Antibacterial Resin Composites with Sustained Chlorhexidine Release: One-Year In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Resin Composites Synthesis
2.2. Physical–Chemical Evaluation of the Experimental Composites
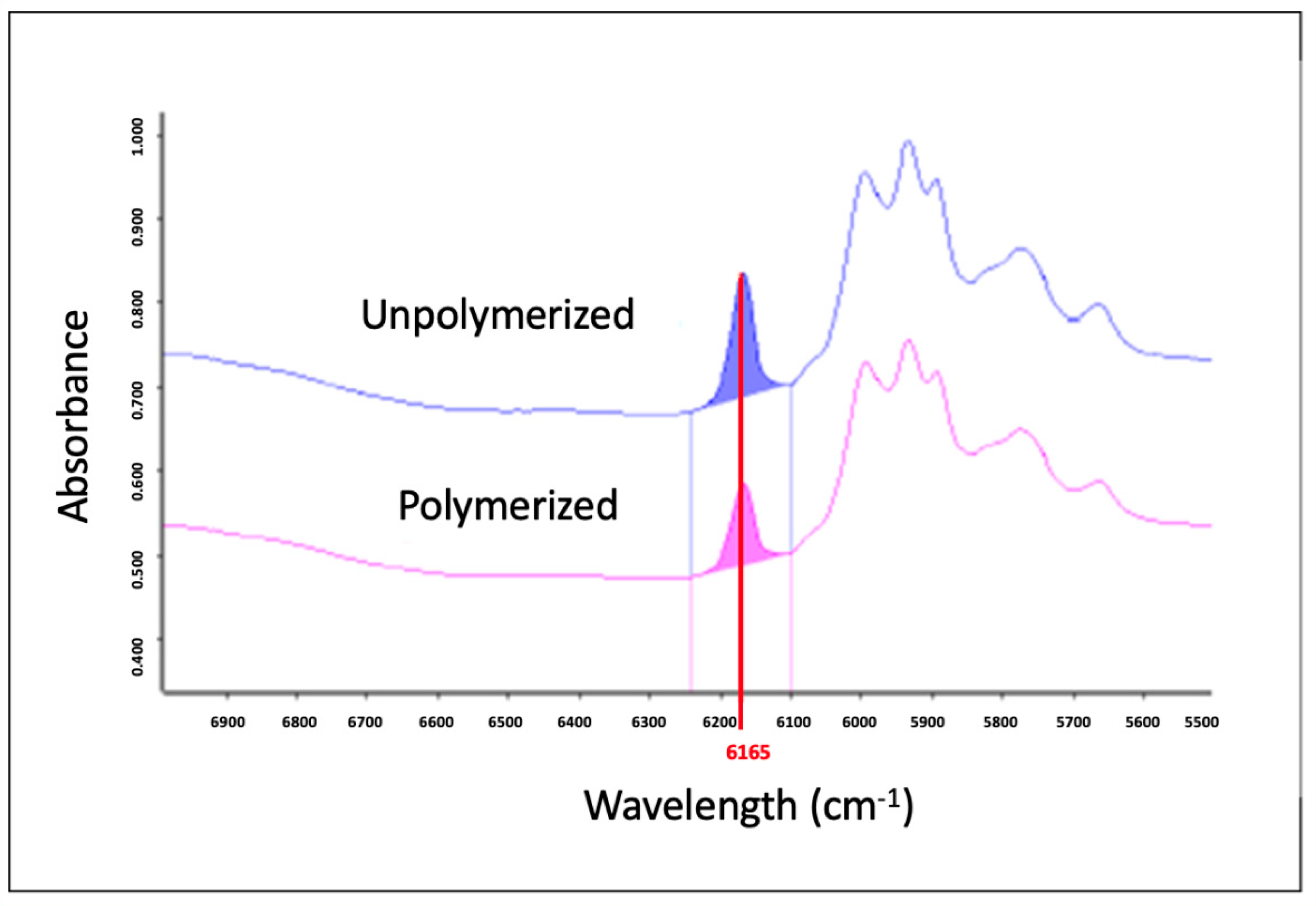
2.2.1. Degree of Conversion
2.2.2. Sorption and Solubility
2.2.3. Flexural Strength and Elastic Modulus
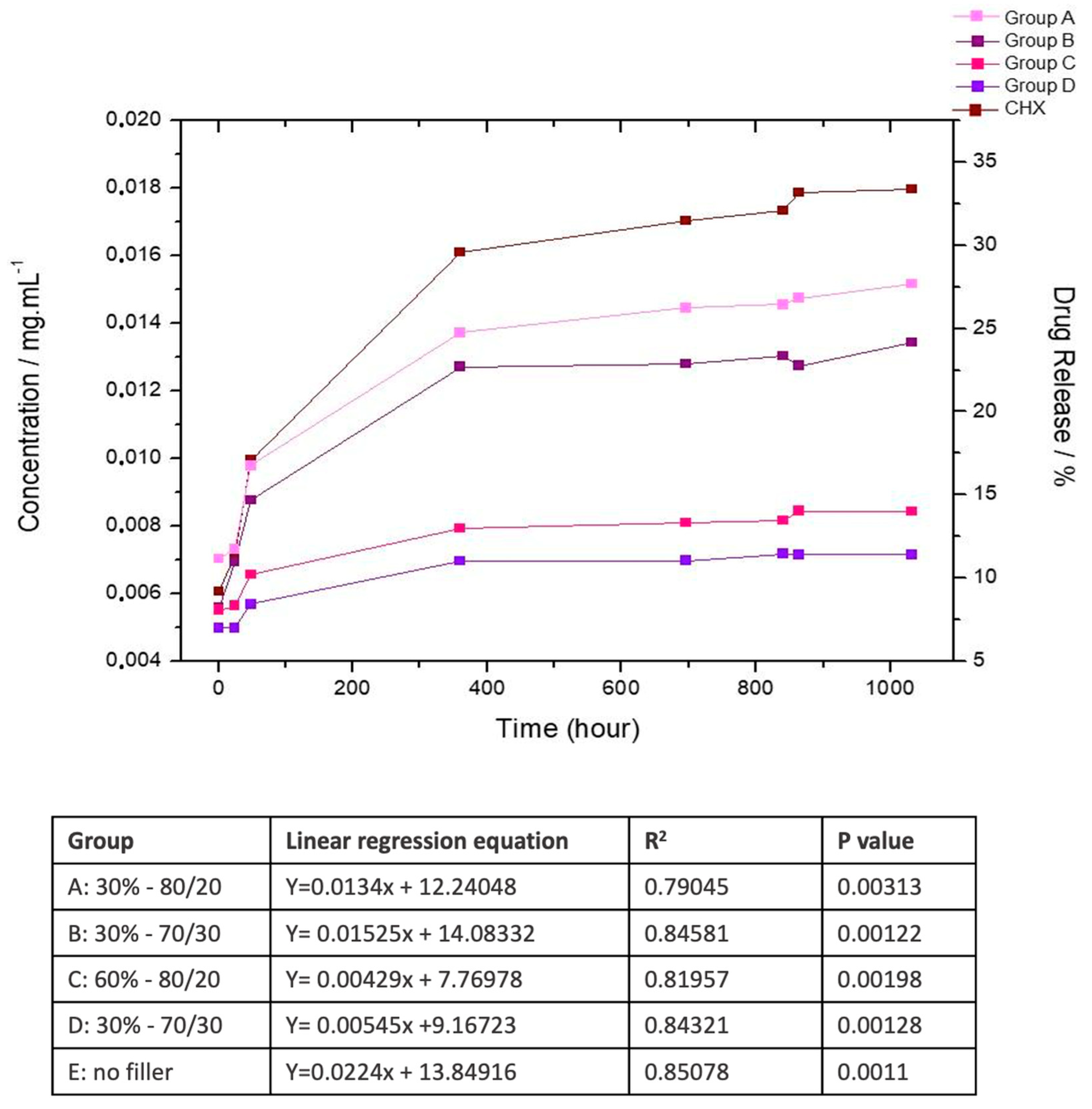
2.2.4. Chlorhexidine Release
2.3. Microbiological Evaluations
2.3.1. Inhibition Halo (12 Months)
2.3.2. CFU–Biofilm Assay (12 Months)
2.3.3. Dentin Demineralization
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varghese, E.J.; Sihivahanan, D.; Venkatesh, K.V. Development of Novel Antimicrobial Dental Composite Resin with Nano Cerium Oxide Fillers. Int. J. Biomater. 2022, 2022, 3912290. [Google Scholar] [CrossRef] [PubMed]
- Boaro, L.C.; Campos, L.M.; Varca, G.H.; Dos Santos, T.M.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, L.N.; Freitas, S.R.; Amorim, A.F.; Delechiave, G.; Catalani, L.H.; Braga, R.R.; Moreira, M.S.; Boaro, L.C.; Goncalves, F. Effects of the crosslinking of chitosan/DCPA particles in the antimicrobial and mechanical properties of dental restorative composites. Dent. Mater. 2022, 38, 1482–1491. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.E.; Laurence, J.S. Proteins, pathogens, and failure at the composite-tooth interface. J. Dent. Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Luo, J. Biopolymer/montmorillonite nanocomposite: Preparation, drug-controlled release property and cytotoxicity. Nanotechnology 2008, 19, 065707. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, N.; Li, W.; Gu, H.; Fan, Y.; Yuan, J. Long-term and controlled release of chlorhexidine-copper(II) from organically modified montmorillonite (OMMT) nanocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R. Rep. 2000, 18, 1–63. [Google Scholar] [CrossRef]
- Campos, L.M.; Boaro, L.C.; Santos, T.M.; Marques, P.A.; Almeida, S.R.; Braga, R.R.; Parra, D.F. Evaluation of flexural modulus, flexural strength and degree of conversion in BISGMA/TEGDMA resin filled with montmorillonite nanoparticles. J. Compos. Mater. 2017, 51, 927–937. [Google Scholar] [CrossRef]
- ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Young, A.M.; Ng, P.Y.; Gbureck, U.; Nazhat, S.N.; Barralet, J.E.; Hofmann, M.P. Characterization of chlorhexidine-releasing, fast-setting, brushite bone cements. Acta Biomater. 2008, 4, 1081–1088. [Google Scholar] [CrossRef]
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef]
- Gonçalves, F.; Kawano, Y.; Braga, R.R. Contraction stress related to composite inorganic content. Dent. Mater. 2010, 26, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Watts, D.C.; Lassila, L.V. Effect of nanofiller fractions and temperature on polymerization shrinkage on glass fiber reinforced filling material. Dent. Mater. 2008, 24, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Atai, M.; Watts, D.C. A new kinetic model for the photopolymerization shrinkage-strain of dental composites and resin-monomers. Dent. Mater. 2006, 22, 785–791. [Google Scholar] [CrossRef]
- Prati, C.; Mongiorgi, R.; Bertocchi, G.; Baldisserotto, G. Dental composite resin porosity and effect on water absorption. Boll. Soc. Ital. Biol. Sper. 1991, 67, 409–414. [Google Scholar]
- Alsharif, S.; Hazizan, M.A.; El-Aziz, N.; Ahmad, Z. Simulated Body Fluid Sorption and Solubility of Silica Reinforced Dental Resin Composites. Adv. Mater. Res. 2013, 795, 626–630. [Google Scholar] [CrossRef]
- Santerre, J.P.; Shajii, L.; Leung, B.W. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit. Rev. Oral Biol. Med. 2001, 12, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, H.; Tenovuo, J.; Huovinen, P. In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob. Agents Chemother. 1993, 37, 1158–1159. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef]
- Shah, M.B.; Ferracane, J.L.; Kruzic, J.J. R-curve behavior and toughening mechanisms of resin-based dental composites: Effects of hydration and post-cure heat treatment. Dent. Mater. 2009, 25, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Berghaus, E.; Muxkopf, G.A.; Feddersen, S.; Eisenburger, M.; Petersen, S. Antimicrobial agents in dental restorative materials: Effect on long-term drug release and material properties. Eur. J. Oral Sci. 2022, 130, e12840. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yan, J.; Luo, T.; Yan, H.; Hua, F.; He, H. Antibacterial and fluorescent clear aligner attachment resin modified with chlorhexidine loaded mesoporous silica nanoparticles and zinc oxide quantum dots. J. Mech. Behav. Biomed. Mater. 2023, 141, 105817. [Google Scholar] [CrossRef]
- Wang, R.; Habib, E.; Zhu, X.X. Evaluation of the filler packing structures in dental resin composites: From theory to practice. Dent. Mater. 2018, 34, 1014–1023. [Google Scholar] [CrossRef]
- Gonçalves, F.; Campos, L.; Sanches, L.; Silva, L.; Santos, T.; Varca, G.; Lopes, D.P.; Cogo-Muller, K.; Parra, D.F.; Braga, R.R.; et al. Antimicrobial activity and physicochemical performance of a modified endodontic sealer. Res. Soc. Dev. 2020, 9, e069119401. [Google Scholar] [CrossRef]
- Mehdawi, I.M.; Kitagawa, R.; Kitagawa, H.; Yamaguchi, S.; Hirose, N.; Kohno, T.; Imazato, S. Incorporation of chlorhexidine in self-adhesive resin cements. Dent. Mater. J. 2022, 41, 675–681. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Benkova, M.; Soukup, O.; Marek, J. Antimicrobial susceptibility testing: Currently used methods and devices and the near future in clinical practice. J. Appl. Microbiol. 2020, 129, 806–822. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Van den Poel, B.; Saegeman, V.; Schuermans, A. Increasing usage of chlorhexidine in health care settings: Blessing or curse? A narrative review of the risk of chlorhexidine resistance and the implications for infection prevention and control. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 349–362. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Fearber, I.; Domb, A.J.; Weiss, E.I. Long-term antibacterial surface properties of composite resin incorporating polyethyleneimine nanoparticles. Quintessence Int. 2010, 41, 827–835. [Google Scholar] [PubMed]
- Zhong, X.; Gao, F.; Wei, H.; Zhou, H.; Zhou, X. Functionalization of mesoporous silica as an effective composite carrier for essential oils with improved sustained release behavior and long-term antibacterial performance. Nanotechnology 2021, 33, 035706. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhang, Y.; Li, Y.; Shi, X.; Gong, H.; Feng, D.; Guo, X.; Shi, Z.; Zhu, S.; Cui, Z. Fabrication of superhydrophobic coating for preventing microleakage in a dental composite restoration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Bryaskova, R.; Philipova, N.; Bakov, V.; Georgiev, N. Innovative Antibacterial Polymer Coatings. Appl. Sci. 2025, 15, 1780. [Google Scholar] [CrossRef]
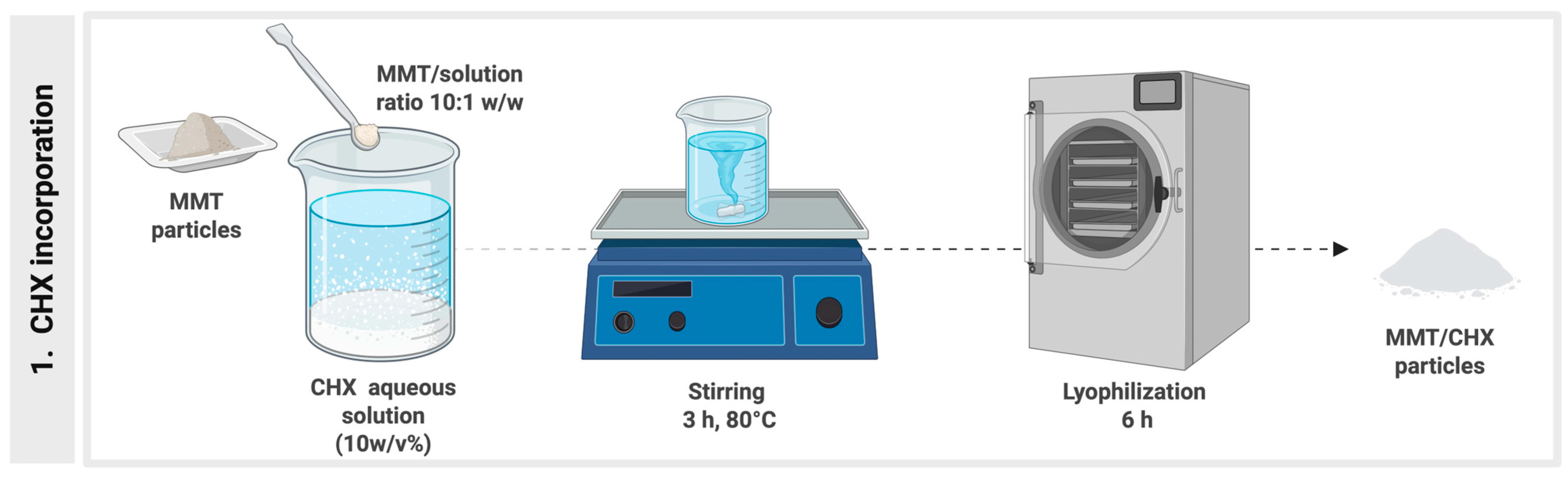

| Group | Inorganic Filler | Montmorillonite Loaded with Chlorhexidine (wt%) | Organic Matrix (wt%) | ||
|---|---|---|---|---|---|
| Total Concentration (wt%) | Barium Glass/Silica Rate | Monomers | Photo-initiator System | ||
| A | 30 | 80/20 | 5 | 31.6 BisGMA 31.6 TEGDMA | 0.8 camphorquinone 1.0 DMAEMA |
| B | 70/30 | ||||
| C | 60 | 80/20 | 16.6 BisGMA 16.6 TEGDMA | ||
| D | 70/30 | ||||
| E | None | 46.6 BisGMA 46.6 TEGDMA | |||
| Groups | Barium Glass/Silica | Sorption (mg/mL) | Solubility (mg/mL) | Degree of Conversion (%) | Flexural Strength (MPa) | Elastic Modulus (GPa) | ||
|---|---|---|---|---|---|---|---|---|
| Concentration (wt%) | Ratio | 10 min | 24 h | |||||
| A | 30 | 80/20 | 0.003 (0.002) | 0.046 (0.021) | 49 (6) | 65 (5) | 27.4 (6.3) BC | 4.0 (1.2) B |
| B | 70/30 | 0.001 (0.001) | 0.039 (0.014) | 54 (10) | 68 (11) | 30.7 (5.4) B | 4.0 (0.8) B | |
| C | 60 | 80/20 | 0.002 (0.004) | 0.037 (0.003) | 47 (6) | 60 (5) | 44.6 (12.2) A | 9.4 (2.4) A |
| D | 70/30 | 0.001 (0.001) | 0.064 (0.033) | 50 (5) | 57 (9) | 42.7 (9.5) A | 9.1 (2.1) A | |
| E | Control * | 0.001 (0.001) | 0.044 (0.018) | 46 (6) | 52 (9) | 18.9 (5.3) C | 3.9 (1.2) B | |
| Groups | Barium Glass/Silica | Month | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (wt%) | Ratio | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| A | 30 | 80/20 | 5.9 (1.7) AB | 4.5 (1.2) A | 1.6 (0.5) B | 2.1 (0.7) A | 3.1 (0.8) A | 5.2 (1.7) A | 3.9 (0.6) A | 3.8 (0.9) A | 1.9 (0.6) AB | 4.5 (1.8) A | 0.0 (0.0) B | 0.0 (0.0) B |
| B | 70/30 | 10.1 (1.7) A | 10.1 (1.7) A | 3.2 (0.4) A | 1.6 (0.5) A | 0.0 (0.0) B | 2.1 (0.6) B | 0.0 (0.0) C | 4.8 (0.8) A | 3.1 (0.7) AB | 3.4 (1.3) A | 4.6 (1.2) A | 0.0 (0.0) B | |
| C | 60 | 80/20 | 6.3 (1.3) AB | 4.3 (1.3) A | 1.6 (0.6) B | 2.7 (0.8) A | 3.2 (1.0) A | 1.3 (0.4) B | 2.6 (0.9) A | 3.8 (1.1) A | 0.9 (0.3) B | 0.0 (0.0) B | 0.0 (0.0) B | 0.0 (0.0) B |
| D | 70/30 | 12.4 (2.5) A | 4.6 (0.8) A | 2.1 (0.2) A | 1.5 (0.3) A | 3.7 (1.1) A | 2.5 (0.8) B | 1.7 (0.5) B | 3.11 (1.1) A | 3.6 (1.1) A | 4.6 (1.2) A | 1.3 (0.3) A | 2.8 (1.0) A | |
| E | Control * | 3.8 (1.0) B | 6.8 (1.4) A | 0.0 (0.0) C | 1.7 (0.5) A | 0.0 (0.0) B | 0.0 (0.0) C | 0.0 (0.0) C | 0.0 (0.0) B | 0.0 (0.0) C | 0.0 (0.0) B | 0.0 (0.0) B | 0.0 (0.0) B | |
| Groups | Barium Glass/Silica | 0 Month | 2nd Month | 4th Month | 6th Month | 8th Month | 10th Month | 12th Month | |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (wt%) | Ratio | ||||||||
| A | 30 | 80/20 | 0 | 2.3 (1.7) A | 23.3 (1.4) A | 1.5 (1.3) AB | 1.8 (2.4) A | 12.8 (2.5) C | 20.8 (5.3) B |
| B | 70/30 | 0 | 0.5 (0.6) A | 27.0 (3.6) A | 1.5 (1.7) AB | 0.8 (1.0) A | 51.8 (4.9) A | 61.7 (7.8) A | |
| C | 60 | 80/20 | 0 | 0.0 (0.0) B | 5.8 (1.7) B | 0.3 (0.5) B | 0.5 (0.6) A | 6.0 (1.3) C | 7.1 (1.9) C |
| D | 70/30 | 0 | 0.0 (0.0) B | 9.0 (1.3) B | 4.5 (1.2) A | 0.8 (1.0) A | 4.3 (1.3) C | 5.6 (1.8) C | |
| E | Control * | 0 | 0.0 (0.0) B | 14.8 (3.7) AB | 0.0 (0.0) B | 0.0 (0.0) A | 24.5 (3.8) B | 40.5 (5.7) AB | |
| Groups | Barium Glass/Silica | Knoop Microhardness | UFC 7 Days | |||
|---|---|---|---|---|---|---|
| Concentration wt% | Ratio | Initial | 7 Days | Reduction (%) | ||
| A | 30 | 80/20 | 82.9 (19.4) Aa | 41.6 (18.7) Ab | 49 | 19.0 (3.8) A |
| B | 70/30 | 72.5 (16.8) Aa | 37.2 (14.3) Ab | 49 | 18.4 (2.4) A | |
| C | 60 | 80/20 | 76.45 (20.1) Aa | 44.21 (16.9) Ab | 42 | 6.2 (1.3) B |
| D | 70/30 | 91.8 (21.2) Aa | 56.6 (13.9) Ab | 38 | 12.0 (1.9) B | |
| E | Control * | 84.1 (19.5) Aa | 38.5 (18.4) Ab | 54 | 22.0 (1.8) A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, F.; Silva, L.S.T.; Roschel, J.N.; de Souza, G.; Campos, L.d.P.M.; Varca, G.H.; Parra, D.; Perez, M.A.; Gordilho, A.C.; Brandt, W.C.; et al. Antibacterial Resin Composites with Sustained Chlorhexidine Release: One-Year In Vitro Study. Pharmaceutics 2025, 17, 1144. https://doi.org/10.3390/pharmaceutics17091144
Gonçalves F, Silva LST, Roschel JN, de Souza G, Campos LdPM, Varca GH, Parra D, Perez MA, Gordilho AC, Brandt WC, et al. Antibacterial Resin Composites with Sustained Chlorhexidine Release: One-Year In Vitro Study. Pharmaceutics. 2025; 17(9):1144. https://doi.org/10.3390/pharmaceutics17091144
Chicago/Turabian StyleGonçalves, Flávia, Larissa Sampaio Tavares Silva, Julia Noborikawa Roschel, Greca de Souza, Luiza de Paiva Mello Campos, Gustavo Henrique Varca, Duclerc Parra, Mirko Ayala Perez, Antonio Carlos Gordilho, William Cunha Brandt, and et al. 2025. "Antibacterial Resin Composites with Sustained Chlorhexidine Release: One-Year In Vitro Study" Pharmaceutics 17, no. 9: 1144. https://doi.org/10.3390/pharmaceutics17091144
APA StyleGonçalves, F., Silva, L. S. T., Roschel, J. N., de Souza, G., Campos, L. d. P. M., Varca, G. H., Parra, D., Perez, M. A., Gordilho, A. C., Brandt, W. C., & Boaro, L. (2025). Antibacterial Resin Composites with Sustained Chlorhexidine Release: One-Year In Vitro Study. Pharmaceutics, 17(9), 1144. https://doi.org/10.3390/pharmaceutics17091144







