Cracking the Blood–Brain Barrier Code: Rational Nanomaterial Design for Next-Generation Neurological Therapies
Abstract
1. Introduction
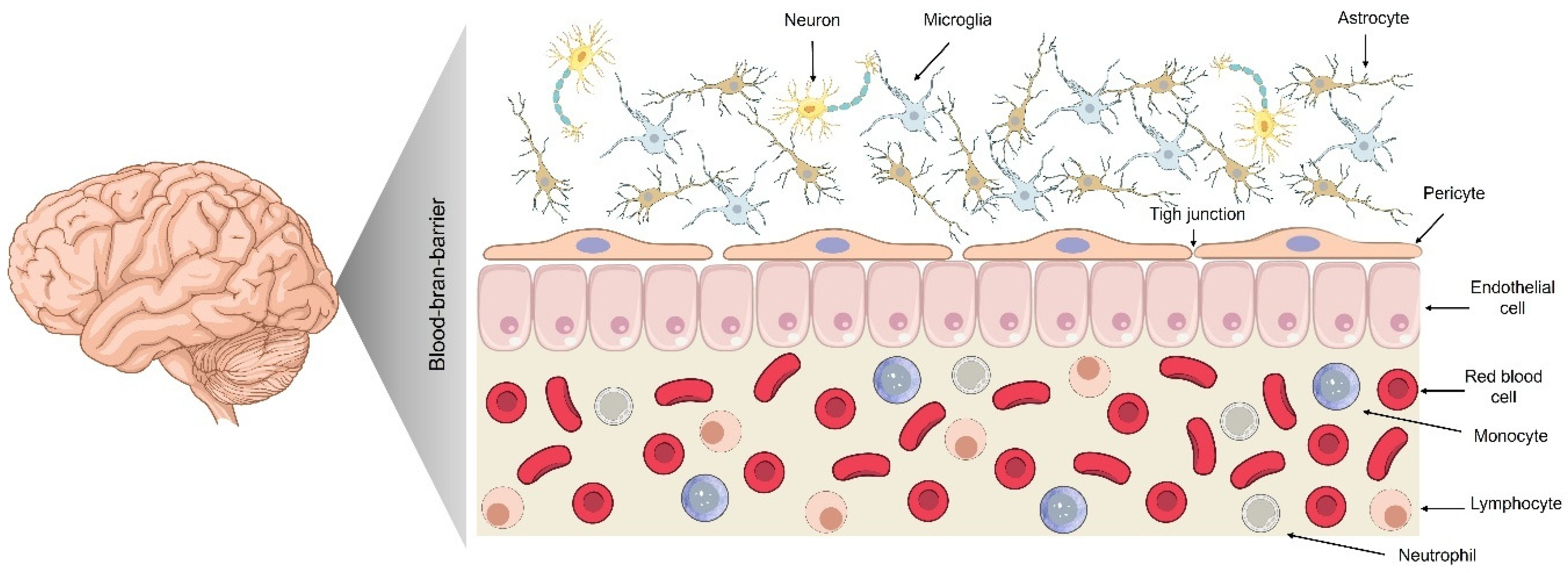
2. The BBB: Physiology and Restrictive Mechanisms
2.1. Composition and Function of the BBB
2.2. Tight Junctions and Paracellular Restriction
2.3. Major Transport Mechanisms Across the BBB
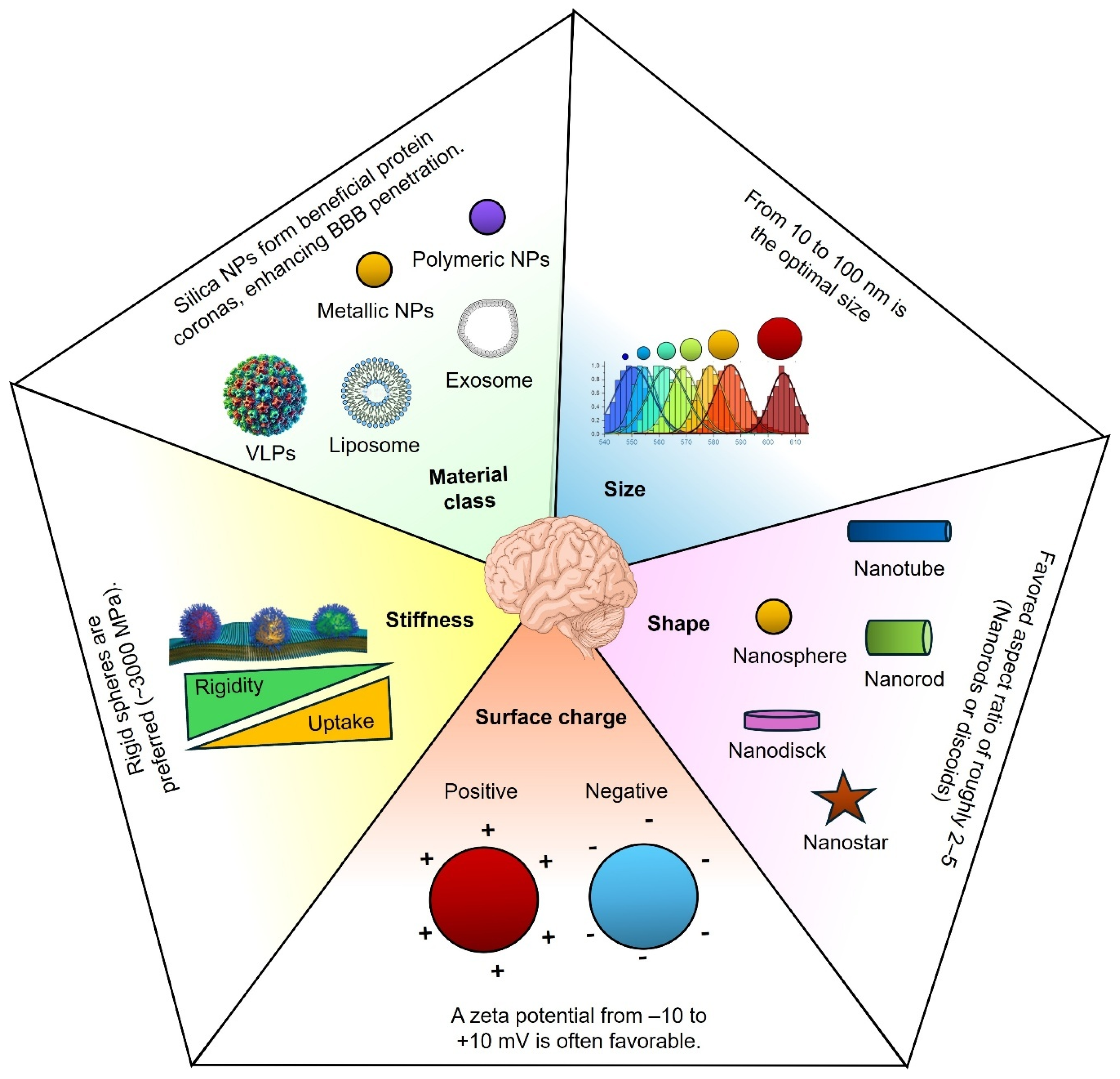
3. Key NP Properties for Crossing the BBB
3.1. Physicochemical Properties
3.2. Composition and Material Class
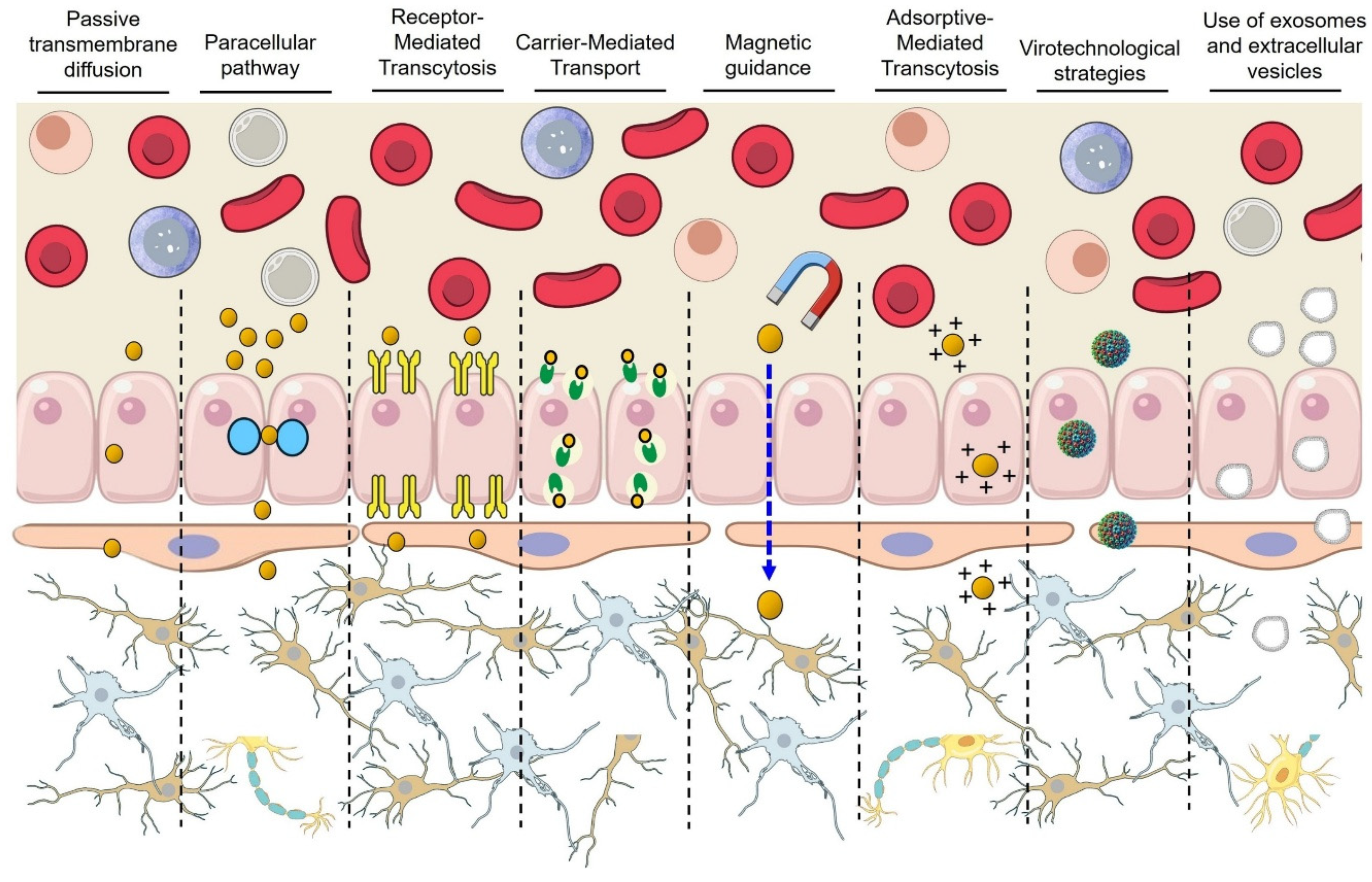
4. Nanotechnological Strategies for Crossing the BBB
4.1. Receptor-Mediated Transcytosis (RMT)
4.2. Adsorptive-Mediated Transcytosis (AMT)
4.3. Magnetically Guided NPs
4.4. Virotechnological Strategies
4.5. Exosomes and Extracellular Vesicles
5. Critical Assessment and Future Perspectives of Nanotechnological Delivery Systems Across the BBB
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–Brain Barrier |
| NP | Nanoparticle |
| CNS | Central Nervous System |
| RMT | Receptor-Mediated Transcytosis |
| AMT | Adsorptive-Mediated Transcytosis |
| TfR | Transferrin Receptor |
| LDLR | Low-Density Lipoprotein Receptor |
| LRP-1 | Low-Density Lipoprotein Receptor-Related Protein 1 |
| GLUT1 | Glucose Transporter 1 |
| LAT1 | Large Neutral Amino Acid Transporter 1 |
| TAT | Trans-Activator of Transcription |
| hCMEC/D3 | Human Cerebral Microvascular Endothelial Cell Line/D3 |
| hiPSC | Human Induced Pluripotent Stem Cell |
| SPION | Superparamagnetic Iron Oxide Nanoparticle |
| GBM | Glioblastoma Multiforme |
| TEER | Transendothelial Electrical Resistance |
| PEG | Polyethylene Glycol |
| RES | Reticuloendothelial System |
| VLP | Virus-Like Particle |
| AuNPs | Gold Nanoparticles |
| APO | Apocynin |
| AAV | Adeno-Associated Virus |
| RVG | Rabies Virus Glycoprotein |
| MSC | Mesenchymal Stem Cell |
| CPP | Cell-Penetrating Peptide |
| NHP | Non-Human Primate |
| CSF | Cerebrospinal Fluid |
| TEM | Transmission Electron Microscopy |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GMP | Good Manufacturing Practice |
| CQAs | Critical Quality Attributes |
| CARPA | Complement Activation-Related Pseudoallergy |
| PK | Pharmacokinetics |
| ADME | Absorption, Distribution, Metabolism, and Excretion |
| PBPK | Physiologically Based Pharmacokinetic |
| ISO | International Organization for Standardization |
| ASTM | American Society for Testing and Materials |
| ICH | International Council for Harmonisation |
| LAL | Limulus Amebocyte Lysate |
| CMC | Chemistry, Manufacturing and Controls |
| PDI | Polydispersity Index |
| ABC | Accelerated Blood Clearance |
References
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. Neurotherapeutics 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Di, L.; Rong, H.; Feng, B. Demystifying Brain Penetration in Central Nervous System Drug Discovery: Miniperspective. J. Med. Chem. 2013, 56, 2–12. [Google Scholar] [CrossRef]
- Chew, K.S.; Wells, R.C.; Moshkforoush, A.; Chan, D.; Lechtenberg, K.J.; Tran, H.L.; Chow, J.; Kim, D.J.; Robles-Colmenares, Y.; Srivastava, D.B.; et al. CD98hc is a target for brain delivery of biotherapeutics. Nat. Commun. 2023, 14, 5053. [Google Scholar] [CrossRef]
- Wang, F.; Qi, L.; Zhang, Z.; Duan, H.; Wang, Y.; Zhang, K.; Li, J. The Mechanism and Latest Research Progress of Blood–Brain Barrier Breakthrough. Biomedicines 2024, 12, 2302. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Kang, Y.-S. The decrease of paclitaxel efflux by pretreatment of interferon-γ and tumor necrosis factor-α after intracerebral microinjection. Brain Res. 2013, 1499, 158–162. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Voth, B.; Nagasawa, D.T.; Pelargos, P.E.; Chung, L.K.; Ung, N.; Gopen, Q.; Tenn, S.; Kamei, D.T.; Yang, I. Transferrin receptors and glioblastoma multiforme: Current findings and potential for treatment. J. Clin. Neurosci. 2015, 22, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Asimakidou, E.; Tan, J.K.S.; Zeng, J.; Lo, C.H. Blood-Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience. Pharmaceuticals 2024, 17, 612. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Estudillo, E.; Guzmán-Ruiz, M.A.; Herrera-Mundo, N.; Victoria-Acosta, G.; Cortés-Malagón, E.M.; López-Ornelas, A. Nanotechnology to Overcome Blood–Brain Barrier Permeability and Damage in Neurodegenerative Diseases. Pharmaceutics 2025, 17, 281. [Google Scholar] [CrossRef]
- Xie, A.; Cheng, G.; Wu, J.; Li, Z.; Yu, G.; Zhu, X.; Chen, T. Highly BBB-permeable nanomedicine reverses neuroapoptosis and neuroinflammation to treat Alzheimer’s disease. Biomaterials 2025, 312, 122749. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.-L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood–Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, F.; Falvo, E.; Mosca, L.; Tisci, G.; Arcovito, A.; Reccagni, A.; Limatola, C.; Bernardini, R.; Ceci, P.; D’Alessandro, G.; et al. Nose-to-brain selective drug delivery to glioma via ferritin-based nanovectors reduces tumor growth and improves survival rate. Cell Death Dis. 2024, 15, 262. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Garcia-Atutxa, I.; Santos, A.; Armendariz-Borunda, J. Toward a New Generation of Bio-Scaffolds for Neural Tissue Engineering: Challenges and Perspectives. Pharmaceutics 2023, 15, 1750. [Google Scholar] [CrossRef]
- ISO 10993-4:2017; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/63448.html (accessed on 10 July 2025).
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- Cockerill, I.; Oliver, J.A.; Xu, H.; Fu, B.M.; Zhu, D. Blood-Brain Barrier Integrity and Clearance of Amyloid-β from the BBB. In Molecular, Cellular, and Tissue Engineering of the Vascular System; Fu, B.M., Wright, N.T., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1097, pp. 261–278. ISBN 978-3-319-96444-7. Available online: http://link.springer.com/10.1007/978-3-319-96445-4_14 (accessed on 26 August 2025).
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Callan, B.; Hawthorne, S. Non-Invasive, Targeted Nanoparticle-Mediated Drug Delivery across a Novel Human BBB Model. Pharmaceutics 2023, 15, 1382. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood–brain barrier endogenous transporters as therapeutic targets: A new model for small molecule CNS drug discovery. Expert Opin. Ther. Targets 2015, 19, 1059–1072. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow. Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Uemura, M.T.; Maki, T.; Ihara, M.; Lee, V.M.Y.; Trojanowski, J.Q. Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front. Aging Neurosci. 2020, 12, 80. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function, and Mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zou, M.-H. Activation of AMP-activated protein kinase alleviates High-glucose-induced dysfunction of brain microvascular endothelial cell tight-junction dynamics. Free Radic. Biol. Med. 2012, 53, 1213–1221. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Zhu, N.; Wei, M.; Yuan, L.; He, X.; Chen, C.; Ji, A.; Zhang, G. Claudin-5 relieves cognitive decline in Alzheimer’s disease mice through suppression of inhibitory GABAergic neurotransmission. Aging 2022, 14, 3554–3568. [Google Scholar] [CrossRef]
- Heffron, T.P.; Salphati, L.; Alicke, B.; Cheong, J.; Dotson, J.; Edgar, K.; Goldsmith, R.; Gould, S.E.; Lee, L.B.; Lesnick, J.D.; et al. The Design and Identification of Brain Penetrant Inhibitors of Phosphoinositide 3-Kinase α. J. Med. Chem. 2012, 55, 8007–8020. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood–brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur. J. Pharm. Biopharm. 2014, 87, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R.; et al. Nano-scale architecture of blood-brain barrier tight-junctions. eLife 2021, 10, e63253. [Google Scholar] [CrossRef]
- Storelli, F.; Billington, S.; Kumar, A.R.; Unadkat, J.D. Abundance of P-Glycoprotein and Other Drug Transporters at the Human Blood-Brain Barrier in Alzheimer’s Disease: A Quantitative Targeted Proteomic Study. Clin. Pharmacol. Ther. 2021, 109, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, F.M.G.; Markert, G.; Deutsch, G.; Antonara, M.; Faaij, N.; Bartelink, I.; Noske, D.; Vandertop, W.P.; Bender, A.; Westerman, B.A. Explaining Blood–Brain Barrier Permeability of Small Molecules by Integrated Analysis of Different Transport Mechanisms. J. Med. Chem. 2023, 66, 7253–7267. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Smit, J.J.M.; Van Tellingen, O.; Beijnen, J.H.; Wagenaar, E.; Van Deemter, L.; Mol, C.A.A.M.; Van Der Valk, M.A.; Robanus-Maandag, E.C.; Te Riele, H.P.J.; et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994, 77, 491–502. [Google Scholar] [CrossRef]
- Oldendorf, W. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am. J. Physiol.-Leg. Content 1971, 221, 1629–1639. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Olivieri Jr, P.H.; Assis, I.F.; Lima, A.F.; Hassan, S.A.; Torquato, R.J.S.; Hayashi, J.Y.; Tashima, A.K.; Nader, H.B.; Salvati, A.; Justo, G.Z.; et al. Glycocalyx Interactions Modulate the Cellular Uptake of Albumin-Coated Nanoparticles. ACS Appl. Bio Mater. 2024, 7, 7365–7377. [Google Scholar] [CrossRef]
- Ozgür, B.; Puris, E.; Brachner, A.; Appelt-Menzel, A.; Oerter, S.; Balzer, V.; Holst, M.R.; Christiansen, R.F.; Hyldig, K.; Buckley, S.T.; et al. Characterization of an iPSC-based barrier model for blood-brain barrier investigations using the SBAD0201 stem cell line. Fluids Barriers CNS 2023, 20, 96. [Google Scholar] [CrossRef]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef]
- Verscheijden, L.F.M.; Koenderink, J.B.; De Wildt, S.N.; Russel, F.G.M. Differences in P-glycoprotein activity in human and rodent blood–brain barrier assessed by mechanistic modelling. Arch. Toxicol. 2021, 95, 3015–3029. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Saengsawang, W.; Ketsawatsomkron, P.; Asavapanumas, N.; Borwornpinyo, S.; Soodvilai, S.; Hongeng, S.; Charoensutthivarakul, S. GDNF and cAMP significantly enhance in vitro blood-brain barrier integrity in a humanized tricellular transwell model. Heliyon 2024, 10, e39343. [Google Scholar] [CrossRef]
- Masuda, T.; Hoshiyama, T.; Uemura, T.; Hirayama-Kurogi, M.; Ogata, S.; Furukawa, A.; Couraud, P.-O.; Furihata, T.; Ito, S.; Ohtsuki, S. Large-Scale Quantitative Comparison of Plasma Transmembrane Proteins between Two Human Blood–Brain Barrier Model Cell Lines, hCMEC/D3 and HBMEC/ciβ. Mol. Pharm. 2019, 16, 2162–2171. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies. Biomedicines 2024, 12, 626. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]
- Fujimoto, T.; Morofuji, Y.; Nakagawa, S.; Kovac, A.; Horie, N.; Izumo, T.; Niwa, M.; Matsuo, T.; Banks, W.A. Comparison of the rate of dedifferentiation with increasing passages among cell sources for an in vitro model of the blood–brain barrier. J. Neural Transm. 2020, 127, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, S.; Yeo, K.R.; Barter, Z.; Jamei, M.; Turner, D.B.; Rostami-Hodjegan, A. Application of permeability-limited physiologically-based pharmacokinetic models: Part I–digoxin pharmacokinetics incorporating P-glycoprotein-mediated efflux. J. Pharm. Sci. 2013, 102, 3145–3160. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Humle, N.; Hede, E.; Moos, T.; Burkhart, A.; Thomsen, L.B. The blood-brain barrier studied in vitro across species. PLoS ONE 2021, 16, e0236770. [Google Scholar] [CrossRef]
- Ribeiro, M.M.B.; Domingues, M.M.; Freire, J.M.; Santos, N.C.; Castanho, M.A.R.B. Translocating the blood-brain barrier using electrostatics. Front. Cell. Neurosci. 2012, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Zidarič, T.; Gradišnik, L.; Velnar, T. Astrocytes and human artificial blood-brain barrier models. Bosn. J. Basic Med. Sci. 2022, 22, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Wakayama, K.; Ohtsuki, S.; Chiba, M.; Ohe, T.; Ishii, Y.; Terasaki, T. Blood-Brain Barrier Pharmacoproteomics-Based Reconstruction of the In Vivo Brain Distribution of P-Glycoprotein Substrates in Cynomolgus Monkeys. J. Pharmacol. Exp. Ther. 2014, 350, 578–588. [Google Scholar] [CrossRef]
- Xia, C.Q.; Xiao, G.; Liu, N.; Pimprale, S.; Fox, L.; Patten, C.J.; Crespi, C.L.; Miwa, G.; Gan, L.-S. Comparison of Species Differences of P-Glycoproteins in Beagle Dog, Rhesus Monkey, and Human Using ATPase Activity Assays. Mol. Pharm. 2006, 3, 78–86. [Google Scholar] [CrossRef] [PubMed]
- O’Brown, N.M.; Megason, S.G.; Gu, C. Suppression of transcytosis regulates zebrafish blood-brain barrier function. eLife 2019, 8, e47326. [Google Scholar] [CrossRef]
- Saleem, S.; Kannan, R.R. Zebrafish: A Promising Real-Time Model System for Nanotechnology-Mediated Neurospecific Drug Delivery. Nanoscale Res. Lett. 2021, 16, 135. [Google Scholar] [CrossRef]
- Xie, J.; Farage, E.; Sugimoto, M.; Anand-Apte, B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev. Biol. 2010, 10, 76. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding cellular interactions with nanomaterials: Towards a rational design of medical nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yu, H.; Zhao, T.; Zhu, G.; Li, X. Nanoparticles with transformable physicochemical properties for overcoming biological barriers. Nanoscale 2023, 15, 13202–13223. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The Effect of Nanoparticle Size on the Ability to Cross the Blood–Brain Barrier: An In Vivo Study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Liu, D.; Lin, B.; Shao, W.; Zhu, Z.; Ji, T.; Yang, C. In Vitro and in Vivo Studies on the Transport of PEGylated Silica Nanoparticles across the Blood–Brain Barrier. ACS Appl. Mater. Interfaces 2014, 6, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.A.; Wu, C.H.; Wu, S.H.; Huang, C.Y.; Mou, C.Y.; Wei, K.C.; Yen, Y.; Chien, I.T.; Runa, S.; Chen, Y.P.; et al. Receptor Ligand-Free Mesoporous Silica Nanoparticles: A Streamlined Strategy for Targeted Drug Delivery across the Blood–Brain Barrier. ACS Nano 2024, 18, 12716–12736. [Google Scholar] [CrossRef]
- Etame, A.B.; Smith, C.A.; Chan, W.C.W.; Rutka, J.T. Design and potential application of PEGylated gold nanoparticles with size-dependent permeation through brain microvasculature. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 992–1000. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Luo, Z.; Wu, Y.-L.; Huo, S.; Liang, X.-J. Gold-based nanomaterials for the treatment of brain cancer. Cancer Biol. Med. 2021, 18, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Brown, T.D.; Graham, A.; Helgeson, M.E.; Mitragotri, S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng. Transl. Med. 2020, 5, e10153. [Google Scholar] [CrossRef]
- Galstyan, A.; Markman, J.L.; Shatalova, E.S.; Chiechi, A.; Korman, A.J.; Patil, R.; Klymyshyn, D.; Tourtellotte, W.G.; Israel, L.L.; Braubach, O.; et al. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 2019, 10, 3850. [Google Scholar] [CrossRef]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C.W. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [CrossRef]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle Surface Charges Alter Blood–Brain Barrier Integrity and Permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Li, G.; Yin, Z.; Fu, B.M. Transcellular Model for Neutral and Charged Nanoparticles Across an In Vitro Blood–Brain Barrier. Cardiovasc. Eng. Technol. 2020, 11, 607–620. [Google Scholar] [CrossRef]
- Kim, B.; Han, G.; Toley, B.J.; Kim, C.; Rotello, V.M.; Forbes, N.S. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat. Nanotechnol. 2010, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.B.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Kolhar, P.; Anselmo, A.C.; Gupta, V.; Pant, K.; Prabhakarpandian, B.; Ruoslahti, E.; Mitragotri, S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl. Acad. Sci. USA 2013, 110, 10753–10758. [Google Scholar] [CrossRef]
- Peralta-Cuevas, E.; Garcia-Atutxa, I.; Huerta-Saquero, A.; Villanueva-Flores, F. The Role of Plant Virus-like Particles in Advanced Drug Delivery and Vaccine Development: Structural Attributes and Application Potential. Viruses 2025, 17, 148. [Google Scholar] [CrossRef]
- Sierri, G.; Saenz-de-Santa-Maria, I.; Renda, A.; Koch, M.; Sommi, P.; Anselmi-Tamburini, U.; Mauri, M.; D’Aloia, A.; Ceriani, M.; Salerno, D.; et al. Nanoparticle shape is the game-changer for blood–brain barrier crossing and delivery through tunneling nanotubes among glioblastoma cells. Nanoscale 2025, 17, 992–1006. [Google Scholar] [CrossRef]
- Fu, L.; Shi, B.; Wen, S.; Morsch, M.; Wang, G.; Zhou, Z.; Mi, C.; Sadraeian, M.; Lin, G.; Lu, Y.; et al. Aspect Ratio of PEGylated Upconversion Nanocrystals Affects the Cellular Uptake In Vitro and In Vivo. Acta Biomater. 2022, 147, 403–413. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- Key, J.; Palange, A.L.; Gentile, F.; Aryal, S.; Stigliano, C.; Di Mascolo, D.; De Rosa, E.; Cho, M.; Lee, Y.; Singh, J.; et al. Soft Discoidal Polymeric Nanoconstructs Resist Macrophage Uptake and Enhance Vascular Targeting in Tumors. ACS Nano 2015, 9, 11628–11641. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.R.; Hernandez, Y.; Miyasaki, K.F.; Kwon, E.J. Engineered nanomaterials that exploit blood-brain barrier dysfunction for delivery to the brain. Adv. Drug Deliv. Rev. 2023, 197, 114820. [Google Scholar] [CrossRef]
- Cai, X.; Drummond, C.J.; Zhai, J.; Tran, N. Lipid Nanoparticles: Versatile Drug Delivery Vehicles for Traversing the Blood Brain Barrier to Treat Brain Cancer. Adv. Funct. Mater. 2024, 34, 2404234. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 2018, 8, 3416–3436. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, M.A.; Dal Magro, R.; Barbieri, L.; Pandolfi, L.; Sguazzini-Viscontini, A.; Truffi, M.; Salvioni, L.; Corsi, F.; Colombo, M.; Re, F.; et al. H-Ferritin nanoparticle-mediated delivery of antibodies across a BBB in vitro model for treatment of brain malignancies. Biomater. Sci. 2021, 9, 2032–2042. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Arora, S.; Kanekiyo, T.; Singh, J. Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res. 2020, 1734, 146738. [Google Scholar] [CrossRef]
- Wehn, A.C.; Krestel, E.; Harapan, B.N.; Klymchenko, A.; Plesnila, N.; Khalin, I. To see or not to see: In vivo nanocarrier detection methods in the brain and their challenges. J. Control. Release 2024, 371, 216–236. [Google Scholar] [CrossRef]
- Somani, S.; Blatchford, D.R.; Millington, O.; Stevenson, M.L.; Dufès, C. Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J. Control. Release 2014, 188, 78–86. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Parashar, A.K.; Saraogi, G.K.; Shrivastava, V.; Bagri, R.; Tyagi, L.K.; Sethi, V.A.; Jain, P.K. Development of Angiopep-2 targeted dendrimer-based nanotheranostic system for enhanced temozolomide delivery to glioblastoma multiforme. Bull. Natl. Res. Cent. 2025, 49, 16. Available online: https://BNRC.springeropen.com/articles/10.1186/s42269-025-01309-3 (accessed on 10 July 2025). [CrossRef]
- Hua, S.; Wu, S.Y. The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharmacol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, Y.; He, M.; Zhang, H.; Yuan, H.; Johnson, M.; Palmisano, M.; Zhou, S.; Sun, D. Distinct biodistribution of doxorubicin and the altered dispositions mediated by different liposomal formulations. Int. J. Pharm. 2017, 519, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Annu; Sartaj, A.; Qamar, Z.; Md, S.; Alhakamy, N.A.; Baboota, S.; Ali, J. An Insight to Brain Targeting Utilizing Polymeric Nanoparticles: Effective Treatment Modalities for Neurological Disorders and Brain Tumor. Front. Bioeng. Biotechnol. 2022, 10, 788128. [Google Scholar] [CrossRef]
- Sulheim, E.; Baghirov, H.; Von Haartman, E.; Bøe, A.; Åslund, A.K.O.; Mørch, Y.; Davies, C.D.L. Cellular uptake and intracellular degradation of poly(alkyl cyanoacrylate) nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Naeem, A.; Xiao, S.; Hu, L.; Zhang, J.; Zheng, Q. Safety Challenges and Application Strategies for the Use of Dendrimers in Medicine. Pharmaceutics 2022, 14, 1292. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Cao, S.; Ma, D.; Ji, S.; Zhou, M.; Zhu, S. Self-Assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications. Molecules 2024, 29, 4221. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Behroozi, Z.; Rahimi, B.; Kookli, K.; Safari, M.S.; Hamblin, M.R.; Razmgir, M.; Janzadeh, A.; Ramezani, F. Distribution of gold nanoparticles into the brain: A systematic review and meta-analysis. Nanotoxicology 2021, 15, 1059–1072. [Google Scholar] [CrossRef]
- Ye, Z.; Gastfriend, B.D.; Umlauf, B.J.; Lynn, D.M.; Shusta, E.V. Antibody-Targeted Liposomes for Enhanced Targeting of the Blood-Brain Barrier. Pharm. Res. 2022, 39, 1523–1534. [Google Scholar] [CrossRef]
- Orii, K.O.; Grubb, J.H.; Vogler, C.; Levy, B.; Tan, Y.; Markova, K.; Davidson, B.L.; Mao, Q.; Orii, T.; Kondo, N.; et al. Defining the Pathway for Tat-mediated Delivery of β-Glucuronidase in Cultured Cells and MPS VII Mice. Mol. Ther. 2005, 12, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Brain Delivery of Nanomedicines: Trojan Horse Liposomes for Plasmid DNA Gene Therapy of the Brain. Front. Med. Technol. 2020, 2, 602236. [Google Scholar] [CrossRef]
- Sarko, D.; Beijer, B.; Garcia Boy, R.; Nothelfer, E.M.; Leotta, K.; Eisenhut, M.; Altmann, A.; Haberkorn, U.; Mier, W. The Pharmacokinetics of Cell-Penetrating Peptides. Mol. Pharm. 2010, 7, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Morovvati, H.; Webster, T.J.; Najaf Asaadi, S.; Rezayat, S.M.; Hadjighassem, M.; Khosravani, M.; Adabi, M. Combination chemotherapy via poloxamer 188 surface-modified PLGA nanoparticles that traverse the blood–brain–barrier in a glioblastoma model. Sci. Rep. 2024, 14, 19516. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sabliov, C. PLA/PLGA nanoparticles for delivery of drugs across the blood-brain barrier. Nanotechnol. Rev. 2013, 2, 241–257. [Google Scholar] [CrossRef]
- Liaw, K.; Zhang, F.; Mangraviti, A.; Kannan, S.; Tyler, B.; Kannan, R.M. Dendrimer size effects on the selective brain tumor targeting in orthotopic tumor models upon systemic administration. Bioeng. Transl. Med. 2020, 5, e10160. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Zhu, Y.; Qian, Y.; Feng, Y.; Li, H.; Hu, G. Preparation and brain targeting effects study of recombinant human ferritin nanoparticles. Biochem. Biophys. Res. Commun. 2024, 712–713, 149939. [Google Scholar] [CrossRef]
- Simon-Santamaria, J.; Rinaldo, C.H.; Kardas, P.; Li, R.; Malovic, I.; Elvevold, K.; McCourt, P.; Smedsrød, B.; Hirsch, H.H.; Sørensen, K.K. Efficient Uptake of Blood-Borne BK and JC Polyomavirus-Like Particles in Endothelial Cells of Liver Sinusoids and Renal Vasa Recta. PLoS ONE 2014, 9, e111762. [Google Scholar] [CrossRef]
- Ye, D.; Zimmermann, T.; Demina, V.; Sotnikov, S.; Ried, C.L.; Rahn, H.; Stapf, M.; Untucht, C.; Rohe, M.; Terstappen, G.C.; et al. Trafficking of JC virus-like particles across the blood-brain barrier. Nanoscale Adv. 2021, 3, 2488–2500. [Google Scholar] [CrossRef]
- Li, X.; Vemireddy, V.; Cai, Q.; Xiong, H.; Kang, P.; Li, X.; Giannotta, M.; Hayenga, H.N.; Pan, E.; Sirsi, S.R.; et al. Reversibly Modulating the Blood-Brain Barrier by Laser Stimulation of Molecular-Targeted Nanoparticles. Nano Lett. 2021, 21, 9805–9815. [Google Scholar] [CrossRef]
- Lim, L.Y.; Koh, P.Y.; Somani, S.; Al Robaian, M.; Karim, R.; Yean, Y.L.; Mitchell, J.; Tate, R.J.; Edrada-Ebel, R.; Blatchford, D.R.; et al. Tumor regression following intravenous administration of lactoferrin- and lactoferricin-bearing dendriplexes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1445–1454. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Shifrina, Z.B. Dendrimers as Antiamyloid Agents. Pharmaceutics 2022, 14, 760. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Zhang, C.; Song, J.; Zheng, Z.; Yan, W. New advances in diagnosis and treatment of nano drug delivery systems across the blood-brain barrier. Nanocomposites 2023, 9, 116–127. [Google Scholar] [CrossRef]
- Lee, M.R.; Jayant, R.D. Penetration of the blood-brain barrier by peripheral neuropeptides: New approaches to enhancing transport and endogenous expression. Cell Tissue Res. 2019, 375, 287–293. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Restricted transport of anti-transferrin receptor antibody (OX26) through the blood–brain barrier in the rat. J. Neurochem. 2001, 79, 119–129. [Google Scholar] [CrossRef]
- Arguello, A.; Mahon, C.S.; Calvert, M.E.K.; Chan, D.; Dugas, J.C.; Pizzo, M.E.; Thomsen, E.R.; Chau, R.; Damo, L.A.; Duque, J.; et al. Molecular architecture determines brain delivery of a transferrin receptor–targeted lysosomal enzyme. J. Exp. Med. 2022, 219, e20211057. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, Y.; Boado, R.J.; Pardridge, W.M. Decline in Exogenous Gene Expression in Primate Brain Following Intravenous Administration Is Due to Plasmid Degradation. Pharm. Res. 2006, 23, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood–brain barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Gong, M.; Zhang, J. Delivery of a peptide-drug conjugate targeting the blood brain barrier improved the efficacy of paclitaxel against glioma. Oncotarget 2016, 7, 79401–79407. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Wang, Y.; Tang, C.; Zhou, Y.; Li, J.; Lu, X.; Wang, Y.; Ma, T.; Xu, H.; et al. Angiopep-2 conjugated biomimetic nano-delivery system loaded with resveratrol for the treatment of methamphetamine addiction. Int. J. Pharm. 2024, 663, 124552. [Google Scholar] [CrossRef]
- Demeule, M.; Currie, J.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef]
- Di Polidoro, A.C.; Cafarchio, A.; Vecchione, D.; Donato, P.; De Nola, F.; Torino, E. Revealing Angiopep-2/LRP1 Molecular Interaction for Optimal Delivery to Glioblastoma (GBM). Molecules 2022, 27, 6696. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Prajapati, N.; DeCoster, M.A.; Poh, S.; Murray, T.A. Efficient LRP1-Mediated Uptake and Low Cytotoxicity of Peptide L57 In Vitro Shows Its Promise as CNS Drug Delivery Vector. J. Pharm. Sci. 2021, 110, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Leite, D.M.; Scarpa, E.; Nyberg, S.; Fullstone, G.; Forth, J.; Matias, D.; Apriceno, A.; Poma, A.; Duro-Castano, A.; et al. On the shuttling across the blood-brain barrier via tubule formation: Mechanism and cargo avidity bias. Sci. Adv. 2020, 6, eabc4397. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Barreira, M.; Cruz, C.; Tomás, J.; Luís, Â.; Pedro, A.Q.; Queiroz, J.A.; Sousa, F. Brain-Targeted Delivery of Pre-miR-29b Using Lactoferrin-Stearic Acid-Modified-Chitosan/Polyethyleneimine Polyplexes. Pharmaceuticals 2020, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.R.; Boraie, N.A.; Ismail, F.A.; Bakr, B.A.; Allam, E.A.; El-Moslemany, R.M. Brain targeted lactoferrin coated lipid nanocapsules for the combined effects of apocynin and lavender essential oil in PTZ induced seizures. Drug Deliv. Transl. Res. 2025, 15, 534–555. [Google Scholar] [CrossRef]
- Sharma, S.; Dang, S. Nanocarrier-Based Drug Delivery to Brain: Interventions of Surface Modification. Curr. Neuropharmacol. 2023, 21, 517–535. [Google Scholar] [CrossRef]
- McCord, E.; Pawar, S.; Koneru, T.; Tatiparti, K.; Sau, S.; Iyer, A.K. Folate Receptors’ Expression in Gliomas May Possess Potential Nanoparticle-Based Drug Delivery Opportunities. ACS Omega 2021, 6, 4111–4118. [Google Scholar] [CrossRef]
- Morshedi, B.; Esfandyari-Manesh, M.; Atyabi, F.; Ghahremani, M.H.; Dinarvand, R. Local delivery of ibrutinib by folate receptor-mediated targeting PLGA–PEG nanoparticles to glioblastoma multiform: In vitro and in vivo studies. J. Drug Target. 2025, 33, 1026–1041. [Google Scholar] [CrossRef]
- Yücel, O.; Aksüt, Y.; Şengelen, A.; Yıldırım, E.; Emik, S.; Arda, N.; Gürdağ, G. Folate receptor-targeted indomethacin-loaded gold nanoparticles enhance drug chemotherapeutic efficacy in glioblastoma cells and spheroids. J. Drug Deliv. Sci. Technol. 2024, 100, 106025. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.K.; Eisenberg, J.B.; Pardridge, W.M. Absorptive-mediated endocytosis of cationized albumin and a beta-endorphin-cationized albumin chimeric peptide by isolated brain capillaries. Model system of blood-brain barrier transport. J. Biol. Chem. 1987, 262, 15214–15219. [Google Scholar] [CrossRef]
- Neves, V.; Aires-da-Silva, F.; Morais, M.; Gano, L.; Ribeiro, E.; Pinto, A.; Aguiar, S.; Gaspar, D.; Fernandes, C.; Correia, J.D.G.; et al. Novel Peptides Derived from Dengue Virus Capsid Protein Translocate Reversibly the Blood–Brain Barrier through a Receptor-Free Mechanism. ACS Chem. Biol. 2017, 12, 1257–1268. [Google Scholar] [CrossRef]
- Szecskó, A.; Mészáros, M.; Simões, B.; Cavaco, M.; Chaparro, C.; Porkoláb, G.; Castanho, M.A.R.B.; Deli, M.A.; Neves, V.; Veszelka, S. PepH3-modified nanocarriers for delivery of therapeutics across the blood-brain barrier. Fluids Barriers CNS 2025, 22, 31. [Google Scholar] [CrossRef]
- Cavaco, M.; Fraga, P.; Valle, J.; Silva, R.D.M.; Gano, L.; Correia, J.D.G.; Andreu, D.; Castanho, M.A.R.B.; Neves, V. Molecular determinants for brain targeting by peptides: A meta-analysis approach with experimental validation. Fluids Barriers CNS 2024, 21, 45. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Teaima, M.; El-Nabarawi, M.; Badawi, N.M. Intranasal delivery of chitosan-based nanoparticles as an innovative way for management of neurodegenerative disorders: A comprehensive review of advanced strategies for CNS targeting. J. Drug Deliv. Sci. Technol. 2024, 99, 105885. [Google Scholar] [CrossRef]
- Khan, I.N.; Navaid, S.; Waqar, W.; Hussein, D.; Ullah, N.; Khan, M.U.A.; Hussain, Z.; Javed, A. Chitosan-Based Polymeric Nanoparticles as an Efficient Gene Delivery System to Cross Blood Brain Barrier: In Vitro and In Vivo Evaluations. Pharmaceuticals 2024, 17, 169. [Google Scholar] [CrossRef]
- Sepand, M.R.; Ghavami, M.; Zanganeh, S.; Stacks, S.; Ghasemi, F.; Montazeri, H.; Corbo, C.; Derakhshankhah, H.; Ostad, S.N.; Ghahremani, M.H.; et al. Impact of plasma concentration of transferrin on targeting capacity of nanoparticles. Nanoscale 2020, 12, 4935–4944. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Xie, S.; Yang, B.; Xu, Q.; Tan, J. Superparamagnetic Iron Oxide Nanoparticles Modified with Tween 80 Pass through the Intact Blood–Brain Barrier in Rats under Magnetic Field. ACS Appl. Mater. Interfaces 2016, 8, 11336–11341. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.G.; Kim, S.; Tran, T.A.T.; Kim, Y.H.; Nagareddy, R.; Jung, T.Y.; Kim, S.K.; Jeong, Y.Y. Magnet-Guided Temozolomide and Ferucarbotran Loaded Nanoparticles to Enhance Therapeutic Efficacy in Glioma Model. Nanomaterials 2024, 14, 939. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Thomsen, M.S.; Moos, T. Targeted Drug Delivery to the Brain Using Magnetic Nanoparticles. Ther. Deliv. 2015, 6, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Savari, M.-N. Fe3O4@Chitosan@ZIF-8@RVG29, an anti-glioma nanoplatform guided by fixed and activated by alternating magnetic field. Sci. Rep. 2024, 14, 7000. [Google Scholar] [CrossRef]
- Chuapoco, M.R.; Flytzanis, N.C.; Goeden, N.; Christopher Octeau, J.; Roxas, K.M.; Chan, K.Y.; Scherrer, J.; Winchester, J.; Blackburn, R.J.; Campos, L.J.; et al. Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain. Nat. Nanotechnol. 2023, 18, 1241–1251. [Google Scholar] [CrossRef]
- Huang, Q.; Chan, K.Y.; Wu, J.; Botticello-Romero, N.R.; Zheng, Q.; Lou, S.; Keyes, C.; Svanbergsson, A.; Johnston, J.; Mills, A.; et al. An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery. Science 2024, 384, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Lee, A.S.; Kim, S.M.; Heo, H.R.; Kim, C.S. Virus-like nanoparticles as a theranostic platform for cancer. Front. Bioeng. Biotechnol. 2023, 10, 1106767. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Khatami, S.H.; Karami, N.; Taheri-Anganeh, M.; Taghvimi, S.; Tondro, G.; Khorsand, M.; Soltani Fard, E.; Sedighimehr, N.; Kazemi, M.; Rahimi Jaberi, K.; et al. Exosomes: Promising Delivery Tools for Overcoming Blood-Brain Barrier and Glioblastoma Therapy. Mol. Neurobiol. 2023, 60, 4659–4678. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Iqbal, Z.; Lu, J.; Wang, J.; Zhang, H.; Chen, X.; Duan, L.; Xia, J. Cell-derived nanovesicle-mediated drug delivery to the brain: Principles and strategies for vesicle engineering. Mol. Ther. 2023, 31, 1207–1224. [Google Scholar] [CrossRef]
- Cui, G.; Guo, H.; Li, H.; Zhai, Y.; Gong, Z.; Wu, J.; Liu, J.; Dong, Y.; Hou, S.; Liu, J. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef]
- Chu, L.; Sun, Y.; Zhao, Y.; Wang, A.; Sun, Y.; Duan, X.; Li, N.; Xia, H.; Liu, W.; Sun, K. Exosome-mediated delivery platform of biomacromolecules into the brain: Cetuximab in combination with doxorubicin for glioblastoma therapy. Int. J. Pharm. 2024, 660, 124262. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Q.; Chai, Y.; Rong, R.; He, L.; Zhang, Y.; Cui, H.; Xu, H.; Zhang, X.; Wang, Z.; et al. An anti-CD19-exosome delivery system navigates the blood–brain barrier for targeting of central nervous system lymphoma. J. Nanobiotechnol. 2025, 23, 173. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- Kuang, Y.; An, S.; Guo, Y.; Huang, S.; Shao, K.; Liu, Y.; Li, J.; Ma, H.; Jiang, C. T7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targeting. Int. J. Pharm. 2013, 454, 11–20. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Jiang, Y.; Lv, W.; Wu, L.; Wang, B.; Lv, L.; Xu, Q.; Xin, H. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci. Rep. 2015, 5, 12651. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Giugliani, L.; De Oliveira Poswar, F.; Donis, K.C.; Corte, A.D.; Schmidt, M.; Boado, R.J.; Nestrasil, I.; Nguyen, C.; Chen, S.; et al. Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): An open label phase 1-2 trial. Orphanet J. Rare Dis. 2018, 13, 110. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, D.; Nyberg, S.; Poma, A.; Cecchin, D.; Jain, S.A.; Kim, K.-A.; Shin, Y.-J.; Kim, E.-H.; Kim, M.; et al. LRP-1 functionalized polymersomes enhance the efficacy of carnosine in experimental stroke. Sci. Rep. 2020, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Y.; Chen, X.; Zhang, Y.; Zhang, K.; Zheng, H.; Wei, Y.; Zheng, H.; Zhu, J.; Wu, F.; et al. Angiopep-2 modified lipid-coated mesoporous silica nanoparticles for glioma targeting therapy overcoming BBB. Biochem. Biophys. Res. Commun. 2021, 534, 902–907. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Zhang, Q.; Jiang, W.; Tang, L.; Liu, J.; He, Q. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur. J. Pharm. Sci. 2011, 44, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qian, Y.; Peng, W.; Qi, X. Functionalized nanoparticles crossing the brain-blood barrier to target glioma cells. PeerJ 2023, 11, e15571. [Google Scholar] [CrossRef]
- Kong, S.D.; Zhang, W.; Lee, J.H.; Brammer, K.; Lal, R.; Karin, M.; Jin, S. Magnetically Vectored Nanocapsules for Tumor Penetration and Remotely Switchable On-Demand Drug Release. Nano Lett. 2010, 10, 5088–5092. [Google Scholar] [CrossRef]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release 2012, 164, 49–57. [Google Scholar] [CrossRef]
- Pitek, A.S.; Wen, A.M.; Shukla, S.; Steinmetz, N.F. The Protein Corona of Plant Virus Nanoparticles Influences their Dispersion Properties, Cellular Interactions, and In Vivo Fates. Small 2016, 12, 1758–1769. [Google Scholar] [CrossRef]
- Kaiser, C.R.; Flenniken, M.L.; Gillitzer, E.; Harmsen, A.L.; Harmsen, A.G.; Jutila, M.A.; Douglas, T.; Young, M.J. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int. J. Nanomed. 2007, 2, 715–733. [Google Scholar]
- Bai, L.; Yu, L.; Ran, M.; Zhong, X.; Sun, M.; Xu, M.; Wang, Y.; Yan, X.; Lee, R.J.; Tang, Y.; et al. Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders. Int. J. Mol. Sci. 2025, 26, 2491. [Google Scholar] [CrossRef]
- Meyer, C.; Losacco, J.; Stickney, Z.; Li, L.; Marriott, G.; Lu, B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int. J. Nanomed. 2017, 12, 3153–3170. [Google Scholar] [CrossRef]
- Chen, J.; Chopp, M. Exosome Therapy for Stroke. Stroke 2018, 49, 1083–1090. [Google Scholar] [CrossRef]
- Drommelschmidt, K.; Serdar, M.; Bendix, I.; Herz, J.; Bertling, F.; Prager, S.; Keller, M.; Ludwig, A.-K.; Duhan, V.; Radtke, S.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 2017, 60, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+ CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Komatsu, H.; Poku, E.K.; Olafsen, T.; Huang, K.X.; Huang, L.A.; Chea, J.; Bowles, N.; Chang, B.; Rawson, J.; et al. Biodistribution of Intra-Arterial and Intravenous Delivery of Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles in a Rat Model to Guide Delivery Strategies for Diabetes Therapies. Pharmaceuticals 2022, 15, 595. [Google Scholar] [CrossRef]
- Ophelders, D.R.M.G.; Wolfs, T.G.A.M.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.-K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Waseem, A.; Saudamini; Haque, R.; Janowski, M.; Raza, S.S. Mesenchymal stem cell-derived exosomes: Shaping the next era of stroke treatment. Neuroprotection 2023, 1, 99–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef]
- Liu, W.; Wang, W.; Dong, X.; Sun, Y. Near-Infrared Light-Powered Janus Nanomotor Significantly Facilitates Inhibition of Amyloid-β Fibrillogenesis. ACS Appl. Mater. Interfaces 2020, 12, 12618–12628. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 2016, 7, 37081–37091. [Google Scholar] [CrossRef]
- Li, J.; Angsantikul, P.; Liu, W.; Esteban-Fernández De Ávila, B.; Chang, X.; Sandraz, E.; Liang, Y.; Zhu, S.; Zhang, Y.; Chen, C.; et al. Biomimetic Platelet-Camouflaged Nanorobots for Binding and Isolation of Biological Threats. Adv. Mater. 2018, 30, 1704800. [Google Scholar] [CrossRef]
- ASTM E2524-08; Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles. ASTM International: West Conshohocken, PA, USA, 2008. Available online: https://www.astm.org/e2524-08r13.html (accessed on 10 July 2025).
- ICH. ICH Harmonised Tripartite Guideline: Safety Pharmacology Studies for Human Pharmaceuticals S7; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). European Medicines Agency (EMA). 2000. Available online: https://database.ich.org/sites/default/files/S7A_Guideline.pdf (accessed on 10 July 2025).
- ASTM F756-17; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2017. Available online: https://www.astm.org/f0756-17.html (accessed on 10 July 2025).
- Braune, S.; Lendlein, A.; Jung, F. Developing standards and test protocols for testing the hemocompatibility of biomaterials. In Hemocompatibility of Biomaterials for Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–76. ISBN 978-0-08-100497-5. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780081004975000045 (accessed on 27 April 2025).
- FDA. Use of International Standard ISO 10993-1, “Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process”; U.S. Food and Drug Administration: Rockville, MD, USA, 2020; U.S. Food and Drug Administration+7. Available online: https://www.fda.gov/media/142959/download (accessed on 10 July 2025).
- Giannakou, C.; Park, M.V.D.Z.; Bosselaers, I.E.M.; de Jong, W.H.; van der Laan, J.W.; van Loveren, H.; Vandebriel, R.J.; Geertsma, R.E. Nonclinical regulatory immunotoxicity testing of nanomedicinal products: Proposed strategy and possible pitfalls. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1633. [Google Scholar] [CrossRef] [PubMed]
- Totea, G.; Ionita, D.; Demetrescu, I.; Mitache, M. In vitro hemocompatibility and corrosion behavior of new Zr-binary alloys in whole human blood. Open Chem. 2014, 12, 796–803. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2009/120/EC of 14 September 2009 Amending Directive 2001/83/EC of the European Parliament and of the Council on the Community Code Relating to Medicinal Products for Human Use as Regards Advanced Therapy Medicinal Products; European Commission: Brussels, Belgium, 2009; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009L0120 (accessed on 10 July 2025).
- Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations; U.S. Department of Health and Human Services, Center for Drug Evaluation and Research: Cincinnati, OH, USA, 1997.
- OECD. Test No. 424: Neurotoxicity Study in Rodents. OECD Guidelines for the Testing of Chemicals, Section 4; Organización para la Cooperación y el Desarrollo Económicos: Paris, France, 1997; Available online: https://www.oecd.org/en/publications/test-no-424-neurotoxicity-study-in-rodents_9789264071025-en.html (accessed on 10 July 2025).
- Vervaeke, P.; Borgos, S.E.; Sanders, N.N.; Combes, F. Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics. Adv. Drug Deliv. Rev. 2022, 184, 114236. [Google Scholar] [CrossRef]
- FDA. Drug Products, Including Biological Products, That Contain Nanomaterials: Guidance for Industry; U.S. Food and Drug Administration: Rockville, MD, USA, 2022. Available online: https://www.fda.gov/media/157812/download (accessed on 10 July 2025).
- Gu, X.; Song, Q.; Zhang, Q.; Huang, M.; Zheng, M.; Chen, J.; Wei, D.; Chen, J.; Wei, X.; Chen, H.; et al. Clearance of two organic nanoparticles from the brain via the paravascular pathway. J. Control. Release 2020, 322, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jia, W.; Wang, Y.; Hu, C.; Yu, W.; Huang, Y.; Wang, L.; Gao, H. Glymphatic System and Subsidiary Pathways Drive Nanoparticles Away from the Brain. Research 2022, 2022, 9847612. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Campagnolo, L.; Fadeel, B. Interactions of Engineered Nanoparticles with Organs Protected by Internal Biological Barriers. Small 2013, 9, 1557–1572. [Google Scholar] [CrossRef]
- S7A Safety Pharmacology Studies for Human Pharmaceuticals; U.S. Department of Health and Human Services. U.S. Food and Drug Administration: Rockville, MD, USA, 2001.
- ICHS8. ICH Harmonised Tripartite Guideline: Immunotoxicity Studies for Human Pharmaceuticals S8. U.S. Food and Drug Administration+7. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-s-8-immunotoxicity-studies-human-pharmaceuticals-step-5_en.pdf (accessed on 10 July 2025).
- Le Meur, M.; Pignatelli, J.; Blasi, P.; Palomo, V. Nanoparticles targeting the central circadian clock: Potential applications for neurological disorders. Adv. Drug Deliv. Rev. 2025, 220, 115561. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A. Lessons learned from immunological characterization of nanomaterials at the Nanotechnology Characterization Laboratory. Front. Immunol. 2022, 13, 984252. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Y.; Chen, Y.; Zhang, C.; Deng, H.; Lu, J.; Chen, W. Emerging strategies against accelerated blood clearance phenomenon of nanocarrier drug delivery systems. J. Nanobiotechnol. 2025, 23, 138. [Google Scholar] [CrossRef]
- Crist, R.M.; Dasa, S.S.K.; Liu, C.H.; Clogston, J.D.; Dobrovolskaia, M.A.; Stern, S.T. Challenges in the development of nanoparticle-based imaging agents: Characterization and biology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1665. [Google Scholar] [CrossRef] [PubMed]
- FDA. Process Validation: General Principles and Practices; U.S. Food and Drug Administration: Rockville, MD, USA, 2011. Available online: www.fda.gov/media/71021/download (accessed on 10 July 2025).
- ICHQ13. ICH Guideline Q13 on Continuous Manufacturing of Drug Substances and Drug Products. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). European Medicines Agency (EMA)+3. 2021. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q13-continuous-manufacturing-drug-substances-drug-products-step-5_en.pdf (accessed on 10 July 2025).
- Gao, J.; Song, Q.; Gu, X.; Jiang, G.; Huang, J.; Tang, Y.; Yu, R.; Wang, A.; Huang, Y.; Zheng, G.; et al. Intracerebral fate of organic and inorganic nanoparticles is dependent on microglial extracellular vesicle function. Nat. Nanotechnol. 2024, 19, 376–386. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Kagan, C.R.; Millstone, J.E. Reproducibility in Nanocrystal Synthesis? Watch Out for Impurities! ACS Nano 2020, 14, 6359–6361. [Google Scholar] [CrossRef] [PubMed]
| Mechanism | Molecule Type | Size Limit | Transport Rate/Efficiency | Key Examples | References |
|---|---|---|---|---|---|
| Passive diffusion | Small, lipophilic | <400–500 Da | ~2–6% small molecules | O2, CO2, ethanol, nicotine | [2,34] |
| Paracellular transport | Small ions, water | ~1–2 nm pore size | 10−7–10−8 cm/s (e.g., sucrose ~340 Da) | Water, sucrose | [4,27,28] |
| Carrier-mediated transport (CMT) | Polar nutrients | Variable (nutrient-specific) | GLUT1: ~0.5 μmol/g/min (glucose uptake) | Glucose (GLUT1), leucine (LAT1) | [33] |
| Receptor-mediated transcytosis (RMT) | Specific peptides/proteins | Extensive (kDa range) | ~0.1–2% injected dose (antibody conjugates) | TfR, LDLR, insulin receptor | [37] |
| Adsorptive-mediated transcytosis (AMT) | Cationic/amphipathic macromolecules | Large (proteins, NPs) | ~10–20-fold increase (cationic albumin) | Cationic albumin, TAT peptide | [39] |
| Model Type | Specific Description | Preparation/Sourcing | Key Characteristics | Advantages | Limitations | Recommended Use Cases | References |
|---|---|---|---|---|---|---|---|
| In Vitro | Immortalized Cell Monolayer (hCMEC/D3), human brain endothelial cell line grown as a monolayer on inserts | Human brain endothelial cells immortalized (hTERT, SV40), cultured on collagen-coated Transwell inserts. | Retains human BBB markers but forms a leaky barrier (TEER ~30–100 Ω·cm2), low tight junction expression, and reduced efflux transporter activity (e.g., P-gp) compared to in vivo. | Simple, robust, high-throughput human model for rapid screening, drug uptake, and toxicity assays; expresses key BBB transporters and enzymes. | Very low barrier tightness (low TEER, high permeability); incomplete tight junctions; limited transporter expression; lacks astrocytes, pericytes, and flow; poor in vivo predictor. | High-throughput initial screening for NP BBB penetration, cytotoxicity, and human-specific transport; limited permeability precision requires validation in more stringent models. | [43,44,45,46] |
| In Vitro | Primary Endothelial Co-culture, e.g., primary rodent or porcine brain endothelial cells with astrocytes/pericytes | Freshly isolated brain endothelial cells seeded on permeable inserts, co-cultured with astrocytes (direct/indirect) to induce BBB phenotype, with optional inclusion of pericytes or primary human cells. | It develops tight junctions and exhibits low permeability, similar to in vivo conditions. Glial co-culture elevates TEER (porcine > rat > mouse). Expresses major BBB transporters (polarized P-gp, BCRP) and influx receptors (transferrin). Mimics in vivo drug exclusion (low paracellular flux). | Physiologically relevant model (high TEER, correct tight junctions, and native transporter activity); supports endothelial–glial interactions; BBB regulation; gold standard for permeability assays matching in vivo results. | Labor-intensive, low yield, requiring fresh tissue and specialized isolation; high batch variability and a short lifespan. Animal cells differ from human BBB; primary human cells are scarce and rapidly lose BBB properties (TEER ~40–50 Ω·cm2). | Mechanistic studies under near-physiological conditions; moderate-throughput screening with a tighter barrier than cell lines; validates simpler models; species selection based on goals; human model confirmation recommended. | [47,48,49] |
| In Vitro | hiPSC-Derived BBB-on-Chip—human iPSC-derived endothelial cells with astrocytes/pericytes in a microfluidic device. | hiPSCs differentiated to endothelial-like cells (Wnt/RA), cultured on Transwell or chip with astrocytes/pericytes. Shear flow and stimuli (e.g., hypoxia) improve maturation. | Human-like BBB phenotype: correct tight junctions (claudin-5, ZO-1, occludin); high TEER (~1000–5000 Ω·cm2); relevant transporter/receptor expression, efflux pumps (P-gp, BCRP; sometimes reduced activity); supports dynamic modulation. | An entirely human BBB model avoids species differences, maintains high barrier integrity (TEER > 20,000 Ω·cm2, >2 weeks), supports patient-derived iPSCs, and enables mechanistic and permeability studies. | Reflects systemic influences (metabolism, protein binding, and immune clearance). Measures real brain uptake and therapeutic effects. Genetically tractable. Cost-effective and accessible. | Human BBB model is ideal for preclinical NP testing, transport mechanism studies, and validating human-specific transporter targeting; suited for focused, low-throughput studies. | [40,41] |
| In Vivo | Mouse Model—rodent in vivo BBB (adult mice, healthy or disease models) | Brain uptake is assessed via post-mortem analysis, imaging (MRI/PET), or genetic models. | Complete neurovascular unit with tight junctions, high TEER (~1000–6000 Ω·cm2), dynamic blood flow, active efflux, and realistic NP biodistribution. Note: Higher P-gp expression in mice than in humans. | Captures systemic factors influencing NP delivery. Directly measures brain uptake and efficacy. Genetically modifiable (e.g., Mdr1a−/−). Cost-effective; suitable for disease modeling. | Species differences may underestimate human BBB uptake; small size complicates surgery and sampling; limited blood volume and rapid metabolism affect NP circulation; low throughput requires ethical approval; results require confirmation in other models. | In vivo proof-of-concept to confirm NP BBB crossing and payload delivery. Mechanistic analyses and preclinical efficacy testing. Intermediate validation step: positive mouse results typically require follow-up in NHPs for human translation. | [42,50,51] |
| In Vivo | Non-Human Primate Model—rhesus or cynomolgus macaque BBB in vivo. | NPs are administered intravenously (often under anesthesia), with brain uptake monitored by MRI, PET, or post-mortem analysis. Allows for repeated blood/CSF sampling. Small sample sizes (N ≤ 4–6) due to cost and ethical constraints. | Most similar to human BBB in structure and function. Monkeys share transporter profiles nearly identical to those of humans (96% amino acid identity in P-gp). Comparable brain anatomy, capillary tight junctions, and pericyte coverage. Enables NP testing in a human-like brain. | Highly predictive of human BBB outcomes. Supports clinical imaging (PET, SPECT) for detailed in vivo tracking of nanoparticles. Captures physiological BBB modulators, ensuring translational relevance. Essential for safety/toxicology evaluations required by regulatory agencies. | High cost, ethical, and logistical complexity limit throughput and statistical power. Requires specialized facilities and veterinary expertise. Minor physiological differences from humans exist. Handling stress and anesthesia may affect BBB properties. Genetic manipulation is impractical. | Late-stage validation confirms NP BBB crossing, safety, and pharmacokinetics before human trials. The final translational step uses primate models with human-like BBB and metabolism. This step is not for screening but informs critical go/no-go decisions. | [52,53] |
| In Vivo | Zebrafish Larval Model—zebrafish embryo/larva with developing BBB | Transparent zebrafish embryos (~3 dpf) with functional BBB. NPs are administered by microinjection or water exposure. Fluorescent transgenic lines visualize NP crossing of the BBB in vivo. | Zebrafish BBB with tight junctions and conserved transporters form by 3–4 dpf, selectively restricting molecules similarly to mammals. Key regulators (e.g., Mfsd2a) share analogous functions. Enables live tracking of NP BBB crossing. | The high-throughput, low-cost in vivo model enables parallel testing, real-time imaging, and genetic manipulation, with fewer ethical constraints. | The non-mammalian model exhibits differences in BBB maturity, immunity, and pharmacokinetics, resulting in limited predictive value, which necessitates mammalian validation and consideration of injection variability for certain NPs. | Rapid, early-stage in vivo screening of NP brain uptake and toxicity. Ideal for visualizing NP–BBB interactions. Helpful intermediate step before rodent studies; positive hits require mammalian validation. | [54,55,56] |
| Material Type (Examples) | Advantages | Limitations | References |
|---|---|---|---|
| Lipid-based NPs (liposomes, solid lipid NPs) | High biocompatibility: Mimic cell membranes; low toxicity. Effective BBB crossing: Ligand functionalization significantly improves brain uptake (~5× vs. untargeted). Efficient genetic cargo delivery: Excellent mRNA transfection; minimal off-target accumulation. | Limited BBB penetration: PEGylated liposomes show poor brain uptake unless ligand-functionalized. Stability issues: Drug leakage and instability (e.g., unsaturated lipids release payload quickly). Require stabilization (e.g., PEGylation, cholesterol) to prevent aggregation and fusion. Rapid RES clearance: Unmodified liposomes are quickly opsonized and removed by macrophages (liver/spleen). PEGylation increases circulation but may reduce cellular uptake. | [90,92,93] |
| Polymeric NPs (PLGA, PLA, etc.) | Biodegradable and biocompatible: FDA-approved PLA/PLGA minimizes toxicity. Tunable release: Polymer matrices allow controlled drug delivery. Surface versatility: Easy PEGylation or ligand attachment for BBB targeting (e.g., LDLR-targeted PLGA: ~4% vs. ~1% untargeted). Customizable shape (spheres, rods) optimizes biodistribution. | Incomplete biodegradation: High-molecular-weight or non-degradable polymers may accumulate. Potential cytotoxicity: Cationic/high-generation polymers risk cell and BBB damage; safety optimization is challenging. Limited BBB crossing: Unmodified polymeric NPs require PEGylation or ligands (e.g., surfactants, ApoE) for effective brain uptake. | [94,95,96] |
| Dendrimers (poly(propylenimine) PPI, PAMAM) | Multivalent BBB crossing: Highly branched polymers utilize both adsorptive (cationic surfaces) and receptor-mediated (ligand-attached) transcytosis mechanisms. Enhanced targeted delivery: Transferrin/lactoferrin-PPI dendrimers achieved a >6-fold higher gene delivery efficiency; Angiopep-2-PPI dendrimers improved paclitaxel uptake in glioma cells. CNS therapeutic efficacy: Specialized dendrimers (e.g., maltose–histidine G4 PPI) crossed the BBB and preserved cognition in Alzheimer’s models; versatile conjugation of drugs/genes for targeted therapy. | Intrinsic cytotoxicity: Higher-generation cationic dendrimers disrupt cell membranes; require surface modifications (e.g., sugars, PEG) to reduce toxicity. Non-biodegradable: Many dendrimers (PPI, PAMAM) persist in the liver/spleen, causing long-term accumulation. Developing degradable dendrimers is challenging. Immunogenicity/biocompatibility: Can induce complement activation or oxidative stress; repeated dosing demands extensive surface engineering for safety. | [97,98] |
| Protein-based NPs (ferritin, albumin, virus-like particles) | Natural transport pathways: Ferritin/transferrin-based NPs use transferrin receptor transcytosis for inherent BBB targeting (e.g., human H-ferritin delivers antibodies into brain tumors). High biocompatibility: Endogenous/recombinant protein NPs (albumin, VLPs) are biodegradable, enzymatically degradable, and non-toxic. Easy surface modification: Can attach peptides or antibodies (e.g., RVG29, angiopep-2, transferrin) for enhanced specificity; naturally multivalent ligands (e.g., VLPs) increase targeting density. | Immunogenicity risk: Protein carriers can trigger immune responses, especially with repeated doses or non-human sources, requiring stealth modifications (PEGylation/humanization). Stability issues: Proteins can easily aggregate or denature during formulation and storage, reducing their efficacy; therefore, cold-chain storage or the use of lyoprotectants is often necessary. Limited drug loading: Protein nanocages have a restricted interior space, limiting the size and amount of the payload, which may mean higher doses are required for a therapeutic effect. | [86,99] |
| Inorganic NPs (gold, iron oxide, silica) | Stable cores: Structurally robust inorganic NPs (metal/mineral) that are precisely tunable (1–100 nm), optimizing BBB crossing (e.g., ultrasmall gold NPs < 10 nm transit intact BBB). Multifunctional theranostics: Intrinsic imaging/therapy capabilities (gold: CT/photothermal; iron oxide: MRI; silica: drug carrier/imaging). Easy functionalization: Surfaces (gold/silica) readily attach ligands/coatings (thiols, silanes, polymers), enhancing targeting (~50 nm gold NPs: ~5× higher brain uptake) and colloidal stability. | Non-degradable: Inorganic cores (gold, silica, metal oxides) persist in organs (such as the liver, spleen, and brain), causing long-term accumulation and potential chronic toxicity. Potential delayed toxicity: Oxidative stress or inflammation from NP degradation, surface exposure, or ion release (e.g., iron oxide > 50 nm: oxidative stress; gold NPs < 5 nm: cellular disruption). Limited BBB transit: Large, unmodified inorganic NPs exhibit poor brain uptake; achieving therapeutic levels requires ultrasmall size (<10 nm), targeted delivery, or BBB disruption techniques (e.g., magnetic guidance, ultrasound). | [100,101] |
| Class | NPs (Formulation and Surface) | Size | Zeta | Brain Uptake | t1/2 | Therapeutic Efficacy | Model | References |
|---|---|---|---|---|---|---|---|---|
| Lipid-based | Liposome—PEGylated (untargeted) | ~90 nm | Negative | 0.023% ID/g (4 h post-IV) | Short (less than targeted) | N/A (no CNS therapy tested; baseline delivery) | Healthy mice (C57BL/6) | [102] |
| Lipid-based | Liposome–scFv antibody-targeted (BBB receptor-specific) | ~90 nm | Negative | 0.24% ID/g (4 h; ~10× over untargeted) | Longer circulation vs. Control | Improved brain drug levels (2-PAM); distribution study (no disease model) | Healthy mice | [102] |
| Lipid-based | Liposome—TAT peptide-functionalized | ~100 nm (est.) | Cationic (+) | ~0.1% ID/g (1 h; ~background level) | NA | N/A (no improved uptake; no efficacy) | Healthy mice | [103,104,105] |
| Polymeric | PLGA NP—Poloxamer 188-coated (MTX + PTX combo) | 133 nm and 221 nm | −29 mV/−18 mV nature.com | 17.2% ID/g (48 h post-IV) | Detected in the brain up to 48 h | ↓ Tumor volume, Ki-67; improved survival vs. control | Rat glioma (C6 orthotopic) | [106] |
| Polymeric | PLGA NP—unmodified (PEG-PLGA) | ~100 nm (typical) | ≈−15 mV (typical) | <1% ID/g (generally low) | Hours (moderate) | N/A (minimal BBB penetration) | Healthy rodents (general) | [107] |
| Polymeric | PAMAM Dendrimer—G4 (OH-terminated) | 4.3 nm | ~0 mV | 1.9 ± 0.3 μg/g in tumor (24 h) | Rapid renal clearance | N/A (carrier targeted to microglia/Mϕ) | Rat 9L gliosarcoma/GL261 GBM | [108] |
| Polymeric | PAMAM Dendrimer—G6 (OH-terminated) | 6.7 nm | ~0 mV | 17.6 ± 4.5 μg/g in tumor (24 h) | Extended (slower clearance) | N/A (selective TAM uptake; immunotherapy vehicle) | Mouse GL261 GBM | [108] |
| Protein-based | H-Ferritin nanocage (human heavy-chain) | ~12 nm | −(native) | Effective BBB penetration; slow clearance in brain | Long (persistent in brain) | N/A (proposed CNS drug carrier; no drug loaded) | Healthy mice | [109] |
| Protein-based | Virus-Like Particle (JC polyomavirus VLP) | ~40 nm | NA | ~0% ID/g (negligible brain uptake after IV) | NA | N/A (gene vector; no therapeutic cargo in study) | Healthy mice (IV vs. carotid) | [110,111] |
| Inorganic | Gold NP—PEGylated (no targeting) | ~15 nm (core) | ~0 mV (PEG-coated) | 0.04% ID/g (baseline) | ~2.3 h | N/A (used as BBB photomodulation agent) | Healthy mice | [112] |
| Inorganic | Gold NP—anti-JAM-A antibody (BV11) coated | ~15 nm (core) | ~0 mV | 0.13% ID/g (baseline; ~3× PEG-NP) | ~0.17 h (≈10 min) | N/A (facilitates laser-induced BBB opening) | Healthy mice | [112] |
| PPI dendrimer | Lactoferrin- and lactoferricin-conjugated PPI dendrimers (Gen.3) complexed with TNF-α plasmid DNA (dual-targeted dendriplex) | ~150 nm (polyplex) | Positive (cationic) at all DNA ratios. | Yes, targeted delivery to tumors; higher tumor uptake vs. non-targeted (lower liver uptake). | NA | Complete tumor regression in 60% of A431 tumors and 50% of B16-F10 tumors (one month) after IV treatment; well-tolerated. | Tumor-bearing mice (A431 xenograft and B16-F10 melanoma). | [113] |
| PPI dendrimer | Histidine–maltose shell PPI dendrimer (G4HisMal) (glyco-modified dendrimer, no drug; neuroprotective agent) | ~6 nm (monomer) | ~Neutral (sugar-modified) | Yes, enhanced BBB penetration (intranasal delivery gave 40% higher brain level vs. non-histidine control) | Not reported | Memory rescue: Treated APP/PS1 Alzheimer’s mice showed significantly improved memory vs. controls and preserved synaptic markers | APP/PS1 transgenic AD mice (±Aβ in vitro) | [114] |
| Strategy and System | Target/Mechanism | NP or Vector | Model Used | Brain Uptake Metrics | Therapeutic Outcome | References |
|---|---|---|---|---|---|---|
| TfR-mediated (RMT), e.g., OX26 antibody NP. | Transferrin receptor on the BBB endothelium. | OX26-conjugated PEGylated liposome or gold NP. | Rat (in vivo) | ~0.3% ID in brain vs. 0.03% for IgG (10-fold increase); parenchymal 0.23% ID/g with optimized affinity. | Enhanced brain drug levels; basis for enzyme therapy (ETV:IDS) yielding 50–76% substrate reduction in CNS. | [117,118,133] |
| T7-PLGA NPs | Transferrin receptor (TfR) targeting, T7 peptide (HAIYPRH) binds TfR on the BBB endothelium, triggering receptor-mediated transcytosis. | PLGA polymer NPs decorated with T7 peptide (often PEGylated; can carry drugs or genes). | Murine brain tumor models (orthotopic glioma) and healthy mice (distribution studies). | T7-functionalized NPs increased brain accumulation by ~6-fold, gene expression by 1.7-fold, and photosensitizer delivery to gliomas by ~6-fold versus untargeted controls. | T7-targeting improved outcomes: T7-liposomes (ZL006) reduced infarct volume and improved neurological recovery in stroke; T7-NPs enhanced tumor suppression and survival in glioma. | [154,155,156] |
| INSR-mediated (RMT), e.g., 29B4 antibody. | Insulin receptor (ubiquitous, BBB, and neurons). | Human insulin receptor mAb (29B4) on HSA NP. | Mouse (in vivo) | Qualitative crossing confirmed (therapeutic levels achieved); clinical fusion protein ~2–3% CSF: plasma ratio in patients (phase 1). | CNS enzyme delivery in Hunter syndrome (valanafusp alpha)—reduced CNS pathology; in rodents, INSR-NPs showed functional neuroprotection. | [119,120,157] |
| LRP1-mediated (RMT): Angiopep-2 peptide. | LRP1 on the endothelium (also in tumors). | Angiopep-2 decorated polymeric NP. | Mouse (in vivo); in vitro BBB models. | ~2–4× higher brain uptake vs. non-targeted NP (biodistribution studies); transcytosis of Ang2-NPs observed in iPSC-derived human BBB model. | In patients, Ang2-NP delivering paclitaxel (ANG1005) showed tumor shrinkage; Ang2-polymersomes with carnosine reduced stroke infarct volume. | [121,122,158] |
| Angiopep-2 lipid–silica NPs | Angiopep-2 targets LRP1 receptors on the BBB and glioma cells, mediating transcytosis into the brain. | Lipid-coated mesoporous silica NPs loaded with paclitaxel and functionalized with Angiopep-2. | Rat intracranial glioma model (C6 glioma-bearing rats; IV administration). | Angiopep-2 NPs enhanced paclitaxel brain delivery (~20.6% vs. ~10.6% targeting efficiency), doubling brain drug concentrations compared to untargeted controls. | Angiopep-2 targeting enhanced brain tumor therapy, prolonging survival and increasing tumor apoptosis compared to untargeted NPs. | [159] |
| Lactoferrin R-mediated (RMT)—Lf-NC. | Lactoferrin receptor (on BBB and glioma cells). | Lactoferrin-coated lipid nanocapsule (Lf-LNC). | Rat (PTZ epilepsy model). | Brain APO concentration ↑ (significant, e.g., 1.5-fold vs. uncoated); Lf coating improved BBB permeability. | Suppressed seizures: ~0.67 Racine score with Lf-LNC vs. ~3 (uncoated); reduced neuroinflammation. | [127,128,160] |
| Folate-mediated (RMT)—FA-NP. | Folate receptor-α (high in glioma, low BBB). | Folic acid-conjugated gold NP (or polymer NP). | Mouse glioma (orthotopic) | Tumor: brain ratio > 5:1 uptake in FR-positive tumor; minimal uptake in normal brain. | Enhanced GBM cell kill and imaging contrast; extended survival in folate-R expressing tumor models. | [131,132,161] |
| Adsorptive (AMT)—PepH3 peptide NP. | Electrostatic adsorptive uptake. | PepH3 (7-aa cationic) tagged vesicular NP. | Rat and human BBB cell culture; Mouse IV. | Endothelial uptake ↑ (~3–5× vs. no peptide); in vivo high brain localization, low off-target (radiotracer). | Delivered anti-Aβ single-domain antibody across the BBB in vitro; potential Alzheimer’s therapy shuttle (in vivo efficacy pending). | [135,136,137] |
| Adsorptive (AMT)—Chitosan NP. | Electrostatic (polycationic polymer) | Chitosan DNA NP (~260 nm) | Mouse (in vivo, i.p. injection). | Confirmed BBB crossing: GFP gene expressed in brain cells; brain transfection efficiency ~53% (FACS, vs. 27% with control vector). | Successfully expressed therapeutic gene (GFP) in brain parenchyma; proof-of-concept for gene therapy in brain tumors or neurodegeneration. | [139] |
| Magnetic Targeting—Liposomal SPION (LTF). | External static magnetic field (SMF) guides NP. | Temozolomide + ferucarbotran liposome (LTF). | Mouse glioma (GL261 in the brain). | The tumor NP concentration was ~2 times higher in the magnet (MRI-based) group; the magnet-guided group showed a p < 0.01 reduction in tumor volume by day 7. | Tumor growth suppressed; median survival ↑ vs. non-magnet (e.g., ~25 days to >31 days with magnet). | [142] |
| Magnetic nanocapsules | Magnetic targeting via an external field enhances the transcytosis of iron oxide nanocapsules across the BBB. | ~100 nm silica-coated magnetic NPs (iron oxide core) with RF-triggered drug-release capability. | Healthy mice with intact BBBs were subjected to localized magnetic targeting post-IV injection. | Localized magnetic fields increased brain NP delivery by ~25–26-fold versus controls; ~30% of peak brain signal persisted at 48 h, while non-magnetized delivery remained near background levels. | Magnetic nanocapsules enabled non-invasive BBB crossing without acute toxicity; histology confirmed vessel integrity. Though therapeutic efficacy remains untested, they allow for on-demand drug release via radio-frequency heating. | [162,163] |
| Magnetic Targeting, Tween-SPION. | Magnetic field induces BBB transport. | 20 nm Tween-80 coated SPIONs. | Rat (normal BBB, iv + EMF). | Crossed intact BBB under EMF; SPIONs detected in brain parenchyma (none without EMF). | No therapeutic payload (diagnostic); demonstrates non-invasive BBB crossing by physical force. | [142] |
| Viral Vector—Engineered AAV (CAP-Mac). | Capsid-mediated transcytosis (evolved tropism). | AAV.CAP-Mac (neurotropic AAV variant). | Non-human primates (marmoset, macaque). | ~1.1–1.3% of all neurons transduced (green monkey) vs. <0.5% with AAV9; broad CNS distribution (11 of 11 regions positive). | Enabled IV gene delivery, e.g., widespread GCaMP expression for imagingnature.com; supports CNS gene therapy (potential for autism, Alzheimer’s). | [145] |
| Viral Vector—RVG-pseudotyped LV. | Viral glycoprotein-mediated entry. | Lentivirus coated with RVG peptide. | Mouse (in vivo). | Qualitative BBB crossing (RVG-LV detected in brain, unlike unmodified LV); transgene in neurons. | Partial motor function restoration in a neurodegenerative mouse model (using RVG-LV to deliver therapeutic gene). | [144,151] |
| TMV-VLPs | Size/shape EPR + ligand (albumin, etc.) | Tobacco Mosaic Virus nanorod + albumin coat | Mouse (brain tumor model) | Accumulated in brain tumor (MRI and NIR imaging); higher tumor: normal brain ratio than spherical NP. | Improved tumor imaging and delivery of photothermal therapy; significant tumor cell apoptosis in combination treatment. | [77,164] |
| CCMV VLPs | None (passive) Natural 28 nm protein cage (plant virus capsid) with no specific targeting; crosses BBB at low levels, possibly via adsorptive transcytosis. | Empty CCMV capsid as a drug nanocarrier. | Healthy mice (IV injection, no disruption of BBB). | Approximately 0.3% ID/g was detected in the brain at one h post-injection, decreasing to <0.01% by 24 h; brain distribution was comparable to other protein-based NPs. | No therapeutic payload was tested; safety studies showed no overt toxicity or immune response in mice. | [165] |
| Exosome—RVG-modified MSC exosomes | Endogenous vesicle uptake + neuron targeting | RVG-peptide engineered exosomes (MSC-derived) | Mouse Alzheimer’s model | Preferential localization to cortex/hippocampus; exosomal cargo (siRNA) in brain increased ~2× vs. free siRNA. | Restored memory function (exosome-treated mice performed significantly better in Morris water maze); reduced brain Aβ and inflammation. | [151,166,167] |
| Exosome—Cetuximab-Exo-Dox | Endogenous vesicle + tumor targeting (EGFR) | Exosomes loaded with doxorubicin + Cetuximab | Mouse glioblastoma model | Brain delivery of cetuximab ↑ (~2-fold) with exosomes vs. free Ab; doxorubicin brain concentration also higher (HPLC quantification). | Enhanced GBM growth inhibition and prolonged survival vs. free drug; exo combo therapy induced greater tumor cell apoptosis (histology). | [152] |
| MSC-derived exosomes | MSC-derived exosomes (~50–150 nm) cross the BBB via endocytosis, with enhanced uptake under inflammatory conditions. | MSC-derived EVs carrying therapeutic cargo (proteins/miRNA or drugs). | Rodent models of CNS injury (stroke, TBI) for therapy; healthy rats for biodistribution. | Baseline brain uptake was low (~0.03–0.04% ID/g) after IV administration; entry increased in neuroinflammatory models with preferential accumulation in injured regions. | MSC-exosomes reduced infarct volume by ~50%, improved neurological function in stroke, and attenuated neuroinflammation with cognitive recovery in TBI models. | [168,169,170,171,172,173,174] |
| Micromotor—NIR Janus nanomotor | Photothermal propulsion (active movement) | Gold–Janus NPs (NIR-responsive) | Mouse (in vivo experiment) | BBB penetration significantly improved under NIR (qualitative: increased dye leakage into brain); no crossing without NIR. | Facilitated brain delivery of a model drug (dye) with spatiotemporal control; concept validated for on-demand BBB opening. | [175] |
| Macrophage-mediated “Trojan Horse” delivery | Monocytes/macrophages naturally cross the BBB, delivering internalized drug-loaded NPs to inflamed or tumor sites. | Macrophages loaded ex vivo with drug-encapsulated NPs, then injected intravenously. | Mouse glioblastoma and neuroinflammation (e.g., Parkinson’s) models using macrophage adoptive transfer. | NP-loaded macrophages greatly enhanced brain tumor localization versus free NPs; intrathecal macrophage transfer achieved ~8.1% ID/g brain uptake, surpassing standard IV delivery. | Macrophage-mediated delivery improved glioma drug deposition, potentially limiting tumor growth, and increased GDNF levels with functional recovery in Parkinson’s, leveraging immune-cell homing to the CNS. | [176] |
| Microrobot—Magnetic spiral (platelet cloaked) | Magnetic rotation (swimming) | Helical nanorobot with Fe coating + platelet membrane | In vitro blood flow; proposed in vivo mouse | Propulsion sustained in blood-mimicking flow; able to navigate and marginate toward vessel walls. (BBB crossing has not yet been directly measured). | Demonstrated long circulation and targeting potential; aims to mechanically traverse the BBB and deliver drugs (studies in progress). | [177] |
| Parameter | Recommended Studies/Assays | Minimal Data Required (Thresholds) | Key Considerations | References |
|---|---|---|---|---|
| Hemocompatibility (blood compatibility of IV nanomedicine) | In vitro blood tests: Hemolysis assay (human RBCs); complement activation (C3a, C5a, SC5b-9); platelet aggregation/coagulation (platelet markers, thrombin, aPTT, PT). | Hemolysis: % hemoglobin release; <5% considered low risk (non-hemolytic). (ISO 10993-4 standard). Ideally, <2% (negligible hemolysis). Complement: No abnormal complement consumption or excessive anaphylatoxin rise (C3a/C5a) compared to negative control (i.e., should not trigger significant CARPA). Platelets/Coagulation: No significant platelet aggregation or >10% change in clotting times relative to baseline. (No official numeric limit; ensure values remain within a normal range of variation.) | Use fresh human blood to avoid species-specific platelets and complement differences. Account for NP interference with assay readouts via proper controls. Follow ISO 10993-4 and ASTM F756 standards to ensure hemocompatibility (to prevent thrombosis, hemolysis, and infusion reactions). If complement activation occurs in vitro, evaluate the risk of infusion reaction in vivo and consider methods for complement inhibition. | [16,178,179,180,181,182,183,184,185] |
| Neurotoxicity (CNS behavioral and histopathological safety) | Safety pharmacology and neurotoxicity: Perform FOB or modified Irwin test (locomotion, reflexes, coordination, sensorimotor responses, convulsions). Include behavioral assays (open field, rotarod, cognition if needed). Assess CNS histopathology (brain, spinal cord) in subchronic/chronic studies for neuronal/glial damage, inflammation, or vacuolization. | Neurobehavioral outcomes: No significant adverse effects on motor activity, gait, reflexes, or behavior at therapeutic levels. Minor changes (<20% compared to the control), reversible, and dose-dependent effects are acceptable. Neuropathology: No significant CNS lesions (neuronal degeneration, gliosis, demyelination) exist beyond background; minimal changes are allowed only at doses above therapeutic exposure. Neurofunctional tests: Grip strength, rotarod, and maze performance impairment should remain minimal (<10–15%) at clinical doses, excluding sedation effects. | FDA S7A Guidance: Evaluate CNS effects (behavior, reflexes, coordination, temperature), especially for CNS-targeted NPs. Assess neuroinflammation (microglia/astrocytes: Iba1, GFAP). Include behavioral tests for chronic CNS exposure. Use recovery groups to check reversibility. | [179,186,187,188,189] |
| Long-term Accumulation (Brain retention and clearance) | Biodistribution studies: Labeled NPs (radioactive/fluorescent) track long-term brain and organ distribution. Multiple time point assessments (weeks to months) are conducted, and imaging methods (PET/MRI) are preferred for non-invasive monitoring. Chronic toxicity: Extended observation periods post-treatment should be included to evaluate CNS persistence and delayed neurotoxicity. Brain and CSF should be analyzed at intervals to assess clearance. | Brain retention half-life: Biodegradable NPs should clear significantly (>50% within weeks); non-biodegradable NPs must plateau without progressive accumulation. Residual brain burden: post-treatment brain levels should substantially decrease (<10% peak) within 1–3 months. Clearance pathways: Identify clearance routes (e.g., glymphatic). Rapid clearance (hours–days) is preferred; persistent presence (>6 months) needs justification. | NP biodegradability: Assess persistence of nonbiodegradable NPs (e.g., gold, silica) and potential chronic neurotoxicity. Biological fate (FDA): Evaluate NP distribution, accumulation, and clearance from the brain and organs. Brain clearance: Examine glymphatic and phagocytic pathways; test in healthy and impaired clearance models. Human translation: Use animal retention data to inform human risk; persistent retention may require clinical imaging or dose adjustments. | [165,187,190,191,192,193] |
| Biodistribution and Pharmacokinetics (PK) (Systemic and CNS distribution, drug exposure) | Animal ADME studies: Radiolabeled or tracer methods track the distribution of NPs and their payloads over time. They measure plasma pharmacokinetics (Cmax, T½, AUC, clearance) and tissue distribution (e.g., percentage of dose in brain vs. organs). Brain penetration metrics: Calculate brain: plasma ratios or brain targeting indices. Include CSF levels if relevant. PK modeling: Apply compartmental or PBPK models using NP properties to predict human PK and dosing. | Animal ADME studies: Radiolabeled/tracer methods measure the distribution of nanoparticles and their payloads in plasma, brain, and organs over time. Report plasma PK (C_max, T_½, AUC, clearance) and tissue distribution (% dose in brain vs. other organs). Brain penetration: Calculate brain: plasma ratios or targeting index; include CSF levels if relevant. PK modeling: Apply compartmental or PBPK models with NP parameters to predict human PK and dosing. | Plasma half-life (T1/2): Report half-life versus free drug; nanoformulations typically extend circulation time (~10× longer than expected). Brain uptake: Higher brain/plasma ratios (>0.1 generally, >1.0 if targeted) indicate improved CNS targeting compared to a free drug. Bioavailability/distribution: Quantify brain delivery fraction. Minimize systemic exposure intrathecally; characterize off-target accumulation intravenously. PK linearity: Confirm dose-proportional exposure; investigate significant non-linearities (e.g., saturation, aggregation). | [190,194,195,196] |
| Immunogenicity and immunotoxicity (Regulatory requirement) | In vitro assays: Cytokine release (IL-6, TNFα, IFNγ) in human PBMCs. Complement activation (C5a, SC5b-9; CARPA risk). Immune cell function (macrophage uptake/ROS, T-cell activation, dendritic cell maturation). Myelosuppression (bone marrow colony assays). In vivo immunotoxicity: Evaluate immune organ histopathology (spleen, lymph nodes) and blood leukocyte subsets. If immunotoxic signals appear, targeted studies should be conducted per ICH S8. Monitor anti-drug antibodies (ADAs), especially against proteins or PEG. | Cytokine induction: Minimal cytokine release (e.g., IL-6 <3× baseline). Use controls for comparison. Complement activation: Low complement activation (<50% positive control). High levels signal hypersensitivity risk. Immunogenic antibodies: Monitor anti-nanoparticle antibodies; incidence typically ≤20%. Significant anti-PEG IgM or clearance changes (ABC phenomenon) require attention. Immune cell counts: Maintain WBC subsets within ±30% of control. Investigate consistent suppression or activation (e.g., T-cell drop, eosinophilia). | Nanomedicine immunogenicity: Assess risks (patient, route, dose). Include NP-specific assays (complement, inflammasome, immune cells). Carrier vs. Payload: Identify carrier (anti-PEG) vs. payload reactions; mitigate significant responses (e.g., ABC phenomenon). Clinical monitoring: Monitor immune reactions (anaphylaxis, complement). Investigate mechanisms; justify and plan mitigation strategies. | [183,190,195,197,198] |
| Scale-up and reproducibility | Chemistry, Manufacturing, and Controls (CMC): Implement robust cGMP processes. Characterize NP CQAs: particle size (DLS, laser diffraction), ζ-potential, morphology (TEM), drug loading/encapsulation (HPLC, spectroscopy), purity, endotoxin (LAL), and sterility (injectables). Conduct stability studies (size, potency, aggregation over time). Scale-up validation: Using statistical quality control, ensure batch consistency (size, PDI, drug content/release) between pilot and production scales. | Particle size and PDI: Maintain consistent size (±10% target), low PDI (<0.3; ideally ≤0.2). Avoid aggregates (>1000 nm). Drug content/release: Drug content within 90–110% of label; consistent batch-to-batch release profiles. Other CQAs: Stable zeta potential, impurities within ICH Q3D, endotoxin below USP limits (<5 EU/kg). Reproducibility: Consistent CQAs batch-to-batch (%RSD < 5–10%). Scale-up should not affect critical attributes. | A QbD approach is recommended to control critical parameters, ensuring consistent NP quality at scale. Analytical methods require NP-specific validation, reporting particle size confirmed by orthogonal methods. Adjustments during scale-up (e.g., homogenization parameters) and documented comparability (FDA/EMA guidelines) are essential. Regulatory compliance requires cGMP manufacturing, aseptic processing (especially if >200 nm), comprehensive CQA testing, and detailed CMC documentation before clinical approval. | [190,199,200,201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera-Maldonado, L.; Parra-González, M.; Peralta-Cuevas, E.; Gutierrez-Onofre, A.J.; Garcia-Atutxa, I.; Villanueva-Flores, F. Cracking the Blood–Brain Barrier Code: Rational Nanomaterial Design for Next-Generation Neurological Therapies. Pharmaceutics 2025, 17, 1169. https://doi.org/10.3390/pharmaceutics17091169
Nájera-Maldonado L, Parra-González M, Peralta-Cuevas E, Gutierrez-Onofre AJ, Garcia-Atutxa I, Villanueva-Flores F. Cracking the Blood–Brain Barrier Code: Rational Nanomaterial Design for Next-Generation Neurological Therapies. Pharmaceutics. 2025; 17(9):1169. https://doi.org/10.3390/pharmaceutics17091169
Chicago/Turabian StyleNájera-Maldonado, Lucio, Mariana Parra-González, Esperanza Peralta-Cuevas, Ashley J. Gutierrez-Onofre, Igor Garcia-Atutxa, and Francisca Villanueva-Flores. 2025. "Cracking the Blood–Brain Barrier Code: Rational Nanomaterial Design for Next-Generation Neurological Therapies" Pharmaceutics 17, no. 9: 1169. https://doi.org/10.3390/pharmaceutics17091169
APA StyleNájera-Maldonado, L., Parra-González, M., Peralta-Cuevas, E., Gutierrez-Onofre, A. J., Garcia-Atutxa, I., & Villanueva-Flores, F. (2025). Cracking the Blood–Brain Barrier Code: Rational Nanomaterial Design for Next-Generation Neurological Therapies. Pharmaceutics, 17(9), 1169. https://doi.org/10.3390/pharmaceutics17091169






