A Bibliometric Analysis of Strategies for Atherosclerosis Treatment with Organic Nanoparticles
Abstract
1. Introduction
2. Bibliometric Analysis
2.1. Data Collection
2.2. Visualization and Analysis
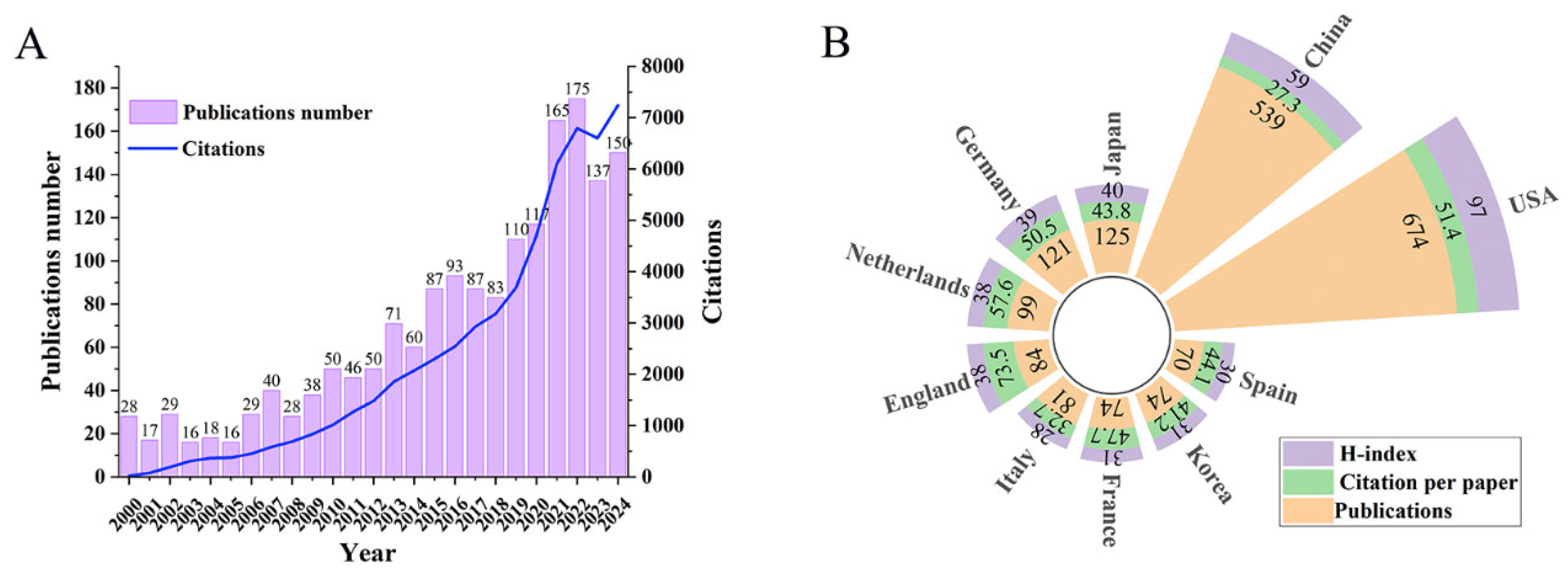
2.2.1. Publications Analysis
2.2.2. Major Research Domains
3. Research Hotspots of Organic Nanoparticles for as Treatment
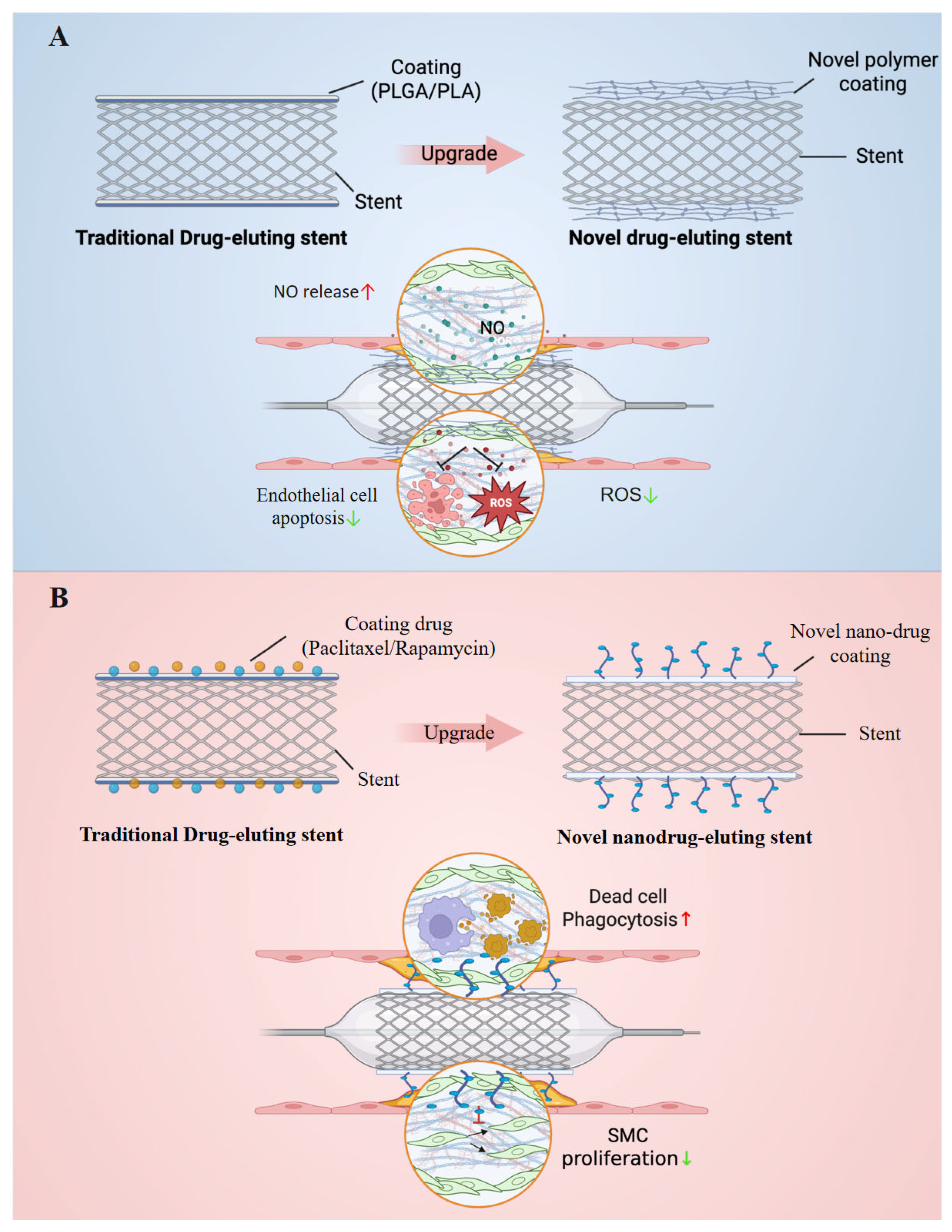
3.1. Organic Coated Stents
3.1.1. New Polymeric Organics as Stent Coatings
Vascular Reendothelialization Coated Stents
Anti-Inflammation Coated Stents
3.1.2. Biomimetic Nanoparticles as Stent Drugs
3.2. Traditional Organic Polymeric Nanoparticles
3.2.1. Targeted Nanoparticles
Ligand-Modified Nanoparticles Designed for Specific Lesion Sites
Cell Membrane-Coated Nanoparticles
Neutrophil Hitchhiking Nanoparticles
Ligand-Free Targeted Nanoparticles
| Target | Targeting Moiety | Nanocarrier | Cargo | Model | Ref. |
|---|---|---|---|---|---|
| VCAM-1 | VHPKQHR | micelles | miR-92a inhibitors | ApoE−/− | [64] |
| CCR2 expressed on synthetic VSMCs | MCP-1 peptide | micelles | miR-145 | ApoE−/− | [71] |
| Macrophage receptor stabilin-2 | Macrophage-targeting peptide ligand S2P | polymer-lipid hybrid nanoparticles | CaMKIIγsiRNA | Ldlr−/− | [78] |
| oxLDL-rich foamy macrophages | Phase-changing peptide (FFFFFFFFFFGDWFKAFYDKVAEKFKEAF) | nanoemulsions | Simvastatin | ApoE−/− | [75] |
| Col IV | Col IV targeted peptide | polyester polymers | IL-10 | Ldlr−/− | [87] |
| Fibrin clots | The peptide CREKA (Cys-Arg-Glu-Lys-Ala) | nanoemulsions | 17-β-estradiol | C57BL/6 | [89] |
| Atherosclerotic macrophages | PtdSer | liposome | PIO | ApoE−/− | [79] |
| Fibronectin and Filamin-A expressed by endothelial cells in the interfered area of blood flow | The peptide GSPREYTSYMPH (PREY) | liposome | tetrahydrobiopterin | ApoE−/− | [68] |
| The p32 receptor expressed on foam cells | Cyclic peptide Lyp-1 (CGNKRTRGC) | liposome | GW3965 | Ldlr−/− | [104] |
| The IL-4 receptor | Atherosclerotic plaque-homing peptide (AP peptide) | hydrophobically modified glycol chitosan | Cy5.5 | Ldlr−/− | [105] |
| Stabilin-2 and CD44 | HA | HA nanoparticles | Cy5.5 | ApoE−/− | [85] |
3.2.2. Stimulus-Responsive Nanoparticles
ROS
pH
Blood Flow Shear Stress
Enzyme
Cholesterol
3.2.3. Multifunctional Synergistic Nanoparticles
Theranostic Nanoparticles
Multi-Target Therapy Nanoparticles
3.3. Organic Biomimetic Nanoparticles
3.3.1. EVs from Specific Cell Sources
Mesenchymal Stem Cells
Efferocytes
M2 Macrophages
EPCs
EVs Generated from Cells Under Stimulation
| The Source of EVs | The Major Cargo | Target Pathway | The Influence on AS | Ref. |
|---|---|---|---|---|
| MSCs | FENDRR | FENDRR targeted miR-28 to increase TEAD1 activation | Reducing HUVEC-C injury and atherosclerotic plaque formation. | [219] |
| MSCs | miR-146a | miR-146a could suppress Src phosphorylation and downstream targets VE cadherin and Caveolin-1. | MSC-sEV mitigated endothelial cell senescence and stimulate angiogenesis. | [206] |
| MSCs | miR-21a-5p | Targeted inhibition of the KLF6 and ERK1/2 pathways | Promote the polarization of macrophages to type M2 and reduce their migration, thereby alleviating the formation of AS plaques and inflammatory responses. | [207] |
| MSCs | miR-145 | Inhibit the expression of JAM-A | Reduce endothelial migration and barrier disruption, thereby inhibiting plaque formation. | [220] |
| MSCs | miR-let7 | Inhibit the HMGA2/NF-κB pathway and down-regulate the IGF2BP1/PTEN pathway | Regulating the phenotype of macrophages alleviates the progression of AS. | [221] |
| BMSCs | long non-coding RNA AU020206 | Block CEBPB-mediated transcriptional activation of NLRP3 | Regulating the phenotype of macrophages alleviates the progression of AS. | [222] |
| BMSCs | - | Inhibiting NLRP3/Caspase-1/GSDMD in the pyroptosis pathway | Alleviate atherosclerosis by regulating the pyroptosis pathway and metabolic/inflammation-related genes. | [223] |
| BMSCs | - | Up-regulate phosphorylated AMPKα and inhibit mTOR activation | Regulating autophagy and polarization of macrophages alleviates diabetic AS. | [224] |
| M2 polarization of naive BMDMs induced by IL-4 | microRNA-99a/146b/378a | Inhibiting NF-kB and TNF-a signaling pathways | Inhibit inflammatory signals, regulate the hematopoietic process, and significantly improve the stability of atherosclerotic plaques. | [211] |
| ADSCs | miR-26 | The upregulation of miR-26 can reduce the mRNA expressions of TNF-α, IL-6 and IL-1β | miR-26 inhibits the progression of carotid atherosclerosis by regulating lipid metabolism and inflammatory responses. | [225] |
| hiPSCs | miR-126 | miR-126 inhibits the PI3K/Akt/mTOR pathway | Activate endothelial autophagy and effectively alleviate arterial stenosis induced by inflammatory injury. | [199] |
| hUCMSC | miR-100-5p | Target FZD5 and inhibit the Wnt/β-catenin pathway | Inhibit eosinophil migration, promote their apoptosis, and alleviate inflammatory responses. | [226] |
| Human fetal aorta-derived EPCs | - | - | Inhibit the formation of new intima after carotid artery injury by promoting endothelial repair. | [213] |
| EPCs | miR-199a-3p | miR-199a-3p reduces the expression of SP1 and upregulates the antioxidant proteins SLC7A11 and GPX4 | Reduce ROS, lipid peroxidation and iron accumulation, thereby reducing endothelial cell ferroptosis and alleviating the progression of AS. | [215] |
| RBCs | heme | Activate the HO-1 pathway by delivering heme | Regulate the transformation of macrophages to anti-inflammatory phenotypes and inhibit the formation of foam cells. | [227] |
| Efferocytes | prosaposin | Activate the GPR37 receptor of macrophages and up-regulate the expression of Tim4 | Enhance the ability of efferocytes macrophages to continuously clear apoptotic cells. | [202] |
| M2 polarization of RAW264.7 macrophages induced by IL-4 | - | - | Inhibit the proliferation, migration and synthetic phenotypic transformation of VSMCs, significantly delay the progression of AS and enhance the stability of plaques. | [212] |
| Baicalin-pretreated MSCs | - | Up-regulate SIRT1 and inhibit the activation of the NF-κB pathway | Reduce the inflammatory response and plaque formation of VSMCs | [217] |
| Endothelial cells sheared by laminar flow | miR-34c-5p | miR-34c-5p inhibits TGIF2, activates the nuclear translocation of Smad3, and promotes the TGF-β signaling pathway | Regulating the M2 polarization of macrophages to achieve anti-atherosclerotic treatment | [218] |
3.3.2. Drug-Loaded EVs
Gene Drugs
Chemical Drugs
3.3.3. EV Mimics
3.3.4. Engineering Strategies for EVs and EV Mimics
4. Prospects for Clinical Translation
Supplementary Materials
Funding
Conflicts of Interest
References
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update A Report From the American Heart Association. Circulation 2017, 135, E146–E603. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef]
- Gui, Y.Z.; Zheng, H.C.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef]
- Crielaard, H.; Wissing, T.B.; Torun, S.G.; Kremers, G.J.; de Miguel, P.; Hengst, R.M.; Gijsen, F.J.H.; Akyildiz, A.C.; van der Heiden, K. Local characterization of collagen architecture and mechanical properties of tissue engineered atherosclerotic plaque cap analogs. Acta Biomater. 2025, 194, 185–193. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Xu, S.W.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.Y.; Luo, S.H.; Li, Z.M.; Liu, P.Q.; Han, J.H.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.M.; Ma, D.; Wang, L.J.; Su, X.Q.; Feng, L.S.; Zhu, L.C.; Chen, Y.; Hao, Y.L.; Wang, X.Y.; Feng, J.C. Metabolic changes with the occurrence of atherosclerotic plaques and the effects of statins. Front. Immunol. 2023, 14, 1301051. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Liepinsh, E.; Fredriksson, R.; Alsehli, A.M.; Williams, M.J.; Dambrova, M.; Jönsson, J.; Schiöth, H.B. Off-target effects of statins: Molecular mechanisms, side effects and the emerging role of kinases. Br. J. Pharmacol. 2024, 181, 3799–3818. [Google Scholar] [CrossRef]
- Zeng, W.; Deng, H.; Luo, Y.; Zhong, S.; Huang, M.; Tomlinson, B. Advances in statin adverse reactions and the potential mechanisms: Asystematic review. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Yoshimura, N.; Kobayashi, A.; Ito, T.; Hara, K.; Tahara, K. Emulsion-electrospun polyvinyl alcohol nanofibers as a solid dispersion system to improve solubility and control the release of probucol, a poorly water-soluble drug. J. Drug Deliv. Sci. Technol. 2022, 67, 102953. [Google Scholar] [CrossRef]
- Cullis, P.R.; Felgner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Gao, C.; He, X.Q.; Ouyang, F.; Zhang, Z.H.; Shen, G.D.; Wu, M.X.; Yang, P.; Ma, L.K.; Yang, F.; Ji, Z.; et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I): An open-label, randomised, non-inferioritytrial. Lancet 2024, 404, 1040–1050. [Google Scholar] [CrossRef]
- Yeh, R.W.; Shlofmitz, R.; Moses, J.; Bachinsky, W.; Dohad, S.; Rudick, S.; Stoler, R.; Jefferson, B.K.; Nicholson, W.; Altman, J.; et al. Paclitaxel-Coated Balloon vs Uncoated Balloon for Coronary In-Stent Restenosis. JAMA 2024, 331, 1015–1024. [Google Scholar] [CrossRef]
- Nakhlband, A.; Eskandani, M.; Omidi, Y.; Saeedi, N.; Ghaffari, S.; Barar, J.; Garjani, A. Combating atherosclerosis with targeted nanomedicines: Recent advances and future prospective. Bioimpacts 2018, 8, 59–75. [Google Scholar] [CrossRef]
- Xu, M.Z.; Qi, Y.M.; Liu, G.S.; Song, Y.Q.; Jiang, X.Y.; Du, B.J. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. Acs Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.Y.; Jiang, X.C.; Gao, J.Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef]
- Zhong, Y.; Zeng, X.P.; Zeng, Y.L.; Yang, L.L.; Peng, J.J.; Zhao, L.Z.; Chang, Y.T. Nanomaterials-based imaging diagnosis and therapy of cardiovascular diseases. Nano Today 2022, 45, 101554. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica-gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.X.; Fang, H.P.; Tian, H.Y. Latest advancements and trends in biomedical polymers for disease prevention, diagnosis, treatment, and clinical application. J. Control. Release 2025, 380, 138–174. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Dou, Y.M.; Liu, Y.; Di, M.Y.; Bian, H.M.; Sun, X.; Yang, Q. Advances in Therapeutic Applications of Extracellular Vesicles. Int. J. Nanomed. 2023, 18, 3285–3307. [Google Scholar] [CrossRef]
- Li, J.H.; Hu, X.L.; Chen, Y.M.; Fan, D.Y.; Tan, C.; Yang, S.H.; Wu, H.M.; Wang, Y.; An, Q.; Xiao, Z.H.; et al. Review of recent progress in vascular stents: From conventional to functional vascular stents. Chin. Chem. Lett. 2025, 36, 107483. [Google Scholar] [CrossRef]
- Jia, B.X.; Zhang, X.L.; Ma, N.; Mo, D.P.; Gao, F.; Sun, X.; Song, L.G.; Liu, L.; Deng, Y.M.; Xu, X.T.; et al. Comparison of Drug-Eluting Stent With Bare-Metal Stent in Patients With Symptomatic High-grade Intracranial Atherosclerotic Stenosis A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 176–184. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Roumeliotis, A.; Tsigas, G.; Soufras, G.; Grapsas, N.; Davlouros, P.; Hahalis, G. Thrombotic responses to coronary stents, bioresorbable scaffolds and the Kounis hypersensitivity-associated acute thrombotic syndrome. J. Thorac. Dis. 2017, 9, 1155–1164. [Google Scholar] [CrossRef]
- Hassan, S.; Ali, M.N.; Ghafoor, B. Evolutionary perspective of drug eluting stents: From thick polymer to polymer free approach. J. Cardiothorac. Surg. 2022, 17, 65. [Google Scholar] [CrossRef]
- Bozsak, F.; Gonzalez-Rodriguez, D.; Sternberger, Z.; Belitz, P.; Bewley, T.; Chomaz, J.M.; Barakat, A.I. Optimization of Drug Delivery by Drug-Eluting Stents. PLoS ONE 2015, 10, e0130182. [Google Scholar] [CrossRef]
- Hamon, M.; Niculescu, R.; Deleanu, D.; Dorobantu, M.; Weissman, N.J.; Waksman, R. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): A prospective, first-in-man study. Eurointervention 2013, 8, 1006–1011. [Google Scholar] [CrossRef]
- Meredith, I.T.; Verheye, S.; Weissman, N.J.; Barragan, P.; Scott, D.; Chávarri, M.V.; West, N.E.J.; Kelbæk, H.; Whitbourn, R.; Walters, D.L.; et al. Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: A randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. Eurointervention 2013, 9, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.Y.; Li, Y.H.; Ren, Y.Z.; Fuad, A.R.M.; Hu, F.F.; Du, R.L.; Wang, Y.; Wang, G.X.; Wang, Y.Z. Phagocytosis of polymeric nanoparticles aided activation of macrophages to increase atherosclerotic plaques in ApoE-/- mice. J. Nanobiotechnol. 2021, 19, 121. [Google Scholar] [CrossRef]
- Wang, X.; He, B. Endothelial dysfunction: Molecular mechanisms and clinical implications. MedComm 2024, 5, e651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Yang, Y.; Xiong, K.Q.; Li, X.Y.; Qi, P.K.; Tu, Q.F.; Jing, F.J.; Weng, Y.J.; Wang, J.; Huang, N. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials 2015, 63, 80–92. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, H.N.; Liu, X.Y.; Yu, T.; Yang, Y.; Dai, Y.; Han, Y.L.; Xu, K.; Yang, L.; Wang, Y.B.; et al. Engineering Immunomodulatory Stents Using Zinc Ion-Lysozyme Nanoparticle Platform for Vascular Remodeling. Acs Nano 2023, 17, 23498–23511. [Google Scholar] [CrossRef] [PubMed]
- Juni, R.P.; Duckers, H.J.; Vanhoutte, P.M.; Virmani, R.; Moens, A.L. Oxidative Stress and Pathological Changes After Coronary Artery Interventions. J. Am. Coll. Cardiol. 2013, 61, 1471–1481. [Google Scholar] [CrossRef]
- Lu, L.; Wang, Y.N.; Sun, W.H.; Liu, Z.H.; Zhang, Q.; Pu, L.J.; Yang, K.; Wang, L.J.; Zhu, Z.B.; Meng, H.; et al. Two-Dimensional Fluorescence In-Gel Electrophoresis of Coronary Restenosis Tissues in Minipigs Increased Adipocyte Fatty Acid Binding Protein Induces Reactive Oxygen Species-Mediated Growth and Migration in Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 572–580. [Google Scholar] [CrossRef] [PubMed]
- van Lith, R.; Gregory, E.K.; Yang, J.; Kibbe, M.R.; Ameer, G.A. Engineering biodegradable polyester elastomers with antioxidant properties to attenuate oxidative stress in tissues. Biomaterials 2014, 35, 8113–8122. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Fu, C.H.; Guo, J.Q.; Dong, C.M.; Wei, Q.; Pan, J.M.; Dai, S.; Yang, P. Baicalin-based composite coating for achieving biological multifunctionality of vascular stents. J. Control. Release 2025, 379, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, N.R.; Garratt, K.N. Clinical Studies with Paclitaxel—Eluting Stent Systems. Curr. Pharm. Des. 2010, 16, 4025–4036. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sukhorukov, V.N.; Zhuravlev, A.; Orekhov, N.A.; Kalmykov, V.; Orekhov, A.N. Modulating mTOR Signaling as a Promising Therapeutic Strategy for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1153. [Google Scholar] [CrossRef]
- Schrijvers, D.M.; De Meyer, G.R.Y.; Kockx, M.M.; Herman, A.G.; Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1256–1261. [Google Scholar] [CrossRef]
- Che, H.L.; Bae, I.H.; Lim, K.S.; Song, I.T.; Lee, H.; Muthiah, M.; Namgung, R.; Kim, W.J.; Kim, D.G.; Ahn, Y.; et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials 2012, 33, 8548–8556. [Google Scholar] [CrossRef]
- Wulf, K.; Arbeiter, D.; Matschegewski, C.; Teske, M.; Huling, J.; Schmitz, K.P.; Grabow, N.; Kohse, S. Smart releasing electrospun nanofibers-poly: L.lactide fibers as dual drug delivery system for biomedical application. Biomed. Mater. 2021, 16, 015022. [Google Scholar] [CrossRef]
- Islam, P.; Schaly, S.; Abosalha, A.K.; Boyajian, J.; Thareja, R.; Ahmad, W.; Shum-Tim, D.; Prakash, S. Nanotechnology in development of next generation of stent and related medical devices: Current and future aspects. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1941. [Google Scholar] [CrossRef]
- Zou, D.; Yang, P.; Liu, J.N.; Dai, F.F.; Xiao, Y.Y.; Zhao, A.S.; Huang, N. Exosome-Loaded Pro-efferocytic Vascular Stent with Lp-PLA2-Triggered Release for Preventing In-Stent Restenosis. Acs Nano 2022, 16, 14925–14941. [Google Scholar] [CrossRef]
- Wei, Y.Z.; Wu, Y.F.; Zhao, R.X.; Zhang, K.Y.; Midgley, A.C.; Kong, D.L.; Li, Z.J.; Zhao, Q. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials 2019, 204, 13–24. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- Sluimer, J.C.; Kolodgie, F.D.; Bijnens, A.; Maxfield, K.; Pacheco, E.; Kutys, B.; Duimel, H.; Frederik, P.M.; van Hinsbergh, V.W.M.; Virmani, R.; et al. Thin-Walled Microvessels in Human Coronary Atherosclerotic Plaques Show Incomplete Endothelial Junctions Relevance of Compromised Structural Integrity for Intraplaque Microvascular Leakage. J. Am. Coll. Cardiol. 2009, 53, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, G.L.; Jorgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Tamargo, I.A.; Baek, K.I.; Kim, Y.; Park, C.; Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 738–753. [Google Scholar] [CrossRef]
- He, L.; Zhang, C.L.; Chen, Q.H.; Wang, L.; Huang, Y. Endothelial shear stress signal transduction and atherogenesis: From mechanisms to therapeutics. Pharmacol. Ther. 2022, 235, 108152. [Google Scholar] [CrossRef]
- Chen, X.L.; Grey, J.Y.; Thomas, S.; Qiu, F.H.; Medford, R.M.; Wasserman, M.A.; Kunsch, C. Sphingosine kinase-1 mediates TNF-α-induced MCP-1 gene expression in endothelial cells:: Upregulation by oscillatory flow. Am. J. Physiol.-Heart Circ. Physiol. 2004, 287, H1452–H1458. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.M.; Zhu, S.N.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Yeh, C.F.; Mellas, M.; Oh, M.J.; Zhu, J.Y.; Li, J.; Huang, R.T.; Harrison, D.L.; Shentu, T.P.; Wu, D.; et al. Targeted polyelectrolyte complex micelles treat vascular complications in vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2114842118. [Google Scholar] [CrossRef] [PubMed]
- Distasio, N.; Dierick, F.; Ebrahimian, T.; Tabrizian, M.; Lehoux, S. Design and development of Branched Poly(ß-aminoester) nanoparticles for Interleukin-10 gene delivery in a mouse model of atherosclerosis. Acta Biomater. 2022, 143, 356–371. [Google Scholar] [CrossRef]
- Lonberg, N. Fully human antibodies from transgenic mouse and phage display platforms. Curr. Opin. Immunol. 2008, 20, 450–459. [Google Scholar] [CrossRef]
- Arap, W.; Kolonin, M.G.; Trepel, M.; Lahdenranta, J.; Cardó-Vila, M.; Giordano, R.J.; Mintz, P.J.; Ardelt, P.U.; Yao, V.J.; Vidal, C.I.; et al. Steps toward mapping the human vasculature by phage display. Nat. Med. 2002, 8, 121–127. [Google Scholar] [CrossRef]
- Hofmeister, L.H.; Lee, S.H.; Norlander, A.E.; Montaniel, K.R.C.; Chen, W.; Harrison, D.G.; Sung, H.J. Phage-Display-Guided Nanocarrier Targeting to Atheroprone Vasculature. Acs Nano 2015, 9, 4435–4446. [Google Scholar] [CrossRef]
- Schober, A.; Zernecke, A.; Liehn, E.A.; von Hundelshausen, P.; Knarren, S.; Kuziel, W.A.; Weber, C. Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets. Circ. Res. 2004, 95, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Taubman, M.B. Chemokines in the pathogenesis of vascular disease. Circ. Res. 2004, 95, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.D.; Poon, C.; Wang, J.N.; Joo, J.; Ong, V.; Jiang, Z.J.; Cheng, K.L.; Plotkin, A.; Magee, G.A.; Chung, E.J. miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype. Biomaterials 2021, 273, 120810. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and Atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med.-Jmm 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Kim, I.; Elliott, J.C.; Lawanprasert, A.; Koehle, A.M., III; Wood, G.M.; Castro, R.; Simon, J.C.; Medina, S.H. Twinkling Peptide Nanoemulsions Enable Precision Ultrasound Detection of Atherosclerotic Plaques. Adv. Funct. Mater. 2025, 35, 2415609. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Kim, J.H.; Oh, G.T.; Lee, B.H.; Kwon, I.C.; Kim, I.S. Molecular targeting of atherosclerotic plaques by a stabilin-2-specific peptide ligand. J. Control. Release 2011, 155, 211–217. [Google Scholar] [CrossRef]
- Huang, X.G.; Liu, C.; Kong, N.; Xiao, Y.F.; Yurdagul, A.; Tabas, I.; Tao, W. Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. 2022, 17, 748–780. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Dai, L.L.; Wang, Q.Q.; Xue, L.J.; Su, Z.G.; Zhang, C. An apoptotic body-biomimic liposome in situ upregulates anti-inflammatory macrophages for stabilization of atherosclerotic plaques. J. Control. Release 2019, 316, 236–249. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Yoo, J.; Keliher, E.J.; Truelove, J.J.; Iwamoto, Y.; Sena, B.; Dutta, P.; Borodovsky, A.; Fitzgerald, K.; Di Carli, M.F.; et al. Polymeric Nanoparticle PET/MR Imaging Allows Macrophage Detection in Atherosclerotic Plaques. Circ. Res. 2013, 112, 755–761. [Google Scholar] [CrossRef]
- Uchida, M.; Kosuge, H.; Terashima, M.; Willits, D.A.; Liepold, L.O.; Young, M.J.; McConnell, M.V.; Douglas, T. Protein Cage Nanoparticles Bearing the LyP-1 Peptide for Enhanced Imaging of Macrophage-Rich Vascular Lesions. Acs Nano 2011, 5, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased Expression of Matrix Metalloproteinases and Matrix-Degrading Activity in Vulnerable Regions of Human Atherosclerotic Plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef]
- Little, P.J.; Tannock, L.; Olin, K.L.; Chait, A.; Wight, T.N. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-β1 exhibit increased binding to LDLs. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Skålén, K.; Gustafsson, M.; Rydberg, E.K.; Hultén, L.M.; Wiklund, O.; Innerarity, T.L.; Borén, J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002, 417, 750–754. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, J.H.; Choi, K.Y.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Choi, K.; Lee, B.H.; Park, J.H.; Kim, I.S. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials 2015, 53, 341–348. [Google Scholar] [CrossRef]
- Lopes, J.; Adiguzel, E.; Gu, S.; Liu, S.L.; Hou, G.P.; Heximer, S.; Assoian, R.K.; Bendeck, M.P. Type VIII Collagen Mediates Vessel Wail Remodeling after Arterial Injury and Fibrous Cap Formation in Atherosclerosis. Am. J. Pathol. 2013, 182, 2241–2253. [Google Scholar] [CrossRef]
- Kamaly, N.; Fredman, G.; Fojas, J.J.R.; Subramanian, M.; Choi, W.I.; Zepeda, K.; Vilos, C.; Yu, M.Y.; Gadde, S.; Wu, J.; et al. Targeted Interleukin-10 Nanotherapeutics Developed with.a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. Acs Nano 2016, 10, 5280–5292. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef]
- Deshpande, D.; Kethireddy, S.; Gattacceca, F.; Amiji, M. Comparative pharmacokinetics and tissue distribution analysis of systemically administered 17-β-estradiol and its metabolites in vivo delivered using a cationic nanoemulsion or a peptide-modified nanoemulsion system for targeting atherosclerosis. J. Control. Release 2014, 180, 117–124. [Google Scholar] [CrossRef]
- Zhu, C.J.; Ma, J.K.; Ji, Z.H.; Shen, J.; Wang, Q.W. Recent Advances of Cell Membrane Coated Nanoparticles in Treating Cardiovascular Disorders. Molecules 2021, 26, 3428. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Rao, L.; Yao, H.M.; Wang, Z.L.; Ning, P.B.; Chen, X.Y. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv. Mater. 2020, 32, e2002054. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Y.; Yan, M.; Ni, S.; Zhao, X.; Wu, S.; Wang, G.X.; Zhang, K.; Chi, Q.J.; Qin, X.; et al. Macrophage Membrane-Encapsulated Dopamine-Modified Poly Cyclodextrin Multifunctional Biomimetic Nanoparticles for Atherosclerosis Therapy. Acs Appl. Mater. Interfaces 2024, 16, 32027–32044. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Li, T.H.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.H.; McGinty, S.; Pontrelli, G.; et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef]
- Liu, Y.; Bühring, H.J.; Zen, K.; Burst, S.L.; Schnell, F.J.; Williams, I.R.; Parkos, C.A. Signal regulatory protein (SIRPα), a cellular ligand for CD47, regulates neutrophil transmigration. J. Biol. Chem. 2002, 277, 10028–10036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Kang, Z.F.; Yin, D.Z.; Gao, J. Role of neutrophils in different stages of atherosclerosis. Innate Immun. 2023, 29, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.X.; Wang, Q.; Zhang, S.; Lan, Q.; Wang, R.J.; Tan, E.C.; Zhou, L.; Wang, C.P.; Wang, H.; Cheng, Y.Y. Polypyridiniums with Inherent Autophagy-Inducing Activity for Atherosclerosis Treatment by Intracellularly Co-Delivering Two Antioxidant Enzymes. Adv. Mater. 2024, 36, e2409015. [Google Scholar] [CrossRef]
- Huilcaman, R.; Veliz-Olivos, N.; Venturini, W.; Olate-Briones, A.; Treuer, A.V.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Endothelial transmigration of platelets depends on soluble factors released by activated endothelial cells and monocytes. Platelets 2021, 32, 1113–1119. [Google Scholar] [CrossRef]
- Kee, M.F.; Myers, D.R.; Sakurai, Y.; Lam, W.A.; Qiu, Y.Z. Platelet Mechanosensing of Collagen Matrices. PLoS ONE 2015, 10, e0126624. [Google Scholar] [CrossRef]
- Wei, X.L.; Ying, M.; Dehaini, D.; Su, Y.Y.; Kroll, A.V.; Zhou, J.R.; Gao, W.W.; Fang, R.H.; Chien, S.; Zhang, L.F. Nanoparticle Functionalization with Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. Acs Nano 2018, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Toner, Y.C.; Meerwaldt, A.E.; van Leent, M.M.T.; Soultanidis, G.; Elschot, M.; Gonai, H.; Grendstad, K.; Flobak, A.; Neckmann, U.; et al. Tumor Targeting by αvβ3-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. Acs Nano 2020, 14, 7832–7846. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, M.; Wu, Y.; Gong, F.L.; Wang, Z.B.; Xue, L.J.; Su, Z.G. An elastase-inhibiting, plaque-targeting and neutrophil-hitchhiking liposome against atherosclerosis. Acta Biomater. 2024, 173, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Ben-Akiva, E.; Karlsson, J.; Hemmati, S.; Yu, H.Z.; Tzeng, S.Y.; Pardoll, D.M.; Green, J.J. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2301606120. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.J.; Allen, S.D.; Liu, Y.G.; Ouyang, B.Z.; Li, X.M.; Augsornworawat, P.; Thorp, E.B.; Scott, E.A. Tailoring Nanostructure Morphology for Enhanced Targeting of Dendritic Cells in Atherosclerosis. Acs Nano 2016, 10, 11290–11303. [Google Scholar] [CrossRef]
- Benne, N.; Cardoso, R.M.; Boyle, A.L.; Kros, A.; Jiskoot, W.; Kuiper, J.; Bouwstra, J.; Van Eck, M.; Slütter, B. Complement Receptor Targeted Liposomes Encapsulating the Liver X Receptor Agonist GW3965 Accumulate in and Stabilize Atherosclerotic Plaques. Adv. Healthc. Mater. 2020, 9, e2000043. [Google Scholar] [CrossRef]
- Park, Y.; Hong, H.Y.; Moon, H.J.; Lee, B.H.; Kim, I.S.; Kwon, I.C.; Rhee, I. A new atherosclerotic lesion probe based on hydrophobically modified chitosan nanoparticles functionalized by the atherosclerotic plaque targeted peptides. J. Control. Release 2008, 128, 217–223. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.B.; Ren, X.Y.; Wang, Y.; Chen, D.X.; Li, Q.; Huo, M.F.; Shi, J.L. Ischemic Microenvironment-Responsive Therapeutics for Cardiovascular Diseases. Adv. Mater. 2021, 33, e2105348. [Google Scholar] [CrossRef]
- Wadström, B.N.; Wulff, A.B.; Pedersen, K.M.; Jensen, G.B.; Nordestgaard, B.G. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: A cohort-based study. Eur. Heart J. 2022, 43, 3258–3269. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; John, R.; Naguib, S.; Siadaty, M.S.; Grasu, R.; Kurian, K.C.; van Winkle, W.B.; Soller, B.; Litovsky, S.; Madjid, M.; et al. pH heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis 2002, 164, 27–35. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Wu, H.X.; Du, Q.N.; Dai, Q.Y.; Ge, J.B.; Cheng, X.W. Cysteine Protease Cathepsins in Atherosclerotic Cardiovascular Diseases. J. Atheroscler. Thromb. 2018, 25, 111–123. [Google Scholar] [CrossRef]
- Tian, K.J.; Yang, Y.; Zhou, K.; Deng, N.H.; Tian, Z.; Wu, Z.F.; Liu, X.Y.; Zhang, F.; Jiang, Z.S. The role of ROS-induced pyroptosis in CVD. Front. Cardiovasc. Med. 2023, 10, 1116509. [Google Scholar] [CrossRef]
- Hou, X.Y.; Lin, H.; Zhou, X.D.; Cheng, Z.T.; Li, Y.; Liu, X.; Zhao, F.; Zhu, Y.P.; Zhang, P.; Chen, D.Q. Novel dual ROS-sensitive and CD44 receptor targeting nanomicelles based on oligomeric hyaluronic acid for the efficient therapy of atherosclerosis. Carbohydr. Polym. 2020, 232, 115787. [Google Scholar] [CrossRef] [PubMed]
- Kannaujiya, V.K.; Qiao, Y.J.; Sheikh, R.H.; Xue, J.Y.; Dargaville, T.R.; Liang, K.; Wich, P.R. pH-Responsive Micellar Nanoparticles for the Delivery of a Self-Amplifying ROS-Activatable Prodrug. Biomacromolecules 2024, 25, 1775–1789. [Google Scholar] [CrossRef]
- Yan, B.K.; Zhang, Y.; Wei, C.; Xu, Y. Facile synthesis of ROS-responsive biodegradable main chain poly(carbonate-thioether) copolymers. Polym. Chem. 2018, 9, 904–911. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, X.W.; Wang, X.L.; Miao, D.D.; Liang, X.Y.; Wang, C.W.; Pang, L.Y.; Sun, H.F.; Kong, D.L.; Yang, J. Hydrogen peroxide-responsive micelles self-assembled from a peroxalate ester-containing triblock copolymer. Biomater. Sci. 2016, 4, 255–257. [Google Scholar] [CrossRef]
- Zhang, T.H.; Chen, X.; Xiao, C.S.; Zhuang, X.L.; Chen, X.S. Synthesis of a phenylboronic ester-linked PEG-lipid conjugate for ROS-responsive drug delivery. Polym. Chem. 2017, 8, 6209–6216. [Google Scholar] [CrossRef]
- Yang, H.Q.; Guo, M.C.; Guan, Q.R.; Zhang, L.X.; Liu, M.; Li, H.Y.; Qiao, G.Y.; Yang, Q.B.; Shen, M.L.; Li, Y.P. ROS-responsive simvastatin nano-prodrug based on tertiary amine-oxide zwitterionic polymer for atherosclerotic therapy. J. Nanobiotechnol. 2025, 23, 176. [Google Scholar] [CrossRef]
- Chen, W.Z.; Zhen, X.; Wu, W.; Jiang, X.Q. Responsive boron biomaterials and their biomedical applications. Sci. China Chem. 2020, 63, 648–664. [Google Scholar] [CrossRef]
- Li, B.Y.; He, M.; Xu, Z.C.; Zhang, Q.T.; Zhang, L.Y.; Zhao, S.; Cao, Y.; Mou, N.L.; Wang, Y.; Wang, G.X. Biomimetic ROS-responsive hyaluronic acid nanoparticles loaded with methotrexate for targeted anti-atherosclerosis. Regen. Biomater. 2024, 11, rbae102. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhu, H.M.; Liu, H.; Liu, D.Y.; Liu, J.H.; Zhang, Y.; Qin, Z.; Xu, Y.J.; Peng, Y.; Ruan, L.H.; et al. Synergistic dual cell therapy for atherosclerosis regression: ROS-responsive Bio-liposomes co-loaded with Geniposide and Emodin. J. Nanobiotechnol. 2024, 22, 129. [Google Scholar] [CrossRef]
- Shen, M.L.; Li, H.L.; Yao, S.Y.; Wu, X.D.; Liu, S.; Yang, Q.B.; Zhang, Y.J.; Du, J.S.; Qi, S.L.; Li, Y.P. Shear stress and ROS-responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 126, 112164. [Google Scholar] [CrossRef]
- Shen, L.; Chen, W.Y.; Ding, J.Y.; Shu, G.F.; Chen, M.J.; Zhao, Z.W.; Xia, S.W.; Ji, J.S. The role of metabolic reprogramming of oxygen-induced macrophages in the dynamic changes of atherosclerotic plaques. Faseb J. 2023, 37, e22791. [Google Scholar] [CrossRef]
- Maruf, A.; Wang, Y.; Yin, T.Y.; Huang, J.L.; Wang, N.; Durkan, C.; Tan, Y.H.; Wu, W.; Wang, G.X. Atherosclerosis Treatment with Stimuli-Responsive Nanoagents: Recent Advances and Future Perspectives. Adv. Healthc. Mater. 2019, 8, e1900036. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, C.Y.; Wang, H.Y.; Ao, M.Y.; Fan, Y.L.; Chen, Y. AFM detects the effects of acidic condition on the size and biomechanical properties of native/oxidized low-density lipoprotein. Colloids Surf. B Biointerfaces 2021, 208, 112053. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Cheraga, N.; Ye, Z.; Xu, M.J.; Zou, L.; Sun, N.C.; Hang, Y.; Shan, C.J.; Yang, Z.Z.; Chen, L.J.; Huang, N.P. Targeted therapy of atherosclerosis by pH-sensitive hyaluronic acid nanoparticles co-delivering all-trans retinal and rapamycin. Nanoscale 2022, 14, 8709–8726. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.Z.; Yang, Z.Z. Benzoic-Imine-Based Physiological-pH-Responsive Materials for Biomedical Applications. Chem. Asian J. 2016, 11, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Z.; Liu, J.J.; Chong, S.Y.; Ting, H.J.; Tang, X.C.; Yang, L.Q.; Zhang, S.T.; Qi, X.Y.; Pei, P.; Yi, Z.G.; et al. Dual-Function Nanoscale Coordination Polymer Nanoparticles for Targeted Diagnosis and Therapeutic Delivery in Atherosclerosis. Small 2024, 20, e2401659. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Shen, L.B.; Zhong, Q.Z.; Li, J.H. Metal-phenolic network coatings for engineering bioactive interfaces. Colloids Surf. B Biointerfaces 2021, 205, 111851. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Yue, T.X.; Wang, H.; Jiang, L.; Huang, Q.L.; Li, F.Z. Biomimetic nanoparticles co-deliver hirudin and lumbrukinase to ameliorate thrombus and inflammation for atherosclerosis therapy. Asian J. Pharm. Sci. 2025, 20, 100990. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef]
- Natsume, T.; Yoshimoto, M. Mechanosensitive Liposomes as Artificial Chaperones for Shear-Driven Acceleration of Enzyme-Catalyzed Reaction. Acs Appl. Mater. Interfaces 2014, 6, 3671–3679. [Google Scholar] [CrossRef]
- Marosfoi, M.G.; Korin, N.; Gounis, M.J.; Uzun, O.; Vedantham, S.; Langan, E.T.; Papa, A.L.; Brooks, O.W.; Johnson, C.; Puri, A.S.; et al. Shear-Activated Nanoparticle Aggregates Combined With Temporary Endovascular Bypass to Treat Large Vessel Occlusion. Stroke 2015, 46, 3507–3513. [Google Scholar] [CrossRef]
- Holme, M.N.; Fedotenko, I.A.; Abegg, D.; Althaus, J.; Babel, L.; Favarger, F.; Reiter, R.; Tanasescu, R.; Zaffalon, P.L.; Ziegler, A.; et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat. Nanotechnol. 2012, 7, 536–543. [Google Scholar] [CrossRef]
- Olejarz, W.; Lacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef] [PubMed]
- Bot, P.T.; Pasterkamp, G.; Goumans, M.J.; Strijder, C.; Moll, F.L.; de Vries, J.P.; Pals, S.T.; de Kleijn, D.P.; Piek, J.J.; Hoefer, I.E. Hyaluronic acid metabolism is increased in unstable plaques. Eur. J. Clin. Investig. 2010, 40, 818–827. [Google Scholar] [CrossRef]
- Zhang, M.Y.; He, J.H.; Jiang, C.P.; Zhang, W.L.; Yang, Y.; Wang, Z.Y.; Liu, J.P. Plaque-hyaluronidase-responsive high-density-lipoprotein-mimetic nanoparticles for multistage intimal-macrophage-targeted drug delivery and enhanced anti-atherosclerotic therapy. Int. J. Nanomed. 2017, 12, 533–558. [Google Scholar] [CrossRef]
- Platt, M.O.; Ankeny, R.F.; Shi, G.P.; Weiss, D.; Vega, J.D.; Taylor, W.R.; Jo, H. Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1479–H1486. [Google Scholar] [CrossRef]
- Barascuk, N.; Skjot-Arkil, H.; Register, T.C.; Larsen, L.; Byrjalsen, I.; Christiansen, C.; Karsdal, M.A. Human macrophage foam cells degrade atherosclerotic plaques through cathepsin K mediated processes. Bmc Cardiovasc. Disord. 2010, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ni, Y.H.; Yu, H.C.; Yin, H.M.; Yang, F.; Li, C.L.; Sun, D.L.; Pei, T.; Ma, J.; Deng, L.; et al. Inflammatory endothelium-targeted and cathepsin responsive nanoparticles are effective against atherosclerosis. Theranostics 2022, 12, 4200–4220. [Google Scholar] [CrossRef]
- Janoudi, A.; Shamoun, F.E.; Kalavakunta, J.K.; Abela, G.S. Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque. Eur. Heart J. 2016, 37, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis 1985, 56, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra350. [Google Scholar] [CrossRef]
- Kim, H.; Kumar, S.; Kang, D.W.; Jo, H.; Park, J.H. Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy. Acs Nano 2020, 14, 6519–6531. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.L.; Chen, Z.; Shin, D.M. Application of nanotechnology in cancer therapy and imaging. CA A Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef]
- Nair, A.; Kuban, B.D.; Tuzcu, E.M.; Schoenhagen, P.; Nissen, S.E.; Vince, D.G. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002, 106, 2200–2206. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef]
- Auerbach, S.R.; Fenton, M.J.; Grutter, G.; Albert, D.C.; Di-Filippo, S.; Burch, M.; Kuhn, M.A. The complication rate of intravascular ultrasound (IVUS) in a multicenter pediatric heart transplant population: A study of the international pediatric IVUS consortium. Clin. Transplant. 2020, 34, e13981. [Google Scholar] [CrossRef]
- Kumamaru, K.K.; Hoppel, B.E.; Mather, R.T.; Rybicki, F.J. CT Angiography: Current Technology and Clinical Use. Radiol. Clin. North Am. 2010, 48, 213–235. [Google Scholar] [CrossRef]
- Wüst, R.C.I.; Calcagno, C.; Daal, M.R.R.; Nederveen, A.J.; Coolen, B.F.; Strijkers, G.J. Emerging Magnetic Resonance Imaging Techniques for Atherosclerosis Imaging High Magnetic Field, Relaxation Time Mapping, and Fluorine-19 Imaging. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 841–849. [Google Scholar] [CrossRef]
- Frias, J.C.; Ma, Y.Q.; Williams, K.J.; Fayad, Z.A.; Fisher, E.A. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006, 6, 2220–2224. [Google Scholar] [CrossRef]
- Qi, J.; Sun, C.W.; Li, D.Y.; Zhang, H.Q.; Yu, W.B.; Zebibula, A.; Lam, J.W.Y.; Xi, W.; Zhu, L.; Cai, F.H.; et al. Aggregation-Induced Emission Luminogen with Near-Infrared-II Excitation and Near-Infrared-I Emission for Ultradeep Intravital Two-Photon Microscopy. Acs Nano 2018, 12, 7936–7945. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, M.D.; Parker, I.; Wei, S.H.; Miller, M.J. Two-photon tissue imaging: Seeing the immune system in a fresh light. Nat. Rev. Immunol. 2002, 2, 872–880. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.X.; Xu, H.; Zhuang, W.H.; Wang, Y.N.; Li, G.C.; Wang, Y.B. ROS Responsive Nanoplatform with Two-Photon AIE Imaging for Atherosclerosis Diagnosis and “Two-Pronged” Therapy. Small 2020, 16, e2003253. [Google Scholar] [CrossRef]
- Nezu, T.; Hosomi, N.; Aoki, S.; Matsumoto, M. Carotid Intima-Media Thickness for Atherosclerosis. J. Atheroscler. Thromb. 2016, 23, 18–31. [Google Scholar] [CrossRef]
- Mehta, K.S.; Lee, J.J.; Taha, A.A.; Avgerinos, E.; Chaer, R.A. Vascular applications of contrast-enhanced ultrasound imaging. J. Vasc. Surg. 2017, 66, 266–274. [Google Scholar] [CrossRef]
- Dayton, P.A.; Rychak, J.J. Molecular ultrasound imaging using microbubble contrast agents. Front. Biosci. 2007, 12, 5124–5142. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.Y.; Li, Y.; Yu, L.D.; Chen, Y.; Zhang, B. A dual-targeting therapeutic nanobubble for imaging-guided atherosclerosis treatment. Mater. Today Bio 2024, 26, 101037. [Google Scholar] [CrossRef]
- Xie, Z.H.; Yang, Y.Q.; He, Y.Q.; Shu, C.Y.; Chen, D.; Zhang, J.K.; Chen, J.Q.; Liu, C.B.; Sheng, Z.H.; Liu, H.D.; et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging. Theranostics 2020, 10, 4694–4704. [Google Scholar] [CrossRef]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical Applications of Photoacoustic Imaging with Exogenous Contrast Agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; She, P.Y.; Zhao, Z.Y.; Ma, B.X.; Li, G.C.; Wang, Y.B. Duplex Responsive Nanoplatform with Cascade Targeting for Atherosclerosis Photoacoustic Diagnosis and Multichannel Combination Therapy. Adv. Mater. 2023, 35, e2300439. [Google Scholar] [CrossRef]
- Fan, J.L.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chilbert, M.R.; VanDuyn, D.; Salah, S.; Clark, C.M.; Ma, Q. Combination Therapy of Ezetimibe and Rosuvastatin for Dyslipidemia: Current Insights. Drug Des. Dev. Ther. 2022, 16, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xu, L.; Zhao, M.X.; Kong, F.; Lu, X.R.; Tang, C.; Yin, C.H. Dual targeted delivery of statins and nucleic acids by chitosan-based nanoparticles for enhanced antiatherosclerotic efficacy. Biomaterials 2022, 280, 121324. [Google Scholar] [CrossRef]
- He, H.L.; Wang, J.; Yannie, P.J.; Korzun, W.J.; Yang, H.; Ghosh, S. Nanoparticle-based “Two-pronged” approach to regress atherosclerosis by simultaneous modulation of cholesterol influx and efflux. Biomaterials 2020, 260, 120333. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; Grp, C.T. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.Y.; Liang, X.Y.; Heger, Z.; Xu, M.; Lu, Q.; Yu, M.; Adam, V.; Li, N. Turning Hot into Cold: Immune Microenvironment Reshaping for Atherosclerosis Attenuation Based on pH-Responsive shSiglec-1 Delivery System. Acs Nano 2022, 16, 10517–10533. [Google Scholar] [CrossRef]
- Li, X.T.; Gu, J.Y.; Xiao, Q.Q.; Liu, Y.; Zhou, P.; Fan, L.F.; Zhang, X.L.; Lu, X.; Wu, J.; Liu, Z.X.; et al. Liposomal codelivery of inflammation inhibitor and collagen protector to the plaque for effective anti-atherosclerosis. Chin. Chem. Lett. 2023, 34, 107483. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; El Andaloussi, S.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.J.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, EAAU6977. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as Cell-Cell Messengers in Cardiovascular Diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.A.A.; van Solinge, W.W.; Wood, M.J.A.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release 2012, 161, 635–644. [Google Scholar] [CrossRef]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Chavda, V.P.; Pandya, A.; Kumar, L.; Raval, N.; Vora, L.K.; Pulakkat, S.; Patravale, V.; Duo, Y.; Tang, B.Z. Exosome nanovesicles: A potential carrier for therapeutic delivery. Nano Today 2023, 49, 101771. [Google Scholar] [CrossRef]
- Mendonca, A.; Acharjee, A.; Kumar, J.S.; Sundaresan, S. Comparative analysis of exosomes isolated by ultracentrifugation and total exosome isolation reagent: A biophysical and physicochemical study. J. Nanoparticle Res. 2025, 27, 183490. [Google Scholar] [CrossRef]
- Asaadi, A.; Dolatabad, N.A.; Atashi, H.; Raes, A.; Van Damme, P.; Hoelker, M.; Hendrix, A.; Pascottini, O.B.; Van Soom, A.; Kafi, M.; et al. Extracellular Vesicles from Follicular and Ampullary Fluid Isolated by Density Gradient Ultracentrifugation Improve Bovine Embryo Development and Quality. Int. J. Mol. Sci. 2021, 22, 578. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, D.; Shah, R.; van Hout, I.; De Jonge, W.; Bunton, R.; Parry, D.; Davis, P.; Katare, R. Combination of precipitation and size exclusion chromatography as an effective method for exosome like extracellular vesicle isolation from pericardial fluids. Nanotheranostics 2023, 7, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.L.; Liu, Y.N.; Yuan, L.J. Exosomes in atherosclerosis: Performers, bystanders, biomarkers, and therapeutic targets. Theranostics 2021, 11, 3996–4010. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Gao, H.L.; Wang, X.L.; Lin, C.L.; An, Z.J.; Yu, J.B.; Cao, H.Y.; Fan, Y.; Liang, X. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol. Chem. 2020, 401, 367–376. [Google Scholar] [CrossRef]
- Wang, F.; Chen, F.F.; Shang, Y.Y.; Li, Y.; Wang, Z.H.; Han, L.; Li, Y.H.; Zhang, L.; Ti, Y.; Zhang, W.; et al. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE−/− mice. Int. J. Cardiol. 2018, 265, 181–187. [Google Scholar] [CrossRef]
- Chen, L.; Hu, L.Q.; Li, Q.; Ma, J.; Li, H.Q. Exosome-encapsulated miR-505 from ox-LDL-treated vascular endothelial cells aggravates atherosclerosis by inducing NET formation. Acta Biochim. Et Biophys. Sin. 2019, 51, 1233–1241. [Google Scholar] [CrossRef]
- Yaker, L.; Tebani, A.; Lesueur, C.; Dias, C.; Jung, V.C.; Bekri, S.; Guerrera, I.C.; Kamel, S.; Ausseil, J.; Boullier, A. Extracellular Vesicles From LPS-Treated Macrophages Aggravate Smooth Muscle Cell Calcification by Propagating Inflammation and Oxidative Stress. Front. Cell Dev. Biol. 2022, 10, 823450. [Google Scholar] [CrossRef]
- Li, K.; Cui, M.Z.; Zhang, K.W.; Wang, G.Q.; Zhai, S.T. M1 macrophages-derived extracellular vesicles elevate microRNA-185-3p to aggravate the development of atherosclerosis in ApoE-/- mice by inhibiting small mothers against decapentaplegic 7. Int. Immunopharmacol. 2021, 90, 107138. [Google Scholar] [CrossRef]
- Yang, H.Q.; Chen, J.Y.; Liu, S.Y.; Xue, Y.F.; Li, Z.W.; Wang, T.; Jiao, L.Q.; An, Q.; Liu, B.; Wang, J.; et al. Exosomes From IgE-Stimulated Mast Cells Aggravate Asthma-Mediated Atherosclerosis Through circRNA CDR1as-Mediated Endothelial Cell Dysfunction in Mice. Arterioscler. Thromb. Vasc. Biol. 2024, 44, E99–E115. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, M.H.; Chen, E.Q.; Jiang, W.; Shi, W.D.; Wang, Z.Y. Bone marrow-derived mesenchymal stem cells microvesicles stabilize atherosclerotic plaques by inhibiting NLRP3-mediated macrophage pyroptosis. Cell Biol. Int. 2021, 45, 820–830. [Google Scholar] [CrossRef]
- Ko, K.W.; Yoo, Y.I.; Kim, J.Y.; Choi, B.; Park, S.B.; Park, W.; Rhim, W.K.; Han, D.K. Attenuation of Tumor Necrosis Factor-α Induced Inflammation by Umbilical Cord-Mesenchymal Stem Cell Derived Exosome-Mimetic Nanovesicles in Endothelial Cells. Tissue Eng. Regen. Med. 2020, 17, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Popov, M.A.; Utkina, A.S.; Babayeva, G.A.; Maksaeva, A.O.; Sukhorukov, V.N.; Orekhov, A.N. Preclinical and mechanistic perspectives on adipose-derived stem cells for atherosclerotic cardiovascular disease treatment. Mol. Cell. Biochem. 2025, 480, 4647–4670. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.H.; Li, Z.C.; Yang, X.; Li, M.Y.; Liu, C.; Pang, Y.J.; Zhang, L.Y.; Li, X.Y.; Liu, G.C.; Xiao, Y.L. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging-Us 2020, 12, 3880–3898. [Google Scholar] [CrossRef] [PubMed]
- He, Y.C.; Li, Q.F.; Feng, F.; Gao, R.P.; Li, H.D.; Chu, Y.X.; Li, S.B.; Wang, Y.; Mao, R.Y.; Ji, Z.Z.; et al. Extracellular vesicles produced by human-induced pluripotent stem cell-derived endothelial cells can prevent arterial stenosis in mice via autophagy regulation. Front. Cardiovasc. Med. 2022, 9, 922790. [Google Scholar] [CrossRef]
- Yan, W.H.; Li, T.H.; Yin, T.Y.; Hou, Z.J.; Qu, K.; Wang, N.; Durkan, C.; Dong, L.Q.; Qiu, J.H.; Gregersen, H.; et al. M2 macrophage-derived exosomes promote the c-KIT phenotype of vascular smooth muscle cells during vascular tissue repair after intravascular stent implantation. Theranostics 2020, 10, 10712–10728. [Google Scholar] [CrossRef]
- Chen, D.X.; Lu, C.H.; Na, N.; Yin, R.X.; Huang, F. Endothelial progenitor cell-derived extracellular vesicles: The world of potential prospects for the treatment of cardiovascular diseases. Cell Biosci. 2024, 14, 72. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dhawan, U.K.; Hussain, M.T.; Singh, P.; Bhagat, K.K.; Singhal, A.; Austin-Williams, S.; Sengupta, S.; Subramanian, M. Efferocytes release extracellular vesicles to resolve inflammation and tissue injury via prosaposin-GPR37 signaling. Cell Rep. 2023, 42, 112808. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Yang, J.X.; Fang, J.K.; Zhou, Y.P.; Candi, E.; Wang, J.H.; Hua, D.; Shao, C.S.; Shi, Y.F. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Dazzi, F.; English, K.; Farge, D.; Galipeau, J.; Horwitz, E.M.; Kadri, N.; Krampera, M.; Lalu, M.M.; Nolta, J.; et al. ISCT MSC committee statement on the US FDA approval of allogenic bone-marrow mesenchymal stromal cells. Cytotherapy 2025, 27, 413–416. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, L.; Zhu, X.; Li, Q.; Hu, L.Q.; Li, H.Q. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim. Et Biophys. Sin. 2021, 53, 1227–1236. [Google Scholar] [CrossRef]
- Mao, Y.W. Apoptotic cell-derived metabolites in efferocytosis-mediated resolution of inflammation. Cytokine Growth Factor Rev. 2021, 62, 42–53. [Google Scholar] [CrossRef]
- Thorp, E.; Subramanian, M.; Tabas, I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur. J. Immunol. 2011, 41, 2515–2518. [Google Scholar] [CrossRef]
- Lin, P.; Ji, H.H.; Li, Y.J.; Guo, S.D. Macrophage Plasticity and Atherosclerosis Therapy. Front. Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef]
- Bouchareychas, L.; Duong, P.; Covarrubias, S.; Alsop, E.; Phu, T.A.; Chung, A.; Gomes, M.; Wong, D.; Meechoovet, B.; Capili, A.; et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020, 32, 107881. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.K.; Lv, Y.H.; Zhang, Z.H.; He, T.; Hao, X.D.; Wang, S.; Wang, C.Q.; Meng, J.; Zhong, K.J.; et al. M2 Macrophage-Derived Exosomes Inhibit Atherosclerosis Progression by Regulating the Proliferation, Migration, and Phenotypic Transformation of Smooth Muscle Cells. Front. Biosci. 2024, 29, 288. [Google Scholar] [CrossRef]
- Kong, J.; Wang, F.; Zhang, J.B.; Cui, Y.Y.; Pan, L.; Zhang, W.J.; Wen, J.Y.; Liu, P. Exosomes of Endothelial Progenitor Cells Inhibit Neointima Formation After Carotid Artery Injury. J. Surg. Res. 2018, 232, 398–407. [Google Scholar] [CrossRef]
- Bai, S.W.; Yin, Q.Q.; Dong, T.; Dai, F.; Qin, Y.; Ye, L.; Du, J.; Zhang, Q.; Chen, H.B.; Shen, B. Endothelial progenitor cell-derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomed. Pharmacother. 2020, 131, 110756. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.N.; Zhang, J.; Chen, X.; Zhang, Z.W.; Li, Q. Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 2021, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.X.; Li, L.J. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.C.; Wu, W.; Huang, W.T.; Fang, J.F.; Chen, Y.L.; Chen, X.Y.; Lin, X.L.; He, Y.B. Exosomes derived from baicalin-pretreated mesenchymal stem cells mitigate atherosclerosis by regulating the SIRT1/NF-κB signaling pathway. Mol. Med. Rep. 2025, 31, 126. [Google Scholar] [CrossRef]
- Li, C.L.; Fang, F.; Wang, E.R.; Yang, H.Q.; Yang, X.R.; Wang, Q.W.; Si, L.L.; Zhang, Z.; Liu, X.H. Engineering extracellular vesicles derived from endothelial cells sheared by laminar flow for anti-atherosclerotic therapy through reprogramming macrophage. Biomaterials 2025, 314, 122832. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, Y.X.; Zhang, H.P.; Zhang, F.; Gao, X.; Shao, J.W. Exosomes Derived from Mesenchymal Stem Cells Ameliorate the Progression of Atherosclerosis in ApoE-/- Mice via FENDRR. Cardiovasc. Toxicol. 2022, 22, 528–544. [Google Scholar] [CrossRef]
- Yang, W.Z.; Yin, R.H.; Zhu, X.Y.; Yang, S.N.; Wang, J.; Zhou, Z.F.; Pan, X.D.; Ma, A.J. Mesenchymal stem-cell-derived exosomal miR-145 inhibits atherosclerosis by targeting JAM-A. Mol. Ther. Nucleic Acids 2021, 23, 119–131. [Google Scholar] [CrossRef]
- Li, J.B.; Xue, H.; Li, T.T.; Chu, X.L.; Xin, D.Q.; Xiong, Y.; Qiu, W.; Gao, X.; Qian, M.Y.; Xu, J.Y.; et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE-/- mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem. Biophys. Res. Commun. 2019, 510, 565–572. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, Y.X.; Shao, J.W.; Sun, H.H.; Ma, K.; Gao, X. Exosomal long non-coding RNA AU020206 alleviates macrophage pyroptosis in atherosclerosis by suppressing CEBPB-mediated NLRP3 transcription. Exp. Cell Res. 2024, 438, 114054. [Google Scholar] [CrossRef]
- Bai, Z.B.; Hu, H.L.; Hu, F.F.; Ji, J.J.; Ji, Z.L. Bone marrow mesenchymal stem cellsderived exosomes stabilize atherosclerosis through inhibiting pyroptosis. Bmc Cardiovasc. Disord. 2023, 23, 441. [Google Scholar] [CrossRef]
- Liu, L.B.; An, Z.Y.; Zhang, H.; Wan, X.Q.; Zhao, X.; Yang, X.Y.; Tian, J.F.; Song, X.T. Bone marrow mesenchymal stem cell-derived extracellular vesicles alleviate diabetes-exacerbated atherosclerosis via AMPK/mTOR pathway-mediated autophagy-related macrophage polarization. Cardiovasc. Diabetol. 2025, 24, 48. [Google Scholar] [CrossRef]
- Han, G.C.; Li, H.; Guo, H.Y.; Yi, C.; Yu, B.G.; Lin, Y.; Zheng, B.J.; He, D.R. The roles and mechanisms of miR-26 derived from exosomes of adipose-derived stem cells in the formation of carotid atherosclerotic plaque. Ann. Transl. Med. 2022, 10, 1134. [Google Scholar] [CrossRef]
- Gao, H.; Yu, Z.B.; Li, Y.Y.; Wang, X. miR-100-5p in human umbilical cord mesenchymal stem cell-derived exosomes mediates eosinophilic inflammation to alleviate atherosclerosis via the FZD5/Wnt/β-catenin pathway. Acta Biochim. Et Biophys. Sin. 2021, 53, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Le, A.H.; Dang, C.P.; Chong, S.Y.; Do, D.V.; Peng, B.Y.; Jayasinghe, M.K.; Ong, H.B.; Hoang, D.V.; Louise, R.A.; et al. Endocytosis of red blood cell extracellular vesicles by macrophages leads to cytoplasmic heme release and prevents foam cell formation in atherosclerosis. J. Extracell. Vesicles 2023, 12, e12354. [Google Scholar] [CrossRef] [PubMed]
- Brancolini, A.; Vago, R. Investigating the Potential of Extracellular Vesicles as Delivery Systems for Chemotherapeutics. Biomedicines 2024, 12, 2863. [Google Scholar] [CrossRef] [PubMed]
- Torabi, C.; Choi, S.E.; Pisanic, T.R.; Paulaitis, M.; Hur, S.C. Streamlined miRNA loading of surface protein-specific extracellular vesicle subpopulations through electroporation. Biomed. Eng. Online 2024, 23, 116. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, L.T.; Guo, H.Y.; Chen, L.L.; Huang, X. Dapagliflozin-Loaded Exosome Mimetics Facilitate Diabetic Wound Healing by HIF-1a-Mediated Enhancement of Angiogenesis. Adv. Healthc. Mater. 2023, 12, e2202751. [Google Scholar] [CrossRef]
- Hajipour, H.; Farzadi, L.; Roshangar, L.; Latifi, Z.; Kahroba, H.; Shahnazi, V.; Hamdi, K.; Ghasemzadeh, A.; Fattahi, A.; Nouri, M. A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci. 2021, 275, 119351. [Google Scholar] [CrossRef]
- Chen, C.X.; Sun, M.D.; Wang, J.L.; Su, L.Y.; Lin, J.J.; Yan, X.M. Active cargo loading into extracellular vesicles: Highlights the heterogeneous encapsulation behaviour. J. Extracell. Vesicles 2021, 10, e12163. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.R.; Wang, Q.Q.; Cai, N.G.; Wu, L.N.; Yan, X.M. Single-particle assessment of six different drug-loading strategies for incorporating doxorubicin into small extracellular vesicles. Anal. Bioanal. Chem. 2023, 415, 1287–1298. [Google Scholar] [CrossRef]
- Lu, X.L.; Fan, S.Y.; Cao, M.; Liu, D.M.; Xuan, K.; Liu, A.Q. Extracellular vesicles as drug delivery systems in therapeutics: Current strategies and future challenges. J. Pharm. Investig. 2024, 54, 785–802. [Google Scholar] [CrossRef]
- Stranford, D.M.; Simons, L.M.; Berman, K.E.; Cheng, L.Y.; Dibiase, B.N.; Hung, M.E.; Lucks, J.B.; Hultquist, J.F.; Leonard, J.N. Genetically encoding multiple functionalities into extracellular vesicles for the targeted delivery of biologics to T cells. Nat. Biomed. Eng. 2024, 8, 397–414. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Encabo-Berzosa, M.D.; Beltrán-Visiedo, M.; Fernández-Messina, L.; Sebastián, V.; Sánchez-Madrid, F.; Arruebo, M.; Santamaría, J.; Martín-Duque, P. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: Leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale 2019, 11, 18825–18836. [Google Scholar] [CrossRef]
- Erana-Perez, Z.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Genetically engineered loaded extracellular vesicles for drug delivery. Trends Pharmacol. Sci. 2024, 45, 350–365. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, H.; Liu, J.Y.; Chen, J.Y.; Cui, Y.X.; Wang, S.M.; Zhang, X.Y.; Yang, Z.G. Extracellular Vesicles: A New Star for Gene Drug Delivery. Int. J. Nanomed. 2024, 19, 2241–2264. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, Z.L.; Hou, Y.; Sun, W.Q.; Zhang, R.X.; Zhao, L.B.; Wei, M.Y.; Yang, G.D.; Yuan, L.J. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef] [PubMed]
- Lennaard, A.J.; Mamand, D.R.; Wiklander, R.J.; El Andaloussi, S.; Wiklander, O.P.B. Optimised Electroporation for Loading of Extracellular Vesicles with Doxorubicin. Pharmaceutics 2022, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.H.; Zhang, J.F.; Zhao, Q.R.; Zhuang, W.R.; Ding, J.J.; Zhang, C.; Gao, H.J.; Pang, D.W.; Pu, K.Y.; Xie, H.Y. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew. Chem. Int. Ed. 2020, 59, 4068–4074. [Google Scholar] [CrossRef]
- Hood, J.L.; Scott, M.J.; Wickline, S.A. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014, 448, 41–49. [Google Scholar] [CrossRef]
- Ma, Q.L.; Fan, Q.; Han, X.; Dong, Z.L.; Xu, J.L.; Bai, J.Y.; Tao, W.W.; Sun, D.D.; Wang, C. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J. Control. Release 2021, 329, 445–453. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, G.B.; Dai, Z.C.; Yang, R.Q.; Zhang, Y.; Zhang, Y.L.; Shen, H.L.; Pu, Z.; Ma, L.Y.; Li, S. Artificial Cell-Derived Vesicles: Extracellular Vesicle Mimetics for Chondrocyte Restoration in TMJOA Therapy. Int. J. Nanomed. 2025, 20, 5393–5405. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.X.; Zhang, R.; Feng, H.X.; Zhou, H.; Luo, Y.; Xiong, W.; Li, J.Y.; He, Y.; Ye, Q.S. A new subtype of artificial cell-derived vesicles from dental pulp stem cells with the bioequivalence and higher acquisition efficiency compared to extracellular vesicles. J. Extracell. Vesicles 2024, 13, e12473. [Google Scholar] [CrossRef]
- Liu, W.S.; Wu, L.L.; Chen, C.M.; Zheng, H.; Gao, J.; Lu, Z.M.; Li, M. Lipid-hybrid cell-derived biomimetic functional materials: A state-of-the-art multifunctional weapon against tumors. Mater. Today Bio 2023, 22, 100751. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Park, B.; Park, J.C.; Jin, H.; Shim, J.S.; Koo, J.; Lee, K.H.; Shim, M.K.; Kim, H. Exosome-Inspired Lipid Nanoparticles for Enhanced Tissue Penetration. Acs Nano 2025, 19, 8882–8894. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Xie, L.; Chen, J.Y.; Hu, H.C.; Zhu, Y.; Wang, X.Y.; Zhou, S.Y.; Wang, F.F.; Xiang, M.X. Engineered M2 macrophage-derived extracellular vesicles with platelet membrane fusion for targeted therapy of atherosclerosis. Bioact. Mater. 2024, 35, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Huang, Z.Y.; Pang, Z.Q.; Wang, Q.Z.; Gao, J.F.; Chen, J.; Wang, Z.M.; Tan, H.P.; Li, S.; Xu, F.; et al. Targeted delivery of platelet membrane modified extracellular vesicles into atherosclerotic plaque to regress atherosclerosis. Chem. Eng. J. 2023, 452, 138992. [Google Scholar] [CrossRef]
- Alter, C.L.; Lotter, C.; Puligilla, R.D.; Bolten, J.S.; Sedzicki, J.; Marchese, J.; Schittny, V.; Rucci, F.; Beverly, M.; Palivan, C.G.; et al. Nano Plasma Membrane Vesicle-Lipid Nanoparticle Hybrids for Enhanced Gene Delivery and Expression. Adv. Healthc. Mater. 2025, 14, e2401888. [Google Scholar] [CrossRef]
- Zheng, J.R.; Li, Y.Y.; Ge, X.F.; Zhang, G.M.; Wang, Y.; Nawsherwan; Yu, S.J.; Dai, C.L.; Sang, M.M. Devouring Atherosclerotic Plaques: The Engineered Nanorobot Rousing Macrophage Efferocytosis by a Two-Pronged Strategy. Adv. Funct. Mater. 2025, 35, 2415477. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, J.H.; Chen, J.M.; Li, Y.N.; Bu, T.; Li, Z.L.; Zhang, L.; Sun, W.Q.; Zhou, T.; Hu, W.; et al. Targeted delivery of MerTK protein via cell membrane engineered nanoparticle enhances efferocytosis and attenuates atherosclerosis in diabetic ApoE-/- Mice. J. Nanobiotechnol. 2024, 22, 178. [Google Scholar] [CrossRef]
- Palmerini, T.; Benedetto, U.; Biondi-Zoccai, G.; Della Riva, D.; Bacchi-Reggiani, L.; Smits, P.C.; Vlachojannis, G.J.; Jensen, L.O.; Christiansen, E.H.; Berencsi, K.; et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents Evidence From a Comprehensive Network Meta-Analysis. J. Am. Coll. Cardiol. 2015, 65, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Korjian, S.; Tricoci, P.; Daaboul, Y.; Yee, M.; Jain, P.; Alexander, J.H.; Steg, P.G.; Lincoff, A.M.; Kastelein, J.J.P.; et al. Safety and Tolerability of CSL112, a Reconstituted, Infusible, Plasma-Derived Apolipoprotein A-I, After Acute Myocardial Infarction The AEGIS-I Trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation 2016, 134, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Duffy, D.; Korjian, S.; Bahit, M.C.; Chi, G.; Alexander, J.H.; Lincoff, A.M.; Heise, M.; Tricoci, P.; Deckelbaum, L.I.; et al. Apolipoprotein A1 Infusions and Cardiovascular Outcomes after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1560–1571. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhao, X.; Xu, X.; A, L.; Liu, Q.; Qu, P. A Bibliometric Analysis of Strategies for Atherosclerosis Treatment with Organic Nanoparticles. Pharmaceutics 2025, 17, 1131. https://doi.org/10.3390/pharmaceutics17091131
Ma J, Zhao X, Xu X, A L, Liu Q, Qu P. A Bibliometric Analysis of Strategies for Atherosclerosis Treatment with Organic Nanoparticles. Pharmaceutics. 2025; 17(9):1131. https://doi.org/10.3390/pharmaceutics17091131
Chicago/Turabian StyleMa, Jizhuang, Xia Zhao, Xinwen Xu, Lixin A, Qiang Liu, and Peng Qu. 2025. "A Bibliometric Analysis of Strategies for Atherosclerosis Treatment with Organic Nanoparticles" Pharmaceutics 17, no. 9: 1131. https://doi.org/10.3390/pharmaceutics17091131
APA StyleMa, J., Zhao, X., Xu, X., A, L., Liu, Q., & Qu, P. (2025). A Bibliometric Analysis of Strategies for Atherosclerosis Treatment with Organic Nanoparticles. Pharmaceutics, 17(9), 1131. https://doi.org/10.3390/pharmaceutics17091131






