google Scholar] [CrossRef]
- Dirersa, W.B.; Kan, T.C.; Chang, J.; Getachew, G.; Ochirbat, S.; Kizhepat, S.; Wibrianto, A.; Rasal, A.; Chen, H.; Ghule, A.V.; et al. Engineering H2O2 self-supplying platform for xdynamic therapies via Ru–Cu peroxide nanocarrier: Tumor microenvironment-mediated synergistic therapy. ACS Appl. Mater. 2024, 16, 24172–24190. [Google Scholar] [CrossRef]
- Yu, P.; Li, X.; Cheng, G.; Zhang, X.; Wu, D.; Chang, J.; Wang, S. Hydrogen peroxide-generating nanomedicine for enhanced chemodynamic therapy. Chin. Chem. Lett. 2021, 32, 2127–2138. [Google Scholar] [CrossRef]
- Yang, B.; Ding, L.; Yao, H.; Chen, Y.; Shi, J. A Metal-Organic Framework (MOF) Fenton Nanoagent-Enabled Nanocatalytic Cancer Therapy in Synergy with Autophagy Inhibition. Adv. Master 2020, 32, e1907152. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, H.; Wang, H.; Xie, Y.; Shang, Y.; Wu, Y.; Guo, X.; Yu, S.; Li, N.; Ding, B. A DNA origami–based enzymatic cascade nanoreactor for chemodynamic cancer therapy and activation of antitumor immunity. Sci. Adv. 2025, 11, eadr9196. [Google Scholar] [CrossRef]
- Huang, P.; Gao, W.; Fu, C.; Wang, M.; Li, Y.; Chu, B.; He, A.; Li, Y.; Deng, X.; Zhang, Y.; et al. Clinical functional proteomics of intercellular signalling in pancreatic cancer. Nature 2025, 637, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Elahi-Gedwillo, K.Y.; Carlson, M.; Zettervall, J.; Provenzano, P.P. Antifibrotic therapy disrupts stromal barriers and modulates the immune landscape in pancreatic ductal adenocarcinoma. Cancer Res. 2019, 79, 372–386. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.X.; Li, X.L.; Chen, H.Y.; Xu, J.J. Core–shell plasmonic nanomaterials toward: Dual-mode imaging analysis of glutathione and enhanced chemodynamic therapy. Anal. Chem. 2021, 93, 10317–10325. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Huang, J.; Ding, J.; Yan, B.; Li, Y.; Gao, X.; Zhou, W. Poly-thymine DNA templated MnO2 biomineralization as a high-affinity anchoring enabling tumor targeting delivery. J. Colloid. Interface Sci. 2023, 637, 441–452. [Google Scholar] [CrossRef]
- Jin, W.; Chen, Z.; Wang, Y.; Li, J.; Li, J.; Pei, Y.; Pei, Z. Nano metal-photosensitizer based on Aza-BODIPY-Cu complex for CDT-enhanced dual phototherapy. Chin. Chem. Lett. 2024, 3, 109328. [Google Scholar] [CrossRef]
- Feng, L.; Xie, R.; Wang, C.; Gai, S.; He, F.; Yang, D.; Yang, P.; Lin, J. Magnetic Targeting, Tumor Microenvironment-Responsive Intelligent Nanocatalysts for Enhanced Tumor Ablation. Angew. Chem. Int. Ed. 2018, 12, 11000–11012. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, F.; Li, W.; Zhang, L.; You, Y.; Deng, Q.; Dong, K.; Ren, J.; Qu, X. Bioinspired Construction of a Nanozyme-Based H2O2 Homeostasis Disruptor for Intensive Chemodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 5177–5183. [Google Scholar] [CrossRef]
- Ren, H.; Bai, Y.; Liu, Z.; Ma, C.; Tao, X.; Wang, Q.; Lian, H.; Li, X. A multifunctional cascade gas-nanoreactor with MnO2 as a gatekeeper to enhance starvation therapy and provoke antitumor immune response. Acta Biomater. 2024, 190, 501–517. [Google Scholar] [CrossRef]
- Wang, L.; Shi, R.; Wang, S.; Duan, Y.; Wang, Z.; Zheng, P.; Sun, X.; Chen, X.; Ji, G.; Shen, Y.; et al. ADSL promotes autophagy and tumor growth through fumarate-mediated Beclin1 dimethylation. Nat. Chem. Biol. 2020, 21, 894–905. [Google Scholar] [CrossRef]
- Dong, K.; Xu, Y.; Tang, Y.; Li, Q. Photoactivated Self-Assembled Nanomicelles Integrating Mitophagy Inhibition to Enhance Pyroptosis for Cancer Immunotherapy. Adv. Funct. Mater. 2025, 2504384. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, X.; Chen, J.; Gao, S.; Wang, L.; Zhao, Y. Perturbation of Autophagy by a Beclin 1-Targeting Stapled Peptide Induces Mitochondria Stress and Inhibits Proliferation of Pancreatic Cancer Cells. Cancers 2023, 15, 953. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, S.J.; Lee, Y.S.; Kim, S. Theranostic iRGD peptide containing cisplatin prodrug: Dual-cargo tumor penetration for improved imaging and therapy. J. Control. Release 2019, 300, 73–80. [Google Scholar] [CrossRef]
- de Mendoza, T.H.; Mose, E.S.; Botta, G.P.; Braun, G.B.; Kotamraju, V.R.; French, R.P.; Suzuki, K.; Miyamura, N.; Teesalu, T.; Ruoslahti, E.; et al. Tumor-penetrating therapy for β5 integrin-rich pancreas cancer. Nat. Commun. 2021, 12, 1541. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Zheng, S.; Mu, J.; Yu, N.; Guo, S. Tumor-penetrating iRGD facilitates penetration of poly(floxuridine-ketal)-based nanomedicine for enhanced pancreatic cancer therapy. J. Control. Release 2024, 369, 444–457. [Google Scholar] [CrossRef]
- Pan, Y.; Xue, Q.; Yang, Y.; Shi, T.; Wang, H.; Song, X.; Luo, Y.; Liu, W.; Ren, S.; Cai, Y.; et al. Glycoengineering-based anti-PD-1-iRGD peptide conjugate boosts antitumor efficacy through T cell engagement. Cell Rep. Med. 2024, 5, 101590. [Google Scholar] [CrossRef]
- Chen, B.; Liu, X.; Li, Y.; Shan, T.; Bai, L.; Li, C.; Wang, Y. iRGD tumor-penetrating peptide-modified nano-delivery system based on a marine sulfated polysaccharide for enhanced anti-tumor efficiency against breast cancer. Int. J. Nanomed. 2022, 17, 617–633. [Google Scholar] [CrossRef]
- Bai, X.; Meng, F.; Wang, X.; He, L.; Fan, C.; Tian, L.; Zhang, Y.; Pan, J.; Wu, Q.; Hao, X.; et al. Photodynamic gel-bombs enhance tumor penetration and downstream synergistic therapies. Signal Transduct. Target. Ther. 2025, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Hu, Y.R.; Qi, C.; He, T.; Jiang, S.; Jiang, C.; He, J.; Qu, J.; Lin, J.; Huang, P. Biodegradable Manganese-Doped Calcium Phosphate Nanotheranostics for Traceable Cascade Reaction-Enhanced Anti-Tumor Therapy. Angew. Chem. Int. Ed. 2019, 13, 13985–13994. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yao, S.Y.; Luo, J.; Ding, C.; Huang, Q.; Yang, Y.; Shi, Z.; Lin, J.; Pan, Y.C.; Zeng, X.; et al. Author Correction: Engineered hypoxia-responsive albumin nanoparticles mediating mitophagy regulation for cancer therapy. Nat. Commun. 2025, 16, 2066. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, F.; Li, Y.; Deng, Y.; Huang, Y.; Liu, W.; Jin, Q.; Ji, J. Nitric oxide-induced stromal depletion for improved nanoparticle penetration in pancreatic cancer treatment. Biomaterials 2020, 246, 119999. [Google Scholar] [CrossRef]
- Forest, V.; Cottier, M.; Pourchez, J. Electrostatic interactions favor the binding of positive nanoparticles on cells: A reductive theory. Nano Today 2015, 10, 677–680. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Huang, F.; Wang, X.; Lu, J.; Jia, F.; Pan, Z.; Cui, X.; Ge, G.; Deng, X.; et al. Complementary autophagy inhibition and glucose metabolism with rattle-structured polydopamine@ mesoporous silica nanoparticles for augmented low-temperature photothermal therapy and in vivo photoacoustic imaging. Theranostics 2020, 10, 7273–7286. [Google Scholar] [CrossRef]
- Ding, J.; Xie, Y.; Liu, Z.; Zhang, Z.; Ni, B.; Yan, J.; Zhou, T.; Hao, J. Hypoxic and Acidic Tumor Microenvironment-Driven AVL9 Promotes Chemoresistance of Pancreatic Ductal Adenocarcinoma via the AVL9-IκBα-SKP1 Complex. Gastroenterology 2025, 168, 539–555.e5. [Google Scholar] [CrossRef]
- Zhang, S.; You, H.; Fan, H.; Chen, Y.; Song, H.; Zhao, Z.; Chen, Q.; Wang, Y.; Tian, Z.; Wu, Y.; et al. Transcytosis-Triggering Nanoparticles for Overcoming Stromal Barriers and Reversing Immunosuppression in Pancreatic Cancer Combinatorial Therapy. Nano Lett. 2025, 25, 2949–2959. [Google Scholar] [CrossRef]
- Chu, Z.; Yang, J.; Zheng, W.; Sun, J.; Wang, W.; Qian, H. Recent advances on modulation of H2O2 in tumor microenvironment for enhanced cancer therapeutic efficacy. Coord. Chem. Rev. 2023, 481, 19. [Google Scholar] [CrossRef]
- Horos, R.; Büscher, M.; Kleinendorst, R.; Alleaume, A.M.; Tarafder, A.K.; Schwarzl, T.; Dziuba, D.; Tischer, C.; Zielonka, E.M.; Adak, A.; et al. The Small Non-coding Vault RNA1-1 Acts as a Riboregulator of Autophagy. Cell 2019, 176, 1054–1067.e12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Feng, L.; Tang, Y.; Yu, N.; Lin, J.; Ji, Y.; Li, H. Pancreatic Cancer-Targeting Cascade Nanoamplifier Enables Self-Replenishing H2O2 Generation and Autophagy Disruption in Chemodynamic Therapy. Pharmaceutics 2025, 17, 1201. https://doi.org/10.3390/pharmaceutics17091201
Yu J, Feng L, Tang Y, Yu N, Lin J, Ji Y, Li H. Pancreatic Cancer-Targeting Cascade Nanoamplifier Enables Self-Replenishing H2O2 Generation and Autophagy Disruption in Chemodynamic Therapy. Pharmaceutics. 2025; 17(9):1201. https://doi.org/10.3390/pharmaceutics17091201
Chicago/Turabian StyleYu, Jiaqi, Lishuai Feng, Yunpeng Tang, Nianhui Yu, Jianning Lin, Yuan Ji, and Hui Li. 2025. "Pancreatic Cancer-Targeting Cascade Nanoamplifier Enables Self-Replenishing H2O2 Generation and Autophagy Disruption in Chemodynamic Therapy" Pharmaceutics 17, no. 9: 1201. https://doi.org/10.3390/pharmaceutics17091201
APA StyleYu, J., Feng, L., Tang, Y., Yu, N., Lin, J., Ji, Y., & Li, H. (2025). Pancreatic Cancer-Targeting Cascade Nanoamplifier Enables Self-Replenishing H2O2 Generation and Autophagy Disruption in Chemodynamic Therapy. Pharmaceutics, 17(9), 1201. https://doi.org/10.3390/pharmaceutics17091201







