Advances in Gold Nanoparticles for the Diagnosis and Management of Alzheimer’s Disease
Abstract
1. Introduction
2. Pathology of Alzheimer’s Disease
3. Gold Nanoparticles, Gut–Brain Axis, and Microbiome
4. Gold Nanoparticles and Alzheimer’s Disease
4.1. Single Gold Nanoparticles
4.2. Dual Metal Gold Nanoparticles
4.3. Hybrid Gold Nanoparticles
4.4. Functionalized Gold Nanoparticles
5. Clinical Applications of Gold Nanoparticles
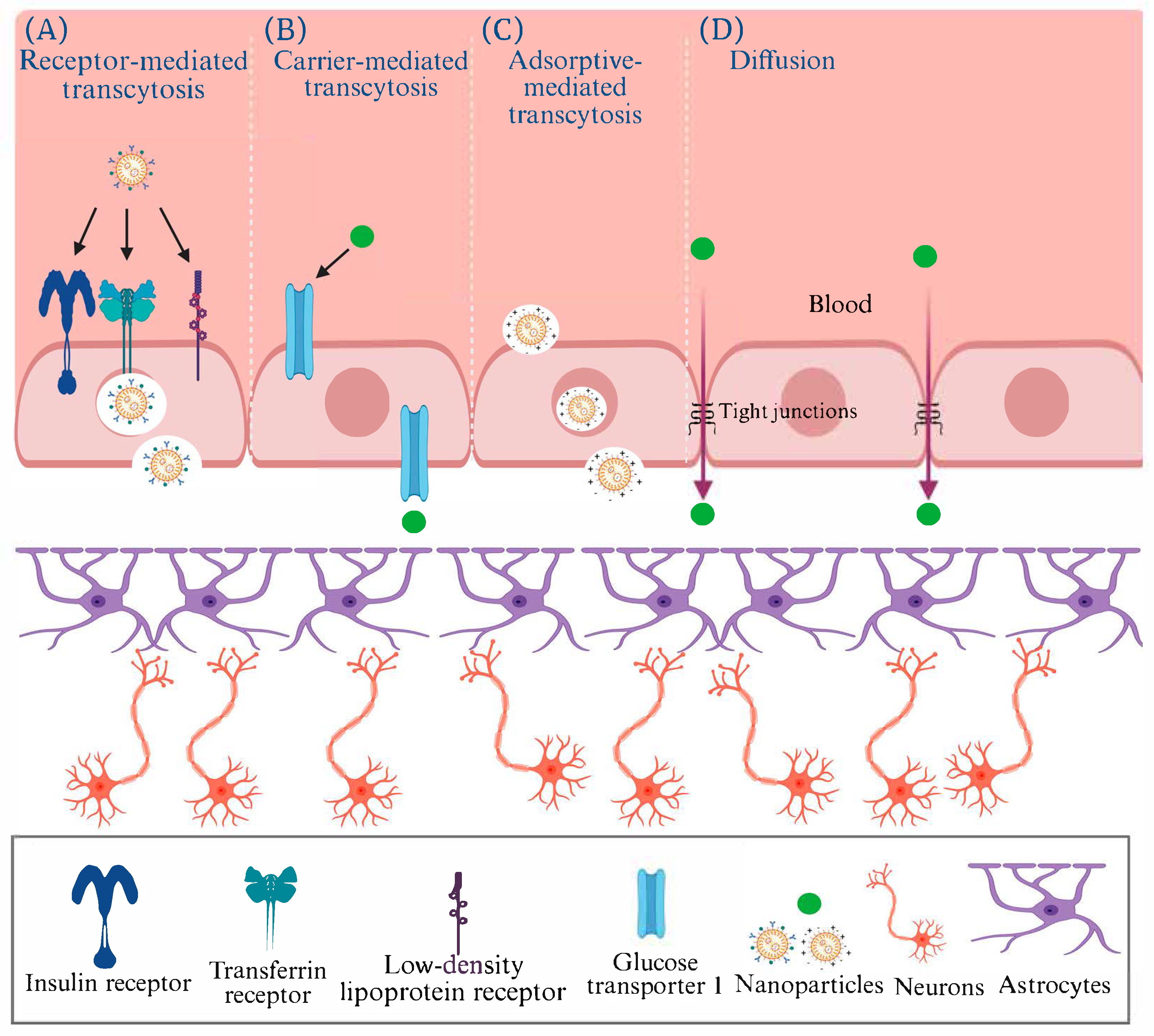
6. Translocation of AuNPs Across the Blood–Brain Barrier
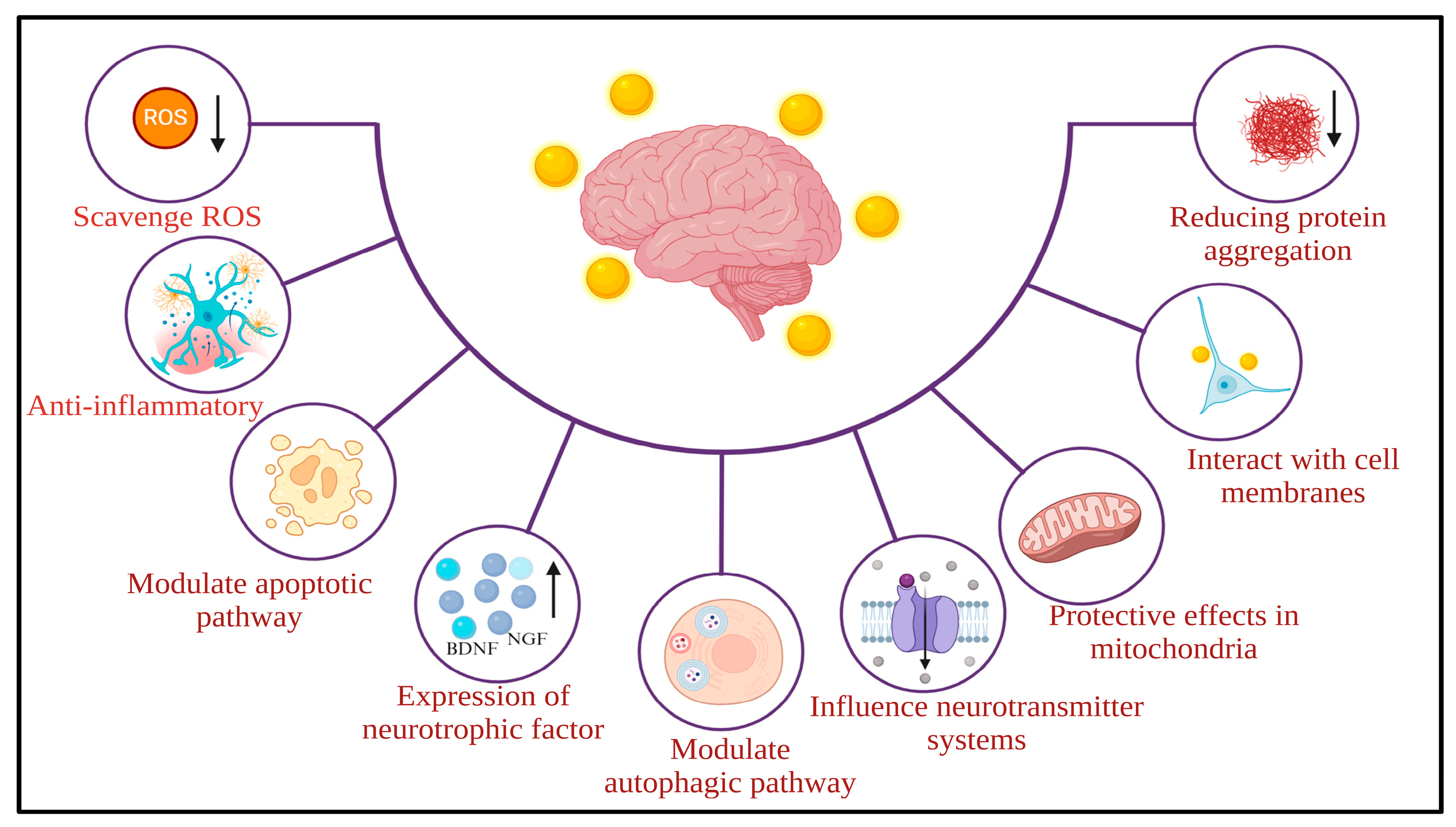
7. Safety and Toxicity of AuNPs
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ↑ | Increase |
| ↓ | Decrease |
| 4HNE | 4-hydroxynonenal |
| Ab | Antibody |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| ADAM10 | A Disintegrin and Metalloproteinase 10 |
| AICD | APP intracellular domain |
| ALS | Amyotrophic lateral sclerosis |
| APH | Aphios |
| ApoE4 | Apolipoprotein E4 |
| APP | Amyloid precursor protein |
| APTMS | Amine (3-aminopropyl) triethoxysilane |
| Au/NiFe2O4@GO-Ch | Gold nanoparticle/nickel ferrite decorated graphene oxide-chitosan nanocomposite |
| Au@Pt/Au core@shell NPs | Gold platinum nanoparticles |
| Au-CeO2 | Gold-cerium oxide |
| AuNCs | AuNPs and nanoclusters |
| AuNPs | Gold nanoparticles |
| AuNR | Gold nanorods |
| Aβ | Amyloid beta |
| AβO | Aβ oligomers |
| BBB | Blood–brain barrier |
| BBB-oC | BBB-on-a-Chip |
| BDNF | Brain-derived neurotrophic factor |
| BDNF-pCREB-pAkt | Brain-derived neurotrophic factor-phosphorylated cyclic AMP response element-binding protein-phosphorylated protein kinase B |
| BSA | Bovine serum albumin |
| C99 | Membrane-bound 99 amino acid comprising CTF-β |
| CA1 | Cornu ammonis 1 |
| Ce (AC)3 | Cerium acetate |
| CeO2 NPs | Ceria (cerium oxide) nanoparticles |
| CNM | Clene nanomedicine |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CTAB | Cetyltrimethylammonium bromide |
| CTF | Carboxy-terminal end |
| Cys-Aβ@AuNP | Cysteine-Aβ peptide-conjugated gold nanoparticles |
| D3.3 | d-glutathione-stabilized gold nanoparticles |
| D-Au | 4,6-diamino-2-pyrimidinethiol-coated Au |
| DG | Dentate gyrus |
| DMF | Dynamic magnetic field |
| DPV | Differential pulse voltammetry; |
| fM | Femtomolar |
| GBA | Gut–brain axis |
| GFAP | Glial fibrillary acidic protein |
| GNR-PEG-Ang2/D1 | Gold nanorods-polyethylene glycol-angiopep-2 peptide/D1 peptide |
| GNS | Gold nanostar |
| GO | Graphene oxide |
| GSH | L-glutathione |
| GSK-3β | Glycogen synthase kinase-3 Beta |
| HAuNS | Hollow gold nanospheres |
| HE-AuNPs | Heliotropium eichwaldi-functionalized AuNPs |
| ih | Iliohypogastric nerve injections |
| IL-1β | Interleukin-1 beta |
| IP3 | Inositol 1,4,5-trisphosphate |
| K2PtCl4 | Potassium tetrachloropropionate |
| KLVFF | Pentapeptide fragments (Aβ16–20) |
| LDL | Low-density lipoprotein |
| MF | Magnetic field |
| Mimo-AuNPs | Mimosine functionalized gold nanoparticles |
| miRNA-137 | microRNA-137 |
| MS | Multiple sclerosis |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFT | Neurofibrillary Tangles. |
| NGF-SPIO-Au NPs | Nerve growth factor functionalized superparamagnetic iron oxide-gold NPs |
| NGFβ | Nerve growth factor-β |
| NIR | Near-Infrared region |
| NPs | Nanoparticles |
| OA | Okadaic acid |
| PC12 | P(heo)C(hromocytoma)12 cells |
| PD | Parkinson’s disease |
| PEG | Polyethylene glycol |
| PEG-COOH | Poly(ethylene glycol) with a Carboxylic Acid group |
| PFGNP | Polypeptid-functionalized gold nanoparticles |
| PSEN1 | Presenilin 1 |
| PSEN2 | Presenilin 2 |
| PTT | Photothermal Therapy |
| Qu@P-80@AuPd | Quercetin-modified gold-palladium nanoparticles |
| ROS | Reactive oxygen species |
| sAPPα | Soluble APPα |
| SCFAs | Short-chain fatty acids |
| SHG-44 | Shanghai Institute of Biochemistry and Cell Biology-44 (Human malignant glioma cell line) |
| SH-SY5Y | Human Neuroblastoma Cell Line |
| SMF | Static magnetic field |
| STIM1 | Stromal interaction molecule-1 |
| STIM2 | Stromal interaction molecule-2 |
| TEER | Trans-endothelial electrical resistance |
| TEM | Transmission electron microscopy |
| TNFα | Tumor necrosis factor-alpha |
| ΨMMP | Mitochondrial membrane potential |
References
- Querfurth, H.W.; LaFerla, F.M. Mechanisms of disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Holtzman, D.M. Biomarker modeling of Alzheimer’s disease. Neuron 2013, 80, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Xiong, C.; Jasielec, M.S.; Bateman, R.J.; Goate, A.M.; Benzinger, T.L.; Ghetti, B.; Martins, R.N.; Masters, C.L.; Mayeux, R.; et al. Longitudinal change in CSF biomarkers in autosomal dominant Alzheimer’s disease. Sci. Transl. Med. 2014, 6, ra30–ra226. [Google Scholar] [CrossRef]
- Patterson, C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018; pp. 32–36. [Google Scholar]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef]
- Sood, S.; Jain, K.; Gowthamarajan, K. Intranasal therapeutic strategies for management of Alzheimer’s disease. J. Drug Target. 2014, 22, 279–294. [Google Scholar] [CrossRef]
- Arias, J.L. (Ed.) Key aspects in nanotechnology and drug delivery. In Nanotechnology and Drug Delivery: Nanoplatforms in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2014; Volume 1, pp. 1–27. [Google Scholar]
- Suri, K.; Wolfram, J.; Shen, H.; Ferrari, M. Advances in nanotechnology-based drug delivery platforms and novel drug delivery systems. In Novel Approaches and Strategies for Biologics, Vaccines and Cancer Therapies; Singh, M., Salnikova, M., Eds.; Elsevier: Holly Springs, NC, USA, 2015; pp. 41–58. [Google Scholar]
- Lu, C.; Lu, G.; Dong, W.; Liu, X. Effect of nano-composite materials on repair of ligament injury in sports detoxification. Adv. Nan. Res. 2022, 13, 247–257. [Google Scholar]
- Zhang, B.; Jin, H.; Duan, X. Sport and exercise impact on the therapy with nanomedicine in drug delivery. Adv. Nano Res. 2022, 13, 269–284. [Google Scholar]
- Prakashkumar, N.; Sivamaruthi, B.S.; Chaiyasut, C.; Suganthy, N. Decoding the neuroprotective potential of methyl gallate-loaded starch nanoparticles against beta amyloid-induced oxidative stress-mediated apoptosis: An in vitro study. Pharmaceutics 2021, 13, 299. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Sisubalan, N.; Varaprasad, K.; Aepuru, R.; Yallapu, M.M.; Viswanathan, M.R.; Sadiku, R. Hybrid nanoparticles from chitosan and nickel for enhanced biocidal activities. New J. Chem. 2022, 46, 13240–13248. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kapoor, D.U.; Kukkar, R.R.; Gaur, M.; Elossaily, G.M.; Prajapati, B.G.; Chaiyasut, C. Mesoporous silica nanoparticles: Types, synthesis, role in the treatment of Alzheimer’s disease, and other applications. Pharmaceutics 2023, 15, 2666. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Thangaleela, S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. Mesoporous silica-based nanoplatforms are theranostic agents for the treatment of inflammatory disorders. Pharmaceutics 2023, 15, 439. [Google Scholar] [CrossRef]
- Mukherjee, S.; Madamsetty, V.S.; Bhattacharya, D.; Roy Chowdhury, S.; Paul, M.K.; Mukherjee, A. Recent advancements of nanomedicine in neurodegenerative disorders theranostics. Adv. Funct. Mater. 2020, 30, 2003054. [Google Scholar] [CrossRef]
- Betancourt, T.; Doiron, A.; Homan, K.A.; Brannon-Peppas, L. Controlled release and nanotechnology. In Nanotechnology in Drug Delivery; De Villiers, M.M., Aramwit, P., Kwon, G.S., Eds.; Biotechnology: Pharmaceutical Aspects, Volume X; Springer: New York, NY, USA, 2009; pp. 283–312. [Google Scholar]
- Safari, J.; Zarnegar, Z. Advanced drug delivery systems: Nanotechnology of health design—A review. J. Saudi Chem. Soc. 2014, 18, 85–99. [Google Scholar] [CrossRef]
- Khan, N.H.; Mir, M.; Ngowi, E.E.; Zafar, U.; Khakwani, M.M.A.K.; Khattak, S.; Zhai, Y.K.; Jiang, E.S.; Zheng, M.; Duan, S.F.; et al. Nanomedicine: A promising way to manage Alzheimer’s disease. Front. Bioeng. Biotechnol. 2021, 9, 630055. [Google Scholar] [CrossRef]
- Kabanov, A.; Gendelman, H.E. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Progr. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef]
- Neely, A.; Perry, C.; Varisli, B.; Singh, A.K.; Arbneshi, T.; Senapati, D.; Kalluri, J.R.; Ray, P.C. Ultrasensitive and highly selective detection of Alzheimer’s disease biomarker using two-photon Rayleigh scattering properties of gold nanoparticle. ACS Nano 2009, 3, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Karaboga, M.N.S.; Sezgintürk, M.K. Analysis of Tau-441 protein in clinical samples using rGO/AuNP nanocomposite-supported disposable impedimetric neuro-biosensing platform: Towards Alzheimer’s disease detection. Talanta 2020, 219, 121257. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Wong, L.R.; Ho, P.C. Role of serum albumin as a nanoparticulate carrier for nose-to-brain delivery of R-flurbiprofen: Implications for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2018, 70, 59–69. [Google Scholar] [CrossRef]
- Zhao, Y.; Ran, W.; He, J.; Huang, Y.; Liu, Z.; Liu, W.; Tang, Y.; Zhang, L.; Gao, D.; Gao, F. High performance asymmetric supercapacitors based on multilayer MnO2/graphene oxide nanoflakes and hierarchical porous carbon with enhanced cycling stability. Small 2015, 11, 1310–1319. [Google Scholar] [CrossRef]
- Gelinas, D.S.; DaSilva, K.; Fenili, D.; St. George-Hyslop, P.; McLaurin, J. Immunotherapy for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. 2), 14657–14662. [Google Scholar] [CrossRef] [PubMed]
- Moretto, N.; Bolchi, A.; Rivetti, C.; Imbimbo, B.P.; Villetti, G.; Pietrini, V.; Polonelli, L.; Del Signore, S.; Smith, K.M.; Ferrante, R.J.; et al. Conformation-sensitive antibodies against Alzheimer amyloid by immunization with a thioredoxin-constrained B-cell epitope peptide. J. Biol. Chem. 2007, 282, 11436–11445. [Google Scholar] [CrossRef]
- Moon, D.W.; Park, Y.H.; Lee, S.Y.; Lim, H.; Kwak, S.; Kim, M.S.; Kim, H.; Kim, E.; Jung, Y.; Hoe, H.S.; et al. Multiplex protein imaging with secondary ion mass spectrometry using metal oxide nanoparticle-conjugated antibodies. ACS Appl. Mater. Interfaces 2020, 12, 18056–18064. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Dong, X.; Sun, Y. Nitrogen-doped carbonized polymer dots: A potent scavenger and detector targeting Alzheimer’s-amyloid plaques. Small 2020, 16, 2002804. [Google Scholar] [CrossRef]
- Navarro Martínez, N.; Toledo Hernández, J.; Morales, J.O. Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach. Nanotechnol. Rev. 2023, 12, 20220548. [Google Scholar] [CrossRef]
- Sivanesan, S.; Rajeshkumar, S. Gold nanoparticles in diagnosis and treatment of Alzheimer’s disease. In Nanobiotechnology in Neurodegenerative Diseases; Mahendra, R., Alka, Y., Eds.; Springer: Cham, Switzerland, 2019; pp. 289–306. [Google Scholar]
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D.; et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 4790. [Google Scholar] [CrossRef] [PubMed]
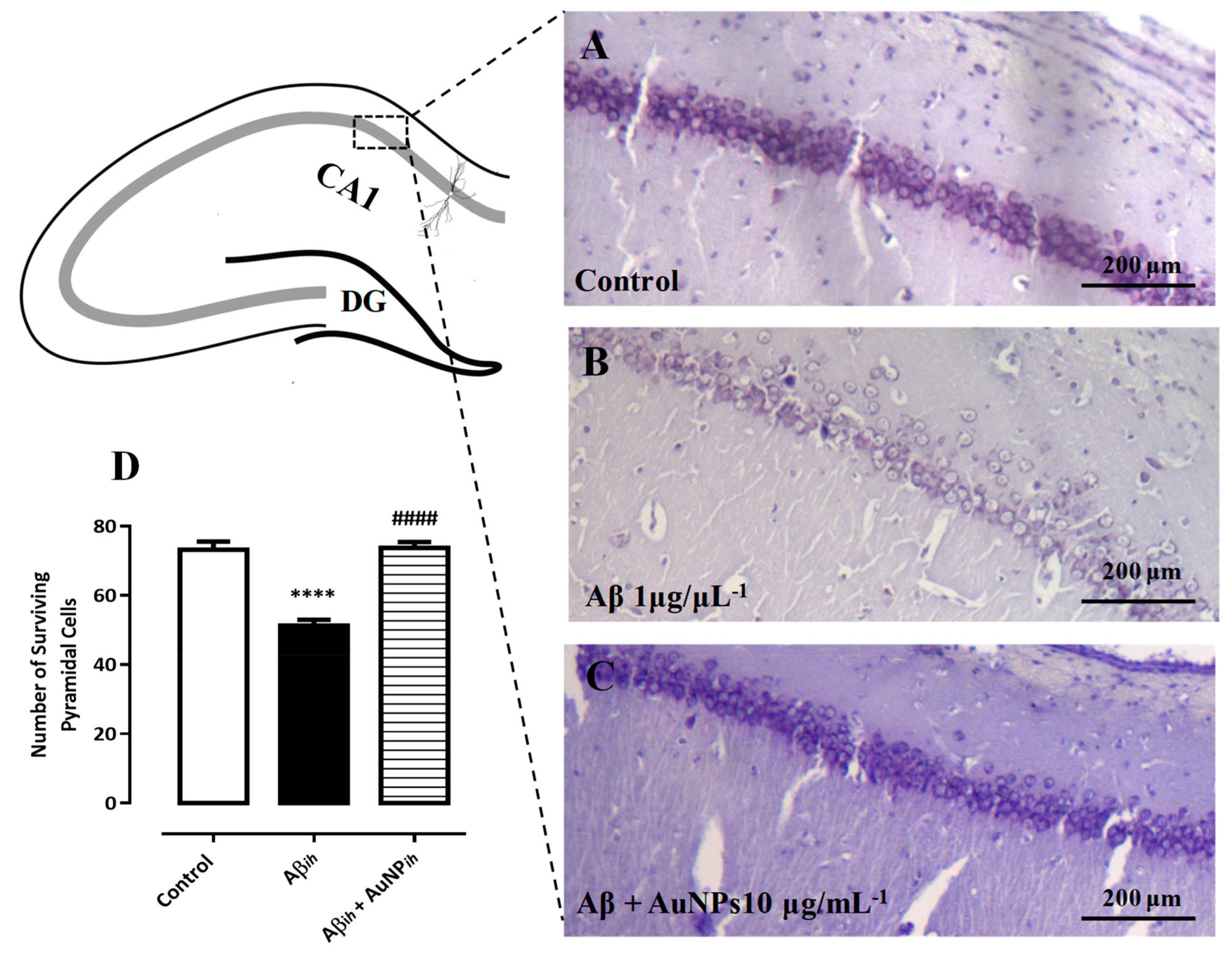
- Sanati, M.; Khodagholi, F.; Aminyavari, S.; Ghasemi, F.; Gholami, M.; Kebriaeezadeh, A.; Sabzevari, O.; Hajipour, M.J.; Imani, M.; Mahmoudi, M.; et al. Impact of gold nanoparticles on amyloid β-induced Alzheimer’s disease in a rat animal model: Involvement of STIM proteins. ACS Chem. Neurosci. 2019, 10, 2299–2309. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ1–42 mouse model of Alzheimer’s disease. Mol. Neurobiol. 2017, 54, 6490–6506. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Zhang, D.; Zhang, Z.; Zhu, J.; Xu, L.; Guo, Y. Neuroprotective effects of maize tetrapeptide-anchored gold nanoparticles in Alzheimer’s disease. Colloids Surf. B Biointerfaces 2021, 200, 111584. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Tramontin, N.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G.; Mendes, C.; Silveira, P.C.L.; Muller, A.P. Gold nanoparticles treatment reverses brain damage in Alzheimer’s disease model. Mol. Neurobiol. 2020, 57, 926–936. [Google Scholar] [CrossRef]
- Chiang, M.C.; Yang, Y.P.; Nicol, C.J.B.; Wang, C.J. Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection. Int. J. Mol. Sci. 2024, 25, 2360. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; El-Batal, A.I.; Amin, Y.M.; Hawas, A.M.; Hassan, S.H.M.; Eid, N.I. Neuroprotective Effect of Gold Nanoparticles and Alpha-Lipoic Acid Mixture against Radiation-Induced Brain Damage in Rats. Int. J. Mol. Sci. 2022, 23, 9640. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Lin, C.H.; Chen, S.J.; Yen, C.; Huang, R.N. Nanogold induces anti-inflammation against oxidative stress induced in human neural stem cells exposed to amyloid-beta peptide. Neurochem. Int. 2021, 145, 104992. [Google Scholar] [CrossRef]
- Aili, M.; Zhou, K.; Zhan, J.; Zheng, H.; Luo, F. Anti-inflammatory role of gold nanoparticles in the prevention and treatment of Alzheimer’s disease. J. Mater. Chem. B 2023, 11, 8605–8621. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Cheng, Y.C.; Yen, C.; Lin, C.H.; Chen, S.J.; Huang, R.N. Nanogold Neuroprotection in Human Neural Stem Cells Against Amyloid-beta-induced Mitochondrial Dysfunction. Neuroscience 2020, 435, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Cascione, M.; Griego, A.; Pellegrino, P.; Moschetti, G.; De Matteis, V. Gold and silver nanoparticles in Alzheimer’s and Parkinson’s diagnostics and treatments. Ibrain 2023, 9, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Rojas-Gutierrez, E.; Muñoz-Arenas, G.; Treviño, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Díaz, A.; Guevara, J. Alzheimer’s Disease and Metabolic Syndrome: A Link from Oxidative Stress and Inflammation to Neurodegeneration. Synapse 2017, 71, e21990. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Du, F.; Yu, Q.; Kanaan, N.M.; Yan, S.S. Mitochondrial Oxidative Stress Contributes to the Pathological Aggregation and Accumulation of Tau Oligomers in Alzheimer’s Disease. Hum. Mol. Genet. 2022, 31, 2498–2507. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Hanrieder, J. Lipid imaging of Alzheimer’s disease pathology. J. Neurochem. 2024, 168, 1175–1178. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Giri, M.; Zhang, M.; Lu, Y. Genes associated with Alzheimer’s disease: An overview and current status. Clin. Interv. Aging 2016, 11, 665–681. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jiang, M.; Trumbauer, M.E.; Sirinathsinghji, D.J.; Hopkins, R.; Smith, D.W.; Heavens, R.P.; Dawson, G.R.; Boyce, S.; Conner, M.W.; et al. Beta-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 1995, 81, 525–531. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Hong, L.; Koelsch, G.; Lin, X.; Wu, S.; Terzyan, S.; Ghosh, A.K.; Zhang, X.C.; Tang, J. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 2000, 290, 150–153. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; De Felice, F.G. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Mina, E.; Glabe, C.; Busciglio, J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J. Neurosci. 2006, 26, 6011–6018. [Google Scholar] [CrossRef] [PubMed]
- Pagani, L.; Eckert, A. Amyloid-beta interaction with mitochondria. Int. J. Alzheimers Dis. 2011, 2011, 925050. [Google Scholar] [CrossRef]
- Zotova, E.; Holmes, C.; Johnston, D.; Neal, J.W.; Nicoll, J.A.; Boche, D. Microglial alterations in human Alzheimer’s disease following Aβ42 immunization. Neuropathol. Appl. Neurobiol. 2011, 37, 513–524. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Singh, H.; Chopra, C.; Singh, H.; Malgotra, V.; Wani, A.K.; Dhanjal, D.S.; Sharma, I.; Nepovimova, E.; Alomar, S.; Singh, R.; et al. Gut-brain axis and Alzheimer’s disease: Therapeutic interventions and strategies. J. Funct. Foods 2024, 112, 105915. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Alzheimer’s disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018, 129, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Frigell, J.; García, I.; Gómez-Vallejo, V.; Llop, J.; Penadés, S. 68Ga-labeled gold glyconanoparticles for exploring blood-brain barrier permeability: Preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J. Am. Chem. Soc. 2014, 136, 449–457. [Google Scholar] [CrossRef]
- Velasco-Aguirre, C.; Morales, F.; Gallardo-Toledo, E.; Guerrero, S.; Giralt, E.; Araya, E.; Kogan, M.J. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: Preclinical approaches. Int. J. Nanomed. 2015, 10, 4919–4936. [Google Scholar] [CrossRef]
- Mercier-Bonin, M.; Despax, B.; Raynaud, P.; Houdeau, E.; Thomas, M. Mucus and microbiota as emerging players in gut nanotoxicology: The example of dietary silver and titanium dioxide nanoparticles. Crit. Rev. Food Sci. Nutr. 2018, 58, 1023–1032. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z.; et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, X.; Boudreau, M.D.; Feng, G.; Miao, Y.; Dong, S.; Wu, H.; Zeng, M.; Yin, J.J. Orally administered gold nanoparticles protect against colitis by attenuating Toll-like receptor 4- and reactive oxygen/nitrogen species-mediated inflammatory responses but could induce gut dysbiosis in mice. J. Nanobiotechnol. 2018, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cha, R.; Zhao, X.; Guo, H.; Luo, H.; Wang, M.; Zhou, F.; Jiang, X. Gold nanoparticles cure bacterial infection with benefit to intestinal microflora. ACS Nano 2019, 13, 5002–5014. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yang, C.; Xiang, T.; Dou, C.; Sun, D.; Dai, Q.; Ling, Z.; Xu, J.; Luo, F.; Chen, Y. Gold nanoparticles exhibit anti-osteoarthritic effects via modulating interaction of the “microbiota-gut-joint” axis. J. Nanobiotechnol. 2024, 22, 157. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Wang, Z. Gold nanoparticle-based dot-blot immunoassay for sensitively detecting Alzheimer’s disease related β-amyloid peptide. Chem. Commun. 2012, 48, 8392–8394. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, D.; Ryu, K.Y.; Choi, I. A gold nanoparticle-mediated rapid in vitro assay of anti-aggregation reagents for amyloid β and its validation. Chem. Commun. 2017, 53, 4449–4452. [Google Scholar] [CrossRef]
- Geng, J.; Qu, K.; Ren, J.; Qu, X. Rapid and efficient screening of Alzheimer’s disease β-amyloid inhibitors using label-free gold nanoparticles. Mol. Biosyst. 2010, 6, 2389–2391. [Google Scholar] [CrossRef]
- Mirsadeghi, S.; Dinarvand, R.; Ghahremani, M.H.; Hormozi-Nezhad, M.R.; Mahmoudi, Z.; Hajipour, M.J.; Atyabi, F.; Ghavami, M.; Mahmoudi, M. Protein corona composition of gold nanoparticles/nanorods affects amyloid beta fibrillation process. Nanoscale 2015, 7, 5004–5013. [Google Scholar] [CrossRef]
- Chang, W.; Zhao, J.; Liu, L.; Xing, X.; Zhang, C.; Meng, H.; Gopinath, S.C.B.; Liu, Y. Graphene oxide-gold star construct on triangular electrodes for Alzheimer’s disease identification. J. Anal. Methods Chem. 2021, 2021, 6661799. [Google Scholar] [CrossRef]
- Hong, Q.; Jin, X.; Zhou, C.; Shao, J. Gold nanoparticles with amyloid-β reduce neurocell cytotoxicity for the treatment and care of Alzheimer’s disease therapy. Gold Bull. 2023, 56, 135–144. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Novelli, A.; Fernández-Sánchez, M.T.; Costa-García, A.; de la Escosura-Muñiz, A. Bifunctional Au@Pt/Au core@shell nanoparticles as novel electrocatalytic tags in immunosensing: Application for Alzheimer’s disease biomarker detection. Anal. Chem. 2020, 92, 7209–7217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid. Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M.; Heli, H. An electrochemical peptide-based biosensor for the Alzheimer biomarker amyloid-β (1-42) using a microporous gold nanostructure. Mikrochim. Acta 2019, 186, 766. [Google Scholar] [CrossRef]
- Devi, R.; Gogoi, S.; Dutta, H.S.; Bordoloi, M.; Sanghi, S.K.; Khan, R. Au/NiFe2O4 nanoparticle-decorated graphene oxide nanosheets for electrochemical immunosensing of amyloid beta peptide. Nanoscale Adv. 2019, 2, 239–248. [Google Scholar] [CrossRef]
- Qiu, Z.; Shen, Q.; Jiang, C.; Yao, L.; Sun, X.; Li, J.; Duan, C.; Li, R.; Li, X.; Gopinath, S.C.B.; et al. Alzheimer’s disease determination by a dual probe on gold nanourchins and nanohorn hybrids. Int. J. Nanomed. 2021, 16, 2311–2322. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, M.; Gong, D.; Chen, R.; Hu, X.; Sun, T. The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation. Nanoscale 2017, 9, 4107–4113. [Google Scholar] [CrossRef]
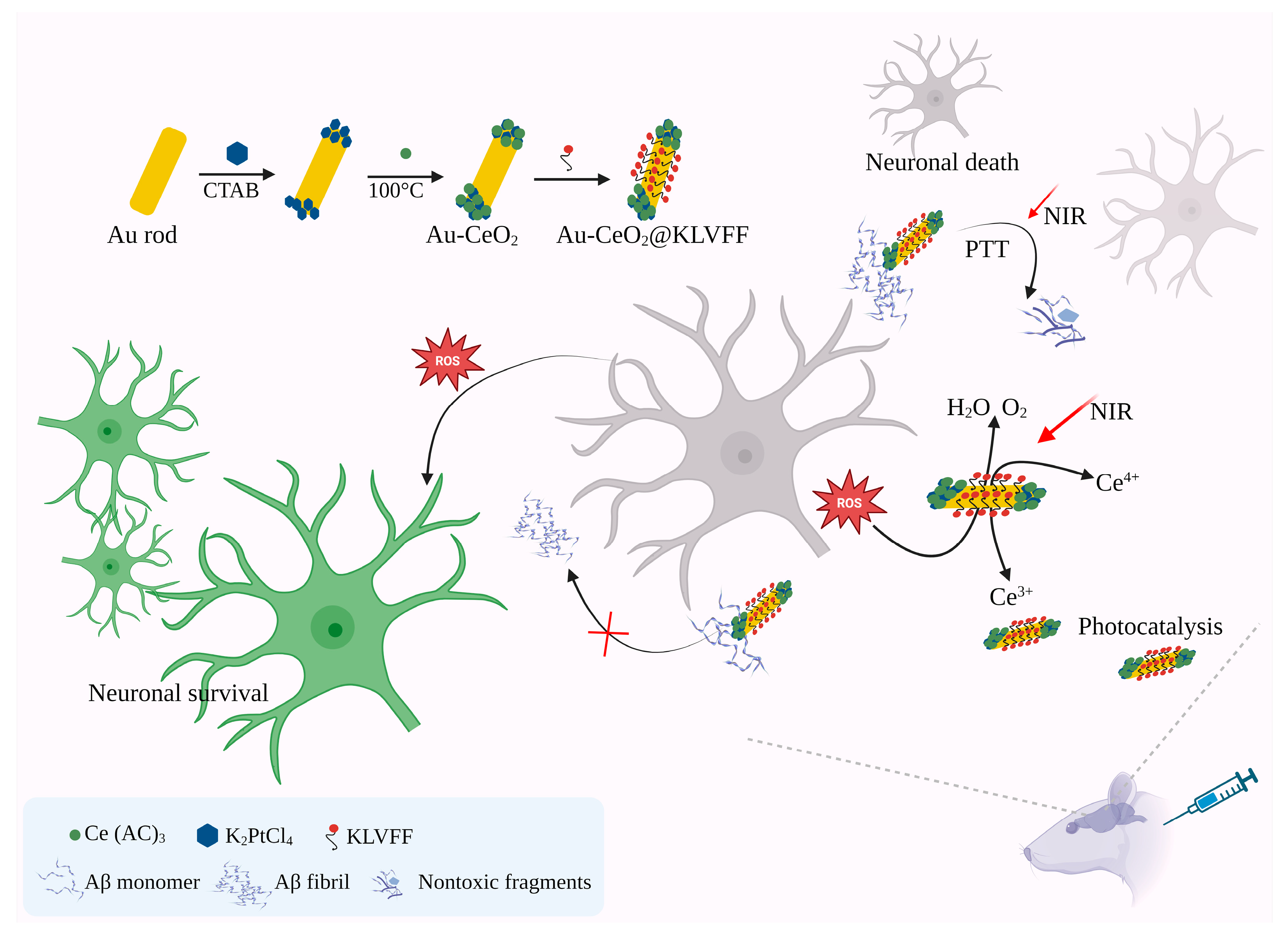
- Ge, K.; Mu, Y.; Liu, M.; Bai, Z.; Liu, Z.; Geng, D.; Gao, F. Gold nanorods with spatial separation of CeO2 deposition for plasmonic-enhanced antioxidant stress and photothermal therapy of Alzheimer’s disease. ACS Appl. Mater. Interfaces 2022, 14, 3662–3674. [Google Scholar] [CrossRef]
- Ruff, J.; Hassan, N.; Morales-Zavala, F.; Steitz, J.; Araya, E.; Kogan, M.J.; Simon, U. CLPFFD-PEG functionalized NIR-absorbing hollow gold nanospheres and gold nanorods inhibit β-amyloid aggregation. J. Mater. Chem. B 2018, 6, 2432–2443. [Google Scholar] [CrossRef]
- Sher, N.; Ahmed, M.; Mushtaq, N. Biogenic synthesis of gold nanoparticles using Heliotropium eichwaldi L and neuroprotective potential via anticholinesterase inhibition in rat brain. Appl. Organomet. Chem. 2023, 37, e7000. [Google Scholar] [CrossRef]
- Anand, B.G.; Wu, Q.; Karthivashan, G.; Shejale, K.P.; Amidian, S.; Wille, H.; Kar, S. Mimosine functionalized gold nanoparticles (Mimo-AuNPs) suppress β-amyloid aggregation and neuronal toxicity. Bioact. Mater. 2021, 6, 4491–4505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sheng, X.; Xie, H.; Zhou, S.; Zhong, M.; Liu, A. Inhibition of Alzheimer’s Aβ1–42 fibrillogenesis and removal of copper ions by polypeptides modified gold nanoparticles. Chem. Biodivers. 2022, 19, e202200342. [Google Scholar] [CrossRef]
- de Oliveira, T.R.; Manzine, P.R.; Cominetti, M.R.; Leite, O.D.; Faria, R.C. Electrochemical magneto-immunoassay for detection of ADAM10 Alzheimer’s biomarker using gold nanoparticles as label. Talanta 2024, 266 Pt 2, 125042. [Google Scholar] [CrossRef]
- Georgas, E.; Yuan, M.; Chen, J.; Wang, Y.; Qin, Y.X. Bioactive superparamagnetic iron oxide-gold nanoparticles regulated by a dynamic magnetic field induce neuronal Ca2+ influx and differentiation. Bioact. Mater. 2023, 26, 478–489. [Google Scholar] [CrossRef]
- Palma-Florez, S.; López-Canosa, A.; Moralez-Zavala, F.; Castaño, O.; Kogan, M.J.; Samitier, J.; Lagunas, A.; Mir, M. BBB-on-a-chip with integrated micro-TEER for permeability evaluation of multi-functionalized gold nanorods against Alzheimer’s disease. J. Nanobiotechnol. 2023, 21, 115. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chien, Y.H.; Chang, C.C.; Wang, P.N.; Chen, Y.R.; Chang, Y.C. Detection of Femtomolar Amyloid-β Peptides for Early-Stage Identification of Alzheimer’s Amyloid-β Aggregation with Functionalized Gold Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, S.; Chai, Q.; Hua, Z. Green synthesis of gold nanoparticles using Acorus calamus leaf extract and study on their anti-Alzheimer potential. Biotechnol. Bioprocess Eng. 2024, 29, 157–163. [Google Scholar] [CrossRef]
- Hao, C.; Meng, D.; Shi, W.; Xu, C.; Wang, Q.; Kuang, H. Chiral Gold Nanostructure Monolayers as SERS Substrates for Ultrasensitive Detection of Enantiomer Biomarkers of Alzheimer’s Disease. Angew. Chem. Int. Ed. Engl. 2025, 64, e202502115. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.H.; Jang, M.J.; Kim, W.S.; Kim, S.S.; Lee, B.; Moon, H.; Oh, S.J.; Ryu, C.H.; Park, K.S.; Cho, I.H.; et al. Enhanced Cognitive and Memory Functions via Gold Nanoparticle-Mediated Delivery of Afzelin through Synaptic Modulation Pathways in Alzheimer’s Disease Mouse Models. ACS Chem. Neurosci. 2025, 16, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Zecca, C.; Tortelli, R.; Panza, F.; Arcuti, S.; Piccininni, M.; Capozzo, R.; Barulli, M.R.; Barone, R.; Cardinali, R.; Abbrescia, D.; et al. Plasma β amyloid1–42 reference values in cognitively normal subjects. J. Neurol. Sci. 2018, 391, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, M.; Lovati, C.; Bertora, P.; Mailland, E.; Galimberti, D.; Scarpini, E.; Quadri, P.; Forloni, G.; Mariani, C. Plasma levels of beta amyloid (1–42) in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 904–905. [Google Scholar] [CrossRef]
- Andreasen, N.; Hesse, C.; Davidsson, P.; Minthon, L.; Wallin, A.; Winblad, B.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Cerebrospinal fluid β-amyloid (1-42) in Alzheimer disease: Differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch. Neurol. 1999, 56, 673–680. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Liu, Y.; Ban, F.; Yang, Z.; Zhang, Y.; Hu, X.; Zhang, Y. Screening for Anti-Aβ Aggregation Activity of Marine Fungal Natural Products Based on a Gold Nanoparticle Method. Chem. Biodivers. 2025, 22, e202401809. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, C.; Yan, F.; Sheng, Z. Synthesis of chiral penicillamine-coated gold nanoparticles and effect on PC12 cells for the treatment of Alzheimer’s disease. J. Clust. Sci. 2020, 31, 1071–1075. [Google Scholar] [CrossRef]
- Nkentsha, Z.; Rambharose, S. Green-synthesized gold nanoparticles exhibit neuroprotective activity against oxidative stress-induced damage in SH-SY5Y cells. J. Nanopart. Res. 2025, 27, 197. [Google Scholar] [CrossRef]
- Hu, L.; Tao, Y.; Jiang, Y.; Qin, F. Recent progress of nanomedicine in the treatment of Alzheimer’s disease. Front. Cell Dev. Biol. 2023, 11, 1228679. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Li, X.; Ji, R.; Hao, Z.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases. Polymers 2023, 15, 2196. [Google Scholar] [CrossRef]
- Sisubalan, N.; Shalini, R.; Ramya, S.; Sivamaruthi, B.S.; Chaiyasut, C. Recent advances in nanomaterials for neural applications: Opportunities and challenges. Nanomedicine 2023, 18, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT03815916 (accessed on 21 August 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04626921 (accessed on 21 August 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04098406 (accessed on 21 August 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT03993171 (accessed on 21 August 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT05299658 (accessed on 21 August 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT03806478 (accessed on 21 August 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT03843710 (accessed on 21 August 2025).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT03536559 (accessed on 21 August 2025).
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef]
- Han, S.; Wang, J.T.; Yavuz, E.; Zam, A.; Rouatbi, N.; Utami, R.N.; Liam-Or, R.; Griffiths, A.; Dickson, W.; Sosabowski, J.; et al. Spatiotemporal tracking of gold nanorods after intranasal administration for brain targeting. J. Control. Release 2023, 357, 606–619. [Google Scholar] [CrossRef]
- Yang, M.Y.; Yu, Y.W.; Li, D.L.; Liu, T.; Wang, Z.X.; Gong, B.F.; Bai, X.X.; He, Y.P.; Liang, H.Y.; Fan, H.Y. Intranasal Administration of a Novel ApoE-Mimetic Peptide-Coated Gold Nanoparticles as Therapy for Ischemic Stroke. CNS Neurosci. Ther. 2025, 31, e70263. [Google Scholar] [CrossRef]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef]
- Jakic, K.; Selc, M.; Razga, F.; Nemethova, V.; Mazancova, P.; Havel, F.; Sramek, M.; Zarska, M.; Proska, J.; Masanova, V.; et al. Long-Term Accumulation, Biological Effects and Toxicity of BSA-Coated Gold Nanoparticles in the Mouse Liver, Spleen, and Kidneys. Int. J. Nanomed. 2024, 19, 4103–4120. [Google Scholar] [CrossRef]
- Niznik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)-Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pan, L.; Touzani, A.; Fernandez-Bertolez, N.; Fraga, S.; Laffon, B.; Valdiglesias, V. Impact of gold nanoparticle exposure on genetic material. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 900, 503827. [Google Scholar] [CrossRef]
- Gerber, A.; Bundschuh, M.; Klingelhofer, D.; Groneberg, D.A. Gold nanoparticles: Recent aspects for human toxicology. J. Occup. Med. Toxicol. 2013, 8, 32. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Abu-Serie, M.M.; Awaad, A.K.; El-Kady, H.; Elwakil, B.H. In vivo bio-distribution and acute toxicity evaluation of greenly synthesized ultra-small gold nanoparticles with different biological activities. Sci. Rep. 2022, 12, 6269. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Shen, X.; Liu, P.; Fan, F.; Fan, S. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials 2012, 33, 4628–4638. [Google Scholar] [CrossRef]
- Zhang, X.D.; Luo, Z.; Chen, J.; Wang, H.; Song, S.S.; Shen, X.; Long, W.; Sun, Y.M.; Fan, S.; Zheng, K.; et al. Storage of gold nanoclusters in muscle leads to their biphasic in vivo clearance. Small 2015, 11, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.C.; Adewale, O.B.; Roux, S.; Cairncross, L.; Davids, H. Biochemical assessment of the neurotoxicity of gold nanoparticles functionalized with colorectal cancer-targeting peptides in a rat model. Hum. Exp. Toxicol. 2021, 40, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, Y.; Jing, G.; Li, K.; Zhao, Y.; Sha, B.; Wang, Q.; Wu, D. Rapid and efficient crossing blood-brain barrier: Hydrophobic drug delivery system based on propionylated amylose helix nanoclusters. Biomaterials 2017, 113, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhary, R.K.; Singh, R.; Singh, S.P.; Wang, S.Y.; Hoe, Z.Y.; Pan, C.T.; Shiue, Y.L.; Wei, D.Q.; Kaushik, A.C.; et al. Nanotheranostic applications for detection and targeting neurodegenerative diseases. Front. Neurosci. 2020, 14, 305. [Google Scholar] [CrossRef]


| Nanoparticle | Classification | Model, Dose, and Route of Administration | Neurological Application | Observations | Study |
|---|---|---|---|---|---|
| Chiral AuNPs | Single metal NPs | In vitro and AD mice model; 25 mg/kg; Intravenous. | Inhibition of Aβ42 aggregation | Prevents Aβ aggregation by adsorbing Aβ monomers. | [31] |
| AuNPs | Single metal NPs | AD rat model; 1 or 10 or 100, or 200 µg/mL; Intrahippocampal and intraperitoneal. | Spatial memory impairment and neural loss | Alleviates spatial memory impairment. | [32] |
| AuNPs | Single metal NPs | AD rat model; 100 µg; Intracerebroventricular. | Spatial memory deficit prevention | Normalize the tau phosphorylation. Prevents spatial memory deficits. AuNPs activate antioxidant defense mechanisms. Preserves normal mitochondrial function. | [35] |
| AuNPs | Single metal NPs | In vitro | Detection of Aβ peptide | Detection of Aβ peptide | [71] |
| AuNPs | Single metal NPs | In vitro | Detection of Aβ peptide | Facilitates the discovery of efficient drug components for the treatment of AD. | [72] |
| Label-free AuNPs | Single metal NPs | In vitro | Detection of Aβ inhibitors | Effective and rapid screening of Aβ inhibitors. | [73] |
| AuNPs | Single metal NPs | In vivo * | Aβ fibrillation process | Prevents the hot spot regions of Aβ monomers from binding to each other and effectively blocks the fibrillation process. | [74] |
| Graphene oxide-gold nanostar | Single metal NPs | In vitro | Detection of miRNA-137 for early diagnosis of AD | It enables specific detection of miRNA-137, achieving a detection limit of 10 fM and a sensitivity of 1 fM, crucial for early AD diagnosis. | [75] |
| AuNPs | Single metal NPs | In vitro | Development of cytotoxic oligomers by binding to proteins in AD | AuNPs with Aβ1–42 oligomers caused a stronger increase in caspase-3 activity compared to control oligomers. | [76] |
| Au@Pt/Au core@shell NPs | Dual metal NPs | In vitro | Quantification of p53 peptide as a biomarker of AD | Aids in quantifying the altered p53 peptide. | [77] |
| Quercetin-modified gold-palladium nanoparticles | Dual metal NPs | In vitro | As a potential autophagy inducer for the treatment of AD | Concave cubic Qu@P-80@AuPd influences the autophagy in SH-SY5Y cells in a dose-dependent manner. | [78] |
| Microporous gold nanostructure | Hybrid AuNPs | In vitro | Peptide-based biosensor fabrication for biomarker Aβ1–42 | Quantifies the Aβ1–42 in artificial CSF and spiked serum samples. | [79] |
| Au/NiFe2O4@GO-Ch | Hybrid AuNPs | In vitro | Immuno-sensing of Aβ peptide | Aids in quantifying Aβ1–42 molecules using DPV. | [80] |
| Gold urchin and hybrid | Hybrid AuNPs | In vitro | Aβ oligomers detection | Enables the selective and sensitive detection of Aβ oligomers (AβOs), offering promise for AD diagnosis. | [81] |
| L-glutathione-coated AuNPs | Hybrid AuNPs | In vivo * | Aβ fibrillation | GSH possesses unbound carboxyl and amino groups capable of interacting with Aβ through non-specific electrostatic and hydrogen bonding interactions. | [82] |
| Gold nanorods (Au NRs) with CeO2 NPs | Hybrid AuNPs | In vitro and mice model; 25 mg/kg; Intravenous. | Photothermal therapy of AD | Enhanced intercellular anti-ROS activity. | [83] |
| HAuNS and AuNR | Functionalized AuNPs | In vitro | Inhibition of Aβ aggregation | Exposing Aβ to irradiation and heating had no impact on its aggregation. Effectively inhibits the Aβ aggregation. | [84] |
| AuNPs | Functionalized AuNPs | In vitro and Ex vivo model (rat brain homogenate) | Anticholinesterase inhibition | The charge distribution on AChE affects how effectively nanoparticles can inhibit its activity. Even when small NPs aggregate into larger particles, gaps between them still allow AChE molecules to interact and bind. | [85] |
| Mimo-AuNPs | Functionalized AuNPs | In vitro | Suppression of Aβ aggregation | Mimo-AuNPs induce disassembly by disrupting the β-sheet structure or interacting with the steric zippers within Aβ1–42 fibers. | [86] |
| PFGNP | Functionalized AuNPs | In vitro | Inhibition of Aβ1–42 fibrillogenesis | Inhibits the Aβ1–42 fibril formation. | [87] |
| AuNPs | Functionalized AuNPs | In vitro and Ex vivo model (Plasma samples from humans) | Detection of the ADAM10 biomarker | Aids in detecting ADAM10 in diluted plasma, demonstrating a low detection limit and a dynamic linear range. | [88] |
| Iron oxide-AuNPs | Dual component-Functionalized AuNPs | In vitro | Neuronal Ca2+ flux regulation | Enhances intracellular calcium influx and facilitates the cellular uptake of superparamagnetic iron oxide-gold NPs. Induces the upregulation of neural differentiation markers and cell adhesive molecules. | [89] |
| Gold nanorods | Functionalized AuNPs | In vitro | Disaggregation of the amyloid | GNR-PEG-Ang2/D1 was able to cross the BBB with the help of the Ang2 peptide. TEER assays and confocal imaging showed increased tight junction expression, indicating stronger barrier integrity. | [90] |
| Cysteine-Aβ Peptide-conjugated AuNPs | Functionalized AuNPs | In vitro and Ex vivo model (Plasma samples from humans) | Aβ Aggregation | Cysteine-functionalized Aβ peptides interact with AuNPs, changing peptide conformation and slowing aggregation. | [91] |
| AuNPs | Functionalized AuNPs | In vitro | Anti-AD | Nanogold aqueous extracts show strong antioxidant and anti-acetylcholinesterase activity. They may cross the BBB, reduce oxidative stress, and improve acetylcholinesterase levels. | [92] |
| Status | Study Title | Classification | Conditions | Interventions | Phase | Details | Identifier |
|---|---|---|---|---|---|---|---|
| Completed | 31P-MRS imaging to assess the effects of CNM-Au8 on impaired neuronal redox state in Parkinson’s disease | Single metal NPs | PD | Drug: Gold nanocrystals | Phase 2 | Duration: From 19 December 2019 to 7 June 2021; No of subjects: 30. | NCT03815916 (https://www.clinicaltrials.gov/study/NCT03815916) (accessed 20 August 2025) [104] |
| Completed | A multi-center, open-label, long-term extension study of CNM-Au8 in patients with stable relapsing multiple sclerosis | Single metal NPs | MS | Drug: CNM-Au8 | Phase 2 Phase 3 | Duration: From 22 October 2020 to 6 September 2023; No of subjects: 55. | NCT04626921 (https://www.clinicaltrials.gov/study/NCT04626921) (accessed 20 August 2025) [105] |
| Completed | Therapeutic nano-catalysis to slow disease progression of ALS | Single metal NPs | ALS | Drug: CNM-Au8 | Phase 2 | Duration: From 19 December 2019 to 13 July 2021; No of subjects: 45. | NCT04098406 (https://www.clinicaltrials.gov/study/NCT04098406) (accessed 20 August 2025) [106] |
| Active and not recruiting | 31P-MRS imaging to assess the effects of CNM-Au8 on impaired neuronal redox state in multiple sclerosis | Single metal NPs | MS | Drug: Gold nanocrystals | Phase 2 | Not applicable | NCT03993171 (https://www.clinicaltrials.gov/study/NCT03993171) (accessed 20 August 2025) [107] |
| Active and not recruiting | An open-label extension for the phase 2 study in early symptomatic amyotrophic lateral sclerosis patients on stable background therapy to assess bioenergetic catalysis with CNM-Au8 to slow disease progression in amyotrophic lateral sclerosis (ALS) | Single metal NPs | ALS | Drug: CNMAu8 (Clene nanomedicine-gold nanocrystal-based compound) | Phase 2 | Not applicable | NCT05299658 (https://www.clinicaltrials.gov/study/NCT05299658) (accessed 20 August 2025) [108] |
| Unknown status | Study of APH-1105 in patients with mild to moderate AD | Functionalized lipid NPs | Mild to moderate AD | Drug: APH-1105 | Phase 2 | Not applicable | NCT03806478 (https://www.clinicaltrials.gov/study/NCT03806478) (accessed 20 August 2025) [109] |
| Withdrawn | 31P-MRS imaging to assess the effects of CNM-Au8 on impaired neuronal redox state in ALS | Single metal NPs | ALS | Drug: Gold nanocrystals | Phase 2 | Not applicable | NCT03843710 (https://www.clinicaltrials.gov/study/NCT03843710) (accessed 20 August 2025) [110] |
| Terminated | Nanocrystalline gold to treat remyelination failure in chronic optic neuropathy in multiple sclerosis | Single metal NPs | MS | Drug: CNM-Au8 | Phase 2 | Not applicable | NCT03536559 (https://www.clinicaltrials.gov/study/NCT03536559) (accessed 20 August 2025) [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Kesika, P.; Sisubalan, N.; Chaiyasut, C. Advances in Gold Nanoparticles for the Diagnosis and Management of Alzheimer’s Disease. Pharmaceutics 2025, 17, 1158. https://doi.org/10.3390/pharmaceutics17091158
Sivamaruthi BS, Kesika P, Sisubalan N, Chaiyasut C. Advances in Gold Nanoparticles for the Diagnosis and Management of Alzheimer’s Disease. Pharmaceutics. 2025; 17(9):1158. https://doi.org/10.3390/pharmaceutics17091158
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Periyanaina Kesika, Natarajan Sisubalan, and Chaiyavat Chaiyasut. 2025. "Advances in Gold Nanoparticles for the Diagnosis and Management of Alzheimer’s Disease" Pharmaceutics 17, no. 9: 1158. https://doi.org/10.3390/pharmaceutics17091158
APA StyleSivamaruthi, B. S., Kesika, P., Sisubalan, N., & Chaiyasut, C. (2025). Advances in Gold Nanoparticles for the Diagnosis and Management of Alzheimer’s Disease. Pharmaceutics, 17(9), 1158. https://doi.org/10.3390/pharmaceutics17091158









