Strategies for Assessing Physical Compatibility of Calcium Folinate with Bicarbonate During Methotrexate Rescue Therapy in Pediatric Patients with Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Modelling Y-Site Concentrations in Rescue Therapy
2.3. Theoretical Analysis
2.3.1. Defining Patient Models
2.3.2. Predicting Y-Site pH and CaCO3 Precipitation Risk
2.3.3. Investigation of Model Representation of Clinical Scenarios
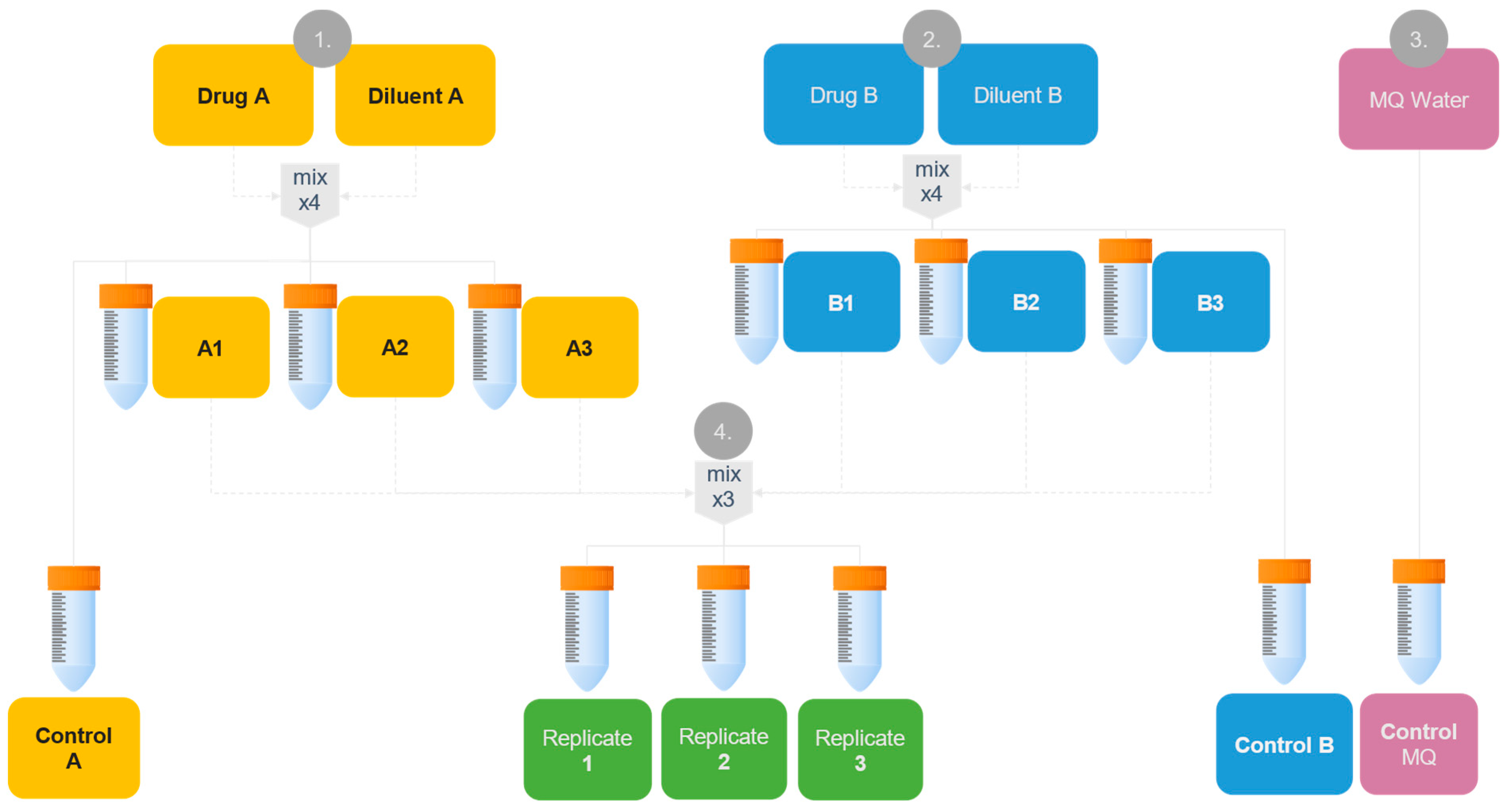
2.4. Physical Compatibility Testing
2.4.1. Preparation of Samples
2.4.2. Analysis of Physical Compatibility Using Validated Methods
2.5. Proofing Precipitate Identity Using Raman Microscopy
2.5.1. Preparation of Samples for Raman Analysis
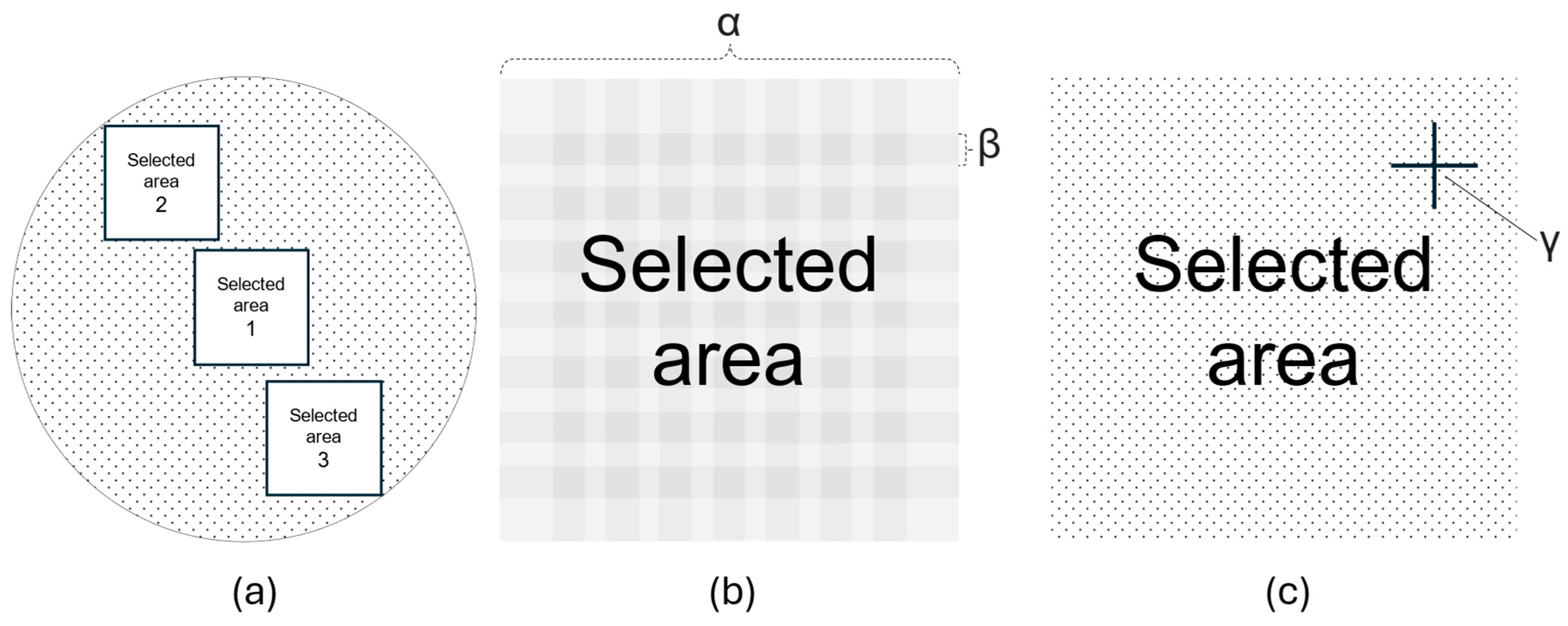
2.5.2. Characterizing Particle Fingerprint for Proof of Identity
3. Results
3.1. Theoretical Analysis
3.1.1. Predicted Ionic Yields, Molar Ratios and Risk of CaCO3 Precipitation
3.1.2. Investigation of Model Representation of Clinical Scenarios
3.2. Physical Compatibility Testing
3.2.1. pH Measurements and Theoretical Consideration
3.2.2. Visual Examination Using Tyndall Light
3.2.3. Turbidimetry
3.2.4. Particle Size Measurement and Quantification
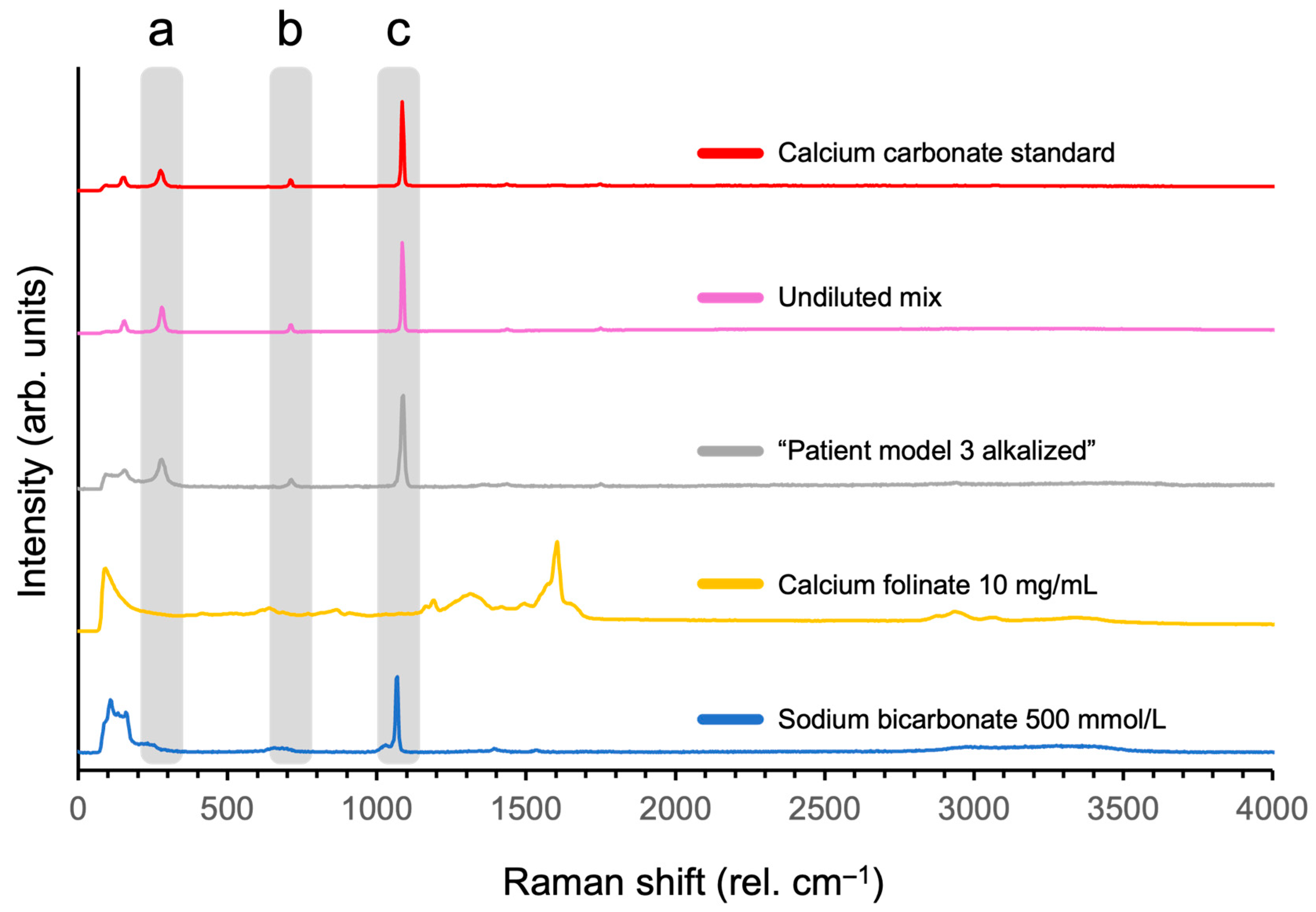
3.2.5. Characterizing Particle Fingerprint for Proof of Identity
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| IV | Intravenous(ly) |
| s-MTX | Serum methotrexate concentrations |
| CVC | Centrally inserted venous catheter |
| PICC | Peripherally inserted central venous catheter |
| MQ | Milli-Q® |
| PES | Polyethersulfone |
| BW | Bodyweight |
| BSA | Body surface area |
| PP | Polypropylene |
| FNU | Formazine Nephelometry Units |
| Ph. Eur. | European Pharmacopoeia |
| PCTE | Track-etched polycarbonate |
| HQI | Hit quality index |
| OL | Overload |
References
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA A Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Surveillance Research Program, National Cancer Institute. SEER*Explorer: An Interactive Website for SEER Cancer Statistics [Internet]; Surveillance Research Program, National Cancer Institute: Rockville, MD, USA, 2024. [Google Scholar]
- Ali, A.A.; Charoo, N.A.; Abdallah, D.B. Pediatric drug development: Formulation considerations. Drug Dev. Ind. Pharm. 2014, 40, 1283–1299. [Google Scholar] [CrossRef]
- Mitchell, C.; Richards, S.; Harrison, C.J.; Eden, T. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980–2001. Leukemia 2010, 24, 406–418. [Google Scholar] [CrossRef]
- UKALL14 Protocol, version 5.0; UCL Cancer Trials Centre: London, UK, 2012.
- UKALL 2019 Interim Guidelines; Children’s Cancer and Leukaemia Group (CCLG): Leicester, UK, 2019.
- ALLTogether Master Protocol; ALLTogether Consortium: Southampton, UK, 2020.
- Acute Lymphoblastic Leukaemia (ALL06) Overview. Available online: https://www.eviq.org.au/haematology-and-bmt/leukaemias/acute-lymphoblastic-leukaemia/3825-acute-lymphoblastic-leukaemia-all06-overview (accessed on 12 August 2023).
- Brown, P.A.; Shah, B.; Advani, A.; Aoun, P.; Boyer, M.W.; Burke, P.W.; DeAngelo, D.J.; Dinner, S.; Fathi, A.T.; Gauthier, J.; et al. Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 1079–1109. [Google Scholar] [CrossRef]
- Shah, B.; Mattison, R.J.; Abboud, R.; Abdelmessieh, P.; Aldoss, I.; Burke, P.W.; DeAngelo, D.J.; Dinner, S.; Fathi, A.T.; Gauthier, J.; et al. Acute Lymphoblastic Leukemia, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2024, 22, 563–576. [Google Scholar] [CrossRef]
- Pediatric Acute Lymphoblastic Leukemia Treatment (PDQ®)—Health Professional Version. Available online: https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq#_22 (accessed on 5 September 2023).
- Hoelzer, D.; Bassan, R.; Boissel, N.; Roddie, C.; Ribera, J.M.; Jerkeman, M. ESMO Clinical Practice Guideline interim update on the use of targeted therapy in acute lymphoblastic leukaemia. Ann. Oncol. 2024, 35, 15–28. [Google Scholar] [CrossRef]
- Hoelzer, D.; Bassan, R.; Dombret, H.; Fielding, A.; Ribera, J.M.; Buske, C. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v69–v82. [Google Scholar] [CrossRef]
- ALLTogether European Treatment Protocol for Children and Young Adults with Acute Lymphoblastic Leukemia (ALL); NordForsk: Oslo, Norway, 2023.
- Vestli, A.; Hellebostad, M. Ped ALL 003acd A2G Mtx 5 g/m2; Oslo University Hospital/Helse Sør-Øst: Oslo, Norway, 2021. [Google Scholar]
- Jiang, R.; Mei, S.; Zhao, Z. Leucovorin (folinic acid) rescue for high-dose methotrexate: A review. J. Clin. Pharm. Ther. 2022, 47, 1452–1460. [Google Scholar] [CrossRef]
- American Society of Health-System Pharmacists. Leucovorin Calcium. In ASHP Injectable Drug Information; American Society of Health-System Pharmacists, Ed.; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2018; pp. 987–991. [Google Scholar]
- Sand, T.E.; Jacobsen, S. Effect of urine pH and flow on renal clearance of methotrexate. Eur. J. Clin. Pharmacol. 1981, 19, 453–456. [Google Scholar] [CrossRef]
- Bedford Laboratories, Leucovorin Calcium Injection USP; Bedford Laboratories: Bedford, OH, USA, 2011.
- Niinimäki, R.; Aarnivala, H.; Banerjee, J.; Pokka, T.; Vepsäläinen, K.; Harila-Saari, A. Reduced dose folinic acid rescue after rapid high-dose methotrexate clearance is not associated with increased toxicity in a pediatric cohort. Support. Care Cancer 2022, 30, 127–133. [Google Scholar] [CrossRef]
- Nunn, J.L.; Takashima, M.D.; Wray-Jones, E.M.; Soosay Raj, T.A.; Hanna, D.M.T.; Ullman, A.J. Central venous access device adverse events in pediatric patients with cancer: A systematic review and meta-analysis. Support. Care Cancer 2024, 32, 662. [Google Scholar] [CrossRef]
- Ullman, A.J.; Gibson, V.; Takashima, M.D.; Kleidon, T.M.; Schults, J.; Saiyed, M.; Cattanach, P.; Paterson, R.; Cooke, M.; Rickard, C.M.; et al. Pediatric central venous access devices: Practice, performance, and costs. Pediatr. Res. 2022, 92, 1381–1390. [Google Scholar] [CrossRef]
- Kamata, Y.; Mizuno, Y.; Okamoto, K.; Okamoto, S.; Ito, Y.; Nishigata, A. Peripherally inserted central catheters can be an alternative to tunneled central venous catheters in chemotherapy for hematological and oncological pediatric patients. Pediatr. Surg. Int. 2023, 39, 264. [Google Scholar] [CrossRef]
- Shulman, R.J.; Smith, E.O.; Rahman, S.; Gardner, P.; Reed, T.; Mahoney, D. Single- vs. double-lumen central venous catheters in pediatric oncology patients. Am. J. Dis. Child. 1988, 142, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Rao, A.; Huang, S.-J.; Chang, C.-Y.; Drechsler, M.; Knaus, J.; Chan, J.C.C.; Raiteri, P.; Gale, J.D.; Gebauer, D. Uncovering the Role of Bicarbonate in Calcium Carbonate Formation at Near-Neutral pH. Angew. Chem. Int. Ed. 2021, 60, 16707–16713. [Google Scholar] [CrossRef] [PubMed]
- Dettori, R.; Donadio, D. Carbon dioxide, bicarbonate and carbonate ions in aqueous solutions under deep Earth conditions. Phys. Chem. Chem. Phys. 2020, 22, 10717–10725. [Google Scholar] [CrossRef] [PubMed]
- Al Omari, M.M.H.; Rashid, I.S.; Qinna, N.A.; Jaber, A.M.; Badwan, A.A. Chapter Two—Calcium Carbonate. In Profiles of Drug Substances, Excipients and Related Methodology, Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 41, pp. 31–132. [Google Scholar]
- Ropp, R.C. Encyclopedia of the Alkaline Earth Compounds; Ropp, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- KGaA, M. Safety Data Sheet: Calcium Carbonate Precipitated for Analysis, EMSURE® Reag. Ph Eur; Merck KGaA: Darmstadt, Germany, 2025. [Google Scholar]
- Kerner, J.A.; Garcia-Careaga, M.G.; Fisher, A.A.; Poole, R.L. Treatment of Catheter Occlusion in Pediatric Patients. J. Parenter. Enter. Nutr. 2006, 30, S73–S81. [Google Scholar] [CrossRef]
- Zheng, L.-y.; Xue, H.; Yuan, H.; Liu, S.-x.; Zhang, X.-y. Efficacy of management for obstruction caused by precipitated medication or lipids in central venous access devices: A systematic review and meta-analysis. J. Vasc. Access 2019, 20, 583–591. [Google Scholar] [CrossRef]
- Sielski, J.; Janion Sadowska, A.; Ciuraszkiewicz, K.; Janion, M. Acute pulmonary embolism following cardiac arrest in a 31 year-old female with long QT syndrome. Kardiol. Pol. 2011, 69, 590–592. [Google Scholar]
- Strickland, S.; Pena, E.; Walker, A.E. Fatal foreign-body granulomatous pulmonary embolization due to microcrystalline cellulose in a patient receiving total parenteral nutrition: All crystals are not what they seem. Forensic Sci. Med. Pathol. 2015, 11, 255–261. [Google Scholar] [CrossRef]
- McNearney, T.; Bajaj, C.; Boyars, M.; Cottingham, J.; Haque, A. Total parenteral nutrition associated crystalline precipitates resulting in pulmonary artery occlusions and alveolar granulomas. Dig. Dis. Sci. 2003, 48, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Steadman, E.; Raisch, D.W.; Bennett, C.L.; Esterly, J.S.; Becker, T.; Postelnick, M.; McKoy, J.M.; Trifilio, S.; Yarnold, P.R.; Scheetz, M.H. Evaluation of a potential clinical interaction between ceftriaxone and calcium. Antimicrob. Agents Chemother. 2010, 54, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Wassel, R.T.; Lee, L.; Nambiar, S. Intravenous ceftriaxone and calcium in the neonate: Assessing the risk for cardiopulmonary adverse events. Pediatrics 2009, 123, e609–e613. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Delegge, M.H.; Kirby, D.F. Pulmonary Embolism as a Complication of Long-Term Total Parenteral Nutrition. J. Parenter. Enter. Nutr. 1993, 17, 578–582. [Google Scholar] [CrossRef]
- Christensen, M.L.; Zareie, P.; Kadiyala, B.; Bursac, Z.; Reed, M.D.; Mattison, D.R.; Davis, R.L. Concomitant ceftriaxone and intravenous calcium therapy in infants. J. Pediatr. Pharmacol. Ther. 2021, 26, 702–707. [Google Scholar] [CrossRef]
- Bruch, H.R.; Esser, M. Catheter occlusion by calcium carbonate during simultaneous infusion of 5-FU and calcium folinate. Onkologie 2003, 26, 469. [Google Scholar] [CrossRef]
- Fackler-Schwalbe, I.; Schwalbe, B.; Epple, M.; Becker, A.; Prügl, L.; Gassel, W.D.; Stoffels, D.; Südhoff, T. Occlusion of central venous port catheters after simultaneous 24 h infusions of 5-fluorouracil and calcium-folinic acid in patients with gastrointestinal cancer. Wien. Med. Wochenschr. 2004, 154, 182–185. [Google Scholar] [CrossRef]
- Ardalan, B.; Flores, M.R. A new complication of chemotherapy administered via permanent indwelling central venous catheter. Cancer 1995, 75, 2165–2168. [Google Scholar] [CrossRef]
- Ratti, M.; Hahne, J.C.; Toppo, L.; Castelli, E.; Petrelli, F.; Passalacqua, R.; Barni, S.; Tomasello, G.; Ghidini, M. Major innovations and clinical applications of disodium-levofolinate: A review of available preclinical and clinical data. Ther. Adv. Med. Oncol. 2019, 11, 1758835919853954. [Google Scholar] [CrossRef] [PubMed]
- Merative. Trissel’s™ 2 Clinical Pharmaceutics Database (Parenteral Compatibility) (Electronic Version); Merative: Ann Arbor, MI, USA, 2025. [Google Scholar]
- Vidal, H. Stabilis: The International Database on Stability and Compatibility of Injectable Drugs, 2025. Available online: https://www.stabilis.org/ (accessed on 17 March 2025).
- Aujoulat, P.; Coze, C.; Braguer, D.; Raybaud, C. Physico-chemical compatibility of methotrexate with co-administered drugs during cancer chemotherapy regimens. J. Pharm. Clin. 1993, 12, 31–35. [Google Scholar]
- Newton, D.W. Crux of drug compatibility and incompatibility. Am. J. Health-Syst. Pharm. 2010, 67, 108–112. [Google Scholar] [CrossRef]
- Castells Lao, G.; Rodríguez Reyes, M.; Roura Turet, J.; Prat Dot, M.; Soy Muner, D.; López Cabezas, C. Compatibility of drugs administered as Y-site infusion in intensive care units: A systematic review. Med. Intensiv. (Engl. Ed.) 2020, 44, 80–87. [Google Scholar] [CrossRef]
- Nilsson, N.; Storesund, I.; Tho, I.; Nezvalova-Henriksen, K. Co-administration of drugs with parenteral nutrition in the neonatal intensive care unit-physical compatibility between three components. Eur. J. Pediatr. 2022, 181, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- D’Huart, E.; Vigneron, J.; Demoré, B. Physical Compatibility of Intravenous Drugs Commonly Used in Intensive Care Units: An Observational Study and Physical Compatibility Laboratory Tests on Anti-Infective Drugs. Pharm. Technol. Hosp. Pharm. 2019, 4, 29–40. [Google Scholar] [CrossRef]
- Staven, V.; Wang, S.; Grønlie, I.; Tho, I. Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr. J. 2016, 15, 29. [Google Scholar] [CrossRef]
- Braun, B. Sodium Hydrogen Carbonate 500 mmol/L Solution for Infusion—Summary of Product Characteristics (SmPC); Electronic Medicines Compendium (EMC): Sheffield, UK, 2024; Available online: https://www.medicines.org.uk/emc/product/15216/smpc (accessed on 17 March 2025).
- Braun, B. Sodium Chloride 0.9% Solution for Infusion—Summary of Product Characteristics (SmPC); Electronic Medicines Compendium (EMC): Sheffield, UK, 2018; Available online: https://www.medicines.org.uk/emc/product/15145/smpc#gref (accessed on 17 March 2025).
- B. Braun Melsungen AG. SmPC for Kalium-Natrium-Glucose Braun Infusjonsvæske; B. Braun Melsungen AG: Melsungen, Germany, 2024. [Google Scholar]
- Heldrup, J.; Bleyer, A.; Ramsey, L.; Schaff, L.; Bernhardt, B.; Schwartz, S.; Chatelut, E.; Hwang, M.; Ten, C.; Guscott, M.; et al. New recommendations for reversal of high-dose methotrexate cytotoxicity with folinic acid. Cancer Chemother. Pharmacol. 2025, 95, 41. [Google Scholar] [CrossRef]
- Jung, Y.; Moe, K.; Torres, E.A.; Kalantar-Zadeh, K.; Hanna, R.M. Unique case of profound iatrogenic hypercalcemia in a patient with recent orthopedic prosthetic infection. Clin. Nephrol. Case Stud. 2020, 8, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Nasjonalt Kompetansenettverk for Legemidler Til Barn. Blandekort: Kalsiumfolinat v3.0. Available online: https://www.legemidlertilbarn.no/blandekort/kalsiumfolinat (accessed on 23 October 2023).
- Centers for Disease Control and Prevention, National Center for Health Statistics. CDC Growth Charts. Available online: https://www.cdc.gov/growthcharts/index.htm (accessed on 15 September 2023).
- WHO. WHO Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards (accessed on 15 September 2023).
- Bois, D.D.; Bois, E.F.D. A height-weight formula to estimate the surface area of man. Exp. Biol. Med. 1916, 13, 77–78. [Google Scholar] [CrossRef]
- Loerting, T.; Bernard, J. Aqueous Carbonic Acid (H2CO3). ChemPhysChem 2010, 11, 2305–2309. [Google Scholar] [CrossRef]
- Eriksson, L. Acid/Base Calculations and Graphical Representations. 2016. Available online: https://www.lerik.se/texter/kort_jmv.pdf (accessed on 11 March 2025).
- NCBI. Sodium Bicarbonate Compound Summary—Dissociation Constants; NCBI: Bethesda, MD, USA, 2005. [Google Scholar]
- Smith, G.B.; Dezeny, G.C.; Hughes, D.L.; King, A.O.; Verhoeven, T.R. Mechanistic studies of the Suzuki cross-coupling reaction. J. Org. Chem. 1994, 59, 8151–8156. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 91st ed.; CRC Press Inc.: Boca Raton, FL, USA, 2010. [Google Scholar]
- Oslo University Hospital (OUS). Aseptisk Arbeidsteknikk [Aseptical Work Technique for Preparing and Administering Medications for Injection and Infusion]; Oslo University Hospital: Oslo, Norway, 2025. [Google Scholar]
- Oslo University Hospital (OUS). Arbeid I Sikkerhetsbenk Og Avtrekksskap Ved Tilberedning Av Legemidler [Work in Safety Cabinets and Fume Hoods for Preparation of Drugs]; Oslo University Hospital: Oslo, Norway, 2025. [Google Scholar]
- Nezvalova-Henriksen, K.; Nilsson, N.; Østerberg, C.T.; Staven Berge, V.; Tho, I. Y-Site Physical Compatibility of Numeta G13E with Drugs Frequently Used at Neonatal Intensive Care. Pharmaceutics 2020, 12, 677. [Google Scholar] [CrossRef]
- EDQM. Ph.Eur. 2.9.19—Particulate Contamination: Sub-Visible Particles; EDQM: Strasbourg, France, 2021. [Google Scholar]
- NCBI. Calcium Carbonate Compound Summary—Raman Spectra; NCBI: Bethesda, MD, USA, 2004. [Google Scholar]
- NCBI. Sodium Bicarbonate Compound Summary—Raman Spectra; NCBI: Bethesda, MD, USA, 2005. [Google Scholar]
- Lewis, I.R.; Edwards, H. (Eds.) Handbook of Raman Spectroscopy: From the Research Laboratory to the Process Line; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Skärby, T.; Jönsson, P.; Hjorth, L.; Behrentz, M.; Björk, O.; Forestier, E.; Jarfelt, M.; Lönnerholm, G.; Höglund, P. High-dose methotrexate: On the relationship of methotrexate elimination time vs. renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother. Pharmacol. 2003, 51, 311–320. [Google Scholar] [CrossRef]
- Skärby, T.V.; Anderson, H.; Heldrup, J.; Kanerva, J.A.; Seidel, H.; Schmiegelow, K. High leucovorin doses during high-dose methotrexate treatment may reduce the cure rate in childhood acute lymphoblastic leukemia. Leukemia 2006, 20, 1955–1962. [Google Scholar] [CrossRef]
- Fong, M.Y.; Nicol, M. Raman spectrum of calcium carbonate at high pressures. J. Chem. Phys. 1971, 54, 575–578. [Google Scholar] [CrossRef]
- Dufresne, W.J.B.; Rufledt, C.J.; Marshall, C.P. Raman spectroscopy of the eight natural carbonate minerals of calcite structure. J. Raman Spectrosc. 2018, 49, 1999–2007. [Google Scholar] [CrossRef]
- Commission, E.P. Revised Chapter 2.2.48 on Raman Spectroscopy, European Pharmacopoeia Supplement 10.7; European Commission: Strasbourg, France, 2021. [Google Scholar]
- Veggeland, T.; Brandl, M. Evaluation of a simple method for visual detection of microprecipitates in blends of parenteral drug solutions using a focused (tyndall) light beam. Int. J. Pharm. Compd. 2010, 14, 78–81. [Google Scholar] [PubMed]
- Dettlaff, K.; Dominiak, K.; Klimaszewska, M.; Gostynska, A. Physical Compatibility of Ibuprofen and Selected Parenteral Drugs During Simulated Y-Site Administration. Acta Pol. Pharm. 2023, 80, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F.; Baker, M.B.; Walter, W.V.; Mirtallo, J.M. Compatibility of parenteral nutrient solutions with selected drugs during simulated Y-site administration. Am. J. Health-Syst. Pharm. 1997, 54, 1295–1300. [Google Scholar] [CrossRef]
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F. Compatibility of doxorubicin hydrochloride liposome injection with selected other drugs during simulated Y-site administration. Am. J. Health-Syst. Pharm. 1997, 54, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F.; Kim, M.C. Compatibility of remifentanil hydrochloride with selected drugs during simulated Y-site administration. Am. J. Health-Syst. Pharm. 1997, 54, 2192–2196. [Google Scholar] [CrossRef]
- Trissel, L.A.; Martinez, J.F. Compatibility of thiotepa (lyophilized) with selected drugs during simulated Y-site administration. Am. J. Health-Syst. Pharm. 1996, 53, 1041–1045. [Google Scholar] [CrossRef]
- Allen, L.V., Jr.; Levinson, R.S.; Phisutsinthop, D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am. J. Hosp. Pharm. 1977, 34, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.V., Jr.; Stiles, M.L. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Part 2. Am. J. Hosp. Pharm. 1981, 38, 380–381. [Google Scholar] [CrossRef]
- Wolf, J.; Milstone, A.M. Vascular access in children to prevent and treat infectious diseases. Pediatrics 2020, 145, S290–S291. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Curtis, N.; Worth, L.J.; Flynn, P.M. Central Line–associated Bloodstream Infection in Children: An Update on Treatment. Pediatr. Infect. Dis. J. 2013, 32, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Flynn, P.M.; Willis, B.; Gaur, A.H.; Shenep, J.L. Catheter Design Influences Recurrence of Catheter-Related Bloodstream Infection in Children With Cancer. J. Clin. Oncol. 2003, 21, 3520–3525. [Google Scholar] [CrossRef]
- Shenep, M.A.; Tanner, M.R.; Sun, Y.; Culley, T.; Hayden, R.T.; Flynn, P.M.; Tang, L.; Wolf, J. Catheter-Related Complications in Children With Cancer Receiving Parenteral Nutrition: Change in Risk Is Moderated by Catheter Type. J. Parenter. Enter. Nutr. 2017, 41, 1063–1071. [Google Scholar] [CrossRef]
- Jack, T.; Boehne, M.; Brent, B.E.; Hoy, L.; Köditz, H.; Wessel, A.; Sasse, M. In-line filtration reduces severe complications and length of stay on pediatric intensive care unit: A prospective, randomized, controlled trial. Intensive Care Med. 2012, 38, 1008–1016. [Google Scholar] [CrossRef]
- Ernst, F.R.; Chen, E.; Lipkin, C.; Tayama, D.; Amin, A.N. Comparison of hospital length of stay, costs, and readmissions of alteplase versus catheter replacement among patients with occluded central venous catheters. J. Hosp. Med. 2014, 9, 490–496. [Google Scholar] [CrossRef]
- Sandberg, K.C.; Lucien, J.N.; Stoll, D.; Yanney, E.; Mezoff, A. Decreasing Door-to-Door Times for Infliximab Infusions in a Children’s Hospital Observation Unit. Pediatr. Qual. Saf. 2019, 4, e131. [Google Scholar] [CrossRef]
- Perez, M.; Décaudin, B.; Abou Chahla, W.; Nelken, B.; Storme, L.; Masse, M.; Barthélémy, C.; Lebuffe, G.; Odou, P. Effectiveness of in-Line Filters to Completely Remove Particulate Contamination During a Pediatric Multidrug Infusion Protocol. Sci. Rep. 2018, 8, 7714. [Google Scholar] [CrossRef]
- Jack, T.; Brent, B.E.; Boehne, M.; Müller, M.; Sewald, K.; Braun, A.; Wessel, A.; Sasse, M. Analysis of particulate contaminations of infusion solutions in a pediatric intensive care unit. Intensive Care Med. 2010, 36, 707–711. [Google Scholar] [CrossRef]
- Falchuk, K.H.; Peterson, L.; McNeil, B.J. Microparticulate-Induced Phlebitis. N. Engl. J. Med. 1985, 312, 78–82. [Google Scholar] [CrossRef]
- van Lingen, R.; Baerts, W.; Marquering, A.; Ruijs, G. The use of in-line intravenous filters in sick newborn infants. Acta Paediatr. 2004, 93, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Norsk barnelegeforenings legemiddelveileder (Norwegian Pediatric Association’s Medication Guide). Bruk av filtre hos barn [Use of Filters in Children]. In Norsk Barnelegeforenings Legemiddelveileder; Norsk Barnelegeforening: Oslo, Norway, 2005. [Google Scholar]
- Oslo University Hospital (OUS). Filtrering Av Injeksjons-og Infusjonsvæsker Til Barn [Filtering of Injection and Infusion Fluids for Children]; Oslo University Hospital: Oslo, Norway, 2023. [Google Scholar]
- Newall, F.; Ranson, K.; Robertson, J. Use of in-line filters in pediatric intravenous therapy. J. Intraven. Nurs. 1998, 21, 166–170. [Google Scholar] [PubMed]
- Foster, J.P.; Richards, R.; Showell, M.G.; Jones, L.J. Intravenous in-line filters for preventing morbidity and mortality in neonates. Cochrane Database Syst. Rev. 2015, 2015, Cd005248. [Google Scholar] [CrossRef]
- Terjung, A.; Kummer, S.; Friedrich, M. Simultaneous 24 h-infusion of high-dose 5-fluorouracil and sodium-folinate as alternative to capecitabine in advanced breast cancer. Anticancer. Res. 2014, 34, 7233–7238. [Google Scholar]
- Qiu, B.; Liu, G.; Wang, C.; Chen, X.; Liu, R.; Huang, Y.; Jia, Y.; Shen, J. Three-Period Bioequivalence Study of Sodium Levofolinate Injection With Calcium Levofolinate for Injection and Sodium Folinate for Injection in Healthy Chinese Subjects. Clin. Pharmacol. Drug Dev. 2023, 12, 416–423. [Google Scholar] [CrossRef] [PubMed]
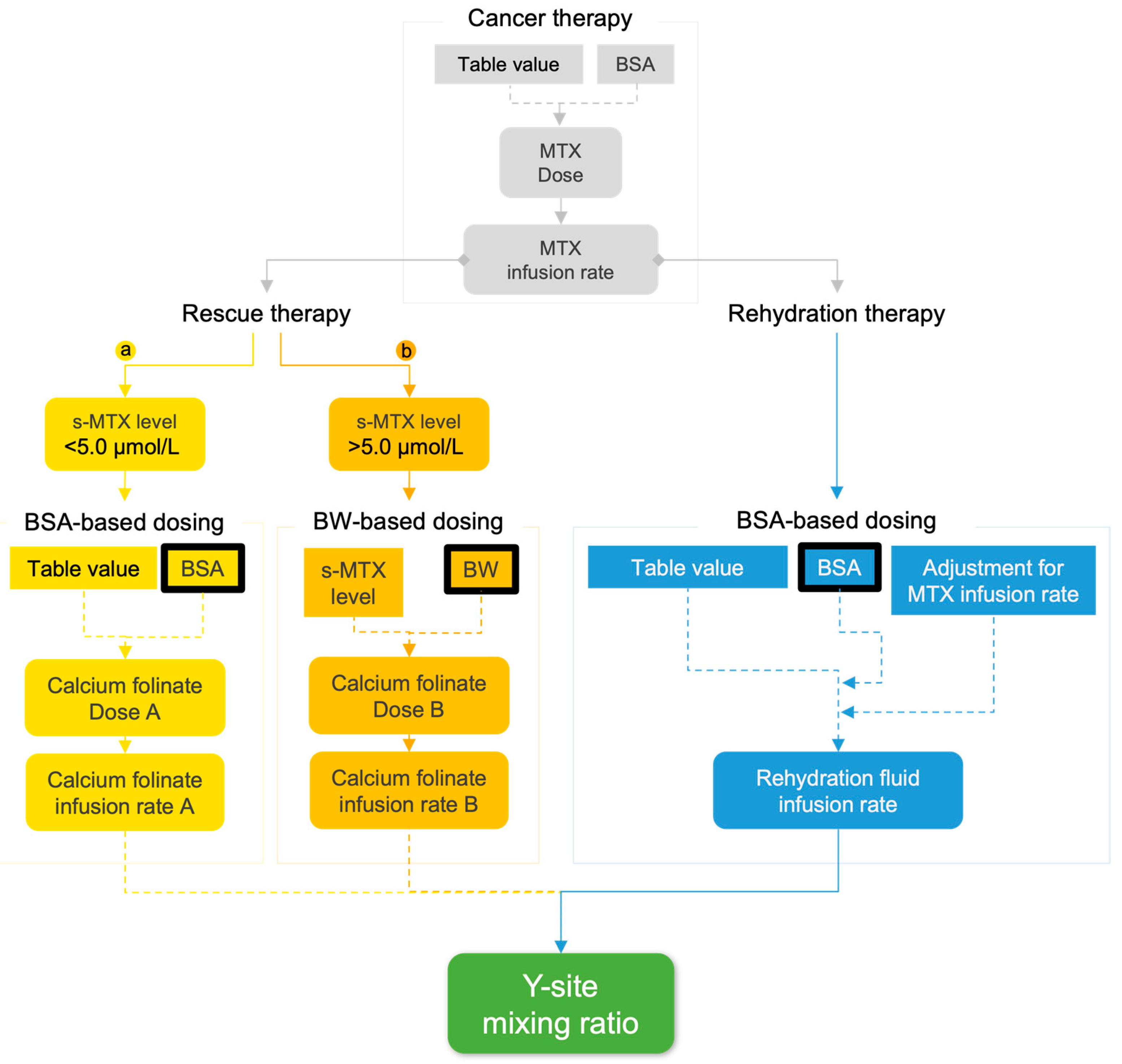

| s-MTX (µmol/L) | Calcium Folinate (mg/m2) |
|---|---|
| <1.0 | 15 |
| 1.0–1.9 | 30 |
| 2.0–2.9 | 45 |
| 3.0–3.9 | 60 |
| 4.0–4.9 | 75 |
| ≥5.0 | s-MTX × BW * |
| Physical and Theoretical Testing | Theoretical Testing | ||||
|---|---|---|---|---|---|
| Patient Model 1 | Patient Model 2 | Patient Model 3 | Undiluted Mix a | Extreme Patient | |
| Age (years) | 1 | 9 | 14 | 11 d | 39 |
| Bodyweight (kg) | 10 | 28 | 50 | 40 d | 118 |
| Body surface area (m2) | 0.49 | 1.0 | 1.5 | 1.3 d | 2.6 |
| s-MTX (µmol/L) | 0.9 | 3.5 | 7.0 | 5.0 d | 9.0 |
| Calcium folinate dose (mg) | 7.35 | 60 | 350 | 200 | 1062 |
| NaHCO3 dose (mmol) | 0.050 | 0.14 | 0.41 | 10.0 | 1.41 |
| Administration time (min) | 2.0 | 2.0 | 5.0 | 60 d | 60 |
| Calcium folinate infusion rate (mL/h) | 1200 | 1200 | 480 | 1200 d | 40.0 |
| Rehydration fluid b infusion rate (mL/h) | 38.3 | 116 | 165 | 151 d | 302 |
| Mixing ratio c | 31.3 | 10.3 | 2.92 | 1.00 | 0.13 |
| Patient Model 1 | Patient Model 2 | Patient Model 3 | Undiluted Mix | Extreme Patient | |
|---|---|---|---|---|---|
| [Calcium folinate](aq) (mg/mL) | 0.18 | 1.37 | 6.52 | 5.00 | 3.10 |
| [Ca2+](aq) (mol/mL) | 3.48 × 10−7 | 2.67 × 10−6 | 1.27 × 10−5 | 1.96 × 10−5 | 6.07 × 10−6 |
| [HCO3−](aq) (mol/mL) | 1.24 × 10−6 | 3.52 × 10−6 | 1.02 × 10−5 | 2.50 × 10−4 | 3.53 × 10−5 |
| Ca2+(aq) amount (mol) | 1.39 × 10−5 | 1.07 × 10−4 | 5.10 × 10−4 | 7.82 × 10−4 | 2.43 × 10−4 |
| HCO3−(aq) amount (mol) | 4.95 × 10−5 | 1.41 × 10−4 | 4.09 × 10−4 | 1.00 × 10−2 | 1.41 × 10−3 |
| HCO3−/Ca2+ molar ratio | 3.56 | 1.32 | 0.80 | 12.8 | 5.82 |
| Predicted pH | 7.35 | 7.81 | 8.27 | 9.66 | 8.81 |
| [CO32−](aq) (mol/mL) * | 1.39 × 10−9 | 1.13 × 10−8 | 9.48 × 10−8 | 5.68 × 10−5 | 1.13 × 10−6 |
| CO32−/Ca2+ molar ratio * | 4.00 × 10−3 | 4.22 × 10−3 | 7.44 × 10−3 | 2.91 | 1.87 × 10−1 |
| Maximum theoretical [CaCO3](aq or s) (mol/mL) ** | 1.39 × 10−9 | 1.13 × 10−8 | 9.48 × 10−8 | 1.96 × 10−5 | 1.13 × 10−6 |
| CaCO3(s) precipitation risk based on solubility *** | no | no | no | yes | yes |
| Ionic product [Ca2+] × [CO32−] (mol2/L2) * | 4.85 × 10−10 | 3.02 × 10−8 | 1.21 × 10−6 | 1.11 × 10−3 | 6.88 × 10−6 |
| CaCO3(s) precipitation risk based on ionic product **** | no | yes | yes | yes | yes |
| Total assessment of CaCO3(s) precipitation risk | no | inconclusive | inconclusive | yes | yes |
| y-Site Mix | A-Control | B-Control | Ionic Product * (mol2/L2) | CaCO3(s) Precipitation Risk ** | ||
|---|---|---|---|---|---|---|
| (n = 3) | (n = 1) | (n = 1) | ||||
| Patient model 1 | t0 | 7.29 ± 0.02 | 6.44 | 8.36 | 4.21 × 10−10 | No |
| t4 | 7.30 ± 0.05 | 6.45 | 8.38 | 4.31 × 10−10 | ||
| pH change | 0.01 | 0.01 | 0.02 | |||
| Patient model 2 | t0 | 7.63 ± 0.05 | 6.71 | 8.20 | 2.01 × 10−8 | Yes |
| t4 | 7.68 ± 0.06 | 6.69 | 8.27 | 2.26 × 10−8 | ||
| pH change | 0.05 | 0.02 | 0.07 | |||
| Patient model 3 | t0 | 7.73 ± 0.01 | 7.07 | 8.20 | 3.50 × 10−7 | Yes |
| t4 | 7.74 ± 0.01 | 7.05 | 8.21 | 3.58 × 10−7 | ||
| pH change | 0.01 | 0.02 | 0.01 | |||
| Undiluted mix | t0 | 7.51 ± 0.03 | 7.34 | 8.18 | 7.93 × 10−6 | Yes |
| t4 | 7.98 ± 0.11 | 7.31 | 8.15 | 2.34 × 10−5 | ||
| pH change | 0.62 | 0.04 | 0.02 |
| y-Site Mix (n = 3) | A-Control (n = 1) | B-Control (n = 1) | ||
|---|---|---|---|---|
| t0 | clear | clear | clear | |
| Patient model 1 | t4 | clear | clear | clear |
| t24 | clear | clear | clear | |
| t0 | clear | clear | clear | |
| Patient model 2 | t4 | clear | clear | clear |
| t24 | clear | clear (dust) | clear (dust) | |
| t0 | clear (yellow) | clear (yellow) | clear | |
| Patient model 3 | t4 | clear (yellow) | clear (yellow) | clear (bubbles) |
| t24 | clear (yellow) | clear (yellow) | clear (bubbles) | |
| t0 | visible particles (yellow) | clear (yellow and bubbles) | clear (bubbles) | |
| Undiluted mix | t4 | visible particles (yellow) | clear (yellow) | clear |
| t24 | visible particles (yellow) | clear (yellow) | clear (dust) |
| y-Site Mix (n = 3) | A-Control (n = 1) | B-Control (n = 1) | MQ Water (n = 1) | ||
|---|---|---|---|---|---|
| Patient model 1 | t0 | 0.13 ± 0.02 | 0.12 | 0.14 | 0.11 |
| t4 | 0.14 ± 0.02 | 0.12 | 0.17 | 0.14 | |
| Patient model 2 | t0 | 0.18 ± 0.10 | 0.29 | 0.16 | 0.19 |
| t4 | 0.16 ± 0.02 | 0.21 | 0.19 | 0.18 | |
| Patient model 3 | t0 | 0.29 ± 0.04 | 0.48 | 0.28 | 0.27 |
| t4 | 0.18 ± 0.06 | 0.18 | 0.17 | 0.16 | |
| Undiluted mix | t0 | 62.4 ± 24.5 | 0.24 | 0.73 | 0.30 |
| t4 | 81.4 ± 96.0 | 0.32 | 0.87 | 0.14 |
| y-Site Mix (n = 9) | A-Control (n = 3) | B-Control (n = 3) | |||||
|---|---|---|---|---|---|---|---|
| t0 | t4 | t0 | t4 | t0 | t4 | ||
| Patient model 1 | ≥5 | 0.22 ± 0.40 | 0.40 ± 0.41 | 0.13 ± 0.12 | 1.20 ± 0.87 | 0.33 ± 0.46 | 0.27 ± 0.46 |
| ≥10 | 0.20 ± 0.40 | 0.27 ± 0.26 | 0.13 ± 0.12 | 0.80 ± 0.69 | 0.20 ± 0.20 | 0.27 ± 0.46 | |
| ≥25 | 0.09 ± 0.18 | 0.09 ± 0.15 | 0.07 ± 0.12 | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Patient model 2 | ≥5 | 1.69 ± 1.29 | 5.71 ± 3.99 | 1.20 ± 0.53 | 6.33 ± 1.10 | 1.20 ± 1.06 | 0.67 ± 0.50 |
| ≥10 | 0.22 ± 0.31 | 2.51 ± 1.98 | 0.27 ± 0.31 | 2.67 ± 1.01 | 0.13 ± 0.12 | 0.27 ± 0.12 | |
| ≥25 | 0.02 ± 0.07 | 0.38 ± 0.34 | 0.07 ± 0.12 | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Patient model 3 | ≥5 | 1.58 ± 1.61 | 1.56 ± 1.06 | 0.53 ± 0.31 | 0.80 ± 0.20 | 0.40 ± 0.35 | 1.00 ± 0.87 |
| ≥10 | 0.53 ± 0.46 | 0.69 ± 0.54 | 0.33 ± 0.31 | 0.20 ± 0.35 | 0.13 ± 0.35 | 0.47 ± 0.64 | |
| ≥25 | 0.11 ± 0.15 | 0.13 ± 0.17 | 0.13 ± 0.23 | 0.13 ± 0.23 | 0.00 ± 0.00 | 0.07 ± 0.12 | |
| Undiluted mix | ≥5 | 4070 ± 2320 | OL | 2.27 ± 1.17 | OL | 6.20 ± 1.51 | OL |
| ≥10 | 345 ± 160 | OL | 1.13 ± 1.01 | OL | 0.60 ± 0.20 | OL | |
| ≥25 | 24.9 ± 15.1 | OL | 0.07 ± 0.12 | OL | 0.07 ± 0.12 | OL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teimori, K.; Larsen, B.S.; Austli, M.B.; Nilsson, N.; Tho, I.; Nezvalova-Henriksen, K. Strategies for Assessing Physical Compatibility of Calcium Folinate with Bicarbonate During Methotrexate Rescue Therapy in Pediatric Patients with Acute Lymphoblastic Leukemia. Pharmaceutics 2025, 17, 1155. https://doi.org/10.3390/pharmaceutics17091155
Teimori K, Larsen BS, Austli MB, Nilsson N, Tho I, Nezvalova-Henriksen K. Strategies for Assessing Physical Compatibility of Calcium Folinate with Bicarbonate During Methotrexate Rescue Therapy in Pediatric Patients with Acute Lymphoblastic Leukemia. Pharmaceutics. 2025; 17(9):1155. https://doi.org/10.3390/pharmaceutics17091155
Chicago/Turabian StyleTeimori, Kaveh, Bjarke Strøm Larsen, Mathias Buaas Austli, Niklas Nilsson, Ingunn Tho, and Katerina Nezvalova-Henriksen. 2025. "Strategies for Assessing Physical Compatibility of Calcium Folinate with Bicarbonate During Methotrexate Rescue Therapy in Pediatric Patients with Acute Lymphoblastic Leukemia" Pharmaceutics 17, no. 9: 1155. https://doi.org/10.3390/pharmaceutics17091155
APA StyleTeimori, K., Larsen, B. S., Austli, M. B., Nilsson, N., Tho, I., & Nezvalova-Henriksen, K. (2025). Strategies for Assessing Physical Compatibility of Calcium Folinate with Bicarbonate During Methotrexate Rescue Therapy in Pediatric Patients with Acute Lymphoblastic Leukemia. Pharmaceutics, 17(9), 1155. https://doi.org/10.3390/pharmaceutics17091155








