Strategic and Chemical Advances in Antibody–Drug Conjugates
Abstract
1. Introduction
2. Historical Development and ADC Overview
3. Chemical Aspects of ADCs
3.1. Antibody–Drug Conjugate (ADC) Engineering
3.2. Linker Technology
4. Conjugation Methods
4.1. Traditional Conjugation (Lysine-Based Conjugation)
4.2. Site-Specific Conjugation (Click Reactions)
4.2.1. Copper-Catalyzed Cycloaddition of Azides and Alkynes
4.2.2. Oxime Formation
4.2.3. Diels–Alder Reaction (DA)
5. Tailoring ADCs to Specific Diseases
5.1. Tailoring ADCs to Cancer
5.2. Tailoring ADCs for Non-Cancer Diseases
6. Advances in Delivery Systems
6.1. Nanoparticle-Based Delivery
6.2. Bioconjugation Techniques
6.2.1. ADC Stability
6.2.2. Precision Conjugation Strategies
6.2.3. Minimizing Off-Target Effects
6.2.4. Outlook for Next-Generation ADCs
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rowland, G.F.; O’Neill, G.J.; Davies, D.A.L. Suppression of tumour growth in mice by a drug–antibody conjugate using a novel approach to linkage. Nature 1975, 255, 487–488. [Google Scholar] [CrossRef]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody–drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. mAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody-Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, B.; Pan, Z.; Mo, C.; Zhao, X.; Liu, G.; Hou, P.; Cui, Q.; Xu, Z.; Wang, W.; et al. Antibody-Drug Conjugates (ADCs): Current and future biopharmaceuticals. J. Hematol. Oncol. 2025, 18, 51. [Google Scholar] [CrossRef]
- Kiss, B.; Borbély, J. Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment. Pharmaceutics 2023, 15, 1761. [Google Scholar] [CrossRef] [PubMed]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload diversification: A key step in the development of antibody-drug conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef]
- Huang, Z.; Braunstein, Z.; Chen, J.; Wei, Y.; Rao, X.; Dong, L.; Zhong, J. Precision Medicine in Rheumatic Diseases: Unlocking the Potential of Antibody-Drug Conjugates. Pharmacol. Rev. 2024, 76, 579–598. [Google Scholar] [CrossRef]
- Theocharopoulos, C.; Lialios, P.-P.; Samarkos, M.; Gogas, H.; Ziogas, D.C. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines 2021, 9, 1111. [Google Scholar] [CrossRef]
- Peters, C.; Brown, S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci. Rep. 2015, 35, e00225. [Google Scholar] [CrossRef]
- McKertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.C.; Ershler, R.; Kanapuru, B.; Xu, Q.; Shen, G.; Li, L.; Ma, L.; Okusanya, O.O.; Simpson, N.E.; Nguyen, W.; et al. FDA Approval Summary: Belantamab Mafodotin for Patients with Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 4629–4633. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Gogia, P.; Ashraf, H.; Bhasin, S.; Xu, Y. Antibody-Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers 2023, 15, 3886. [Google Scholar] [CrossRef]
- Su, Z.; Xiao, D.; Xie, F.; Liu, L.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Antibody-drug conjugates: Recent advances in linker chemistry. Acta Pharm. Sin. B 2021, 11, 3889–3907. [Google Scholar] [CrossRef]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs 2021, 13, 1951427. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 39. [Google Scholar] [CrossRef]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef]
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 20. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Tarantino, P.; Rich, J.R.; LoRusso, P.M.; de Vries, E.G.E. The Journey of Antibody–Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov. 2024, 14, 2089–2108. [Google Scholar] [CrossRef] [PubMed]
- Perez, H.L.; Cardarelli, P.M.; Deshpande, S.; Gangwar, S.; Schroeder, G.M.; Vite, G.D.; Borzilleri, R.M. Antibody–drug conjugates: Current status and future directions. Drug Discov. Today 2014, 19, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Geethika, L.S.; Sneha, P. Antibody-drug Conjugates in Cancer Treatment: An Overview. J. Cancer Tumor Int. 2024, 14, 33–45. [Google Scholar] [CrossRef]
- Pettinato, M.C. Introduction to Antibody-Drug Conjugates. Antibodies 2021, 10, 42. [Google Scholar] [CrossRef]
- Hammam, S.N. Antibody drug conjugate, historical and future overview. Sohag Med. J. 2023, 27, 59–75. [Google Scholar] [CrossRef]
- Baah, S.; Laws, M.; Rahman, K.M. Antibody–Drug Conjugates—A Tutorial Review. Molecules 2021, 26, 2943. [Google Scholar] [CrossRef]
- Lucas, A.T.; Moody, A.; Schorzman, A.N.; Zamboni, W.C. Importance and Considerations of Antibody Engineering in Antibody-Drug Conjugates Development from a Clinical Pharmacologist’s Perspective. Antibodies 2021, 10, 30. [Google Scholar] [CrossRef]
- Wiggins, B.; Liu-Shin, L.; Yamaguchi, H.; Ratnaswamy, G. Characterization of cysteine-linked conjugation profiles of immunoglobulin G1 and immunoglobulin G2 antibody-drug conjugates. J. Pharm. Sci. 2015, 104, 1362–1372. [Google Scholar] [CrossRef]
- Hoffmann, R.M.; Coumbe, B.G.T.; Josephs, D.H.; Mele, S.; Ilieva, K.M.; Cheung, A.; Tutt, A.N.; Spicer, J.F.; Thurston, D.E.; Crescioli, S.; et al. Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs). Oncoimmunology 2018, 7, e1395127. [Google Scholar] [CrossRef] [PubMed]
- McCombs, J.R.; Owen, S.C. Antibody drug conjugates: Design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Wang, D.; Yin, F.; Li, Z.; Zhang, Y.; Shi, C. Current progress and remaining challenges of peptide-drug conjugates (PDCs): Next generation of antibody-drug conjugates (ADCs)? J. Nanobiotechnol. 2025, 23, 305. [Google Scholar] [CrossRef]
- Yang, X.; Pan, Z.; Choudhury, M.R.; Yuan, Z.; Anifowose, A.; Yu, B.; Wang, W.; Wang, B. Making smart drugs smarter: The importance of linker chemistry in targeted drug delivery. Med. Res. Rev. 2020, 40, 2682–2713. [Google Scholar] [CrossRef]
- Najminejad, Z.; Dehghani, F.; Mirzaei, Y.; Mer, A.H.; Saghi, S.A.; Abdolvahab, M.H.; Bagheri, N.; Meyfour, A.; Jafari, A.; Jahandideh, S.; et al. Clinical perspective: Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Mol. Ther. 2023, 31, 1874–1903. [Google Scholar] [CrossRef]
- Jain, N.; Smith, S.W.; Ghone, S.; Tomczuk, B. Current ADC Linker Chemistry. Pharm. Res. 2015, 32, 3526–3540. [Google Scholar] [CrossRef]
- Kölmel, D.K.; Kool, E.T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, D. Linker Design Impacts Antibody-Drug Conjugate Pharmacokinetics and Efficacy via Modulating the Stability and Payload Release Efficiency. Front. Pharmacol. 2021, 12, 687926. [Google Scholar] [CrossRef]
- Bai, C.; Reid, E.E.; Wilhelm, A.; Shizuka, M.; Maloney, E.K.; Laleau, R.; Harvey, L.; Archer, K.E.; Vitharana, D.; Adams, S.; et al. Site-Specific Conjugation of the Indolinobenzodiazepine DGN549 to Antibodies Affords Antibody–Drug Conjugates with an Improved Therapeutic Index as Compared with Lysine Conjugation. Bioconjugate Chem. 2020, 31, 93–103. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef]
- Haque, M.; Forte, N.; Baker, J.R. Site-selective lysine conjugation methods and applications towards antibody-drug conjugates. Chem. Commun. 2021, 57, 10689–10702. [Google Scholar] [CrossRef]
- Wang, L.; Amphlett, G.; Blättler, W.A.; Lambert, J.M.; Zhang, W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci. 2005, 14, 2436–2446. [Google Scholar] [CrossRef]
- Hwang, D.; Rader, C. Site-Specific Antibody–Drug Conjugates in Triple Variable Domain Fab Format. Biomolecules 2020, 10, 764. [Google Scholar] [CrossRef]
- Matos, M.J.; Oliveira, B.L.; Martínez-Sáez, N.; Guerreiro, A.; Cal, P.M.S.D.; Bertoldo, J.; Maneiro, M.; Perkins, E.; Howard, J.; Deery, M.J.; et al. Chemo- and Regioselective Lysine Modification on Native Proteins. J. Am. Chem. Soc. 2018, 140, 4004–4017. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.P.; Fang, S.; Benjamin, S.R.; Alayi, T.; Hathout, Y.; Gillen, S.M.; Handel, J.P.; Brems, B.M.; Howe, J.M.; Tumey, L.N. Evaluation of an ester-linked immunosuppressive payload: A case study in understanding the stability and cleavability of ester-containing ADC linkers. Bioorganic Med. Chem. Lett. 2022, 75, 128953. [Google Scholar] [CrossRef]
- Fujii, T.; Matsuda, Y.; Seki, T.; Shikida, N.; Iwai, Y.; Ooba, Y.; Takahashi, K.; Isokawa, M.; Kawaguchi, S.; Hatada, N.; et al. AJICAP Second Generation: Improved Chemical Site-Specific Conjugation Technology for Antibody–Drug Conjugate Production. Bioconjugate Chem. 2023, 34, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gao, Y.; Liu, D.; Tan, X.; Hu, L.; Qiu, Z.; Liu, J.; He, H.; Liu, Y. Study on the Heterogeneity of T-DM1 and the Analysis of the Unconjugated Linker Structure under a Stable Conjugation Process. ACS Omega 2019, 4, 8834–8845. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Bertozzi, C.R. Site-Specific Antibody–Drug Conjugates: The Nexus of Bioorthogonal Chemistry, Protein Engineering, and Drug Development. Bioconjugate Chem. 2015, 26, 176–192. [Google Scholar] [CrossRef]
- Dosio, F.; Stella, B.; Milla, P.; Della Pepa, C.; Gastaldi, D.; Arpicco, S. Targeted Cancer Therapy: The Roles Played by Antibody-Drug and Antibody-Toxin Conjugates. In Topics in Anti-Cancer Research: Volume 4; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015; pp. 3–103. [Google Scholar]
- Selby, C.; Yacko, L.R.; Glode, A.E. Gemtuzumab Ozogamicin: Back Again. J. Adv. Pract. Oncol. 2019, 10, 68–82. [Google Scholar]
- Zhang, J.; Liu, R.; Gao, S.; Li, W.; Chen, Y.; Meng, Y.; Liu, C.; Jin, W.; Wu, J.; Wang, Y.; et al. Phase I study of A166, an antibody–drug conjugate in advanced HER2-expressing solid tumours. npj Breast Cancer 2023, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Purushottam, L.; Adusumalli, S.R.; Singh, U.; Unnikrishnan, V.B.; Rawale, D.G.; Gujrati, M.; Mishra, R.K.; Rai, V. Single-site glycine-specific labeling of proteins. Nat. Commun. 2019, 10, 2539. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.Y. Processes for Constructing Homogeneous Antibody Drug Conjugates. Org. Process Res. Dev. 2016, 20, 852–866. [Google Scholar] [CrossRef]
- Spirig, T.; Weiner, E.M.; Clubb, R.T. Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 2011, 82, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Peng, H. Perspectives on the development of antibody-drug conjugates targeting ROR1 for hematological and solid cancers. Antib. Ther. 2021, 4, 222–227. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Tong, Z.; Liu, Y.; Wang, X.; Yan, M.; Chang, J.; Wang, S.; Du, C.; Li, L.; et al. HER2-targeting antibody drug conjugate FS-1502 in HER2-expressing metastatic breast cancer: A phase 1a/1b trial. Nat. Commun. 2024, 15, 5158. [Google Scholar] [CrossRef]
- Hussain, A.F.; Grimm, A.; Sheng, W.; Zhang, C.; Al-Rawe, M.; Bräutigam, K.; Abu Mraheil, M.; Zeppernick, F.; Meinhold-Heerlein, I. Toward Homogenous Antibody Drug Conjugates Using Enzyme-Based Conjugation Approaches. Pharmaceuticals 2021, 14, 343. [Google Scholar] [CrossRef]
- Dennler, P.; Chiotellis, A.; Fischer, E.; Brégeon, D.; Belmant, C.; Gauthier, L.; Lhospice, F.; Romagne, F.; Schibli, R. Transglutaminase-Based Chemo-Enzymatic Conjugation Approach Yields Homogeneous Antibody–Drug Conjugates. Bioconjugate Chem. 2014, 25, 569–578. [Google Scholar] [CrossRef]
- Dudchak, R.; Podolak, M.; Holota, S.; Szewczyk-Roszczenko, O.; Roszczenko, P.; Bielawska, A.; Lesyk, R.; Bielawski, K. Click chemistry in the synthesis of antibody-drug conjugates. Bioorganic Chem. 2024, 143, 106982. [Google Scholar] [CrossRef]
- McKay, C.S.; Finn, M.G. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef]
- Scinto, S.L.; Bilodeau, D.A.; Hincapie, R.; Lee, W.; Nguyen, S.S.; Xu, M.; Am Ende, C.W.; Finn, M.G.; Lang, K.; Lin, Q.; et al. Bioorthogonal chemistry. Nat. Rev. Methods Primers 2021, 1, 30. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.J.; Bargh, J.D.; Dannheim, F.M.; Hanby, A.R.; Seki, H.; Counsell, A.J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y. Site-selective modification strategies in antibody–drug conjugates. Chem. Soc. Rev. 2021, 50, 1305–1353. [Google Scholar] [PubMed]
- Abrahams, C.L.; Li, X.; Embry, M.; Yu, A.; Krimm, S.; Krueger, S.; Greenland, N.Y.; Wen, K.W.; Jones, C.; DeAlmeida, V. Targeting CD74 in multiple myeloma with the novel, site-specific antibody-drug conjugate STRO-001. Oncotarget 2018, 9, 37700. [Google Scholar]
- Hong, V.; Steinmetz, N.F.; Manchester, M.; Finn, M.G. Labeling live cells by copper-catalyzed alkyne--azide click chemistry. Bioconjugate Chem. 2010, 21, 1912–1916. [Google Scholar] [CrossRef]
- Hong, V.; Presolski, S.I.; Ma, C.; Finn, M.G. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew. Chem. Int. Ed. Engl. 2009, 48, 9879–9883. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef]
- Collins, J.; Xiao, Z.; Müllner, M.; Connal, L.A. The emergence of oxime click chemistry and its utility in polymer science. Polym. Chem. 2016, 7, 3812–3826. [Google Scholar] [CrossRef]
- Rashidian, M.; Mahmoodi, M.M.; Shah, R.; Dozier, J.K.; Wagner, C.R.; Distefano, M.D. A highly efficient catalyst for oxime ligation and hydrazone-oxime exchange suitable for bioconjugation. Bioconjugate Chem. 2013, 24, 333–342. [Google Scholar] [CrossRef]
- Agten, S.M.; Dawson, P.E.; Hackeng, T.M. Oxime conjugation in protein chemistry: From carbonyl incorporation to nucleophilic catalysis. J. Pept. Sci. 2016, 22, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Hossini, H.; Ashraf, G.M.; Limoee, M. Molecular Mechanism of Aniline Induced Spleen Toxicity and Neuron Toxicity in Experimental Rat Exposure: A Review. Curr. Neuropharmacol. 2019, 17, 201–213. [Google Scholar] [CrossRef]
- Larsen, D.; Pittelkow, M.; Karmakar, S.; Kool, E.T. New Organocatalyst Scaffolds with High Activity in Promoting Hydrazone and Oxime Formation at Neutral pH. Org. Lett. 2015, 17, 274–277. [Google Scholar] [CrossRef]
- Crisalli, P.; Kool, E.T. Water-soluble organocatalysts for hydrazone and oxime formation. J. Org. Chem. 2013, 78, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, U.; Parakh, S.; Gan, H.K.; Scott, A.M. Antibody-Drug Conjugates for Cancer Therapy. Molecules 2020, 25, 4764. [Google Scholar] [CrossRef]
- Hermanson, G.T.; van Delft, F.L. Antibody Conjugation Technologies. In Chemical Linkers in Antibody–Drug Conjugates (ADCs); van Delft, F., Lambert, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Litz, K.E. Aqueous Ti(IV)-catalyzed Diels-Alder reaction. Molecules 2007, 12, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef]
- Gregoritza, M.; Brandl, F.P. The Diels–Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef] [PubMed]
- St Amant, A.H.; Lemen, D.; Florinas, S.; Mao, S.; Fazenbaker, C.; Zhong, H.; Wu, H.; Gao, C.; Christie, R.J.; Read de Alaniz, J. Tuning the Diels-Alder Reaction for Bioconjugation to Maleimide Drug-Linkers. Bioconjugate Chem. 2018, 29, 2406–2414. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, F.; Dal Bo, M.; Macor, P.; Toffoli, G. A comprehensive overview on antibody-drug conjugates: From the conceptualization to cancer therapy. Front. Pharmacol. 2023, 14, 1274088. [Google Scholar] [CrossRef]
- Wehrmüller, J.E. Developing Stoichiometrically Well-Defined Antibody-Drug Conjugates by Enzymatic Conjugation of Native Antibodies. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2021. [Google Scholar]
- Hallam, T.J.; Wold, E.; Wahl, A.; Smider, V.V. Antibody Conjugates with Unnatural Amino Acids. Mol. Pharm. 2015, 12, 1848–1862. [Google Scholar] [CrossRef]
- Moore, E.J.; Rice, M.; Roy, G.; Zhang, W.; Marelli, M. Emerging conjugation strategies and protein engineering technologies aim to improve ADCs in the fight against cancer. Xenobiotica 2024, 54, 469–491. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. reviews. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Barreca, M.; Lang, N.; Tarantelli, C.; Spriano, F.; Barraja, P.; Bertoni, F. Antibody-drug conjugates for lymphoma patients: Preclinical and clinical evidences. Explor. Target. Anti-Tumor Ther. 2022, 3, 763–794. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Wang, X.; Yuan, K.; Wang, G.; Hu, L.; Zhang, G.; Pei, W.; Wang, L.; Sun, C.; et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm. Sin. B 2023, 13, 3583–3597. [Google Scholar] [CrossRef]
- Krebs, S.K.; Stech, M.; Jorde, F.; Rakotoarinoro, N.; Ramm, F.; Marinoff, S.; Bahrke, S.; Danielczyk, A.; Wüstenhagen, D.A.; Kubick, S. Synthesis of an Anti-CD7 recombinant immunotoxin based on PE24 in CHO and E. coli cell-free systems. Int. J. Mol. Sci. 2022, 23, 13697. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Connors, J.M.; Park, S.I.; Fanale, M.; O’Meara, M.M.; Hunder, N.N.; Huebner, D.; Ansell, S.M. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: A phase 1, open-label, dose-escalation study. Lancet Oncol. 2013, 14, 1348–1356. [Google Scholar] [CrossRef]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M. Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef]
- Castellino Sharon, M.; Pei, Q.; Parsons Susan, K.; Hodgson, D.; McCarten, K.; Horton, T.; Cho, S.; Wu, Y.; Punnett, A.; Dave, H.; et al. Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 1649–1660. [Google Scholar] [CrossRef]
- Kondrashov, A.; Sapkota, S.; Sharma, A.; Riano, I.; Kurzrock, R.; Adashek, J.J. Antibody-Drug Conjugates in Solid Tumor Oncology: An Effectiveness Payday with a Targeted Payload. Pharmaceutics 2023, 15, 2160. [Google Scholar] [CrossRef]
- Zhou, D.-d.; Zhai, X.-t.; Zhang, L.-w.; Xie, Z.-h.; Wang, Y.; Zhen, Y.-s.; Gao, R.-j.; Miao, Q.-f. A new TROP2-targeting antibody-drug conjugate shows potent antitumor efficacy in breast and lung cancers. npj Precis. Oncol. 2024, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Medina, P.; Miao, M.; Moore, K.N. A review of mirvetuximab soravtansine-gynx in folate receptor alpha-expressing platinum-resistant ovarian cancer. Am. J. Health-Syst. Pharm. AJHP 2025, 82, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Shitara, K. Trastuzumab deruxtecan for the treatment of HER2-positive gastric cancer. Expert. Opin. Biol. Ther. 2021, 21, 825–830. [Google Scholar] [CrossRef]
- Lewis, G.D.; Li, G.; Guo, J.; Yu, S.-F.; Fields, C.T.; Lee, G.; Zhang, D.; Dragovich, P.S.; Pillow, T.; Wei, B.; et al. The HER2-directed antibody-drug conjugate DHES0815A in advanced and/or metastatic breast cancer: Preclinical characterization and phase 1 trial results. Nat. Commun. 2024, 15, 466. [Google Scholar] [CrossRef]
- Geyer Charles, E.; Untch, M.; Huang, C.-S.; Mano Max, S.; Mamounas Eleftherios, P.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; Fischer Hans, H.; et al. Survival with Trastuzumab Emtansine in Residual HER2-Positive Breast Cancer. N. Engl. J. Med. 2025, 392, 249–257. [Google Scholar] [CrossRef]
- Wang, T.; Quijada, D.; Ahmedna, T.; Castillo, J.R.; Naji, N.S.; Peske, J.D.; Karakousis, P.C.; Paul, S.; Karantanos, T.; Karanika, S. Targeting CCRL2 enhances therapeutic outcomes in a tuberculosis mouse model. Front. Immunol. 2025, 16, 1501329. [Google Scholar] [CrossRef]
- Umotoy, J.C.; de Taeye, S.W. Antibody Conjugates for Targeted Therapy Against HIV-1 as an Emerging Tool for HIV-1 Cure. Front. Immunol. 2021, 12, 708806. [Google Scholar] [CrossRef]
- Cavaco, M.; Castanho, M.A.R.B.; Neves, V. The Use of Antibody-Antibiotic Conjugates to Fight Bacterial Infections. Front. Microbiol. 2022, 13, 835677. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, Z.; Ma, Z.; Chen, J.; Lin, S.; Yang, X.; Gong, Q.; Braunstein, Z.; Wei, Y.; Rao, X.; et al. The next frontier in antibody-drug conjugates: Challenges and opportunities in cancer and autoimmune therapy. Cancer Drug Resist. 2025, 8, 34. [Google Scholar] [CrossRef]
- Castiello, M.C.; Bosticardo, M.; Sacchetti, N.; Calzoni, E.; Fontana, E.; Yamazaki, Y.; Draghici, E.; Corsino, C.; Bortolomai, I.; Sereni, L.; et al. Efficacy and safety of anti-CD45–saporin as conditioning agent for RAG deficiency. J. Allergy Clin. Immunol. 2021, 147, 309–320.e306. [Google Scholar] [CrossRef]
- Darbandi, A.; Abdi, M.; Dashtbin, S.; Yaghoubi, S.; Sholeh, M.; Kouhsari, E.; Darbandi, T.; Ghanavati, R.; Taheri, B. Antibody-Antibiotic Conjugates: A Comprehensive Review on Their Therapeutic Potentials Against Bacterial Infections. J. Clin. Lab. Anal. 2024, 38, e25071. [Google Scholar] [CrossRef]
- Li, X.; Patterson, J.T.; Sarkar, M.; Pedzisa, L.; Kodadek, T.; Roush, W.R.; Rader, C. Site-Specific Dual Antibody Conjugation via Engineered Cysteine and Selenocysteine Residues. Bioconjugate Chem. 2015, 26, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.; Rothenberg, M.E.; Deng, R.; Lewin-Koh, N.; She, G.; Kamath, A.V.; Carrasco-Triguero, M.; Saad, O.; Castro, A.; Teufel, L.; et al. A Phase 1, Randomized, Single-Ascending-Dose Study To Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcus aureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- VanOtterloo, L.M.; Trent, M.S. Microbial Primer: Lipopolysaccharide—A remarkable component of the Gram-negative bacterial surface. Microbiology 2024, 170. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Delaney, J.C.; Guillard, T.; Reffuveille, F.; Varin-Simon, J.; Li, K.; Wollacott, A.; Frapy, E.; Mong, S.; Tissire, H.; et al. Development of an antibody fused with an antimicrobial peptide targeting Pseudomonas aeruginosa: A new approach to prevent and treat bacterial infections. PLoS Pathog. 2023, 19, e1011612. [Google Scholar] [CrossRef]
- Tvilum, A.; Johansen, M.I.; Glud, L.N.; Ivarsen, D.M.; Khamas, A.B.; Carmali, S.; Mhatre, S.S.; Søgaard, A.B.; Faddy, E.; de Vor, L.; et al. Antibody-Drug Conjugates to Treat Bacterial Biofilms via Targeting and Extracellular Drug Release. Adv. Sci. 2023, 10, 2301340. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Analytical Techniques for Characterizing Tumor-Targeted Antibody-Functionalized Nanoparticles. Life 2024, 14, 489. [Google Scholar] [CrossRef]
- Fatima, S.W.; Khare, S.K. Benefits and challenges of antibody drug conjugates as novel form of c hemotherapy. J. Control. Release 2022, 341, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Chen, I.A. Antibody-Nanoparticle Conjugates in Therapy: Combining the Best of Two Worlds. Small 2025, 21, 2409635. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Fojtu, M.; Gumulec, J.; Stracina, T.; Raudenska, M.; Skotakova, A.; Vaculovicova, M.; Adam, V.; Babula, P.; Novakova, M.; Masarik, M. Reduction of Doxorubicin-Induced Cardiotoxicity Using Nanocarriers: A Review. Curr. Drug Metab. 2017, 18, 237–263. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Mamot, C.; Wicki, A.; Hasler-Strub, U.; Riniker, S.; Li, Q.; Holer, L.; Bärtschi, D.; Zaman, K.; Moos, R.; Dedes, K.J.; et al. A multicenter phase II trial of anti-EGFR-immunoliposomes loaded with doxorubicin in patients with advanced triple negative breast cancer. Sci. Rep. 2023, 13, 3705. [Google Scholar] [CrossRef]
- Miller, K.; Cortes, J.; Hurvitz, S.A.; Krop, I.E.; Tripathy, D.; Verma, S.; Riahi, K.; Reynolds, J.G.; Wickham, T.J.; Molnar, I.; et al. HERMIONE: A randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016, 16, 352. [Google Scholar] [CrossRef]
- Munster, P.; Krop, I.E.; LoRusso, P.; Ma, C.; Siegel, B.A.; Shields, A.F.; Molnár, I.; Wickham, T.J.; Reynolds, J.; Campbell, K.; et al. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody–liposo mal doxorubicin conjugate, in patients with advanced HER2-positive bre ast cancer: A phase 1 dose-escalation study. Br. J. Cancer 2018, 119, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; Sahly, H.M.E.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal escape: A bottleneck for LNP-mediated therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kang, S.J.; Jeong, H.Y.; Kim, M.W.; Park, S.I.; Lee, Y.K.; Kim, H.S.; Kim, K.S.; Park, Y.S. Anti-EGFR immunonanoparticles containing IL12 and salmosin genes for targeted cancer gene therapy. Int. J. Oncol. 2016, 49, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chan, C.; Peterson, N.; Hanna, R.N.; Alfaro, A.; Allen, K.L.; Wu, H.; Dall’Acqua, W.F.; Borrok, M.J.; Santos, J.L. Engineering Caveolae-Targeted Lipid Nanoparticles To Deliver mRNA to t he Lungs. ACS Chem. Biol. 2020, 15, 830–836. [Google Scholar] [CrossRef]
- Tombácz, I.; Laczkó, D.; Shahnawaz, H.; Muramatsu, H.; Natesan, A.; Yadegari, A.; Papp, T.E.; Alameh, M.-G.; Shuvaev, V.; Mui, B.L.; et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol. Ther. 2021, 29, 3293–3304. [Google Scholar] [CrossRef]
- Debotton, N.; Zer, H.; Parnes, M.; Harush-Frenkel, O.; Kadouche, J.; Benita, S. A quantitative evaluation of the molecular binding affinity between a monoclonal antibody conjugated to a nanoparticle and an antigen by sur face plasmon resonance. Eur. J. Pharm. Biopharm. 2010, 74, 148–156. [Google Scholar] [CrossRef]
- Cirstoiu-Hapca, A.; Buchegger, F.; Bossy, L.; Kosinski, M.; Gurny, R.; Delie, F. Nanomedicines for active targeting: Physico-chemical characterization of paclitaxel-loaded anti-HER2 immunonanoparticles and in vitro functional studies on target cells. Eur. J. Pharm. Sci. 2009, 38, 230–237. [Google Scholar] [CrossRef]
- Choi, J.-S.; Jang, W.S.; Park, J.-S. Comparison of adsorption and conjugation of Herceptin on poly(lactic-co-glycolic acid) nanoparticles–Effect on cell internalization in breast cancer cells. Mater. Sci. Eng. C 2018, 92, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Sousa, F.; Kennedy, P.; Sarmento, B. Carcinoembryonic antigen-targeted nanoparticles potentiate the delivery of anticancer drugs to colorectal cancer cells. Int. J. Pharm. 2018, 549, 397–403. [Google Scholar] [CrossRef]
- Kalogera, E.; Nevala, W.K.; Finnes, H.D.; Suman, V.J.; Schimke, J.M.; Strand, C.A.; Kottschade, L.A.; Kudgus, R.A.; Buhrow, S.A.; Becher, L.R.; et al. A Phase I Trial of Nab-Paclitaxel/Bevacizumab (AB160) Nano-Immunoconju gate Therapy for Gynecologic Malignancies. Clin. Cancer Res. 2024, 30, 2623–2635. [Google Scholar] [CrossRef]
- Dinauer, N.; Balthasar, S.; Weber, C.; Kreuter, J.; Langer, K.; Briesen, H. Selective targeting of antibody-conjugated nanoparticles to leukemic c ells and primary T-lymphocytes. Biomaterials 2005, 26, 5898–5906. [Google Scholar] [CrossRef]
- Stuchinskaya, T.; Moreno, M.; Cook, M.J.; Edwards, D.R.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochem. Photobiol. Sci. 2011, 10, 822–831. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Fonge, H.; Cai, Z.; Scollard, D.; Lechtman, E.; Done, S.J.; Pignol, J.-P.; Reilly, R.M. Role of Antibody-Mediated Tumor Targeting and Route of Administration in Nanoparticle Tumor Accumulation in Vivo. Mol. Pharm. 2012, 9, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer targeting with biomolecules: A comparative study of photodynamic therapy efficacy using antibody or lectin conjugated phthalocyanine-PEG gold nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef]
- Kubota, T.; Kuroda, S.; Kanaya, N.; Morihiro, T.; Aoyama, K.; Kakiuchi, Y.; Kikuchi, S.; Nishizaki, M.; Kagawa, S.; Tazawa, H.; et al. HER2-targeted gold nanoparticles potentially overcome resistance to tr astuzumab in gastric cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1919–1929. [Google Scholar] [CrossRef]
- Yook, S.; Cai, Z.; Jeong, J.J.; Lu, Y.; Winnik, M.A.; Pignol, J.-P.; Reilly, R.M. Dual-Receptor-Targeted (DRT) Radiation Nanomedicine Labeled with 177Lu Is More Potent for Killing Human Breast Cancer Cells That Coexpress H ER2 and EGFR Than Single-Receptor-Targeted (SRT) Radiation Nanomedicin es. Mol. Pharm. 2020, 17, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.M.; Chang, Y.L.; Chen, M.S.; Wu, H.C. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials 2011, 32, 3265–3274. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for o ligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Mane, V.; Muro, S. Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice. Int. J. Nanomed. 2012, 7, 4223–4237. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.-R.; Heo, J.H. Simultaneous Stabilization and Functionalization of Gold Nanoparticles via Biomolecule Conjugation: Progress and Perspectives. ACS Appl. Mater. Interfaces 2021, 13, 42311–42328. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advanc es in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Truong, T.T.; Mondal, S.; Doan, V.H.M.; Tak, S.; Choi, J.; Oh, H.; Nguyen, T.D.; Misra, M.; Lee, B.; Oh, J. Precision-engineered metal and metal-oxide nanoparticles for biomedica l imaging and healthcare applications. Adv. Colloid. Interface Sci. 2024, 332, 103263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Z.; Yang, B.; Wang, J.; Li, Y. Synthesis of dual-functional targeting probes for cancer theranostics based on iron oxide nanoparticles coated by centipede-like polymer connected with pH-responsive anticancer drug. J. Biomater. Sci. Polym. Ed. 2015, 26, 1178–1189. [Google Scholar] [CrossRef]
- Yang, J.; Lee, C.-H.; Park, J.; Seo, S.; Lim, E.-K.; Song, Y.J.; Suh, J.-S.; Yoon, H.-G.; Huh, Y.-M.; Haam, S. Antibody conjugated magnetic PLGA nanoparticles for diagnosis and treatment of breast cancer. J. Mater. Chem. 2007, 17, 2695–2699. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radiolabeled Mesoporous Silica Nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef]
- Lu, M.W.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Hong, Y.W.; Hong, C.Y.; Yang, H.C.; Wu, J.L. Immunomagnetic reduction assay for nervous necrosis virus extracted from groupers. J. Virol. Methods 2012, 181, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Kaempffe, A.; Dickgiesser, S.; Rasche, N.; Paoletti, A.; Bertotti, E.; De Salve, I.; Sirtori, F.R.; Kellner, R.; Könning, D.; Hecht, S.; et al. Effect of Conjugation Site and Technique on the Stability and Pharmaco kinetics of Antibody-Drug Conjugates. J. Pharm. Sci. 2021, 110, 3776–3785. [Google Scholar] [CrossRef]
- Ouyang, A.; Chiang, S.; Wang, C. Accelerating the Development of Novel Antibody-Drug Conjugates Through Site-Specific Conjugation Methods. Antib. Ther. 2023, 6, tbad014.021. [Google Scholar] [CrossRef]
- Qian, L.; Lin, X.; Gao, X.; Khan, R.U.; Liao, J.-Y.; Du, S.; Ge, J.; Zeng, S.; Yao, S.Q. The Dawn of a New Era: Targeting the “Undruggables” with Antibody-Based Therapeutics. Chem. Rev. 2023, 123, 7782–7853. [Google Scholar] [CrossRef] [PubMed]
- Donk, N.W.C.J.; Dhimolea, E. Brentuximab vedotin. mAbs 2012, 4, 458–465. [Google Scholar] [CrossRef]
- Dai, L.J.; Li, Y.W.; Ma, D.; Shao, Z.M.; Jiang, Y.Z. Next-generation antibody-drug conjugates revolutionize the precise classification and treatment of HER2-expressing breast cancer. Cancer Biol. Med. 2023, 20, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Gorovits, B.; Krinos-Fiorotti, C. Proposed mechanism of off-target toxicity for antibody–drug conjugates driven by mannose receptor uptake. Cancer Immunol. Immunother. CII 2012, 62, 217–223. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef]
- Huang, Q.; Ravindra Pilvankar, M.; Dixit, R.; Yu, H. Approaches to improve the translation of safety, pharmacokinetics and therapeutic index of ADCs. Xenobiotica 2024, 54, 533–542. [Google Scholar] [CrossRef]
- Mahalingaiah, P.K.; Ciurlionis, R.; Durbin, K.R.; Yeager, R.L.; Philip, B.K.; Bawa, B.; Mantena, S.R.; Enright, B.P.; Liguori, M.J.; Van Vleet, T.R. Potential mechanisms of target-independent uptake and toxicity of anti body-drug conjugates. Pharmacol. Ther. 2019, 200, 110–125. [Google Scholar] [CrossRef]
- Autio, K.A.; Boni, V.; Humphrey, R.W.; Naing, A. Probody Therapeutics: An Emerging Class of Therapies Designed to Enhance On-Target Effects with Reduced Off-Tumor Toxicity for Use in Immuno-Oncology. Clin. Cancer Res. 2020, 26, 984–989. [Google Scholar] [CrossRef]
- Ballestín, P.; López de Sá, A.; Díaz-Tejeiro, C.; Paniagua-Herranz, L.; Sanvicente, A.; López-Cade, I.; Pérez-Segura, P.; Alonso-Moreno, C.; Nieto-Jiménez, C.; Ocaña, A. Understanding the Toxicity Profile of Approved ADCs. Pharmaceutics 2025, 17, 258. [Google Scholar] [CrossRef]
- Hinrichs, M.J.M.; Dixit, R. Antibody Drug Conjugates: Nonclinical Safety Considerations. AAPS J. 2015, 17, 1055–1064. [Google Scholar] [CrossRef]
- Grairi, M.; Le Borgne, M. Antibody–drug conjugates: Prospects for the next generation. Drug Discov. Today 2024, 29, 104241. [Google Scholar] [CrossRef]
- D’Arienzo, A.; Verrazzo, A.; Pagliuca, M.; Napolitano, F.; Parola, S.; Viggiani, M.; Caputo, R.; Puglisi, F.; Giuliano, M.; Del Mastro, L.; et al. Toxicity profile of antibody-drug conjugates in breast cancer: Practical considerations. EClinicalMedicine 2023, 62, 102113. [Google Scholar] [CrossRef]
- Loganzo, F.; Sung, M.; Gerber, H.-P. Mechanisms of Resistance to Antibody–Drug Conjugates. Mol. Cancer Ther. 2016, 15, 2825–2834. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Makhesana, M.; Heidarinassab, S.; Plouze, A.; Vazquez, M.; Brabazon, D. A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage. Adv. Colloid. Interface Sci. 2023, 321, 103010. [Google Scholar] [CrossRef]
- Zhou, Q. Site-Specific Antibody Conjugation with Payloads beyond Cytotoxins. Molecules 2023, 28, 917. [Google Scholar] [CrossRef]
- von Witting, E.; Hober, S.; Kanje, S. Affinity-Based Methods for Site-Specific Conjugation of Antibodies. Bioconjugate Chem. 2021, 32, 1515–1524. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Z.; Wang, Y. Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharm. Sin. B 2024, 14, 1965–1986. [Google Scholar] [CrossRef]
- Lv, Y.; Cui, X.; Li, T.; Liu, C.; Wang, A.; Wang, T.; Zhou, X.; Li, R.; Zhang, F.; Hu, Y.; et al. Mechanism of action and future perspectives of ADCs in combination with immune checkpoint inhibitors for solid tumors. Clin. Exp. Med. 2025, 25, 139. [Google Scholar] [CrossRef]
- Li, R.; Liu, M.; Yang, Z.; Li, J.; Gao, Y.; Tan, R. Proteolysis-Targeting Chimeras (PROTACs) in Cancer Therapy: Present and Future. Molecules 2022, 27, 8828. [Google Scholar] [CrossRef]
- Fong, J.Y.; Phuna, Z.; Chong, D.Y.; Heryanto, C.M.; Low, Y.S.; Oh, K.C.; Lee, Y.H.; Ng, A.W.R.; In, L.L.A.; Teo, M.Y.M. Advancements in antibody-drug conjugates as cancer therapeutics. J. Natl. Cancer Cent. 2025, 5, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Sun, L.; Jia, X.; Wang, K.; Li, M. Unveiling the future of breast cancer therapy: Cutting-edge antibody-drug conjugate strategies and clinical outcomes. Breast 2024, 78, 103830. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Antrás, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody–drug conjugates: In search of partners of choice. Trends Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef] [PubMed]



| Drugs | Chemical Structures | IC50 | Approved Date |
|---|---|---|---|
| Belantamab |  | ≈6.0 nM | 5 August 2020 |
| Cetuximab sarotalocan | 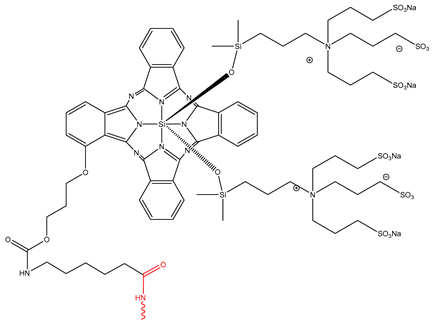 | 2.2–5.2 nM | 25 September 2020 |
| Fam-trastuzumab deruxtecan |  | ≈0.03 nM | 20 December 2019 |
| A do-trastuzumab Emtansine |  | ≈0.24 nM | 22 February 2013 |
| Polatuzumab vedotin |  | ≈0.07 nM | 10 June 2019 |
| Loncastuximab tesirine | 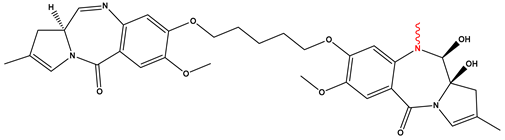 | ≈0.03 nM | 23 April 2021 |
| Gemtuzumab ozogamicin |  | ≈0.5 nM | 1 September 2017 |
| Conjugation Method | Type | Reactive Group | Advantages | Limitations | Examples | References |
|---|---|---|---|---|---|---|
| Lysine-Based Conjugation | Traditional (non-site-specific) | ε-Amine (–NH2) of lysine | Clinically validated; widely used; multiple FDA-approved ADCs | Heterogeneous DAR; off-target variability; batch-to-batch inconsistency | Uses NHS esters, isothiocyanates, squaramate esters; ~5 approved ADCs | [41,43,44,45] |
| Cysteine-Based Conjugation | Traditional and site-selective | Thiol (–SH) group of cysteine | More controlled DAR than lysine; accessible residues upon disulfide reduction | Risk of disulfide bond disruption; partial heterogeneity | Thiol–maleimide chemistry; some FDA-approved ADCs (e.g., brentuximab vedotin) | [48,49,50] |
| Enzyme-Mediated Conjugation | Site-specific | Engineered peptide sequences (e.g., glutamine, lysine tags) | Precise site control; minimal heterogeneity; high reproducibility | Requires antibody engineering and enzyme handling | Transglutaminase, sortase A, formylglycine-generating enzymes | [52,53,55,56] |
| Genetically Encoded Unnatural Amino Acids (UAAs) | Site-specific | Non-natural functional groups (e.g., azides, alkynes) | High precision; enables click chemistry; stable constructs | Requires codon reassignment and expression optimization | Azide–alkyne click; oxime ligation; stable and homogeneous ADCs | [58,62,63] |
| Diels–Alder Cycloaddition (DA) | Site-specific | Cyclopentadiene and maleimide (diene–dienophile pair) | Mild aqueous conditions; high yields; avoids thiol–maleimide issues | Limited in vivo validation; slower at room temperature | Furan–maleimide system; DA-based trastuzumab–vedotin synthesis | [80,81,82,84] |
| Nanoparticle Type | Antibody Target | Disease | Cargo | Outcomes | References |
|---|---|---|---|---|---|
| Liposomes | EGFR, HER2, c-Met, TfR, NS, pRBCs, E. coli, actin | Cancer (e.g., breast, lung, GBM), Alzheimer’s, malaria, bacterial infections, stroke | Doxorubicin, plasmid DNA (p53), chloroquine, fosmidomycin, PmB, TMZ | Improved targeting and reduced tumor size; 50% hemorrhage reduction; 10× drug efficacy against pRBCs; selective antibacterial delivery | [117,118,119,120,121,122,141] |
| Lipid Nanoparticles (LNPs) | EGFR, PV1, CD4 | Cancer, lung diseases, immunotherapy | mRNA, gene constructs | 40× lung-specific protein expression; 30× mRNA uptake in CD4+ T cells; 100× transfection efficiency in vitro | [114,115,116] |
| Polymeric Nanoparticles | HER2, H-ferritin, CEA, ICAM-1, Staphylococcus aureus | Cancer (breast, colorectal), infectious diseases, GI inflammation | Paclitaxel, docetaxel, antibiotics | Enhanced tumor targeting and cytotoxicity; improved oral GI delivery and bacterial clearance | [127,128,142,143,144] |
| Protein Nanoparticles | VEGF, CD3, CD40 | Gynecologic cancers, T-cell leukemia, and viral infections | Paclitaxel, p53, immune modulators | 50% increased drug delivery; specific immune cell targeting; enhanced immune signaling via nanocages | [132,133,145] |
| Gold/Metal Oxide Nanoparticles | EGFR, HER2, EpCAM, VEGF, CD105, NNV, CD63 | Cancer (breast, colorectal, liver), cardiac repair, viral infections | Paclitaxel, doxorubicin, bortezomib, mRNA | Photothermal killing; enhanced imaging; tumor targeting; 40× exosome redirection to heart | [136,137,138,139,140,146,147,148,149,150,151,152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alradwan, I.A.; Alnefaie, M.K.; AL Fayez, N.; Aodah, A.H.; Majrashi, M.A.; Alturki, M.; Fallatah, M.M.; Almughem, F.A.; Tawfik, E.A.; Alshehri, A.A. Strategic and Chemical Advances in Antibody–Drug Conjugates. Pharmaceutics 2025, 17, 1164. https://doi.org/10.3390/pharmaceutics17091164
Alradwan IA, Alnefaie MK, AL Fayez N, Aodah AH, Majrashi MA, Alturki M, Fallatah MM, Almughem FA, Tawfik EA, Alshehri AA. Strategic and Chemical Advances in Antibody–Drug Conjugates. Pharmaceutics. 2025; 17(9):1164. https://doi.org/10.3390/pharmaceutics17091164
Chicago/Turabian StyleAlradwan, Ibrahim A., Meshal K. Alnefaie, Nojoud AL Fayez, Alhassan H. Aodah, Majed A. Majrashi, Meshael Alturki, Mohannad M. Fallatah, Fahad A. Almughem, Essam A. Tawfik, and Abdullah A. Alshehri. 2025. "Strategic and Chemical Advances in Antibody–Drug Conjugates" Pharmaceutics 17, no. 9: 1164. https://doi.org/10.3390/pharmaceutics17091164
APA StyleAlradwan, I. A., Alnefaie, M. K., AL Fayez, N., Aodah, A. H., Majrashi, M. A., Alturki, M., Fallatah, M. M., Almughem, F. A., Tawfik, E. A., & Alshehri, A. A. (2025). Strategic and Chemical Advances in Antibody–Drug Conjugates. Pharmaceutics, 17(9), 1164. https://doi.org/10.3390/pharmaceutics17091164






