Optimizing Dose Conversion from IR-Tac to LCP-Tac Formulations in Renal Transplant Recipients: A Population Pharmacokinetic Modeling Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Blood Sampling and Data Recording
2.3. Tacrolimus Measurement
2.4. Genotyping
2.5. Statistical Analysis
2.6. Population Pharmacokinetic Analysis
2.6.1. Base Model Development
2.6.2. Covariate Analysis
2.7. Model Evaluation and Internal Validation
2.8. Simulations
3. Results
3.1. Patient Characteristics and Datasets
3.2. Population PK Analysis
3.2.1. Base Model
3.2.2. Covariate Model
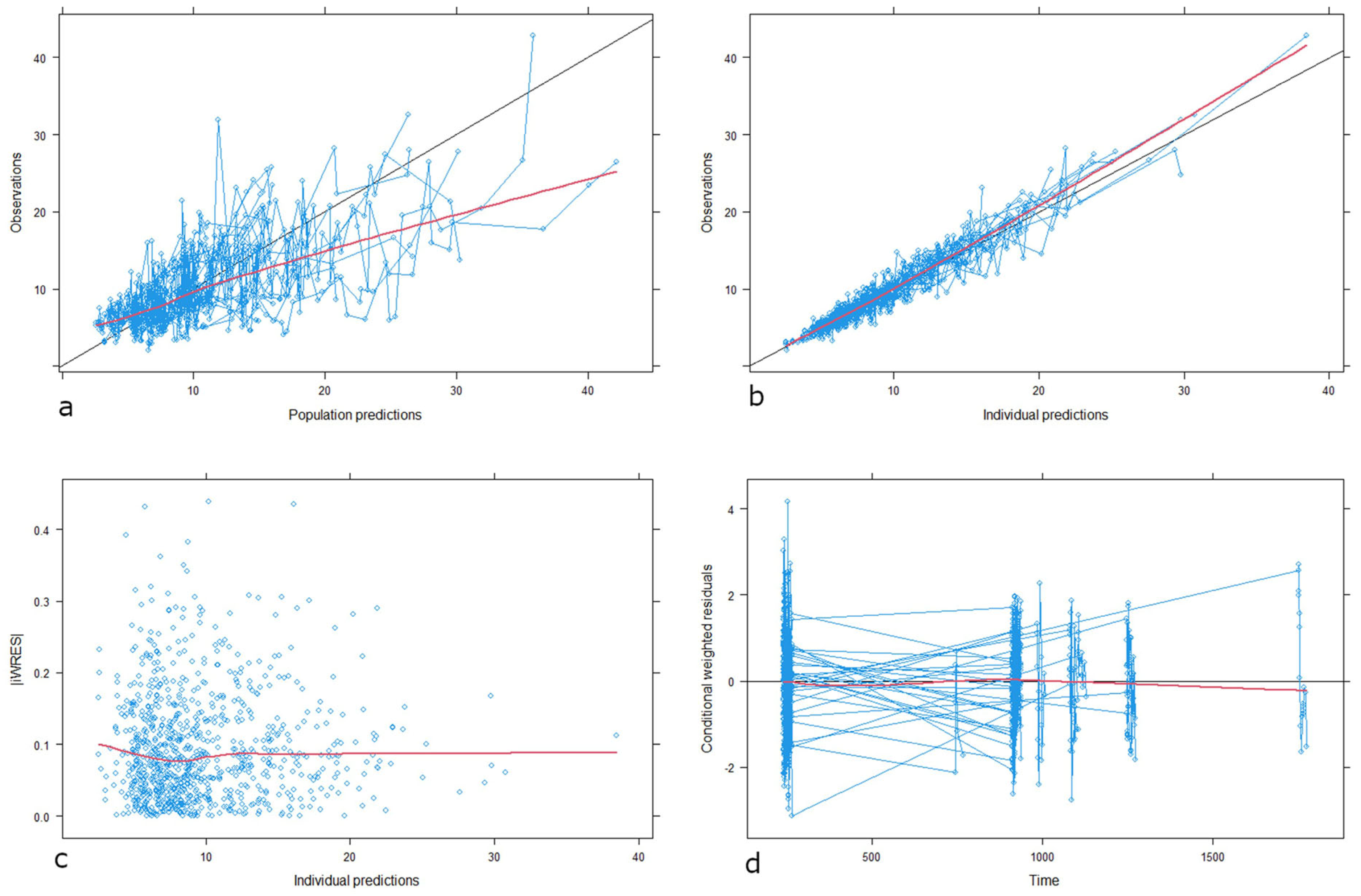
3.2.3. Model Evaluation
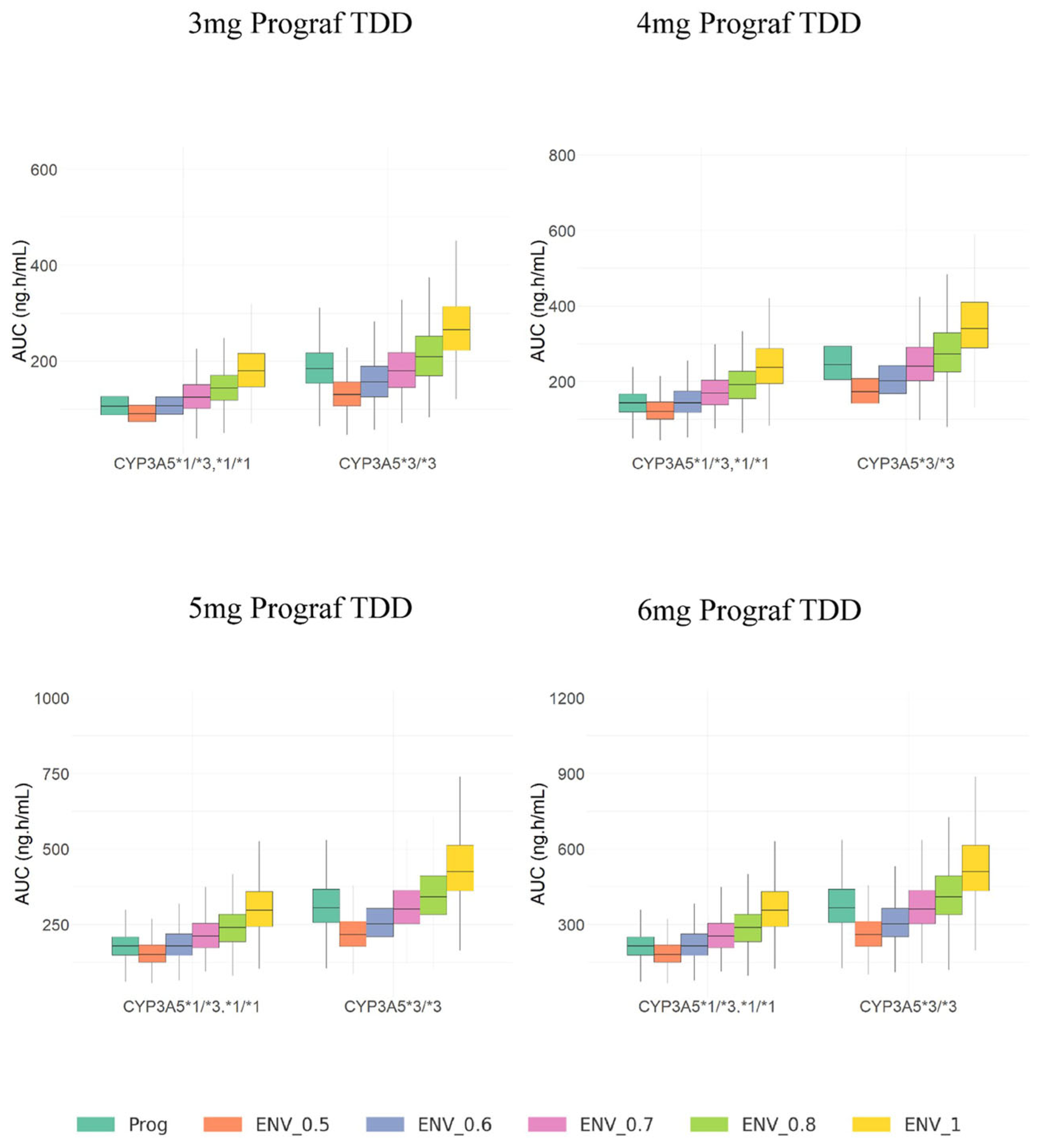
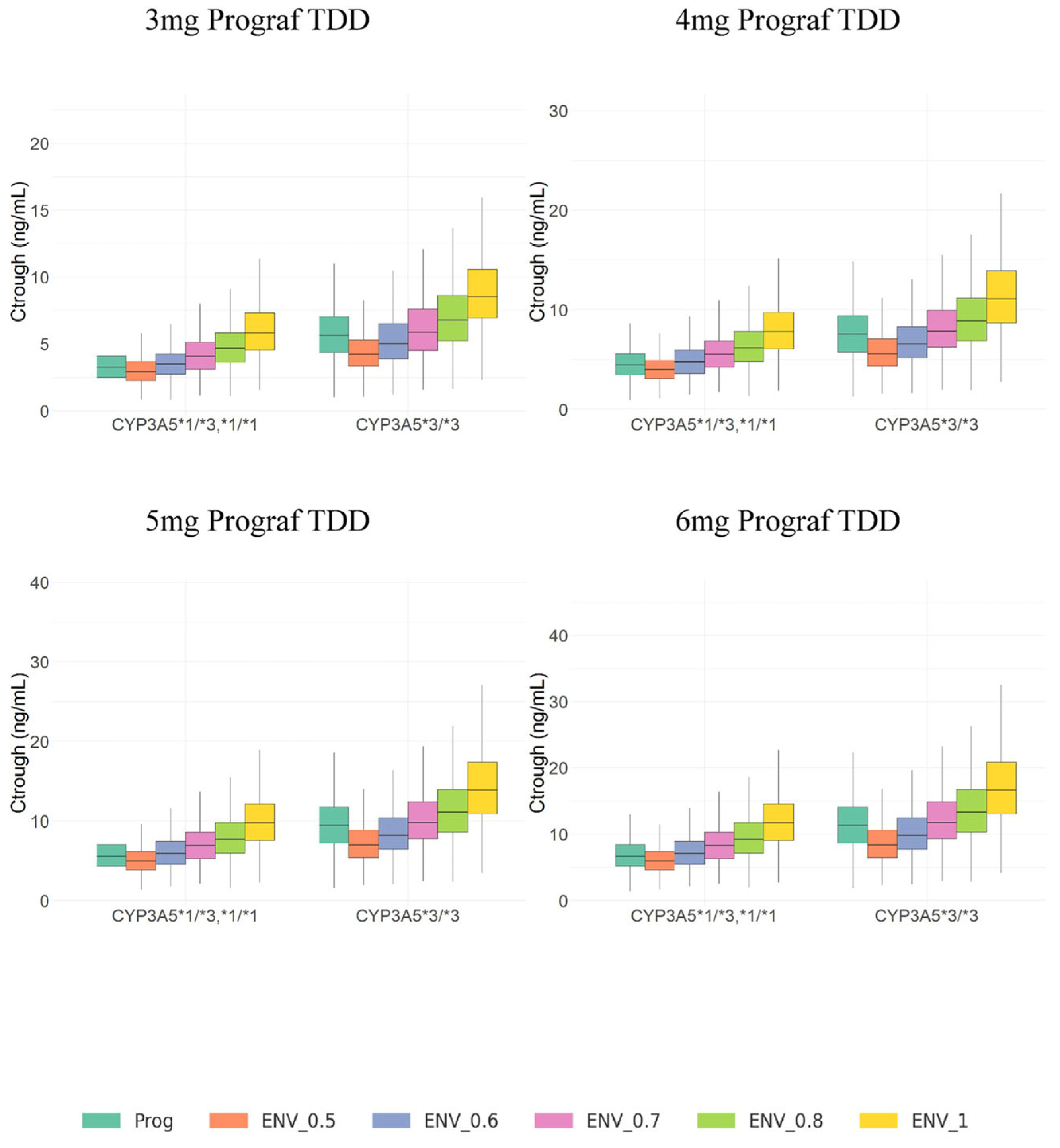
3.3. Model Simulations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuypers, D.R.J. Intrapatient Variability of Tacrolimus Exposure in Solid Organ Transplantation: A Novel Marker for Clinical Outcome. Clin. Pharmacol. Ther. 2020, 107, 347–358. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin. Pharmacokinet. 2004, 43, 623–653. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Swaminathan, A.; Prasad, T.; Jain, A.; Zuckerman, S.; Warty, V.; McMichael, J.; Lever, J.; Burckart, G.; Starzl, T. Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 1995, 29, 404–430. [Google Scholar] [CrossRef]
- Katari, S.R.; Magnone, M.; Shapiro, R.; Jordan, M.; Scantlebury, V.; Vivas, C.; Gritsch, A.; McCauley, J.; Starzl, T.; Demetris, A.J.; et al. Clinical features of acute reversible tacrolimus (FK 506) nephrotoxicity in kidney transplant recipients. Clin. Transplant. 1997, 11, 237. [Google Scholar] [CrossRef]
- Tremblay, S.; Nigro, V.; Weinberg, J.; Woodle, E.S.; Alloway, R.R. A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am. J. Transplant. 2017, 17, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Wallemacq, P.E.; Verbeeck, R.K. Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin. Pharmacokinet. 2001, 40, 283–295. [Google Scholar] [CrossRef]
- Wallemacq, P.E.; Furlan, V.; Möller, A.; Schäfer, A.; Stadler, P.; Firdaous, I.; Taburet, A.-M.; Reding, R.; Clement De Clety, S.; De Ville De Goyet, J.; et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur. J. Drug Metab. Pharmacokinet. 1998, 23, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Glicklich, A.; Weinberg, J. Improved Bioavailability of MELTDOSE Once-Daily Formulation of Tacrolimus (LCP-Tacro) with Controlled Agglomeration Allows for Consistent Absorption over 24 Hrs: A Scintigraphic and Pharmacokinetic Evaluation [abstract]. Am. J. Transpl. 2013, 13 (Suppl. 5), 339. [Google Scholar]
- Rostaing, L.; Bunnapradist, S.; Grinyó, J.M.; Ciechanowski, K.; Denny, J.E.; Silva, H.T., Jr.; Budde, K.; Envarsus Study Group. Novel Once-Daily Extended-Release Tacrolimus Versus Twice-Daily Tacrolimus in De Novo Kidney Transplant Recipients: Two-Year Results of Phase 3, Double-Blind, Randomized Trial. Am. J. Kidney Dis. 2016, 67, 648–659. [Google Scholar] [CrossRef]
- Trofe-Clark, J.; Brennan, D.C.; West-Thielke, P.; Milone, M.C.; Lim, M.A.; Neubauer, R.; Nigro, V.; Bloom, R.D. Results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients. Am. J. Kidney Dis. 2018, 71, 315–326. [Google Scholar] [CrossRef]
- Langone, A.; Steinberg, S.M.; Gedaly, R.; Chan, L.K.; Shah, T.; Sethi, K.D.; Nigro, V.; Morgan, J.C.; STRATO Investigators. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP-TacrO (STRATO): An open-label, multicenter, prospective phase 3b study. Clin. Transplant. 2015, 29, 796–805. [Google Scholar] [CrossRef]
- Gaber, A.O.; Alloway, R.R.; Bodziak, K.; Kaplan, B.; Bunnapradist, S. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): A phase 2 trial of stable renal transplant recipients. Transplantation 2013, 96, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fontova, P.; Colom, H.; Rigo-Bonnin, R.; Bestard, O.; Vidal-Alabró, A.; van Merendonk, L.N.; Cerezo, G.; Polo, C.; Montero, N.; Melilli, E.; et al. Sustained Inhibition of Calcineurin Activity With a Melt-Dose Once-daily Tacrolimus Formulation in Renal Transplant Recipients. Clin. Pharmacol. Ther. 2021, 110, 238–247. [Google Scholar] [CrossRef]
- Bunnapradist, S.; Ciechanowski, K.; West-Thielke, P.; Mulgaonkar, S.; Rostaing, L.; Vasudev, B.; Budde, K. Conversion From Twice-Daily Tacrolimus to Once-Daily Extended Release Tacrolimus (LCPT): The Phase III Randomized MELT Trial. Am. J. Transplant. 2013, 13, 760. [Google Scholar] [CrossRef]
- Budde, K.; Bunnapradist, S.; Grinyó, J.M.; Ciechanowski, K.; Denny, J.E.; Silva, H.T.; Rostaing, L.; Envarsus Study Group. Novel Once-Daily Extended-Release Tacrolimus (LCPT) Versus Twice-Daily Tacrolimus in De Novo Kidney Transplants: One-Year Results of Phase III, Double-Blind, Randomized Trial. Am. J. Transplant. 2014, 14, 2796–2806. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Clinical Pharmacokinetics of Once-Daily Tacrolimus in Solid-Organ Transplant Patients. Clin. Pharmacokinet. 2015, 54, 993–1025. [Google Scholar] [CrossRef]
- Lampen, A.; Christians, U.; Guengerich, F.P.; Watkins, P.B.; Kolars, J.C.; Bader, A.; Gonschior, A.K.; Dralle, H.; Hackbarth, I.; Sewing, K.F. Metabolism of the immunosuppressant tacrolimus in the small intestine: Cytochrome P450, drug interactions, and interindividual variability. Drug Metab. Dispos. 1995, 23, 1315–1324. [Google Scholar] [CrossRef]
- Iwasaki, K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab. Pharmacokinet. 2007, 22, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Shiraga, T.; Matsuda, H.; Teramura, Y.; Kawamura, A.; Hata, T.; Ninomiya, S.; Esumi, Y. Absorption, Distribution, Metabolism and Excretion of Tacrolimus (FK506) in the Rat. Drug Metab. Pharmacokinet. 1998, 13, 259–265. [Google Scholar] [CrossRef]
- Lloberas, N.; Vidal-Alabró, A.; Colom, H. Customizing Tacrolimus Dosing in Kidney Transplantation: Focus on Pharmacogenetics. Ther. Drug Monit. 2025, 47, 141–151. [Google Scholar] [CrossRef]
- Dai, Y.; Hebert, M.F.; Isoherranen, N.; Davis, C.L.; Marsh, C.; Shen, D.D.; Thummel, K.E. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab. Dispos. 2006, 34, 836–847. [Google Scholar] [CrossRef]
- De Jonge, H.; De Loor, H.; Verbeke, K.; Vanrenterghem, Y.; Kuypers, D.R. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin. Pharmacol. Ther. 2012, 92, 366–375. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef]
- Andreu, F.; Colom, H.; Elens, L.; van Gelder, T.; van Schaik, R.H.N.; Hesselink, D.A.; Bestard, O.; Torras, J.; Cruzado, J.M.; Grinyó, J.M.; et al. A New CYP3A5*3 and CYP3A4*22 Cluster Influencing Tacrolimus Target Concentrations: A Population Approach. Clin. Pharmacokinet. 2017, 56, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Woillard, J.B.; De Winter, B.C.M.; Kamar, N.; Marquet, P.; Rostaing, L.; Rousseau, A. Population pharmacokinetic model and Bayesian estimator for two tacrolimus formulations--twice daily Prograf and once daily Advagraf. Br. J. Clin. Pharmacol. 2011, 71, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Henin, E.; Govoni, M.; Cella, M.; Laveille, C.; Piotti, G. Therapeutic Drug Monitoring Strategies for Envarsus in De Novo Kidney Transplant Patients Using Population Modelling and Simulations. Adv. Ther. 2021, 38, 5317–5332. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Ali, Z.; Meertens, M.; Fernández, B.; Fontova, P.; Vidal-Alabró, A.; Rigo-Bonnin, R.; Melilli, E.; Cruzado, J.M.; Grinyó, J.M.; Colom, H.; et al. CYP3A5*3 and CYP3A4*22 Cluster Polymorphism Effects on LCP-Tac Tacrolimus Exposure: Population Pharmacokinetic Approach. Pharmaceutics 2023, 15, 2699. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- Crespo, E.; Vidal-Alabró, A.; Jouve, T.; Fontova, P.; Stein, M.; Mocka, S.; Meneghini, M.; Sefrin, A.; Hruba, P.; Gomà, M.; et al. Tacrolimus CYP3A Single-Nucleotide Polymorphisms and Preformed T- and B-Cell Alloimmune Memory Improve Current Pretransplant Rejection-Risk Stratification in Kidney Transplantation. Front. Immunol. 2022, 13, 869554. [Google Scholar] [CrossRef]
- Vidal-Alabró, A.; Fontova, P.; Rigo-Bonnin, R.; Mohammed Ali, Z.; Melilli, E.; Montero, N.; Manonelles, A.; Coloma, A.; Grinyó, J.M.; Cruzado, J.M.; et al.; Nephrology Department, Hospital Universitari de Bellvitge-IDIBELL, Barcelona, Spain 2025; manuscript in preparation; to be submitted.
- Rigo-Bonnin, R.; Arbiol-Roca, A.; de Aledo-Castillo, J.M.G.; Alía, P. Simultaneous Measurement of Cyclosporine A, Everolimus, Sirolimus and Tacrolimus Concentrations in Human Blood by UPLC–MS/MS. Chromatographia 2015, 78, 1459–1474. [Google Scholar] [CrossRef]
- Denney, W.; Duvvuri, S.; Buckeridge, C. Simple, Automatic Noncompartmental Analysis: The PKNCA R Package. J. Pharmacokinet. Pharmacodyn. 2015, 42, 11–107. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Investigation of Bioequivalence, Rev. 1. 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 19 August 2025).
- Davit, B.M.; Chen, M.-L.; Conner, D.P.; Haidar, S.H.; Kim, S.; Lee, C.H.; Lionberger, R.A.; Makhlouf, F.T.; Nwakama, P.E.; Patel, D.T.; et al. Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products by the US Food and Drug Administration. AAPS J. 2012, 14, 915–924. [Google Scholar] [CrossRef]
- Fernández-Alarcón, B.; Mohammed Ali, Z.; Fontova, P.; Vidal-Alabró, A.; Rigo-Bonnin, R.; Melilli, E.; Montero, N.; Manonelles, A.; Favà, A.; Coloma, A.; et al.; Nephrology Department, Hospital Universitari de Bellvitge-IDIBELL, Barcelona, Spain 2025; manuscript in preparation; to be submitted.
- Fontova, P.; Colom, H.; Rigo-Bonnin, R.; van Merendonk, L.N.; Vidal-Alabró, A.; Montero, N.; Melilli, E.; Meneghini, M.; Manonelles, A.; Cruzado, J.M.; et al. Influence of the Circadian Timing System on Tacrolimus Pharmacokinetics and Pharmacodynamics After Kidney Transplantation. Front. Pharmacol. 2021, 12, 636048. [Google Scholar] [CrossRef]
- Van Rongen, A.; Kervezee, L.; Brill, M.J.E.; van Meir, H.; den Hartigh, J.; Guchelaar, H.-J.; Meijer, J.H.; Burggraaf, J.; van Oosterhout, F. Population Pharmacokinetic Model Characterizing 24-Hour Variation in the Pharmacokinetics of Oral and Intravenous Midazolam in Healthy Volunteers. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef]
- Savic, R.M.; Karlsson, M.O. Importance of Shrinkage in Empirical Bayes Estimates for Diagnostics: Problems and Solutions. AAPS J. 2009, 11, 558–569. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- Comets, E.; Brendel, K.; Mentré, F. Computing Normalised Prediction Distribution Errors to Evaluate Nonlinear Mixed-Effect Models: The npde Add-On Package for R. Comput. Methods Programs Biomed. 2008, 90, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Andreu, F.; Colom, H.; Grinyó, J.M.; Torras, J.; Cruzado, J.M.; Lloberas, N. Development of a population PK model of tacrolimus for adaptive dosage control in stable kidney transplant patients. Ther. Drug Monit. 2015, 37, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alarcón, B.; Nolberger, O.; Vidal-Alabró, A.; Rigo-Bonnin, R.; Grinyó, J.M.; Melilli, E.; Montero, N.; Manonelles, A.; Coloma, A.; Favà, A.; et al. Guiding the starting dose of the once-daily formulation of tacrolimus in “de novo” adult renal transplant patients: A population approach. Front. Pharmacol. 2024, 15, 1456565. [Google Scholar] [CrossRef]
- Andrews, L.M.; Hesselink, D.A.; van Schaik, R.H.N.; van Gelder, T.; de Fijter, J.W.; Lloberas, N.; Elens, L.; Moes, D.J.A.R.; de Winter, B.C.M. A population pharmacokinetic model to predict the individual starting dose of tacrolimus in adult renal transplant recipients. Br. J. Clin. Pharmacol. 2019, 85, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Åsberg, A.; Midtvedt, K.; van Guilder, M.; Størset, E.; Bremer, S.; Bergan, S.; Jelliffe, R.; Hartmann, A.; Neely, M.N. Inclusion of CYP3A5 genotyping in a nonparametric population model improves dosing of tacrolimus early after transplantation. Transplant. Int. 2013, 26, 1198. [Google Scholar] [CrossRef]
- Sanghavi, K.; Brundage, R.C.; Miller, M.B.; Schladt, D.P.; Israni, A.K.; Guan, W.; Oetting, W.S.; Mannon, R.B.; Remmel, R.P.; Matas, A.J.; et al. Genotype-guided tacrolimus dosing in African-American kidney transplant recipients. Pharmacogenomics J. 2017, 17, 61–68. [Google Scholar] [CrossRef]
- Baccarani, U.; Velkoski, J.; Pravisani, R.; Adani, G.L.; Lorenzin, D.; Cherchi, V.; Falzone, B.; Baraldo, M.; Risaliti, A. MeltDose Technology vs. Once-Daily Prolonged Release Tacrolimus in De Novo Liver Transplant Recipients. Transplant. Proc. 2019, 51, 2971–2973. [Google Scholar] [CrossRef]
- Baraldo, M. Meltdose Tacrolimus Pharmacokinetics. Transplant. Proc. 2016, 48, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Tsunashima, D.; Kawamura, A.; Murakami, M.; Sawamoto, T.; Undre, N.; Brown, M.; Groenewoud, A.; Keirns, J.J.; Holman, J.; Connor, A.; et al. Assessment of tacrolimus absorption from the human intestinal tract: Open-label, randomized, 4-way crossover study. Clin. Ther. 2014, 36, 748–759. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | IR-Tac | LCP-Tac |
|---|---|---|
| Patients (n) | 30 | 30 |
| Samplings (n) | 481 | 451 |
| Gender Male/Female, (n/n) | 22/8 | 22/8 |
| Weight (Kg) | 72 (64–80) | 73 (64–80) |
| Age (Years) | 58 (48–68) | 58 (48–68) |
| BMI (Kg·m−2) | 26 (21.5–29.3) | 27 (21.5–29.3) |
| HTC (%) | 40.9 (37.6–44.8) | 40.1 (37.1–43) |
| GFR (mL·min−1) | 49.6 (34–57) | 49.3 (42–58) |
| Cr (μmol·L−1) | 141.9 (108–166) | 147.6 (111–155) |
| CYP3A5 Genotype | ||
| *1/*3 n (%) | 9 (30%) | 9 (30%) |
| *1/*1 n (%) | 1 (3%) | 1 (3%) |
| *3/*3 n (%) | 20 (67%) | 20 (67%) |
| Formulation/ Genotype Group | Dose (mg·day−1) | N | Ctrough (ng·mL−1) | Ctrough/D | AUC24 (ng·h·mL−1) | AUC24/D | Relative Bioavailability | p-Value |
|---|---|---|---|---|---|---|---|---|
| IR-Tac | ||||||||
| CYP3A5 *1/*1, *1/*3 | 5 (3–12) | 20 | 4.9 (4.6–5.2) | 1.6(1.4–2) | 195 (184–224) | 32 (27–43) | ||
| LCP-Tac | 0.60 | <0.001 * | ||||||
| CYP3A5 *1/*1, *1/*3 | 3.75 (2–8.5) | 10 | 5.6 (4.5–6.7) | 1.28 (0.9–1.8) | 232 (173–286) | 53 (38–71) | ||
| IR-Tac | ||||||||
| CYP3A5 *3/*3 | 3 (1.5–8) | 20 | 5.7 (4.7–7.2) | 3.6 (2.9–4.6) | 212 (169–250) | 68 (56–81) | <0.001 # | |
| LCP-Tac | 0.72 | |||||||
| CYP3A5 *3/*3 | 2 (1–4.75) | 10 | 5.7 (4.7–6.7) | 2.7 (2.2–3.3) | 199 (163–265) | 94 (76–122) |
| Final Model Parameter Estimates (RSE%) | Bootstrap Results * | |||
|---|---|---|---|---|
| Parameter | Description | Value | Bootstrap Median | 90% CI |
| Disposition PK parameters | ||||
| CL/F (L·h−1) | Apparent Elimination Clearance | 11.9 (8.5%) | 11.85 | 10.34–13.53 |
| Vc/F (L) | Apparent Distribution Volume of central compartment | 78 (14.7%) | 81 | 63–100.22 |
| CLd/F (L·h−1) | Apparent Distributional Clearance | 25.8 (8.5%) | 25.75 | 22.08–29.39 |
| Vp/F (L) | Apparent Distribution Volume of peripheral compartment | 500 FIX | - | - |
| Absorption parameters | ||||
| Ka IR-Tac | Absorption rate constant (IR-Tac) | 2.04 (40%) | 2.17 | 1.23–3.72 |
| Ka LCP_Tac | Absorption rate constant (LCP-Tac) | 0.111 (16.9%) | 0.115 | 0.08–0.15 |
| F LCP-Tac_PM | Reference group for Relative bioavailability (LCP-Tac_CYP3A5*1 non-expresser) | 1 FIX | - | - |
| F IR-Tac_PM | Relative bioavailability of IR-Tac_CYP3A5*1 non-expresser compared to reference | 0.745 (7.6%) | 0.757 | 0.66–0.84 |
| F LCP-Tac_HM | Relative bioavailability of LCP-Tac_CYP3A5*1 expresser compared to reference | 0.693 (13.7%) | 0.695 | 0.52–0.85 |
| F IR-Tac_HM | Relative bioavailability of IR-Tac_CYP3A5*1 expresser compared to reference | 0.427 (13.4%) | 0.428 | 0.34–0.52 |
| Lag-Time IR-Tac (h) | lag time for IR-Tac formulation in hours | 0.465 (0.1%) | 0.465 | 0.42–0.47 |
| Lag-Time LCP-Tac (h) | lag time for LCP-Tac formulation in hours | 1.4 (2.4%) | 1.39 | 1.32–1.57 |
| Circadian rhythms parameters | ||||
| AcrophaseCL/F (h) | peak time of the cosine function | 17 (3.6%) | 16.94 | 15.94–17.98 |
| AmpCL/F | Amplitude | 3.42 (17.1%) | 3.41 | 2.33–4.39 |
| Acrophaseka (h) | peak time of the cosine function | 3.13 (18.3%) | 3.17 | 1.82–4.52 |
| Ampka | Amplitude | 1.55 (44.5%) | 1.64 | 0.91–2.97 |
| RE. (-) | Combined residual error | 13.30 (8.2%) | 13.11 | 11.83–14.14 |
| Interindividual patient variability | Description | CV% (RSE%) | ||
| IIVCL/F | IIV associated with Elimination Clearance | 26.49 (29.1%) | 25.49 | 18.7–31.14 |
| IIVVc/F | IIV associated with Distribution Volume of central compartment | 53.47 (42%) | 52.15 | 33.46–72.20 |
| Vc/F/Ka IR-Tac Correlation | Correlation between IIV of Vc/F and Ka of IR-Tac | 75.63 (16%) | 72.3 | 43–92.33 |
| Vc/F/Ka LCP-Tac Correlation | Correlation between IIV of Vc/F and Ka of LCP-Tac | 44.38 (10%) | 44.11 | 12.76–65.68 |
| IIVKa IR-Tac | IIV associated with Absorption rate constant (IR-Tac) | 150.66 (25.6%) | 146.62 | 87.6–184.44 |
| Ka IR-Tac/Ka LCP-Tac Correlation | Correlation between IIV of Ka IR-Tac and Ka LCP-Tac | 45 (20.3%) | 41.24 | 38.69–75.55 |
| IIVKa LCP_Tac | IIV associated with Absorption rate constant (LCP-Tac) | 67.23 (46.5%) | 72.25 | 46.96–88.67 |
| IOVCL | IOV associated with Elimination Clearance | 20.85 (23.9%) | 20 | 16.9–24.51 |
| IOVVc | IOV associated with Distribution Volume of central compartment | 58.82 (28.9%) | 58.05 | 38.47–72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed Ali, Z.; Fernández-Alarcón, B.; Fontova, P.; Vidal-Alabró, A.; Rigo-Bonnin, R.; Melilli, E.; Montero, N.; Manonelles, A.; Coloma, A.; Favà, A.; et al. Optimizing Dose Conversion from IR-Tac to LCP-Tac Formulations in Renal Transplant Recipients: A Population Pharmacokinetic Modeling Study. Pharmaceutics 2025, 17, 1185. https://doi.org/10.3390/pharmaceutics17091185
Mohammed Ali Z, Fernández-Alarcón B, Fontova P, Vidal-Alabró A, Rigo-Bonnin R, Melilli E, Montero N, Manonelles A, Coloma A, Favà A, et al. Optimizing Dose Conversion from IR-Tac to LCP-Tac Formulations in Renal Transplant Recipients: A Population Pharmacokinetic Modeling Study. Pharmaceutics. 2025; 17(9):1185. https://doi.org/10.3390/pharmaceutics17091185
Chicago/Turabian StyleMohammed Ali, Zeyar, Beatriz Fernández-Alarcón, Pere Fontova, Anna Vidal-Alabró, Raul Rigo-Bonnin, Edoardo Melilli, Nuria Montero, Anna Manonelles, Ana Coloma, Alexandre Favà, and et al. 2025. "Optimizing Dose Conversion from IR-Tac to LCP-Tac Formulations in Renal Transplant Recipients: A Population Pharmacokinetic Modeling Study" Pharmaceutics 17, no. 9: 1185. https://doi.org/10.3390/pharmaceutics17091185
APA StyleMohammed Ali, Z., Fernández-Alarcón, B., Fontova, P., Vidal-Alabró, A., Rigo-Bonnin, R., Melilli, E., Montero, N., Manonelles, A., Coloma, A., Favà, A., Grinyó, J. M., Cruzado, J. M., Colom, H., & Lloberas, N. (2025). Optimizing Dose Conversion from IR-Tac to LCP-Tac Formulations in Renal Transplant Recipients: A Population Pharmacokinetic Modeling Study. Pharmaceutics, 17(9), 1185. https://doi.org/10.3390/pharmaceutics17091185








