1. Introduction
In recent years, numerous strategies have been developed for the synthesis of nanogels (NGs), reflecting their broad applicability in drug delivery, diagnostics, and biotechnology [
1]. These three-dimensional nanoscale hydrophilic polymeric networks offer unique advantages, such as high water absorption capacity, biocompatibility, and functional versatility. Both natural polymers, including polysaccharides and proteins, and synthetic polymers have been employed to produce NGs with tailored characteristics, such as particle size, colloidal stability, and the ability to encapsulate a wide range of therapeutic molecules (e.g., small drugs, proteins, and nucleic acids) and release them in a controlled manner in response to specific physiological stimuli, including changes in pH, temperature, ionic strength, or enzymatic activity. The most commonly used polymers are chitosan, hyaluronic acid, and dextran or derivatives, each possessing distinct physicochemical and biological properties that favor their use in various clinical contexts [
2,
3,
4]. The development of nanostructured drug delivery systems requires precise control over key parameters, such as size, internal structure, and chemical composition. This control is essential for optimizing therapeutic efficacy, biodistribution, and safety. Although conventional batch synthesis is widely used, it often suffers from poor control over particle size distribution, low encapsulation efficiency, and batch-to-batch variability. These limitations are especially critical when designing nanocarriers for biomedical applications, where uniformity and reproducibility are essential [
5]. Therefore, the development of alternative strategies that allow precise control over the synthesis parameters has become a major area of interest.
Flow chemistry, also known as continuous processing, involves the continuous pumping of reagents through microreactors, enabling reactions to occur under steady-state conditions [
6]. Compared with batch synthesis, this approach offers superior control over the reaction parameters, improved safety, enhanced mass and heat transfer, and increased scalability. These characteristics make flow chemistry particularly well-suited for the synthesis of soft nanomaterials like NGs [
7], which benefit from mild and controllable reaction environments. Importantly, flow reactors provide the opportunity to exploit synthesis routes that are difficult to reproduce in batch, such as those requiring rapid temperature cycling or hazardous intermediates, in a safe and reproducible manner. Small reactor volumes, excellent heat exchange surfaces, and efficient mixing allow for better selectivity, improved thermal control (e.g., for isothermal operation), and real-time process control [
8]. In addition, the modular nature of flow chemistry systems allows for the easy integration of multiple synthesis steps in a continuous sequence, further increasing the efficiency and scalability of the process [
9].
Microfluidic technology represents a specialized subfield of flow chemistry, enabling the manipulation of fluids in channels with dimensions typically ranging from tens to hundreds of micrometers [
10]. This miniaturization allows for reduced reagent consumption, lower waste production, and better compatibility with automation and high-throughput screening. Microfluidics has demonstrated great potential in the synthesis of organic and inorganic nanoparticles [
11,
12], including metal-organic frameworks [
13,
14], and, in particular, of organic NGs [
15], owing to its ability to generate highly uniform nanostructures with narrow size distributions, high encapsulation efficiencies, and more controlled release profiles [
16]. This is due to the confined geometries, possibility of laminar flow conditions, and continuous and highly reproducible nature of microfluidic manufacturing, which enables precise control over mixing regimes, leading to improved reproducibility and scale-down feasibility for early-stage formulation screening [
11]. Several microfluidic configurations have been developed for nanoparticle and nanogel production, including hydrodynamic flow focusing (HFF) [
17] and staggered herringbone micromixers (SHM) [
18]. HFF leverages the laminar nature of microfluidic flows to create a narrow central stream of a nanoparticle precursor, flanked by streams of an antisolvent (a fluid in which the precursor is poorly soluble), thus promoting controlled precipitation and self-assembly. In this setup, mixing occurs via diffusion at the interface between the streams, allowing for control over nucleation and particle growth. In contrast, SHM introduces microstructures within the channels, which induce chaotic advection and enhance mixing efficiency. This passive micromixing technique reduces synthesis time and minimizes dilution effects. Furthermore, active micromixing techniques, such as those based on ultrasonic waves [
19], have been employed to accelerate mixing, enhance nucleation rates, and reduce particle size and polydispersity. The ability to combine passive and active mixing strategies offers a high level of versatility for tuning the characteristics of NGs.
These approaches provide not only greater reproducibility but also adaptability for encapsulating sensitive biological cargoes under mild conditions; for these reasons, microfluidics has emerged as a powerful tool in drug delivery. For example, several studies have shown that microfluidic synthesis can yield NGs with narrower size distributions and more consistent internal architectures, enabling tunability in drug release profiles, compared to conventional batch methods [
11,
12,
20,
21]. As already stated, microfluidic systems also facilitate real-time monitoring and data acquisition, which are critical for establishing robust quality control protocols in pharmaceutical production [
22]. Despite these advantages, challenges such as channel clogging, limited solvent compatibility, and material absorption (e.g., with PDMS devices) must be addressed to ensure long-term reliability and scalability [
23,
24]. To further enhance the structural precision, reproducibility, and functional versatility of NGs synthesized via microfluidics, the incorporation of advanced, highly selective crosslinking chemistries beyond conventional approaches is highly advantageous. Indeed, the combination of microfluidic technology with click chemistry strategies has been explored for microgel production [
25,
26]. These studies demonstrated the reproducible synthesis of microgels with tunable mechanical and structural properties under mild conditions, which is particularly advantageous for encapsulating sensitive biological payloads, such as proteins or nucleic acids. Nonetheless, this approach remains underexplored for NGs production.
Click chemistry encompasses a class of highly selective modular reactions that proceed under mild conditions and yield stable products [
27]. Among the various crosslinking strategies, strain-promoted azide–alkyne cycloaddition (SPAAC) is notable for its efficiency, bioorthogonality, and catalyst-free reaction conditions [
28]. Thus, the integration of SPAAC within microfluidic platforms represents a strategic approach for the reproducible and efficient production of functional nanomaterials. This synergy enables precise spatial and temporal control over crosslinking, contributing to improved homogeneity, enhanced stability, and tunable degradation rates of the resulting NGs.
In this work, we report a microfluidic approach for the synthesis of NGs based on poly(α-glutamic acid) (PGA) crosslinked via SPAAC chemistry. PGA is a water-soluble, biodegradable, and biocompatible polymer known for its ability to encapsulate hydrophilic drugs [
29]. Its tunable chemical structure and favorable biological profile make it an ideal candidate for developing targeted drug delivery systems [
30]. Here, we investigate mostly the effect of flow rate ratios within the microfluidic device on the physicochemical properties of the resulting NGs. We demonstrate that by modulating only these fluidic conditions, it is possible to reproducibly tune the size and morphology of NGs, offering a promising route for formulation optimization and process scalability. Furthermore, our platform lays the groundwork for the future integration of additional on-chip functionalities, such as purification, real-time characterization, or multi-step synthesis workflows, aimed at producing next-generation therapeutic NGs.
2. Materials and Methods
2.1. Materials
All reagents used in this study were of analytical or reagent grade and were used as received unless specified otherwise. Poly(L-glutamic acid) sodium salt (PGA, MW 15,000–50,000) was obtained from Sigma-Aldrich(St. Louis, MO, USA). Azide-functionalized PGA (PGA-N3, MW 34,000, 10% azide-modified side chains) was purchased from Alamanda Polymers, Inc. (Huntsville, AL, USA). Dibenzocyclooctyne-amine (DBCO-NH2) and doxorubicin hydrochloride (Dox·HCl) were acquired from MedChemExpress (South Brunswick, NJ, USA). The carbodiimide activator N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) was supplied by Sigma-Aldrich. All solvents used in this study were of analytical quality. Ultrapure water (Milli-Q system; Merck Millipore, Darmstadt, Germany) was used to prepare all aqueous solutions. NGs purification procedures involved the use of MicroSpin G-50 Columns (GE Healthcare, Chicago, IL, USA) and 0.45 μm nylon centrifugal filters (VWR®, Radnor, PA, USA), operated according to the respective manufacturers’ protocols.
2.2. Synthesis of DBCO-Functionalized PGA
As previously described in detail and characterized in our previous work [
31], the functionalization of PGA with dibenzocyclooctyne groups (DBCO) was carried out using carbodiimide chemistry. Briefly, 100 µL of poly(L-glutamic acid) (MW 15–50 kDa) was dissolved at a concentration of 10 mg/mL in MES-buffered saline (MBS, pH 6.5). To activate the carboxyl groups, EDC was added in a 1.5-fold molar excess with respect to the glutamate residues, and the reaction mixture was incubated at room temperature for 20 min with gentle stirring. DBCO-NH
2 was prepared as a 70 mg/mL solution in DMSO and added to the activated polymer solution. Different feed ratios of DBCO-NH
2 were tested to modulate the extent of functionalization: a 14 mol% feed ratio relative to glutamate monomers resulted in ~10% substitution, whereas a 30 mol% input yielded ~20% functionalization. The coupling reaction was allowed to proceed for 4 h at room temperature with continuous agitation. To eliminate unreacted DBCO-NH
2, the reaction mixture was purified using 10 kDa molecular weight cut-off Amicon
® Ultra centrifugal filters. The sample was centrifuged at 8000×
g for 10 min at room temperature and washed 3 to 5 times with ultrapure water. The successful attachment of DBCO moieties to the PGA backbone was confirmed by high-performance liquid chromatography (HPLC) using a Dionex Ultra 3000 system equipped with a PolySep-GFC-P 4000 analytical column and a corresponding guard column. The mobile phase consisted of phosphate-buffered saline (1×, pH 7.4). The incorporation of DBCO was quantified by measuring the absorbance at 310 nm using UV-Vis spectroscopy.
2.3. Configuration of the Microfluidic System Setup
NGs synthesis was carried out using a modular automated flow chemistry platform (Asia, Syrris Ltd., Royston, UK) designed to ensure controlled fluid mixing, temperature regulation, and automated sample collection. The system is composed of a syringe pump module with two independent channels (Pump A and Pump B), a reagent injector equipped with a six-port valve and a 1 mL loop, a glass microfluidic chip (250 μL internal volume, three inlets) inserted into a temperature-controlled Chip Climate Controller, a backpressure regulator, and an automated collector (
Figure 1). Pump A was loaded with ultrapure water and connected to the reagent injector, which was connected to a 1 mL loop. This was preloaded with a solution containing the polymers of interest (see next section), which was then transferred to inlet 3 of the microfluidic chip. Pump B, containing acetone, was connected directly to inlet 1 of the chip. Inlet 2 was sealed with a cap because it was not used during the experiments. The outlet of the chip was connected in series to the backpressure controller and then to the automated collector. The chip was operated at controlled temperatures of 25 °C or 50 °C, depending on the experimental conditions (
Table 1). All tubing and wettable components are made of polytetrafluoroethylene (PTFE), selected for its high chemical resistance and compatibility with both aqueous and organic phases. The fluidic path was assembled using PTFE tubes with defined lengths and internal diameters (ID) to regulate residence time and flow profiles. Specifically, the segment from Pump A to the injector is 611 mm long with an ID of 0.5 mm; from Pump B to the chip, 614 mm with an ID of 0.3 mm; from the injector to the chip, 509 mm with an ID of 0.5 mm; from the chip outlet to the backpressure controller, 611 mm with an ID of 0.5 mm; and from the backpressure controller to the collector, 710 mm with an ID of 0.3 mm.
Through this configuration, precise and reproducible mixing under laminar flow conditions is achieved, allowing for accurate and scalable nanoparticle formulation.
2.4. Microfluidics-Assisted Inverse-Nanoprecipitation
NGs were formulated using the microfluidic system described in the previous section. The two polymers, azide-functionalized polyglutamic acid (PGA-N
3) and DBCO-modified PGA (PGA-DBCO), were premixed and diluted in ultrapure water to a final concentration of 0.5%
w/
v. This polymer mixture was loaded into a syringe and injected through one of the ports of a six-port valve to fill a 1 mL loop of the reagent injector. Meanwhile, Pump A and Pump B of the syringe pump module of the system were loaded with Milli-Q water and acetone, respectively. Flow rates were varied systematically to screen different conditions, as reported in
Table 1. The resulting NG dispersions were collected into Eppendorf tubes using an automated fraction collector connected to the chip outlet. Samples were formulated at two different temperatures (25 °C and 50 °C), as regulated by the chip climate controller. Although the synthesis procedure remained identical, the post-synthesis treatment varied depending on the temperature condition. The samples synthesized at 25 °C were sealed and agitated in a thermomixer at 25 °C and 500 rpm for at least 5 h to ensure complete crosslinking. Subsequently, the tubes were placed under a chemical fume hood and allowed to evaporate overnight with continuous agitation under the same conditions. In contrast, samples synthesized at 50 °C were immediately agitated in a thermomixer at 50 °C and 500 rpm under a fume hood for 2 h to allow solvent evaporation. After synthesis and post-treatment, NGs were filtered through 0.45 μm nylon membrane filters and resuspended in PBS 1×. Purified samples were stored at 4 °C until further characterization. The same synthesis protocol was used to formulate doxorubicin (Dox)-loaded nanogels (NGs Dox). In this case, Dox was added to the polymer mixture during the premixing step before loading into the loop at a drug/polymer mass ratio of 1:50. The flow conditions and post-synthesis processing were identical to those described previously. However, the purification procedure for the drug-loaded NGs included an additional size-exclusion step using MicroSpin G-50 columns to remove unencapsulated Dox. The purified samples were then stored at 4 °C until further characterization.
2.5. Experimental Design
The experimental design involved systematic variation of the flow conditions within the microfluidic system to optimize NGs formation and investigate their effects on the physicochemical properties of the particles. Specifically, the solvent streams of acetone and Milli-Q water had varying flow rates, resulting in different flow rate ratios (FRR) and total flow rates (flux) inside the chip mixing and reaction channels, as listed in
Table 1. The flow rate ratio, defined as the volumetric flow rate of acetone to that of water, varied from 1 to 7 to explore a wide range of solvent environments and their effects on polymer self-assembly and crosslinking kinetics. For each FRR, total flow rates ranging from 100 to 300 mL/min were tested to assess the impact of shear forces and residence time on the size and uniformity of the NGs. To ensure stable and reproducible flow conditions, each experiment was initiated with the injection of a priming volume (50 µL), introduced prior to the polymer solution to stabilize flow within the microfluidic channels and allow the system to reach steady-state conditions. At the end of each run, a flushing segment of equal volume (50 µL) was injected to clear the channels and minimize the transient effects of residual reagents. These volumes were composed of the same solvents used in the main experiment and were injected through the same inlet channels as the reaction fluid. The resulting NG suspensions were collected automatically using an integrated fraction collector connected to the chip outlet. This setup allowed for continuous, hands-free sampling, reducing the risk of contamination and ensuring consistent sample volumes across experiments. The collected samples were then subjected to downstream processing, including agitation for complete crosslinking, acetone evaporation, and purification. This comprehensive screening approach allowed the identification of optimal flow parameters that balance efficient mixing, reaction kinetics, and particle stability. The data generated guided subsequent optimization steps and provided insights into the relationship between microfluidic flow conditions and NGs characteristics, such as size distribution and encapsulation efficiency.
2.6. Physicochemical Characterization
2.6.1. Particle Size and Polydispersity
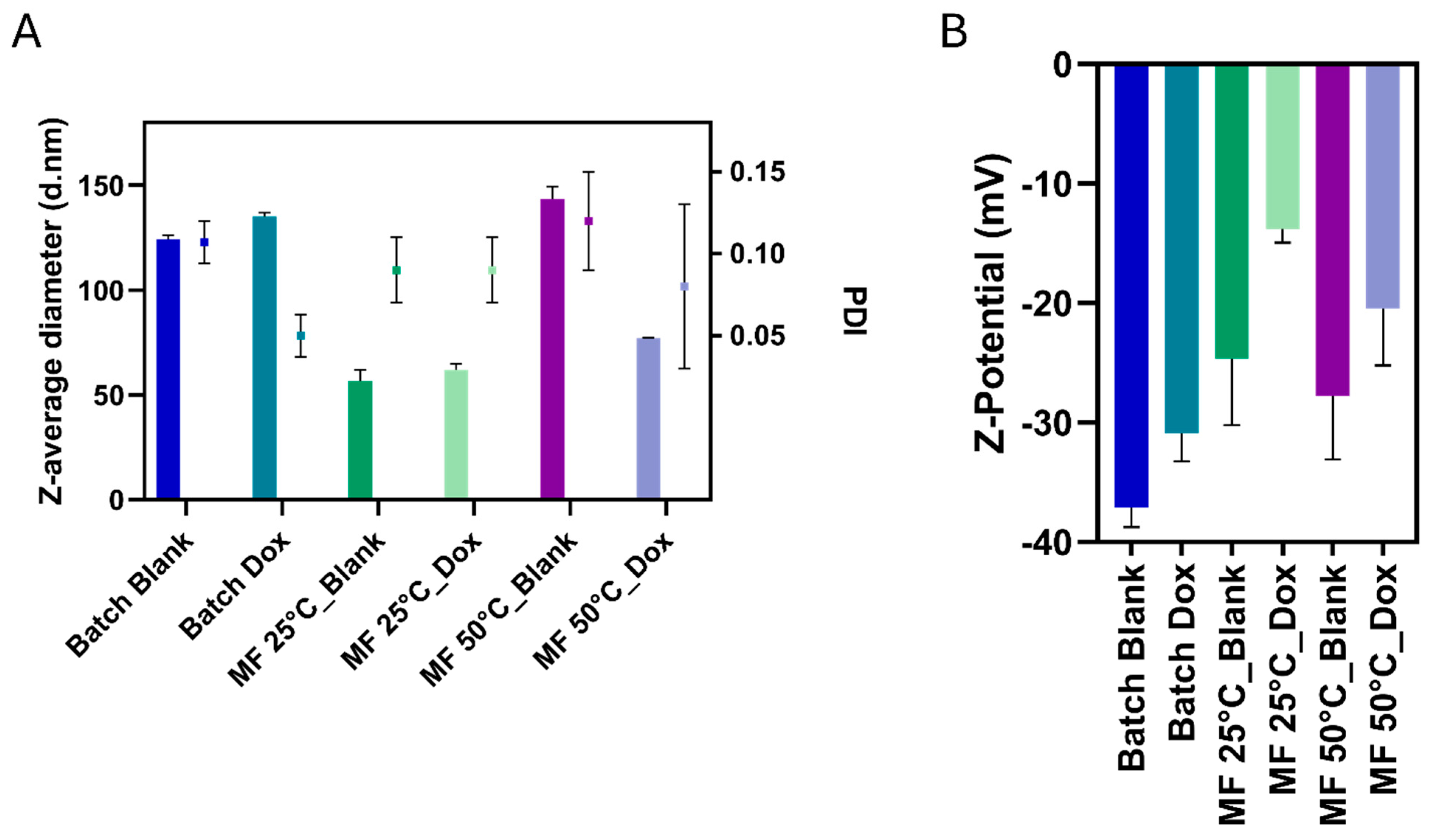
The hydrodynamic diameter and polydispersity index (PDI) of the NGs were assessed via Dynamic Light Scattering (DLS) using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) equipped with a 633 nm laser and operating at a backscattering angle of 173°. NG samples were diluted in 1× PBS to a final concentration of 5 mg/mL, briefly sonicated (60 s), and filtered using 0.45 µm nylon membrane filters (VWR®) prior to analysis. Three independent batches of each formulation were prepared and measured. Each batch was analyzed in triplicate with five consecutive acquisitions per run. Reported values for mean diameter (Z-Average) and PDI were calculated using the cumulant method and are expressed as mean ± standard deviation (SD). Instrument settings for laser attenuation and beam alignment were automatically optimized before each measurement.
2.6.2. Zeta Potential
The zeta potential (ζ) of NGs was measured using the same Zetasizer Nano ZS instrument (Malvern Instruments, UK) operating at 25 °C with a 633 nm laser and disposable folded capillary cells. Samples were diluted in 0.1× PBS (pH 7.4) and filtered through 0.45 μm nylon membrane filters (VWR®) before analysis. Three independent replicates were analyzed for each formulation, with five measurements taken per replicate. Electrophoretic mobility was determined and automatically converted to zeta potential values using the instrument software (Malvern Zetasizer, Version 7.13, Malvern Instruments Ltd. Malvern, UK). Results are expressed as mean ± standard deviation.
2.6.3. Drug Encapsulation
To quantify the Dox HCl encapsulated within the NGs, the drug was added directly to the polymer mixture prior to injection, as previously described. A fixed drug-to-polymer mass ratio of 1:50 was maintained. After formulation via the microfluidic protocol, the resulting dispersion underwent solvent evaporation, followed by purification using MicroSpin G-50 columns to remove unencapsulated drugs and low-molecular-weight species. Purified NGs were resuspended in 1× PBS to a final volume of 100 μL. Dox content was determined via ultraviolet-visible (UV-Vis) spectrophotometry at 480 nm using a Cary 3500 instrument (Agilent Technologies) with 1 cm path-length quartz cuvettes. Quantification was performed using a calibration curve constructed with Dox standards prepared in ultrapure water, which showed linearity in the range of 0.78 to 50 μg/mL (Figure S7 in the ESI of [
31]). To determine the nanogel mass, samples were lyophilized after resuspension in PBS 1× containing trehalose (10 mg/mL). The resulting dry product was weighed using an analytical balance (Sartorius Cubis
®). To calculate the drug loading (DL%), the measured lyophilized mass was corrected by subtracting the known amounts of trehalose and residual PBS salts introduced with the suspension. The encapsulation efficiency (EE%) and drug loading capacity (DL%) were calculated using the following equations:
2.7. Data Analysis
To analyze the dependence of size, PDI, and EE% on FRR and flux, we employed a non-parametric interpolation approach based on Thin Plate Spline (TPS) fitting implemented in R (v4.5.0) using the “fields” package (v16.3.1); this method smooths and interpolates multidimensional data. After data cleaning, aggregation, and averaging, the model was evaluated over a regular grid to generate three-dimensional response surfaces, enabling a continuous representation of trends across the experimental space. Graphics were produced using R and GraphPad (version 8.0, GraphPad Software, San Diego, CA, USA).
4. Discussion
The precise regulation of NGs size is widely recognized in the literature as a fundamental requirement for developing advanced drug delivery systems, as size significantly influences transport across biological barriers, cellular internalization, and drug pharmacokinetics [
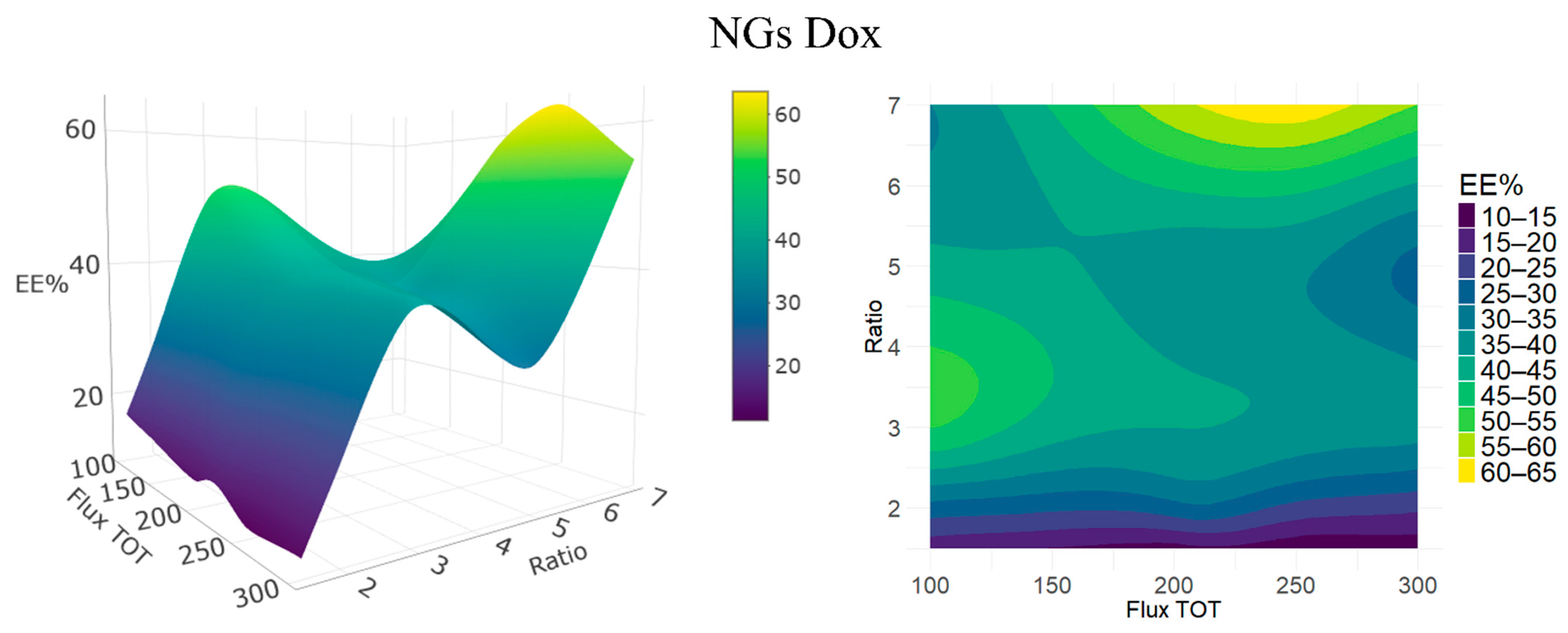
33]. In our study, the synthesis via microfluidics combined with SPAAC click chemistry allowed for accurate control over the physicochemical properties of NGs by varying the acetone/water ratio and total flow rate. Very high acetone:water ratios like 7:1 tend to generate larger and less homogeneous particles, probably due to inefficient desolvation and less-compact polymer crosslinking, while intermediate ratios (between 5:1 and 2:1), especially 3:1, favored the formation of small and uniform NGs, owing to rapid nucleation and efficient mixing. Increasing the total flow rate was correlated with a decrease in size, attributable to shorter residence times and improved hydrodynamic configuration, which are key elements for synthesis control [
32].
Particularly relevant is the role of doxorubicin encapsulation, which does not compromise colloidal stability but seems to lead to further compaction of the particles. This effect is explained by the electrostatic interaction between the amino groups of doxorubicin and the carboxylic residues of the polyglutamate backbone within the NGs, with the drug molecules acting as nucleation centers for a denser and more homogeneous polymer network. This phenomenon opens the possibility of exploiting specific drug-polymer interactions or adding cationic artificial additives in the synthesis to achieve more controlled nucleation and to modulate carrier properties, thereby improving stability and controlled release from a therapeutic perspective. Microfluidic platforms have shown clear advantages over traditional batch methods in NGs synthesis, improving reproducibility, size uniformity, and encapsulation efficiency. For example, Majedi et al. produced chitosan-based NGs with narrow size distributions (100–200 nm) and high protein encapsulation efficiency [
34], while Whiteley et al. optimized protein-loaded NGs with sizes around 84 nm and encapsulation efficiencies of up to 94.6% using microfluidics and “design-of-experiment” (DoE) approaches [
35]. Compared to these studies, our PGA NGs show highly uniform sizes (75–105 nm, PDI 0.08–0.10) and reproducible drug loading (EE ~40%, DL ~0.90%), while offering a fully covalently crosslinked structure. The integration of microfluidics and SPAAC click chemistry enables mild reaction conditions compatible with sensitive payloads and rapid, continuous exploration of formulation parameters to fine-tune physicochemical properties. This approach overcomes the limitations of traditional batch synthesis, including high batch variability and difficulty in real-time process optimization, demonstrating the high reproducibility and tunability achievable with our platform [
22,
28]. Regarding the cited technical challenges in microfluidics, in our case: the use of a carefully controlled polymer mixture at a relatively low concentration (0.5%
w/
w, as reported in the Materials and Methods section) prevented clogging in all runs; all tubing and connections were made of PTFE, fully compatible with both water and acetone; we did not observe any effect attributable to non-specific adsorption on the device surfaces. Regarding the last point, it should be noted that the observed ~20% loss of polymers (most probably attributable to the purification steps rather than the microfluidic process itself) was lower than that in the batch synthesis (~35%) [
31]. Finally, the modularity offered by microfluidics suggests the future integration of on-chip steps, such as automated purification and multi-step synthesis, which are crucial for efficient industrial-scale production.
Poly(α-L-glutamic acid) has previously been explored for the formulation of covalently crosslinked NGs capable of encapsulating doxorubicin, demonstrating promising stability and anticancer efficacy under physiological conditions [
36]. However, such systems were typically produced via batch methods and lacked control over size tunability and scalability. In contrast, our approach integrates SPAAC click chemistry into a microfluidic framework, enabling the continuous production of covalently crosslinked PGA NGs with precise size control and reproducible drug loading, with EE% and DL% values of approximately 40% and 0.90%, respectively, consistent with the high polymer-to-drug ratio used. This allowed us to focus on particle size, uniformity, and reproducibility while maintaining stable drug incorporation.
Future perspectives thus focus on implementing automated optimization strategies and high-throughput screening for increasingly performant NG formulations, including the use of possible additives affecting nucleation and network compactness (e.g., cationic species). Expanding the platform to new crosslinking chemistries and bioactive payloads and experimenting with coprecipitation with high-molecular-weight compounds like proteins, oligomers, or ultrasmall nanoparticles represents a promising path to increase the versatility and applications of nanocarriers in nanomedicine. Moreover, targeted in vivo studies to assess the biodistribution, biocompatibility, and therapeutic efficacy of these nanoplatforms should be conducted. Overall, our work strengthens the established literature framework regarding the central role of process control in NGs design for drug delivery, emphasizing the value of an integrated microfluidics-click chemistry approach for the development of safer, more efficient, and scalable systems.
5. Conclusions
The development of advanced NGs platforms for biomedical applications requires not only innovative materials but also precise and scalable manufacturing strategies to address challenges in size distribution, encapsulation efficiency, and reproducibility, which are typical of traditional batch methods. In this study, we addressed these needs by establishing a microfluidic approach for the synthesis of poly(α-glutamic acid) (PGA) NGs using strain-promoted azide–alkyne cycloaddition (SPAAC) click chemistry as a highly efficient and biocompatible crosslinking method. By automatically modulating the microfluidic flow rates, we rapidly explored formulation parameters and fine-tuned the physicochemical properties of the NGs. Our results demonstrate that this approach enables the reproducible formation of PGA nanogels with highly uniform and tunable sizes ranging from approximately 75 to 105 nm, narrow size distributions (PDI 0.08–0.10), and controlled drug loading, with encapsulation efficiencies around 40%. The resulting nanogels were stable and robust, and capable of encapsulating sensitive therapeutic agents under mild reaction conditions.
These findings confirm that integrating microfluidic technology with SPAAC click chemistry overcomes common limitations of conventional batch synthesis, such as broad size distributions, variable encapsulation efficiencies, and limited scalability. The wide parameter space explored owing to the fast, automatic modulation of flow parameters within the microfluidic device allowed fine control over the physicochemical properties of NGs, most notably size, dispersion, and drug loading capacity, demonstrating the versatility and robustness of the platform. This tunability, demonstrated through our experimental results and supported by the literature on other hydrophilic polymer NGs [
20,
37,
38], highlights the versatility and robustness of the system and its adaptability to diverse application requirements. While microfluidic synthesis and advanced crosslinking methods have been widely applied to NGs based on polymers like chitosan and hyaluronic acid, PGA-based NGs remain relatively underexplored.
Overall, our work lays a solid foundation for the next generation of NG technologies at the intersection of polymer chemistry, microfluidics, and biomedical engineering, offering a versatile and scalable platform for producing reproducible, customizable, and clinically translatable nanogels.