A Preliminary Study on the Accuracy of MRI-Guided Thalamic Infusion of AAV2-GFP and Biodistribution Analysis Using Cryo-Fluorescence Tomography in Nonhuman Primates
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Test Article
2.4. MRI-Guided Intracerebral Infusion
2.5. Clinical Observations
2.6. Tissue Processing
2.7. Histological Staining and Analysis (Chromogenic Detection)
2.8. Cryo-Fluorescence Tomography
2.9. MRI and 3D Volume Reconstruction
3. Results
3.1. Performance of ClearPoint Navigational Software
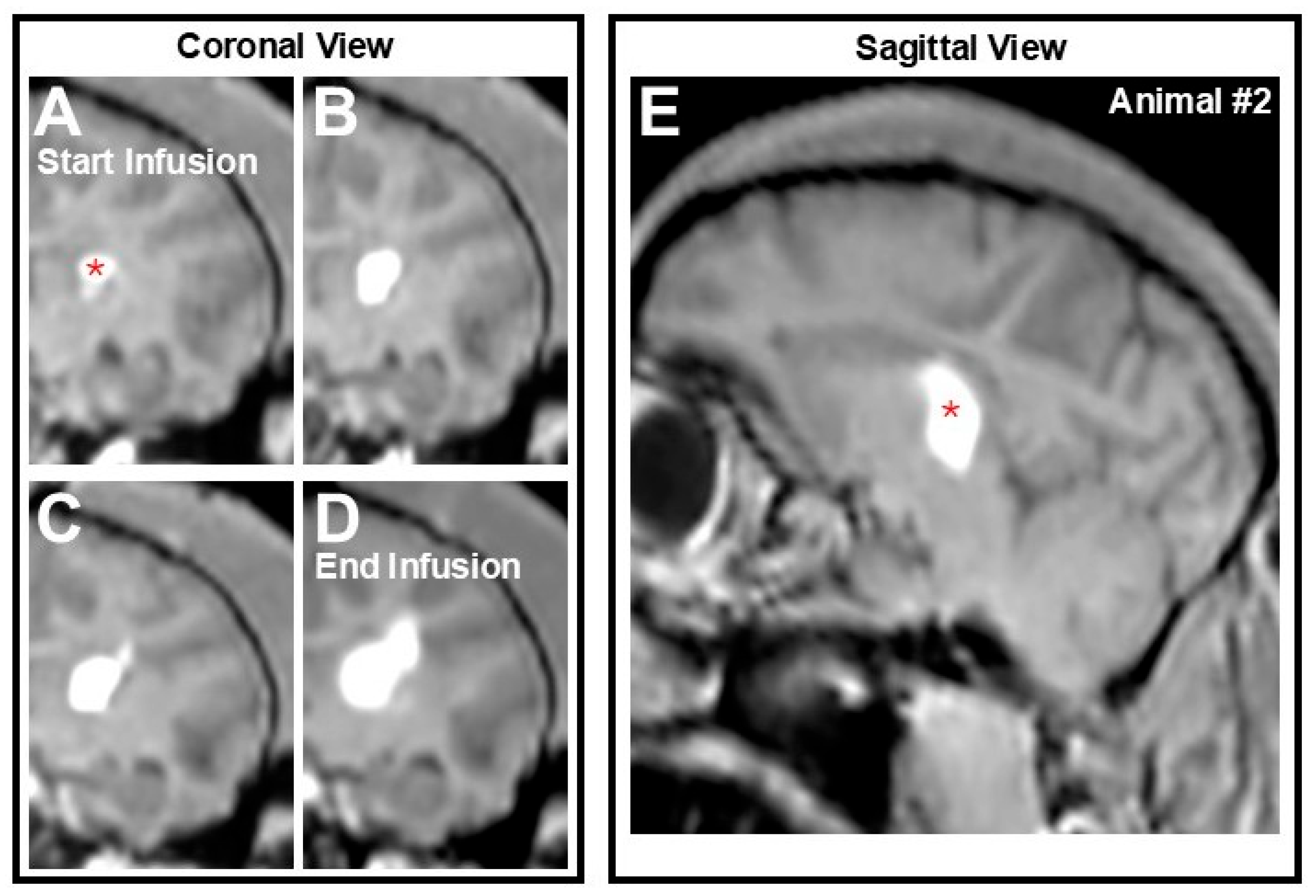
3.2. AAV2-GFP Vector Distribution
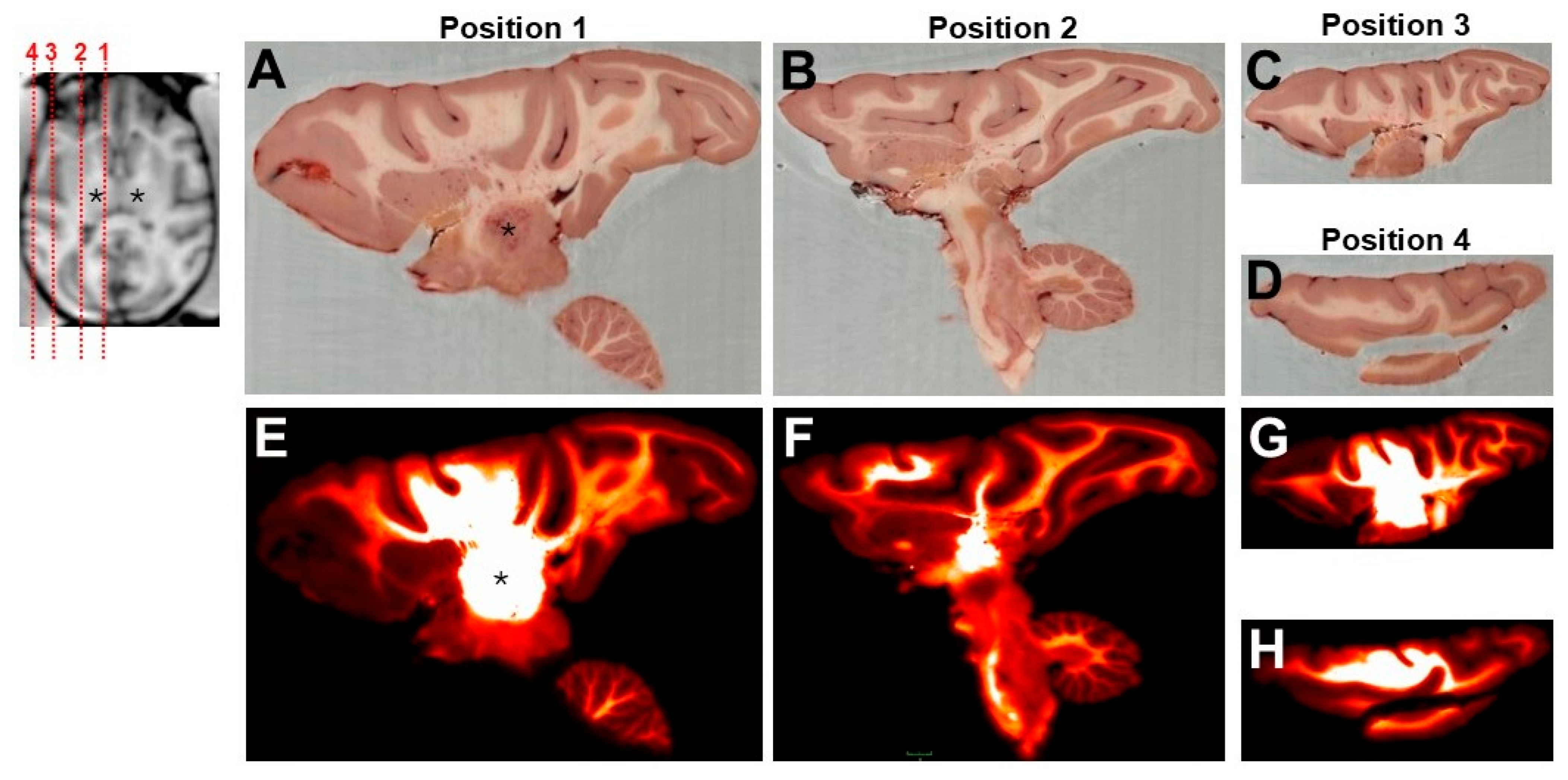
3.3. Biodistribution of GFP Transgene
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAALAC | Association for assessment and accreditation of laboratory animal care |
| AAV | Adeno-associated viral |
| AAV2 | Adeno-associated viral serotype 2 |
| CED | Convection-enhanced delivery |
| CFT | Cryo-fluorescence tomography |
| CMV | Cytomegalovirus |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| DAB | 3, 3′-Diaminobenzidine |
| ECG | Electrocardiogram |
| EMA | European medicines agency |
| FDA | U.S. Food and drug administration |
| GFP | Green fluorescent protein |
| IACUC | Institutional animal care and use committee |
| MRI | Magnetic resonance imaging |
| ms | milliseconds |
| NHP | Nonhuman primate |
| OCT | Optimal cutting temperature |
| ROI | Region-of-interest |
| T1w | Time-1-weighted |
References
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Liu, D.; Li, T.; Liu, L.; Che, X.; Li, X.; Liu, C.; Wu, G. Adeno-Associated Virus Therapies: Pioneering Solutions for Human Genetic Diseases. Cytokine Growth Factor. Rev. 2024, 80, 109–120. [Google Scholar] [CrossRef]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Samaranch, L.; Salegio, E.A.; San Sebastian, W.; Kells, A.P.; Bringas, J.R.; Forsayeth, J.; Bankiewicz, K.S. Strong Cortical and Spinal Cord Transduction after AAV7 and AAV9 Delivery into the Cerebrospinal Fluid of Nonhuman Primates. Hum. Gene Ther. 2013, 24, 526–532. [Google Scholar] [CrossRef]
- Han, S.J.; Bankiewicz, K.; Butowski, N.A.; Larson, P.S.; Aghi, M.K. Interventional MRI-Guided Catheter Placement and Real Time Drug Delivery to the Central Nervous System. Expert. Rev. Neurother. 2016, 16, 635–639. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Aghi, M.K. Convection-Enhanced Delivery for the Treatment of Glioblastoma. Neuro Oncol. 2015, 17 (Suppl. 2), ii3–ii8. [Google Scholar] [CrossRef] [PubMed]
- Kells, A.P.; Hadaczek, P.; Yin, D.; Bringas, J.; Varenika, V.; Forsayeth, J.; Bankiewicz, K.S. Efficient Gene Therapy-Based Method for the Delivery of Therapeutics to Primate Cortex. Proc. Natl. Acad. Sci. USA 2009, 106, 2407–2411. [Google Scholar] [CrossRef] [PubMed]
- Yazdan-Shahmorad, A.; Tian, N.; Kharazia, V.; Samaranch, L.; Kells, A.; Bringas, J.; He, J.; Bankiewicz, K.; Sabes, P.N. Widespread Optogenetic Expression in Macaque Cortex Obtained with MR-Guided, Convection Enhanced Delivery (CED) of AAV Vector to the Thalamus. J. Neurosci. Methods 2018, 293, 347–358. [Google Scholar] [CrossRef]
- Yin, D.; Richardson, R.M.; Fiandaca, M.S.; Bringas, J.; Forsayeth, J.; Berger, M.S.; Bankiewicz, K.S. Cannula Placement for Effective Convection-Enhanced Delivery in the Nonhuman Primate Thalamus and Brainstem: Implications for Clinical Delivery of Therapeutics. J. Neurosurg. 2010, 113, 240–248. [Google Scholar] [CrossRef]
- Salegio, E.A.; Kells, A.P.; Richardson, R.M.; Hadaczek, P.; Forsayeth, J.; Bringas, J.; Sardi, S.P.; Passini, M.A.; Shihabuddin, L.S.; Cheng, S.H.; et al. Magnetic Resonance Imaging-Guided Delivery of Adeno-Associated Virus Type 2 to the Primate Brain for the Treatment of Lysosomal Storage Disorders. Hum. Gene Ther. 2010, 21, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. AAV2-GAD Gene Therapy for Advanced Parkinson’s Disease: A Double-Blind, Sham-Surgery Controlled, Randomised Trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Buttery, P.C.; Barker, R.A. Gene and Cell-Based Therapies for Parkinson’s Disease: Where Are We? Neurotherapeutics 2020, 17, 1539–1562. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.M.; Bankiewicz, K.S.; Christine, C.W.; Van Laar, A.D.; Gross, R.E.; Lonser, R.; Factor, S.A.; Kostyk, S.K.; Kells, A.P.; Ravina, B.; et al. Data-Driven Evolution of Neurosurgical Gene Therapy Delivery in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1210–1218. [Google Scholar] [CrossRef]
- Leach, B.I.; Lister, D.; Adams, S.R.; Bykowski, J.; Schwartz, A.B.; McConville, P.; Dimant, H.; Ahrens, E.T. Cryo-Fluorescence Tomography as a Tool for Visualizing Whole-Body Inflammation Using Perfluorocarbon Nanoemulsion Tracers. Mol. Imaging Biol. 2024, 26, 888–898. [Google Scholar] [CrossRef]
- Roy, D.; Steyer, G.J.; Gargesha, M.; Stone, M.E.; Wilson, D.L. 3D Cryo-Imaging: A Very High-Resolution View of the Whole Mouse. Anat. Rec. 2009, 292, 342–351. [Google Scholar] [CrossRef]
- Deng, L.; Chen, J.; Li, Y.; Han, Y.; Fan, G.; Yang, J.; Cao, D.; Lu, B.; Ning, K.; Nie, S.; et al. Cryo-Fluorescence Micro-Optical Sectioning Tomography for Volumetric Imaging of Various Whole Organs with Subcellular Resolution. iScience 2022, 25, 104805. [Google Scholar] [CrossRef]
- Richardson, R.M.; Kells, A.P.; Rosenbluth, K.H.; Salegio, E.A.; Fiandaca, M.S.; Larson, P.S.; Starr, P.A.; Martin, A.J.; Lonser, R.R.; Federoff, H.J.; et al. Interventional MRI-Guided Putaminal Delivery of AAV2-GDNF for a Planned Clinical Trial in Parkinson’s Disease. Mol. Ther. 2011, 19, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.M.; Kells, A.P.; Martin, A.J.; Larson, P.S.; Starr, P.A.; Piferi, P.G.; Bates, G.; Tansey, L.; Rosenbluth, K.H.; Bringas, J.R.; et al. Novel Platform for MRI-Guided Convection-Enhanced Delivery of Therapeutics: Preclinical Validation in Nonhuman Primate Brain. Stereotact. Funct. Neurosurg. 2011, 89, 141–151. [Google Scholar] [CrossRef]
- Salegio, E.A.; Campagna, M.V.; Allen, P.C.; Stockinger, D.E.; Song, Y.; Hwa, G.G.C. Targeted Delivery and Tolerability of MRI-Guided CED Infusion into the Cerebellum of Nonhuman Primates. Hum. Gene Ther. Methods 2018, 29, 169–176. [Google Scholar] [CrossRef]
- Salegio, E.A.; Cukrov, M.; Lortz, R.; Green, A.; Lambert, E.; Copeland, S.; Gonzalez, M.; Stockinger, D.E.; Yeung, J.M.; Hwa, G.G.C. Feasibility of Targeted Delivery of AAV5-GFP into the Cerebellum of Nonhuman Primates Following a Single Convection-Enhanced Delivery Infusion. Hum. Gene Ther. 2022, 33, 86–93. [Google Scholar] [CrossRef]
- Dubach, M.; Bowden, D. BrainInfo Online 3D Macaque Brain Atlas: A Database in the Shape of a Brain. In Proceedings of the Society for Neuroscience Annual Meeting, Chicago, IL, USA, 17–21 October 2009. 199.5. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-Enhanced Delivery of Macromolecules in the Brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Sudhakar, V.; Naidoo, J.; Samaranch, L.; Bringas, J.R.; Lonser, R.R.; Fiandaca, M.S.; Bankiewicz, K.S. Infuse-as-You-Go Convective Delivery to Enhance Coverage of Elongated Brain Targets: Technical Note. J. Neurosurg. 2020, 133, 530–537. [Google Scholar] [CrossRef]
- Brady, M.; Raghavan, R.; Sampson, J. Determinants of Intraparenchymal Infusion Distributions: Modeling and Analyses of Human Glioblastoma Trials. Pharmaceutics 2020, 12, 895. [Google Scholar] [CrossRef]
- Chittiboina, P.; Heiss, J.D.; Lonser, R.R. Accuracy of Direct Magnetic Resonance Imaging-Guided Placement of Drug Infusion Cannulae. J. Neurosurg. 2015, 122, 1173–1179. [Google Scholar] [CrossRef]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-Enhanced Delivery in Glioblastoma: A Review of Preclinical and Clinical Studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.T.; Yin, D.; Martin, A.J.; Coppes, V.G.; Mann, P.; Larson, P.S.; Starr, P.A.; Zeng, X.; Gupta, N.; Panter, S.S.; et al. Interventional Magnetic Resonance Imaging-Guided Cell Transplantation into the Brain with Radially Branched Deployment. Mol. Ther. 2015, 23, 119–129. [Google Scholar] [CrossRef]
- Green, F.; Samaranch, L.; Zhang, H.S.; Manning-Bog, A.; Meyer, K.; Forsayeth, J.; Bankiewicz, K.S. Axonal Transport of AAV9 in Nonhuman Primate Brain. Gene Ther. 2016, 23, 520–526. [Google Scholar] [CrossRef]
- Samaranch, L.; Blits, B.; San Sebastian, W.; Hadaczek, P.; Bringas, J.; Sudhakar, V.; Macayan, M.; Pivirotto, P.J.; Petry, H.; Bankiewicz, K.S. MR-Guided Parenchymal Delivery of Adeno-Associated Viral Vector Serotype 5 in Non-Human Primate Brain. Gene Ther. 2017, 24, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S.; Ball, G. A Phylogenetically-Conserved Axis of Thalamocortical Connectivity in the Human Brain. Nat. Commun. 2023, 14, 6032. [Google Scholar] [CrossRef] [PubMed]
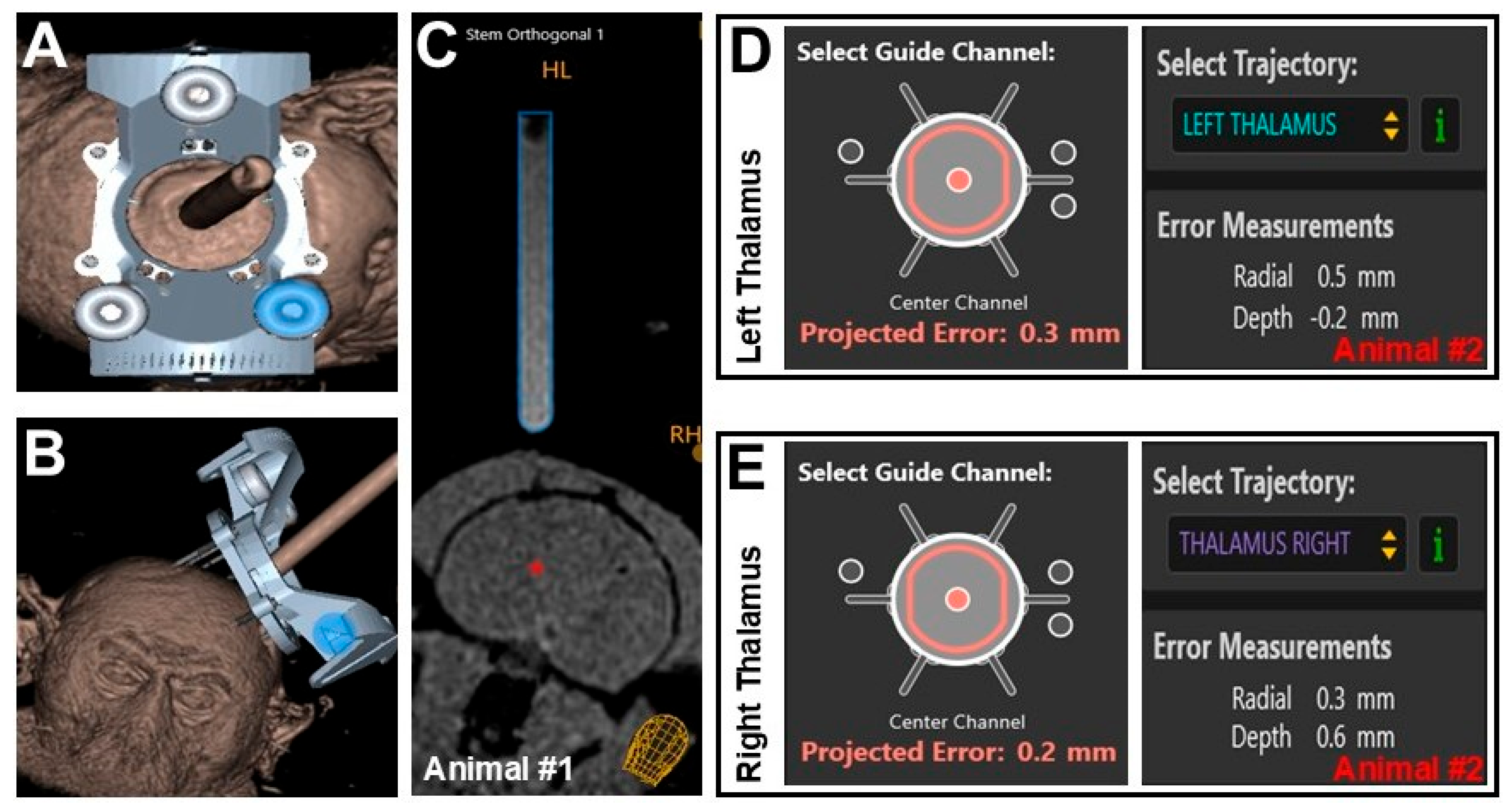

| Metric | Animal #1 | Animal #2 | |
|---|---|---|---|
| Right Side | Right Side | Left Side | |
| Projected Radial Error | 0.7 mm | 0.3 mm | 0.5 mm |
| Actual Radial Error | 0.6 mm | 0.2 mm | 0.3 mm |
| Difference in Radial Error | 0.1 mm | 0.1 mm | 0.2 mm |
| Final Depth Error | −0.1 mm | 0.6 mm | −0.2 mm |
| Brain Region | Structures Transduced | GFP Expression |
|---|---|---|
| Cerebral gray/white matter | Axons, neurons | Strong, 1 or 3 * |
| Internal capsule | Axons | Strong, 1 or 3 * |
| Lateral ventricle | Ependymal cells | Moderate, 1 |
| Hypothalamus | Axons, neurons | Light to strong, 2 |
| Thalamus | Axons, neurons | Light to strong, 2 |
| Caudate nucleus | Axons | Light to moderate, 1 |
| Pons | Axons, neurons | Light to strong, 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salegio, E.A.; Espinosa, R.; Smith, G.R.; Shoshan, D.; Silva, M.; White, E.; McDonald, J. A Preliminary Study on the Accuracy of MRI-Guided Thalamic Infusion of AAV2-GFP and Biodistribution Analysis Using Cryo-Fluorescence Tomography in Nonhuman Primates. Pharmaceutics 2025, 17, 1167. https://doi.org/10.3390/pharmaceutics17091167
Salegio EA, Espinosa R, Smith GR, Shoshan D, Silva M, White E, McDonald J. A Preliminary Study on the Accuracy of MRI-Guided Thalamic Infusion of AAV2-GFP and Biodistribution Analysis Using Cryo-Fluorescence Tomography in Nonhuman Primates. Pharmaceutics. 2025; 17(9):1167. https://doi.org/10.3390/pharmaceutics17091167
Chicago/Turabian StyleSalegio, Ernesto A., Reinier Espinosa, Geary R. Smith, David Shoshan, Matthew Silva, Eli White, and Jacob McDonald. 2025. "A Preliminary Study on the Accuracy of MRI-Guided Thalamic Infusion of AAV2-GFP and Biodistribution Analysis Using Cryo-Fluorescence Tomography in Nonhuman Primates" Pharmaceutics 17, no. 9: 1167. https://doi.org/10.3390/pharmaceutics17091167
APA StyleSalegio, E. A., Espinosa, R., Smith, G. R., Shoshan, D., Silva, M., White, E., & McDonald, J. (2025). A Preliminary Study on the Accuracy of MRI-Guided Thalamic Infusion of AAV2-GFP and Biodistribution Analysis Using Cryo-Fluorescence Tomography in Nonhuman Primates. Pharmaceutics, 17(9), 1167. https://doi.org/10.3390/pharmaceutics17091167





