Development of Timed Release Vaginal Mucosal Cloprostenol for Farrowing Management in Sows
Abstract
1. Introduction
2. Materials and Methods
2.1. HPLC Method for Cloprostenol Quantification
2.2. Preparation of the Immediate Release (IR) Formulations
2.3. Preparation of Delayed Release (DR) Coating Formulations
2.4. Physical Characteristics of Tablets
2.4.1. Weight Variation Test
2.4.2. Hardness Test
2.4.3. Thickness Test
2.4.4. Friability Test
2.4.5. In Vitro Disintegration Time
2.4.6. Content Uniformity
2.4.7. Dissolution Test
2.4.8. Kinetic Analysis
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Scanning Electron Microscopy (SEM)
2.8. In Vivo Experiment
2.9. Statistics
3. Results
3.1. Physical Properties
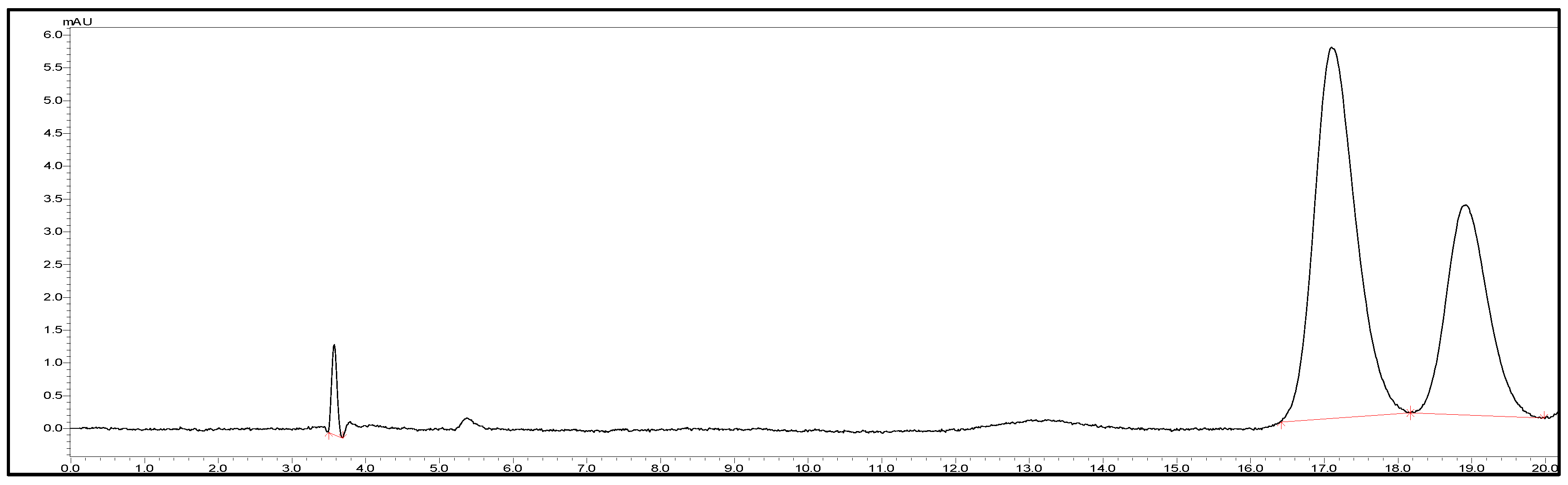
3.2. Drug Uniformity Test
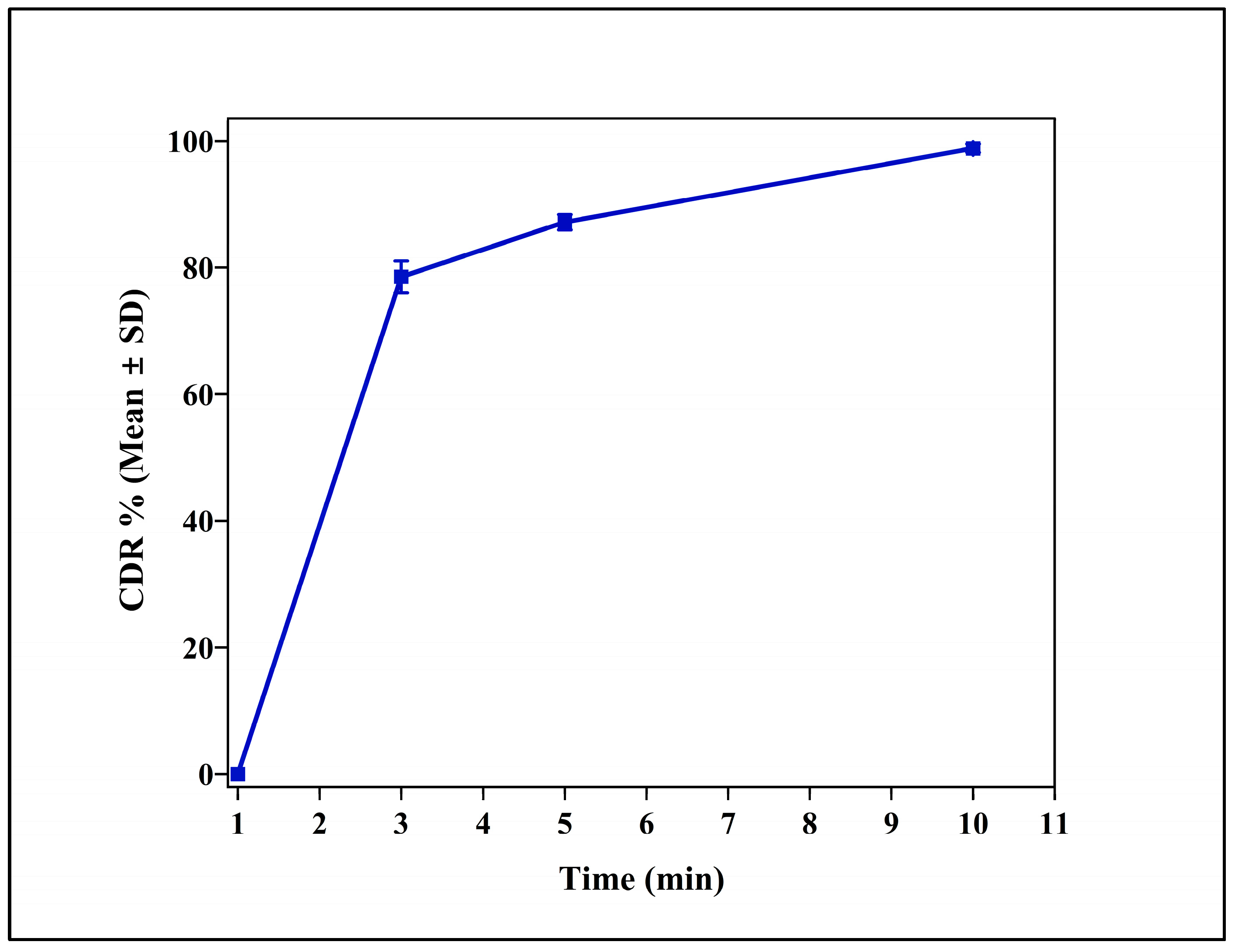
3.3. In Vitro Release Studies
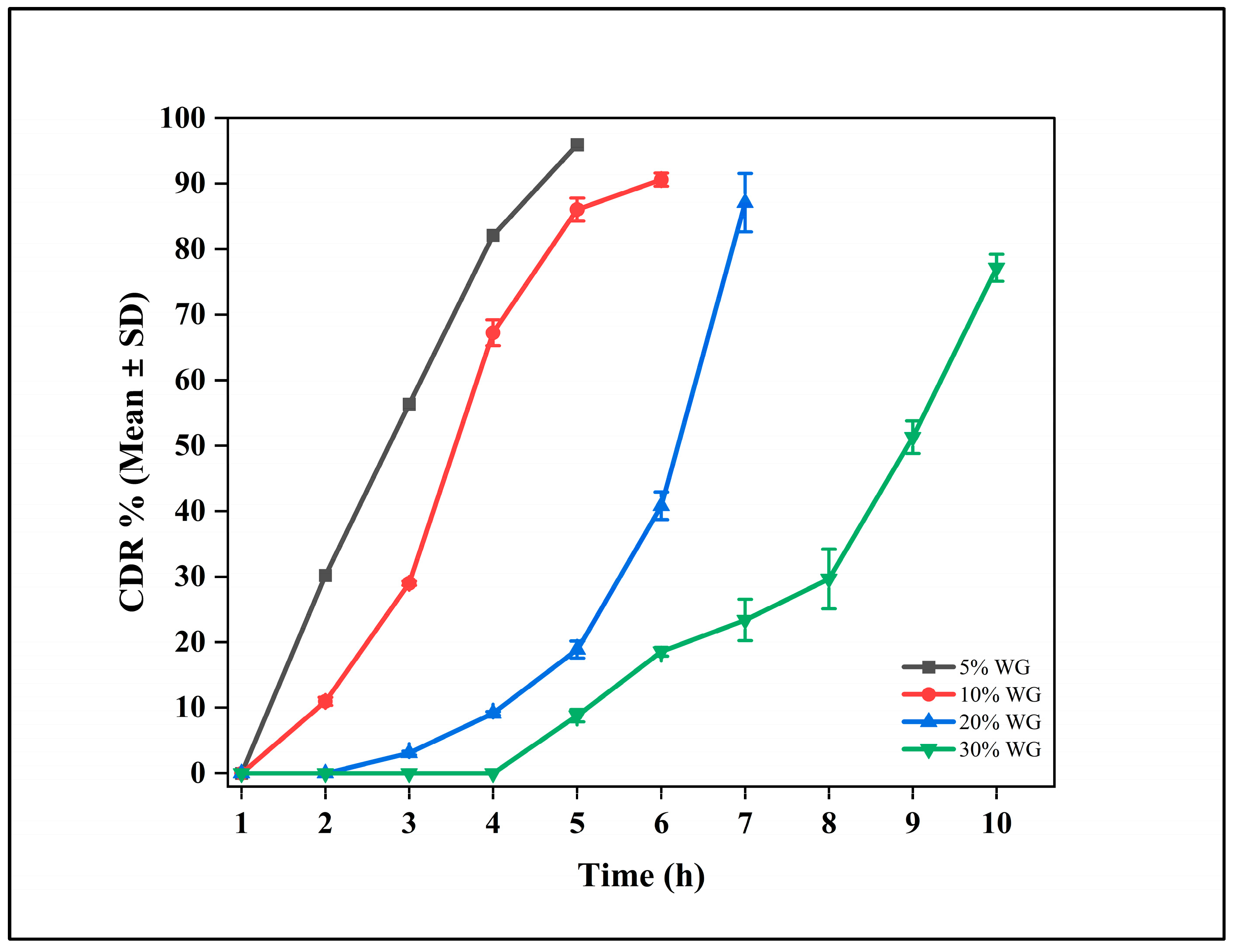
3.4. Delayed Release Formulation
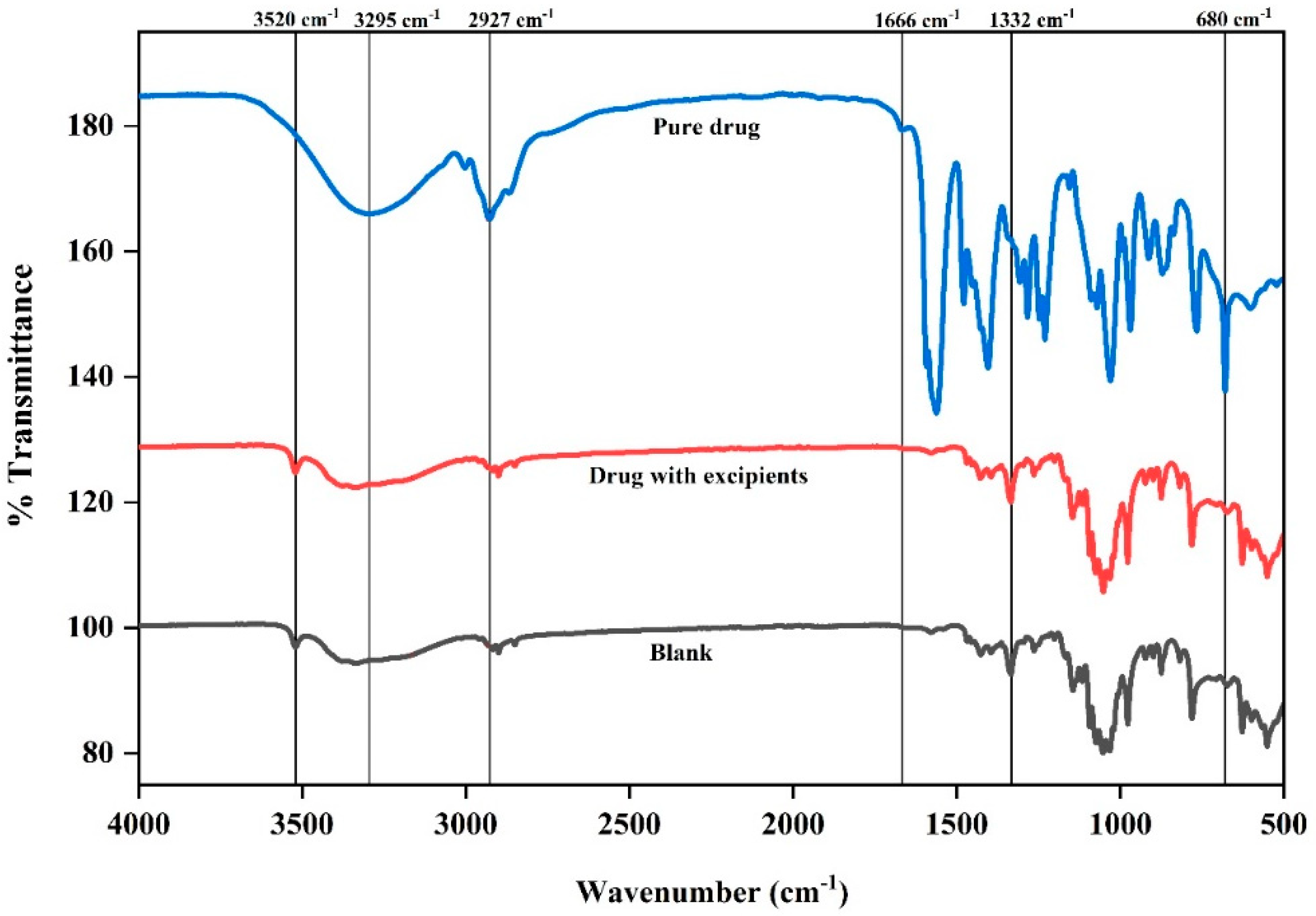
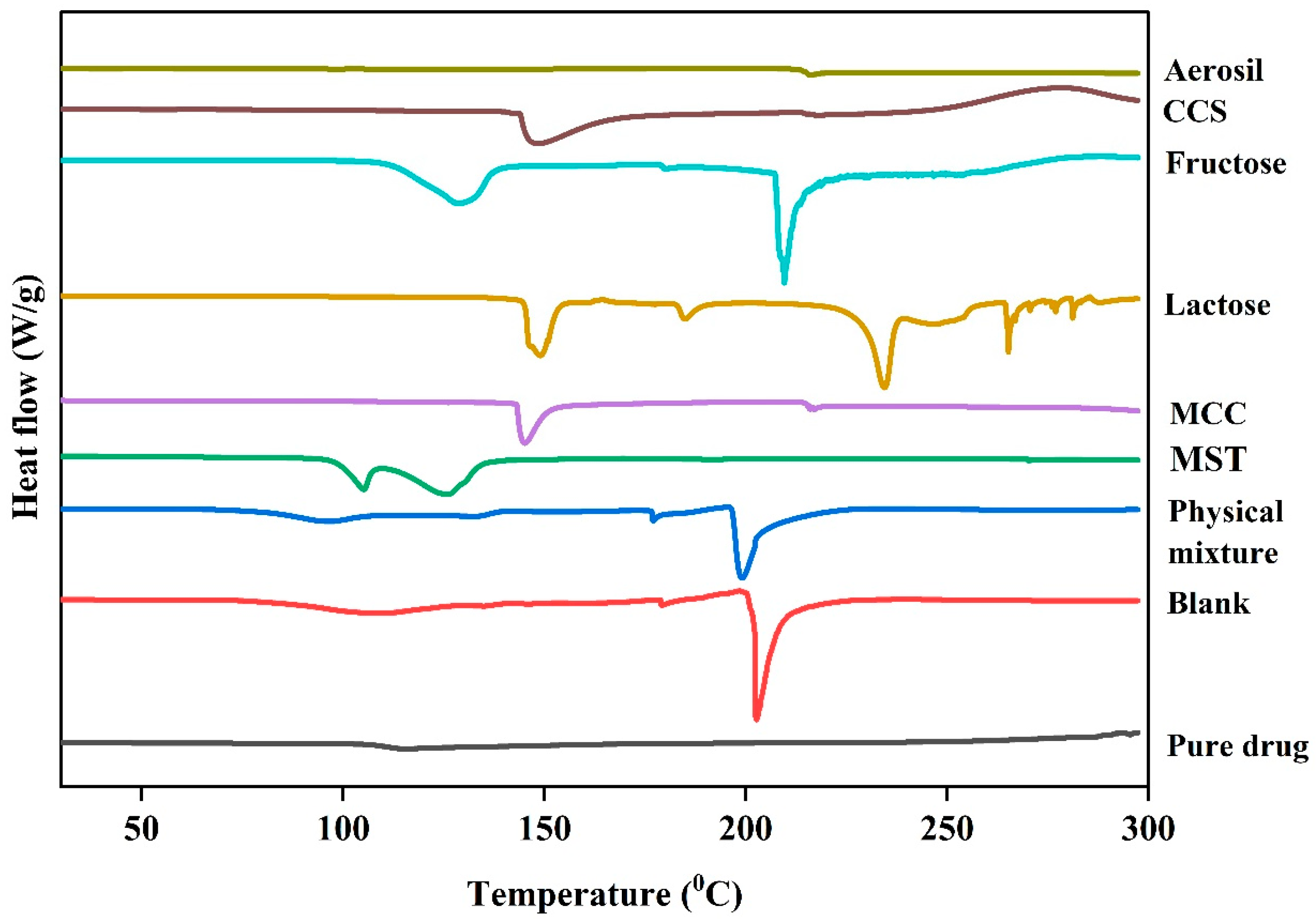
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.6. Differential Scanning Calorimetry (DSC)
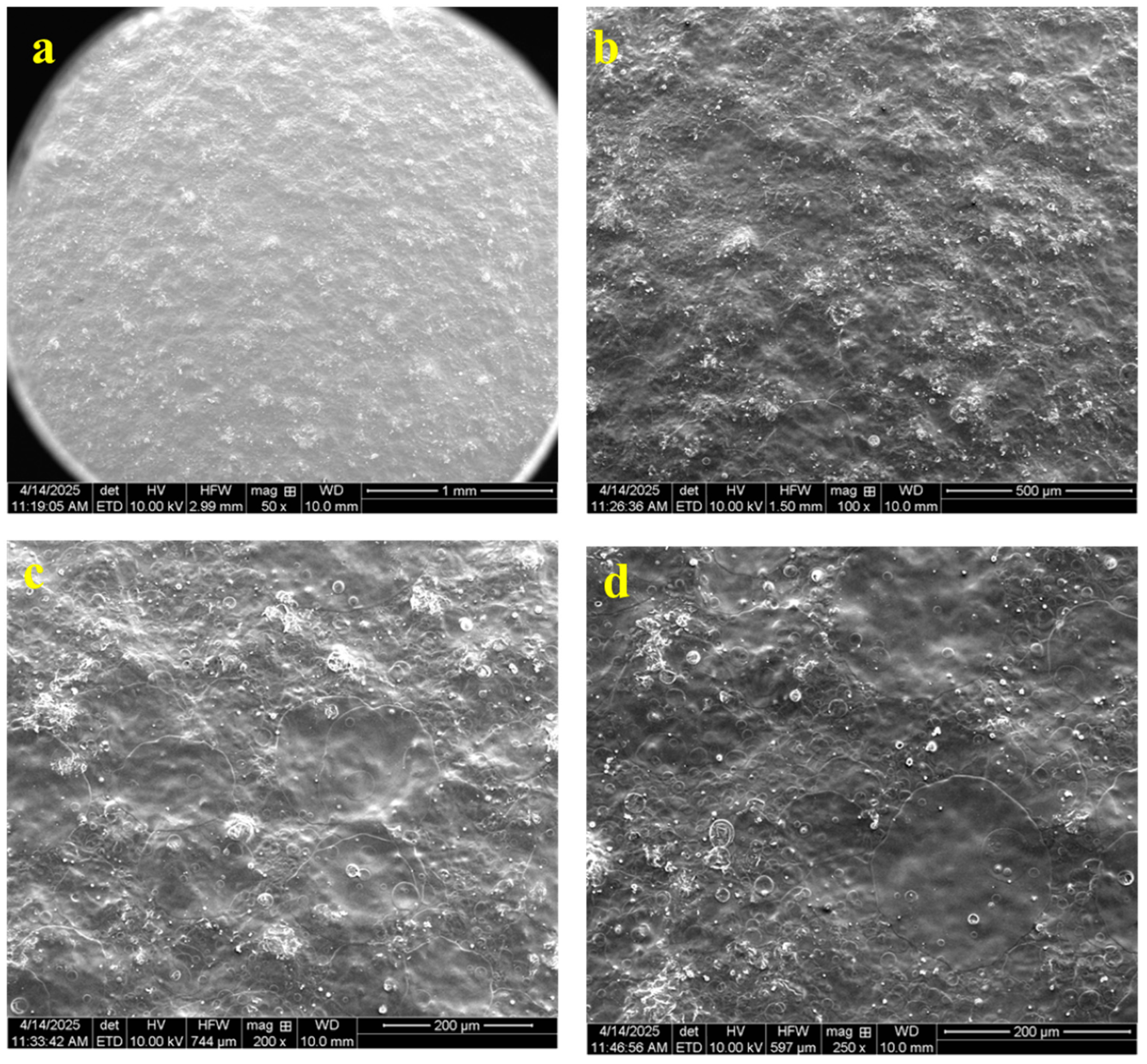
3.7. Scanning Electron Microscopy (SEM)
3.8. Mathematical Model for Drug Release Mechanism
3.9. Immediate Release (IR) Tablets
3.10. Delayed Release (DR) Tablets
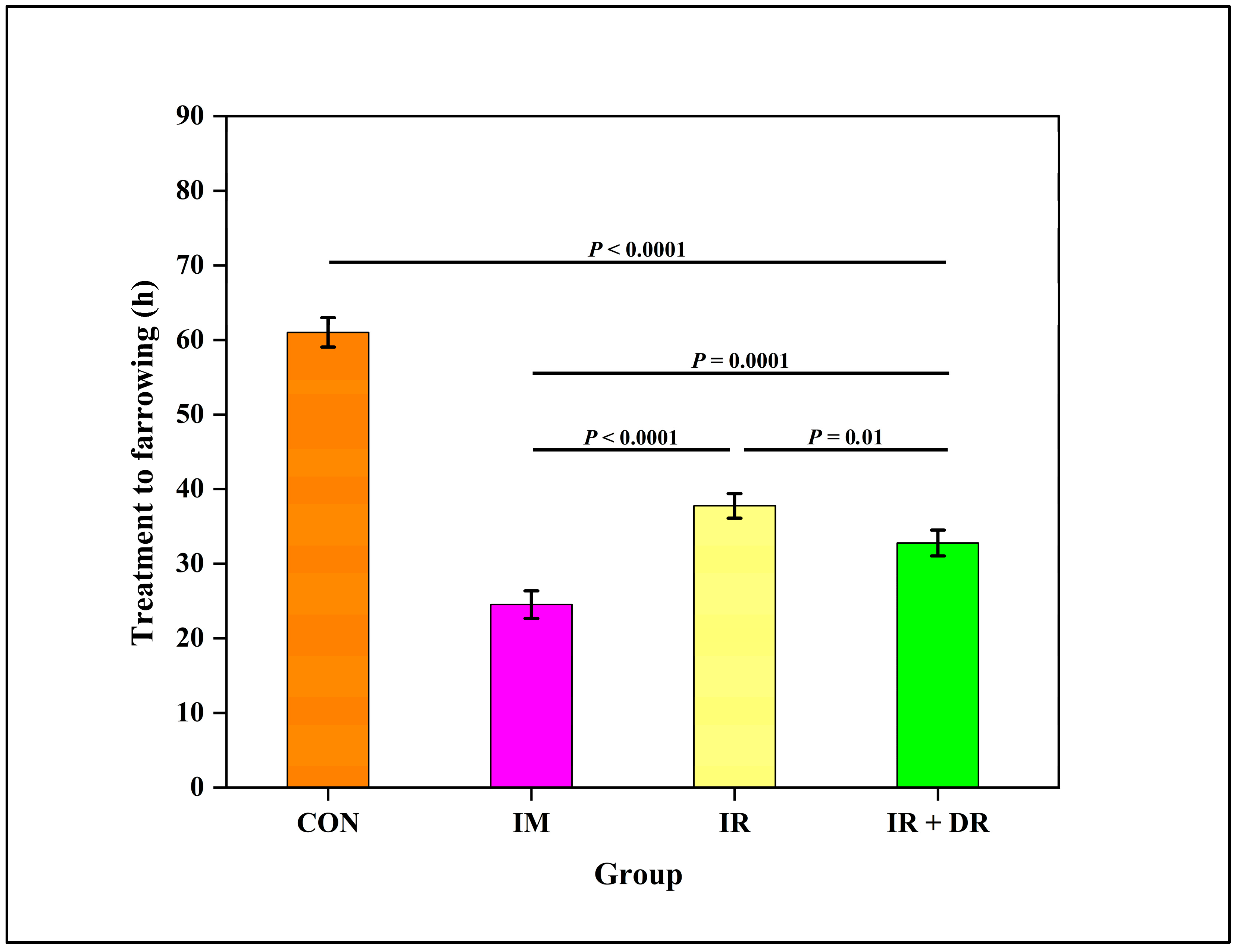
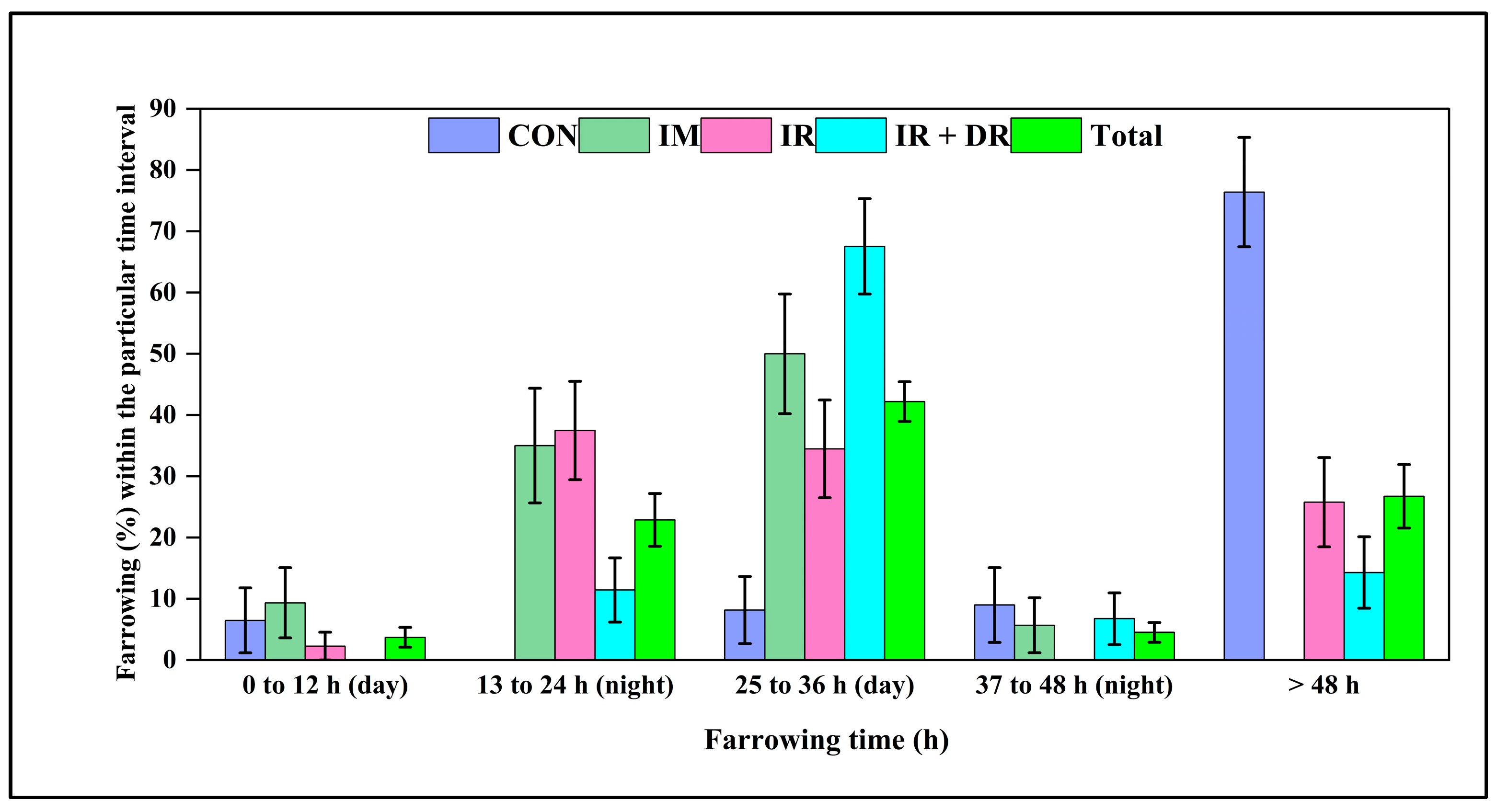
3.11. In Vivo Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero, M.; Calvo, L.; Morales, J.I.; Rodriguez, A.I.; Escudero, R.M.; Olivares, A.; Lopez-Bote, C. Short-and Long-Term Effects of Birth Weight and Neonatal Care in Pigs. Animals 2022, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Edwards, S. Improving the performance of neonatal piglets. Animal 2022, 16, 100350. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, R.N.; Thacker, P.A.; Aherne, F.X.; Goonewardene, L.A. The effect of dose and route of administration of prostaglandin F2α on the parturient response of sows. J. Swine Health Prod. 1996, 4, 123–126. [Google Scholar]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Theil, P.K.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Zhuo, Y.; Wu, F.; Jiang, X.; et al. The differences in energy metabolism and redox status between sows with short and long farrowing duration. Animal 2021, 15, 100355. [Google Scholar] [CrossRef]
- Schoos, A.; Muro, B.B.D.; Carnevale, R.F.; Chantziaras, I.; Biebaut, E.; Janssens, G.P.J.; Maes, D. Relationship between piglets’ survivability and farrowing kinetics in hyper-prolific sows. Porc. Health Manag. 2023, 9, 37. [Google Scholar] [CrossRef]
- Nguyen, K.; Cassar, G.; Friendship, R.; Dewey, C.; Farzan, A.; Kirkwood, R. Stillbirth and preweaning mortality in litters of sows induced to farrow with supervision compared to litters of naturally farrowing sows with minimal supervision. J. Swine Health Prod. 2011, 19, 214–217. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Muro, B.B.; Poor, A.P.; Leal, D.F.; Carnevale, R.F.; Shiroma, M.P.; Almond, G.W.; Garbossa, C.A.; Moreno, A.M.; Viana, C.H. Effects of farrowing induction with prostaglandins on farrowing traits and piglet performance: A systematic review and meta-analysis. Theriogenology 2022, 180, 1–16. [Google Scholar] [CrossRef]
- Kirkwood, R.N. Induction of parturition in sows. Thai J. Vet. Med. 2015, 45, 487–491. [Google Scholar] [CrossRef]
- Kirkwood, R.N.; Aherne, F.X. Increasing the predictability of cloprostenol-induced farrowing in sows. J. Swine Health Prod. 1998, 6, 57–59. [Google Scholar]
- Cassar, G.; Kirkwood, R.N.; Friendship, R.; Poljak, Z. Sow and litter performance following farrowing induction with prostaglandin: Effect of adjunct treatments with dexamethasone or oxytocin. J. Swine Health Prod. 2005, 13, 81–85. [Google Scholar] [CrossRef]
- Robert, S.; Passille, A.-M.D.; St-Pierre, N.; Pelletier, G.; Petitclerc, D.; Dubreuil, P.; Brazeau, P. Effect of the stress of injections on the serum concentration of cortisol, prolactin, and growth hormone in gilts and lactating sows. Can. J. Anim. Sci. 1989, 69, 663–672. [Google Scholar] [CrossRef]
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef]
- Sahoo, C.K.; Nayak, P.K.; Sarangi, D.K.; Sahoo, T.K. Intra vaginal drug delivery system: An overview. Am. J. Adv. Drug Deliv. 2013, 1, 43–55. [Google Scholar]
- Kalíková, K.; Tesařová, E.; Bosáková, Z. HPLC method for enantioselective analysis of cloprostenol. J. Pharm. Biomed. Anal. 2008, 46, 892–897. [Google Scholar] [CrossRef]
- Ward, S.A.; Kirkwood, R.N.; Plush, K.J.; Abdella, S.; Song, Y.; Garg, S. Development of a novel vaginal drug delivery system to control time of farrowing and allow supervision of piglet delivery. Pharmaceutics 2022, 14, 340. [Google Scholar] [CrossRef]
- El-Badry, M.; Alanazi, F.K.; Mahrous, G.M.; Alsarra, I.A. Effects of Kollicoat IR and hydroxypropyl-β-cyclodextrin on the dissolution rate of omeprazole from its microparticles and enteric-coated capsules. Pharm. Dev. Technol. 2010, 15, 500–510. [Google Scholar] [CrossRef]
- Beissner, B.; Porter, S.C.; Williamson, J.; Greenamoyer, J.; Dürig, T. Delayed-release diclofenac sodium tablets: Scaling up the coating process (Pharmaceutical Technology Report PTR-100). Ashland Consum. Spec. 2016. [Google Scholar]
- Nair, A.B.; Gupta, R.; Kumria, R.; Jacob, S.; Attimarad, M. Formulation and evaluation of enteric coated tablets of proton pump inhibitor. J. Basic Clin. Pharm. 2010, 1, 215. [Google Scholar] [PubMed]
- USP. Uniformity of Dosage Units; United States Pharmacopeia: Rockville, MD, USA, 2023. [Google Scholar]
- Battu, S.K.; Repka, M.A.; Majumdar, S.; Rao Y, M. Formulation and evaluation of rapidly disintegrating fenoverine tablets: Effect of superdisintegrants. Drug Dev. Ind. Pharm. 2007, 33, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Gohel, M.; Patel, M.; Amin, A.; Agrawal, R.; Dave, R.; Bariya, N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPS PharmSciTech 2004, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.R.; Zhou, J.P.; Zou, A.F.; Li, W.Z.; Yao, C.L.; Xie, S.F. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Abdella, S.; Kim, S.; Afinjuomo, F.; Song, Y.; Upton, R.; Garg, S. Combining the potential of 3D printed buccal films and nanostructured lipid carriers for personalised cannabidiol delivery. Drug. Deliv. Transl. Res. 2024, 14, 984–1004. [Google Scholar] [CrossRef]
- Hue, D.T.; Williams, J.L.; Petrovski, K.; Bottema, C.D. Predicting colostrum and calf blood components based on refractometry. J. Dairy Res. 2021, 88, 194–200. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Xie, J.; Xu, J.; Yue, P.; Yang, M. Novel nanocrystal-based solid dispersion with high drug loading, enhanced dissolution, and bioavailability of andrographolide. Int. J. Nanomed. 2018, 13, 3763–3779. [Google Scholar] [CrossRef] [PubMed]
- Kalinkova, G.N. Studies of beneficial interactions between active medicaments and excipients in pharmaceutical formulations. Int. J. Pharm. 1999, 187, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J. Evaluation of mathematical models describing drug release from estradiol transdermal systems. Drug Dev. Ind. Pharm. 2003, 29, 89–97. [Google Scholar] [CrossRef]
- Khamanga, S.M.; Walker, R.B. In vitro dissolution kinetics of captopril from microspheres manufactured by solvent evaporation. Dissolut. Technol. 2012, 19, 42–51. [Google Scholar] [CrossRef]
- Biswas, S.; Chattopadhyay, M.; Sen, K.K.; Saha, M.K. Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice. Carbohydr. Polym. 2015, 121, 403–410. [Google Scholar] [CrossRef]
- Li-Fang, F.; Wei, H.; Yong-Zhen, C.; Bai, X.; Qing, D.; Feng, W.; Min, Q.; De-Ying, C. Studies of chitosan/Kollicoat SR 30D film-coated tablets for colonic drug delivery. Int. J. Pharm. 2009, 375, 8–15. [Google Scholar] [CrossRef]
- Palmer, D.; Levina, M.; Douroumis, D.; Maniruzzaman, M.; Morgan, D.J.; Farrell, T.P.; Rajabi-Siahboomi, A.R.; Nokhodchi, A. Mechanism of synergistic interactions and its influence on drug release from extended release matrices manufactured using binary mixtures of polyethylene oxide and sodium carboxymethylcellulose. Colloids Surf. B Biointerfaces 2013, 104, 174–180. [Google Scholar] [CrossRef]
- Rojas, J.; Guisao, S.; Ruge, V. Functional assessment of four types of disintegrants and their effect on the spironolactone release properties. AAPS PharmSciTech 2012, 13, 1054–1062. [Google Scholar] [CrossRef]
- Zhao, N.; Augsburger, L.L. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets. AAPS PharmSciTech 2005, 6, E120–E126. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.; McGinity, J.W. Aqueous polymeric film coating. In Pharmaceutical Dosage Forms-Tablets; CRC Press: Boca Raton, FL, USA, 2008; pp. 415–454. [Google Scholar]
- Fan, T.; Wei, S.; Yan, W.; Chen, D.; Li, J. An investigation of pulsatile release tablets with ethylcellulose and Eudragit L as film coating materials and cross-linked polyvinylpyrrolidone in the core tablets. J. Control Release 2001, 77, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Friendship, R.M.; Templeton, C.L.; Deckert, A.E. An evaluation of vulvomucosal injections of prostaglandins for induction of parturition in swine. Can. Vet. J. 1990, 31, 433. [Google Scholar] [PubMed]
| Compositions | List of Formulation | |||||||
|---|---|---|---|---|---|---|---|---|
| CLIR1 | CLIR2 | CLIR3 | CLIR4 | CLIR5 | CLIR6 | CLIR7 | CLIR8 | |
| Fructose (mg) | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 |
| Lactose (mg) | 24.0 | 21.5 | 18.5 | 16.5 | 24.0 | 21.5 | 18.5 | 16.5 |
| MCC (mg) | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 |
| CCS (mg) | 2.5 | 5.0 | 8.0 | 10.0 | 0 | 0 | 0 | 0 |
| CPV (mg) | 0 | 0 | 0 | 0 | 2.5 | 5.0 | 8.0 | 10.0 |
| Aerosil 200 (mg) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| MST (mg) | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Weight per tablet (mg/tab) | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 |
| Formulations No. | List of Polymers | ||
|---|---|---|---|
| Eudragit RS 100 (%) | Eudragit RL 100 (%) | HPMC E4M (%) | |
| CLDR1 | – | 70 | 30 |
| CLDR2 | – | 50 | 50 |
| CLDR3 | – | 30 | 70 |
| CLDR4 | 70 | – | 30 |
| CLDR5 | 50 | – | 50 |
| CLDR6 | 30 | – | 70 |
| CLDR7 | 55 | 22.5 | 22.5 |
| CLDR8 | 50 | 25 | 25 |
| Formulations | Weight Variations (mg) | Hardness (kgf) | Thickness (mm) | Friability (%) | Disintegration Time (min) |
|---|---|---|---|---|---|
| CLIR1 | 113.16 ± 2.34 | 6.40 ± 0.11 | 3.01 ± 0.05 | 0.33 | 3.50 ± 0.04 |
| CLIR2 | 122.60 ± 2.76 | 5.92 ± 0.18 | 3.24 ± 0.08 | 0.37 | 2.10 ± 0.04 |
| CLIR3 | 124.83 ± 6.10 | 6.01 ± 0.10 | 3.45 ± 0.15 | 0.36 | 1.27 ± 0.02 |
| CLIR4 | 121.87 ± 2.46 | 5.75 ± 0.09 | 3.20 ± 0.04 | 0.35 | 0.62 ± 0.04 |
| CLIR5 | 130.24 ± 2.02 | 5.95 ± 0.09 | 3.51 ± 0.05 | 0.62 | 5.29 ± 0.08 |
| CLIR6 | 131.51 ± 1.99 | 5.98 ± 0.08 | 3.56 ± 0.04 | 0.57 | 4.26 ± 0.02 |
| CLIR7 | 123.71 ± 2.21 | 5.87 ± 0.08 | 3.22 ± 0.03 | 0.73 | 2.94 ± 0.17 |
| CLIR8 | 124.37 ± 2.72 | 5.90 ± 0.11 | 3.29 ± 0.07 | 0.73 | 1.37 ± 0.02 |
| Formulations | Batch No | Desired Drug Amount (µg) | Average Drug Amount (µg) | Acceptance Value (L1 ≤ 15) |
|---|---|---|---|---|
| CL55IR4 | CL55IR4B1 | 100 | 99.75 ± 0.12 | 0.33 |
| CL55IR4B2 | 100 | 100.98 ± 2.09 | 5.44 | |
| CL55IR4B3 | 100 | 100.03 ± 0.90 | 2.17 |
| Model Name | Parameter | IR Tablet | DR Tablet |
|---|---|---|---|
| Zero order | R2-adjusted | –19.22 | 0.56 |
| AIC | 36.66 | 57.41 | |
| MSC | –3.50 | 0.35 | |
| First order | R2-adjusted | 0.94 | 0.48 |
| AIC | 13.33 | 58.55 | |
| MSC | 2.32 | 0.19 | |
| Higuchi | R2-adjusted | –3.87 | 0.32 |
| AIC | 30.96 | 60.45 | |
| MSC | –2.08 | −0.07 | |
| Hixson–Crowell | R2-adjusted | –0.06 | 0.50 |
| AIC | 24.89 | 58.23 | |
| MSC | –0.56 | 0.24 | |
| Hopfenberg | R2-adjusted | 0.91 | 0.96 |
| AIC | 15.33 | 40.18 | |
| MSC | 1.82 | 2.81 | |
| Makoid–Banakar | R2-adjusted | 0.99 | 0.99 |
| AIC | 2.32 | 11.31 | |
| MSC | 5.07 | 6.943 | |
| Peppas–Sahlin | R2-adjusted | 0.99 | 0.99 |
| AIC | 2.38 | 12.31 | |
| MSC | 5.06 | 6.80 | |
| Weibull | R2-adjusted | 0.97 | 0.98 |
| AIC | 9.05 | 35.56 | |
| MSC | 3.39 | 3.47 | |
| Korsmeyer–Peppas | R2-adjusted | 0.88 | 0.99 |
| AIC | 16.48 | 20.30 | |
| MSC | 1.53 | 5.65 |
| Parameter | Control | IM | IR | IR + DR |
|---|---|---|---|---|
| Total born | 13.55 ± 0.79 | 13.09 ± 0.71 | 14.72 ± 0.65 | 13.22 ± 0.69 |
| Stillbirth | 0.39 ± 0.27 | 0.40 ± 0.24 | 0.88 ± 0.21 | 0.37 ± 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, A.M.; Taechamaeteekul, P.; Petrovski, K.R.; Tummaruk, P.; Song, Y.; Garg, S.; Kirkwood, R.N. Development of Timed Release Vaginal Mucosal Cloprostenol for Farrowing Management in Sows. Pharmaceutics 2025, 17, 1198. https://doi.org/10.3390/pharmaceutics17091198
Uddin AM, Taechamaeteekul P, Petrovski KR, Tummaruk P, Song Y, Garg S, Kirkwood RN. Development of Timed Release Vaginal Mucosal Cloprostenol for Farrowing Management in Sows. Pharmaceutics. 2025; 17(9):1198. https://doi.org/10.3390/pharmaceutics17091198
Chicago/Turabian StyleUddin, AHM Musleh, Preechaphon Taechamaeteekul, Kiro R. Petrovski, Padet Tummaruk, Yunmei Song, Sanjay Garg, and Roy N. Kirkwood. 2025. "Development of Timed Release Vaginal Mucosal Cloprostenol for Farrowing Management in Sows" Pharmaceutics 17, no. 9: 1198. https://doi.org/10.3390/pharmaceutics17091198
APA StyleUddin, A. M., Taechamaeteekul, P., Petrovski, K. R., Tummaruk, P., Song, Y., Garg, S., & Kirkwood, R. N. (2025). Development of Timed Release Vaginal Mucosal Cloprostenol for Farrowing Management in Sows. Pharmaceutics, 17(9), 1198. https://doi.org/10.3390/pharmaceutics17091198










