Compatibility Studies of Sildenafil-HPBCD Inclusion Complex with Pharmaceutical Excipients
Abstract
1. Introduction
1.1. Current Level of Knowledge Regarding the ICs of SC
1.2. Purpose of the Study
2. Materials and Methods
2.1. Molecular Modeling
2.2. Samples and Preparation
2.3. Stoichiometry and Stability Constant Determination
2.4. ATR–FTIR Investigations
2.5. Thermal Investigations
3. Results and Discussion
3.1. Molecular Modeling
3.2. Stoichiometry and Stability Constant Determination
3.3. ATR–FTIR Investigations
3.4. Thermal Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAH | Pulmonary arterial hypertension |
| SC | Sildenafil citrate |
| HPBCD | 2-Hydroxypropyl-beta-cyclodextrin |
| CD | Cyclodextrin |
| IC | Inclusion complex |
| BM | Binary mixture |
| SiO2 | Aerosil |
| PVP | Polyvinylpyrrolidone K30 |
| CaL | Calcium lactate pentahydrate |
| Met | Methocel |
| MNT | D-Mannitol |
| LGA | Lamarckian Genetic Algorithm |
References
- Ghofrani, H.A.; Osterloh, I.H.; Grimminger, F. Sildenafil: From Angina to Erectile Dysfunction to Pulmonary Hypertension and Beyond. Nat. Rev. Drug Discov. 2006, 5, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A. Revised Definition of Pulmonary Hypertension and Approach to Management: A Clinical Primer. J. Am. Heart Assoc. 2023, 12, e029024. [Google Scholar] [CrossRef]
- Galiè, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil Citrate Therapy for Pulmonary Arterial Hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Ewert, R.; Jansa, P.; Sirenko, Y.; Skride, A.; Balagtas, C.; Hackley, S.; Vogt, S.; Abreu, P.; Haughie, S.; et al. Randomized, Multicenter Study to Assess the Effects of Different Doses of Sildenafil on Mortality in Adults With Pulmonary Arterial Hypertension. Circulation 2024, 149, 1949–1959. [Google Scholar] [CrossRef]
- Sastry, B.K.S.; Narasimhan, C.; Reddy, N.K.; Raju, B.S. Clinical Efficacy of Sildenafil in Primary Pulmonary Hypertension: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. J. Am. Coll. Cardiol. 2004, 43, 1149–1153. [Google Scholar] [CrossRef]
- Michelakis, E.; Tymchak, W.; Lien, D.; Webster, L.; Hashimoto, K.; Archer, S. Oral Sildenafil Is an Effective and Specific Pulmonary Vasodilator in Patients with Pulmonary Arterial Hypertension: Comparison with Inhaled Nitric Oxide. Circulation 2002, 105, 2398–2403. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Respir. J. 2023, 61, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- REVATIO (Sildenafil) Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021845s20_022473s11_203109s11lbl.pdf (accessed on 2 February 2025).
- Barnett, C.F.; Machado, R.F. Sildenafil in the Treatment of Pulmonary Hypertension. Vasc. Health Risk Manag. 2006, 2, 411–422. [Google Scholar] [CrossRef]
- Bertici, R.A.; Bertici, N.S.; Ridichie, A.; Fira-Mladinescu, O. Comorbidities, Treatment and Survival Rates of Chronic Thromboembolic Pulmonary Hypertension in a Regional Centre. J. Clin. Med. 2024, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.P.; Babos, M. Sildenafil. [Updated 14 February 2023]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558978/ (accessed on 2 February 2025).
- Webb, A.J.S.; Birks, J.S.; Feakins, K.A.; Lawson, A.; Dawson, J.; Rothman, A.M.K.; Werring, D.J.; Llwyd, O.; Stewart, C.R.; Thomas, J. Cerebrovascular Effects of Sildenafil in Small Vessel Disease: The OxHARP Trial. Circ. Res. 2024, 135, 320–331. [Google Scholar] [CrossRef]
- Xiong, Y.; Wintermark, P. The Role of Sildenafil in Treating Brain Injuries in Adults and Neonates. Front. Cell Neurosci. 2022, 16, 879649. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Zhang, L.; Zhang, Z.; Tsang, W.; Lu, M.; Zhang, L.; Chopp, M. Sildenafil (Viagra) Induces Neurogenesis and Promotes Functional Recovery after Stroke in Rats. Stroke 2002, 33, 2675–2680. [Google Scholar] [CrossRef]
- Bertici, N.S.; Tudoran, C.; Bertici, R.A.; Fira-Mladinescu, O.; Jianu, D.C.; Streian, C.G.; Staicu, R.E.; Manzur, A.R.; Lascu, A. Concomitance of Pericardial Tamponade and Pulmonary Embolism in an Invasive Mucinous Lung Adenocarcinoma with Atypical Presentation: Diagnostic and Therapeutic Pitfalls—Case Report and Literature Review. Int. J. Mol. Sci. 2024, 25, 8413. [Google Scholar] [CrossRef]
- Chaumais, M.C.; Perrin, S.; Sitbon, O.; Simonneau, G.; Humbert, M.; Montani, D. Pharmacokinetic Evaluation of Sildenafil as a Pulmonary Hypertension Treatment. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1193–1205. [Google Scholar] [CrossRef]
- Muirhead, G.J.; Rance, D.J.; Walker, D.K.; Wastall, P. Comparative Human Pharmacokinetics and Metabolism of Single-Dose Oral and Intravenous Sildenafil Citrate. Br. J. Clin. Pharmacol. Suppl. 2002, 53, 13–20. [Google Scholar] [CrossRef]
- Miranda, C.; Pérez-Rodríguez, Z.; Hernández-Armengol, R.; Quiñones-García, Y.; Betancourt-Purón, T.; Cabrera-Pérez, M.Á. Biowaiver or Bioequivalence: Ambiguity in Sildenafil Citrate BCS Classification. AAPS PharmSciTech 2018, 19, 1693–1698. [Google Scholar] [CrossRef]
- Almutairi, M.; Hefnawy, A.; Almotairy, A.; Alobaida, A.; Alyahya, M.; Althobaiti, A.; Adel Ali Youssef, A.; Elkanayati, R.M.; Ashour, E.A.; Smyth, H.D.C.; et al. Formulation and Evaluation of Inhaled Sildenafil-Loaded PLGA Microparticles for Treatment of Pulmonary Arterial Hypertension (PAH): A Novel High Drug Loaded Formulation and Scalable Process via Hot Melt Extrusion Technology (Part I). Int. J. Pharm. 2024, 655, 124044. [Google Scholar] [CrossRef]
- Shahin, H.I.; Vinjamuri, B.P.; Mahmoud, A.A.; Shamma, R.N.; Mansour, S.M.; Ammar, H.O.; Ghorab, M.M.; Chougule, M.B.; Chablani, L. Design and Evaluation of Novel Inhalable Sildenafil Citrate Spray-Dried Microparticles for Pulmonary Arterial Hypertension. J. Control. Release 2019, 302, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Nurul Fitri, A.M.; Elim, D.; Sya’ban Mahfud, M.A.; Fitri Sultan, N.A.; Saputra, M.D.; Afika, N.; Friandini, R.A.; Natsir Djide, N.J.; Permana, A.D. Polymeric Hydrogel Forming Microneedle-Mediated Transdermal Delivery of Sildenafil Citrate from Direct-Compressed Tablet Reservoir for Potential Improvement of Pulmonary Hypertension Therapy. Int. J. Pharm. 2023, 631, 122549. [Google Scholar] [CrossRef] [PubMed]
- Almutairy, B.K.; Khafagy, E.S.; Aldawsari, M.F.; Alshetaili, A.; Alotaibi, H.F.; Abu Lila, A.S. Tailoring of Bilosomal Nanogel for Augmenting the Off-Label Use of Sildenafil Citrate in Pediatric Pulmonary Hypertension. ACS Omega 2024, 9, 19536–19547. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; El-Say, K.M.; Ahmed, O.A. Optimized Sildenafil Citrate Fast Orodissolvable Film: A Promising Formula for Overcoming the Barriers Hindering Erectile Dysfunction Treatment. Drug Deliv. 2016, 23, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Sawatdee, S.; Phetmung, H.; Srichana, T. Sildenafil Citrate Monohydrate-Cyclodextrin Nanosuspension Complexes for Use in Metered-Dose Inhalers. Int. J. Pharm. 2013, 455, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Sulistiawati, S.; Kristina Enggi, C.; Wiyulanda Iskandar, I.; Rachmad Saputra, R.; Sartini, S.; Rifai, Y.; Rahman, L.; Aswad, M.; Dian Permana, A. Bioavailability Enhancement of Sildenafil Citrate via Hydrogel-Forming Microneedle Strategy in Combination with Cyclodextrin Complexation. Int. J. Pharm. 2024, 655, 124053. [Google Scholar] [CrossRef]
- Al Omari, M.M.; Zughul, M.B.; Davies, J.E.D.; Badwan, A.A. Sildenafil/Cyclodextrin Complexation: Stability Constants, Thermodynamics, and Guest-Host Interactions Probed by 1H NMR and Molecular Modeling Studies. J. Pharm. Biomed. Anal. 2006, 41, 857–865. [Google Scholar] [CrossRef]
- Swart, H.; Steyn, K. Inclusion Complexes of Alpha-Cyclodextrin and Sildenafil Salt. U.S. Patent WO2010/070617AI, 24 June 2010. [Google Scholar]
- Matilainen, L.; Toropainen, T.; Vihola, H.; Hirvonen, J.; Järvinen, T.; Jarho, P.; Järvinen, K. In Vitro Toxicity and Permeation of Cyclodextrins in Calu-3 Cells. J. Control. Release 2008, 126, 10–16. [Google Scholar] [CrossRef]
- Atipairin, A.; Sawatdee, S. Development of Spray-Dried Sildenafil Citrate -α-Cyclodextrin Complexes for Use in Dry Powder Inhalers. Int. J. Pharm. Investig. 2020, 10, 93–95. [Google Scholar] [CrossRef]
- Suwandecha, T.; Rungnim, C.; Namuangruk, S.; Ruktanonchai, U.; Sawatdee, S.; Dechraksa, J.; Srichana, T. Host-Guest Interactions between Sildenafil and Cyclodextrins: Spectrofluorometric Study and Molecular Dynamic Modeling. J. Mol. Graph. Model. 2017, 77, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Júlio, T.A.; Zâmara, I.F.; Garcia, J.S.; Trevisan, M.G. Compatibility of Sildenafil Citrate and Pharmaceutical Excipients by Thermal Analysis and LC-UV. J. Therm. Anal. Calorim. 2013, 111, 2037–2044. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B.; Bajaj, A.N. Interactions and Incompatibilities of Pharmaceutical Excipients with Active Pharmaceutical Ingredients: A Comprehensive Review. J. Excip. Food Chem. 2016, 1, 3–26. [Google Scholar]
- MacCarthy, P. Simplified Experimental Route for Obtaining Job’s Curves. Anal. Chem. 1978, 50, 2165. [Google Scholar] [CrossRef]
- Hill, Z.D.; MacCarthy, P. Novel Approach to Job’s Method: An Undergraduate Experiment. J. Chem. Educ. 1986, 63, 162–167. [Google Scholar] [CrossRef]
- Andor, M.; Temereancă, C.; Sbârcea, L.; Ledeți, A.; Man, D.E.; Mornoș, C.; Ridichie, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; et al. Host–Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives. Molecules 2024, 29, 2209. [Google Scholar] [CrossRef]
- Toader, A.M.; Dascalu, I.; Ionita, G.; Angelescu, D.G.; Enache, M. Encapsulation of Quinizarin into Cucurbit [8] Uril Macrocycle. J. Mol. Liq. 2025, 434, 128044. [Google Scholar] [CrossRef]
- Agatić, Z.F.; Tepavčević, V.; Puača, G.; Poša, M. Interaction of Drug Molecules with Surfactants below (Benesi-Hildebrand Equation) and above the Critical Micelle Concentration (Kawamura Equation). Int. J. Pharm. 2024, 665, 124675. [Google Scholar] [CrossRef]
- Niţu, E.T.; Ridichie, A.; Temereancă, C.; Mitrofan, I.; Buliga, L.; Simu, S.; Muntean, C.; Rusu, G.; Ledeţi, I.; Ledeţi, A.; et al. Study of Carvedilol–β-Cyclodextrin Derivatives Interactions. Processes 2025, 13, 1141. [Google Scholar] [CrossRef]
- Bezerra, G.S.N.; Pereira, M.A.V.; Ostrosky, E.A.; Barbosa, E.G.; de Moura, M.D.F.V.; Ferrari, M.; Aragão, C.F.S.; Gomes, A.P.B. Compatibility Study between Ferulic Acid and Excipients Used in Cosmetic Formulations by TG/DTG, DSC and FTIR. J. Therm. Anal. Calorim. 2017, 127, 1683–1691. [Google Scholar] [CrossRef]
- Ridichie, A.; Bengescu, C.; Ledeţi, A.; Rusu, G.; Bertici, R.; Vlase, T.; Vlase, G.; Peter, F.; Ledeţi, I.; Rădulescu, M. Thermal Stability, Preformulation, and Kinetic Degradation Studies for Gestrinone. J. Therm. Anal. Calorim. 2024, 150, 6785–6799. [Google Scholar] [CrossRef]
- de Barros Lima, Í.P.; Lima, N.G.P.; Barros, D.M.; Oliveira, T.S.; Barbosa, E.G.; Gomes, A.P.B.; Ferrari, M.; do Nascimento, T.G.; Aragão, C.F. Compatibility Study of Tretinoin with Several Pharmaceutical Excipients by Thermal and Non-Thermal Techniques. J. Therm. Anal. Calorim. 2015, 120, 733–747. [Google Scholar] [CrossRef]
- Ridichie, A.; Ledeţi, A.; Sbârcea, L.; Rusu, G.; Muntean, C.; Cîrcioban, D.; Peter, F.; Ledeţi, I. Preformulation Studies of Levonorgestrel. J. Therm. Anal. Calorim. 2024, 150, 6717–6730. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, Y.; Su, D.; Wang, H.; Huang, X.; Niu, Y.; Ke, Q.; Xiao, Z.; Meng, Q. Study on Host-Guest Interaction of Aroma Compounds/γ-Cyclodextrin Inclusion Complexes. Lwt 2023, 178, 114589. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xia, N.; Wang, F.; Xie, C.; Ye, R.; Tang, H.; Zhang, H.; Liu, Y. Preparation and Characterization of Curcumin/β-Cyclodextrin Nanoparticles by Nanoprecipitation to Improve the Stability and Bioavailability of Curcumin. Lwt 2022, 171, 114149. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, T.-P.; Zou, W.; Wang, Y.; Wang, K.; Yang, Y.; Liu, C.; Tu, Z.; Liu, Q.; Yuan, Y. Formation of β-Cyclodextrin Inclusion Complexes with a Series of Structurally Related Parabens: Preparation, Physicochemical Characterization and Antifungal Properties. Carbohydr. Polym. Technol. Appl. 2025, 11, 100924. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical Properties of Catechin/β-Cyclodextrin Inclusion Complex Obtained via Co-Precipitation. CYTA–J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef]
- Melnikov, P.; Corbi, P.P.; Cuin, A.; Cavicchioli, M.; Guimarães, W.R. Physicochemical Properties of Sildenafil Citrate (Viagra) and Sildenafil Base. J. Pharm. Sci. 2003, 92, 2140–2143. [Google Scholar] [CrossRef]
- PubChem Mannitol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Mannitol (accessed on 1 January 2025).

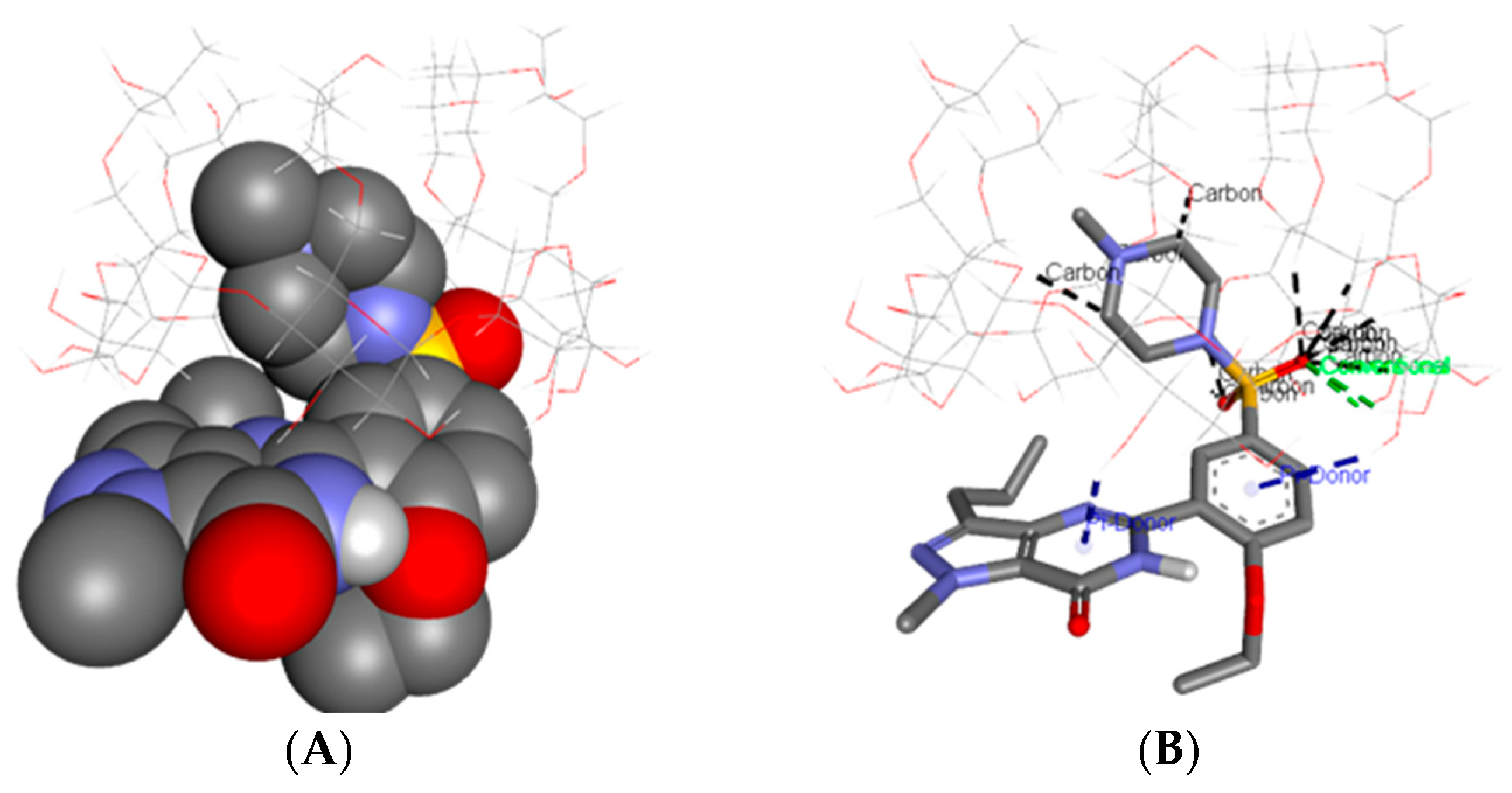
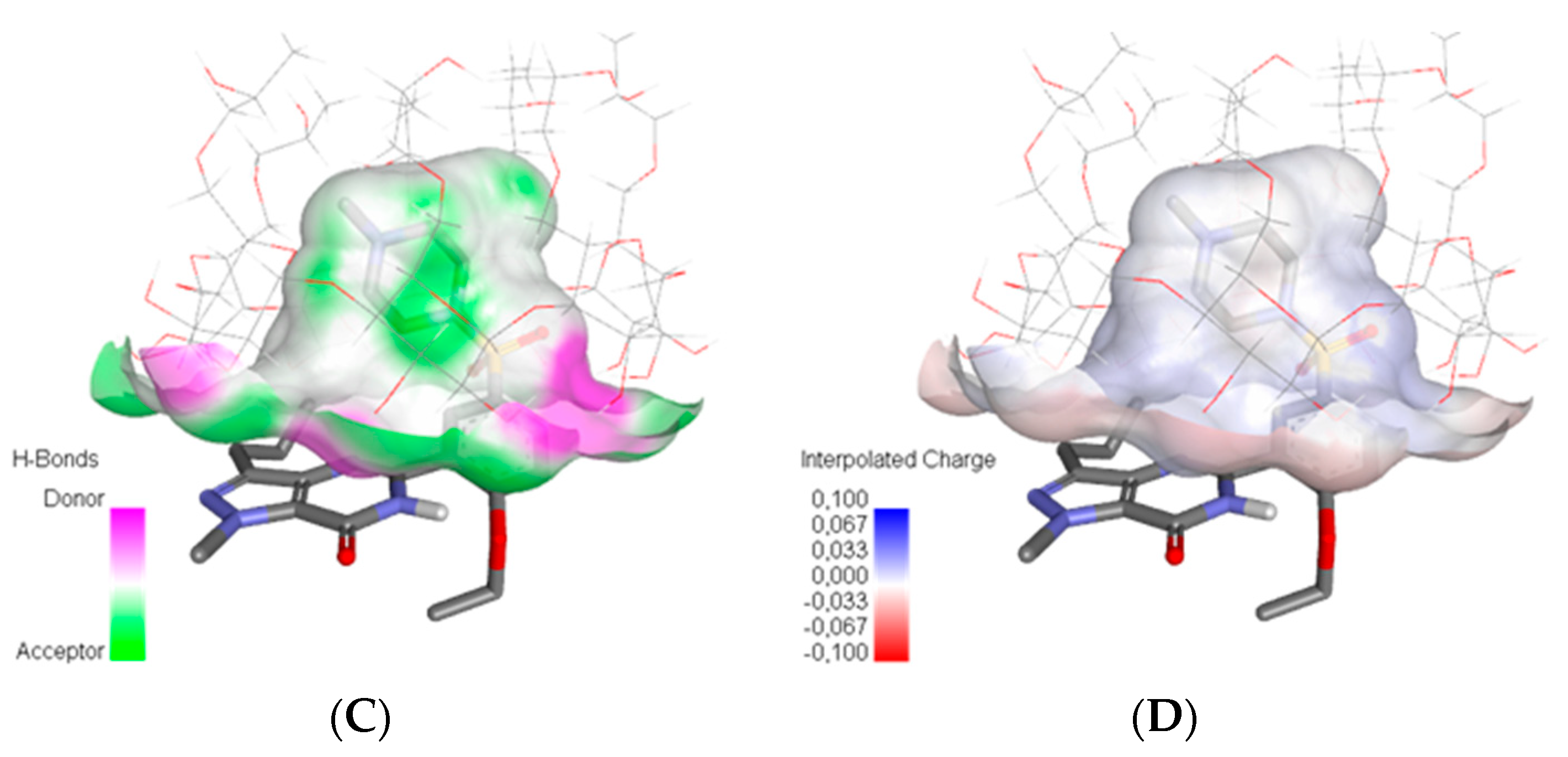
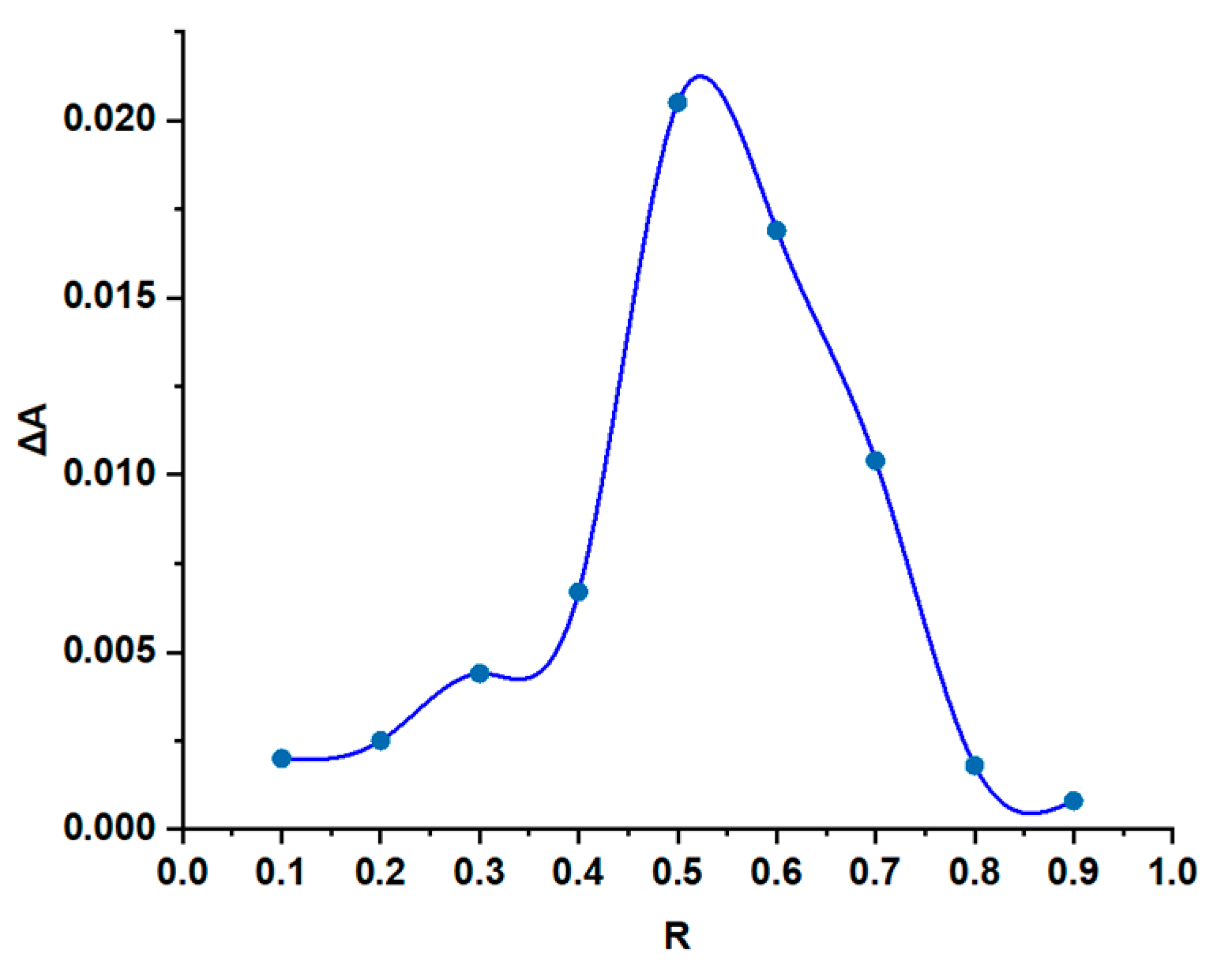
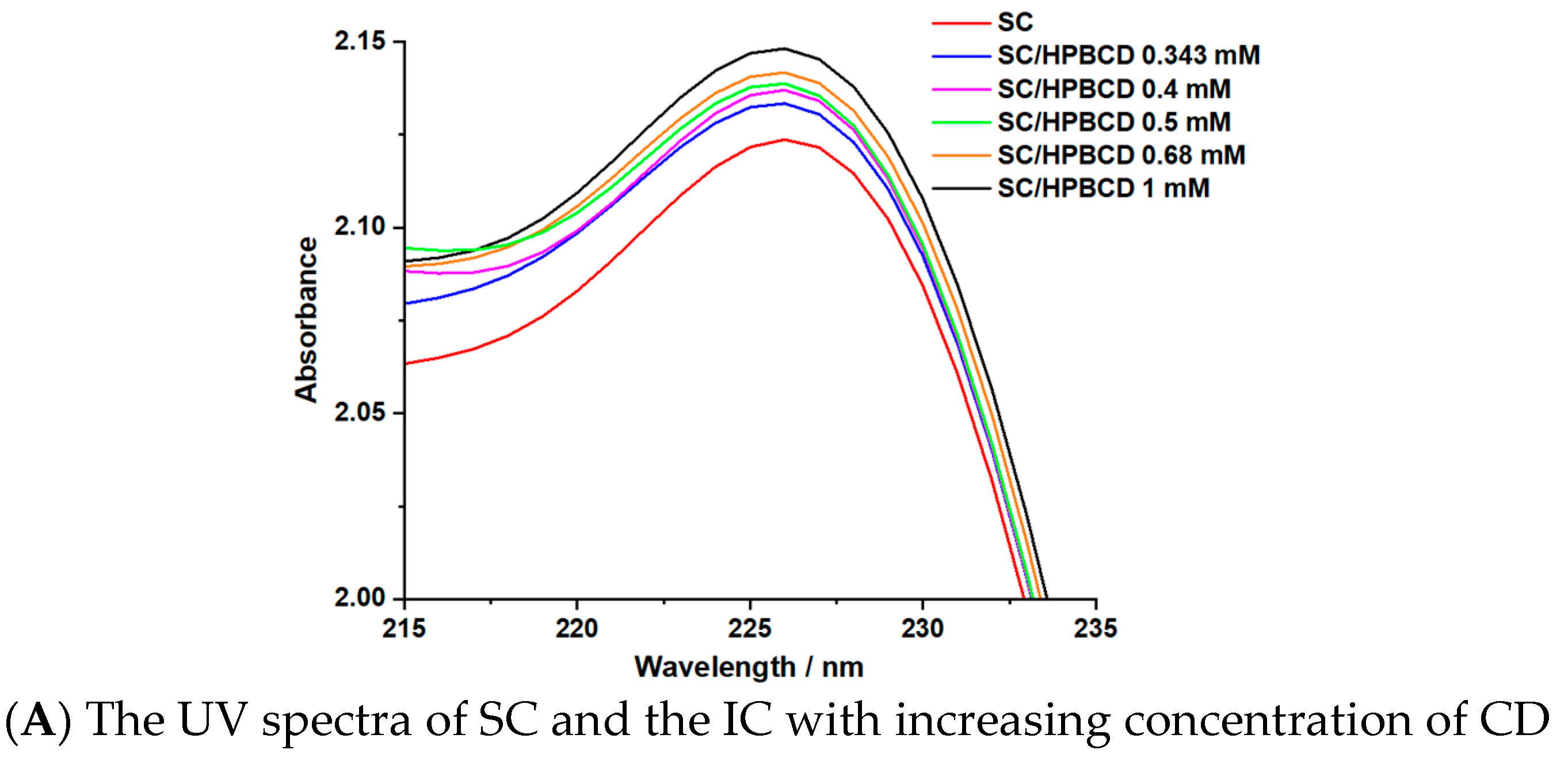
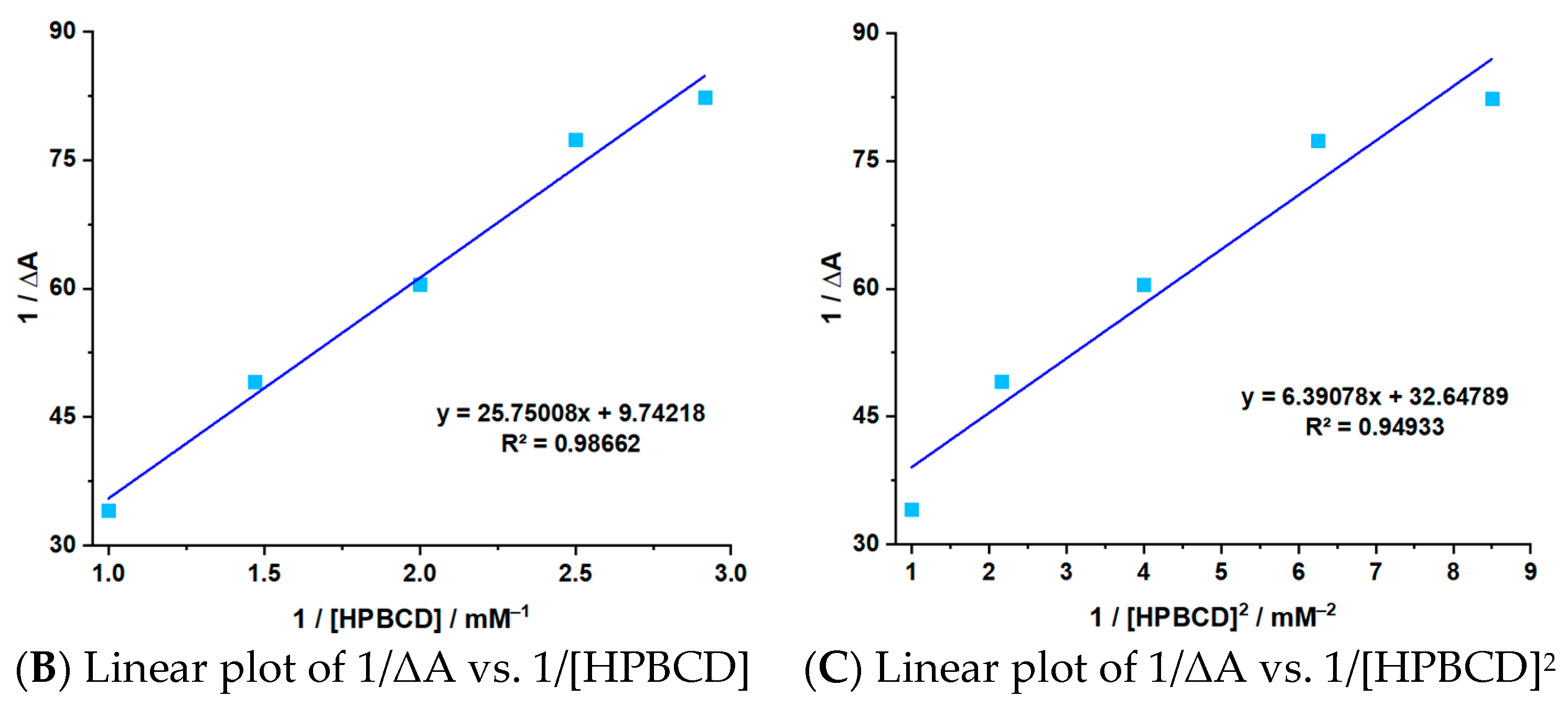
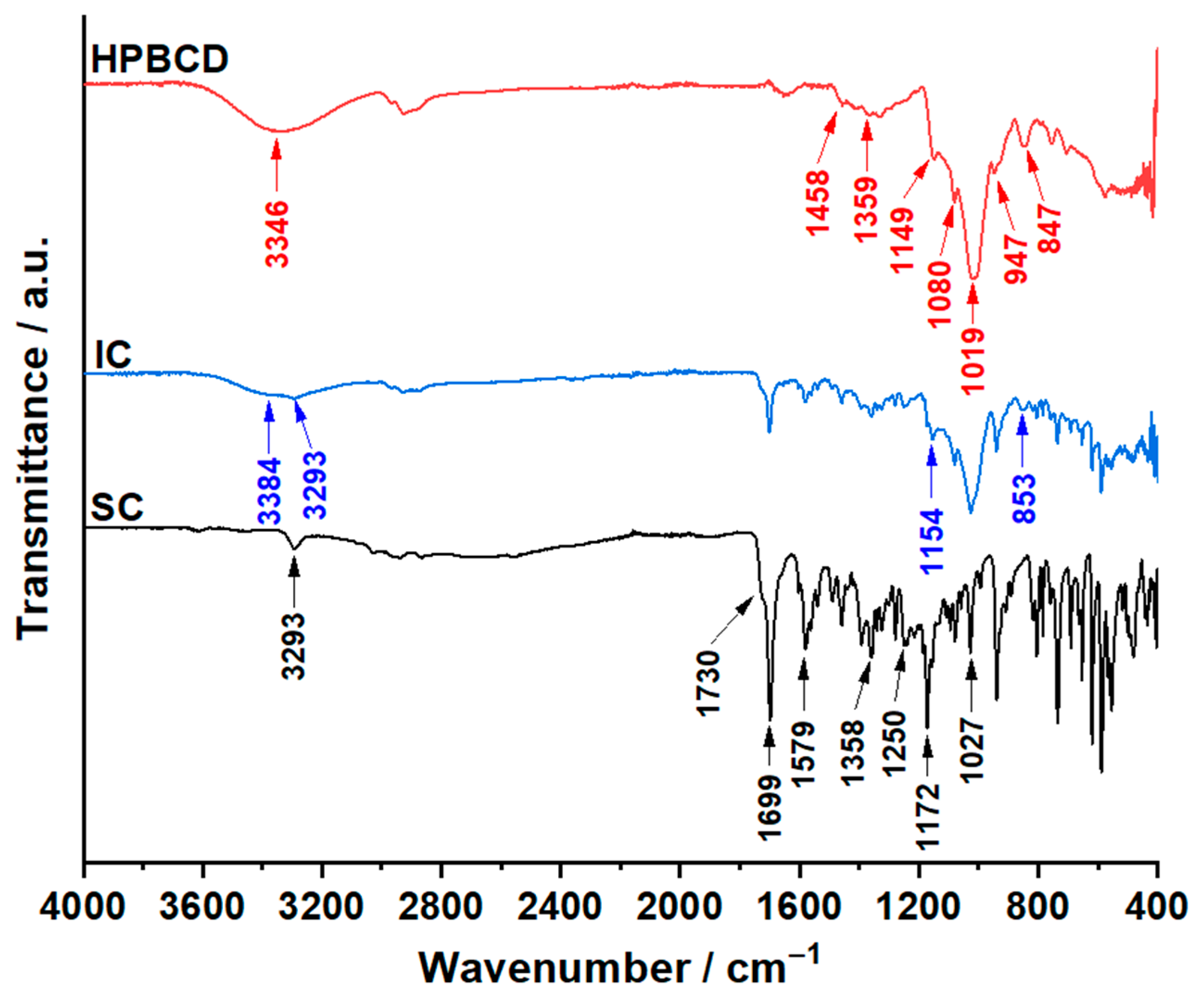
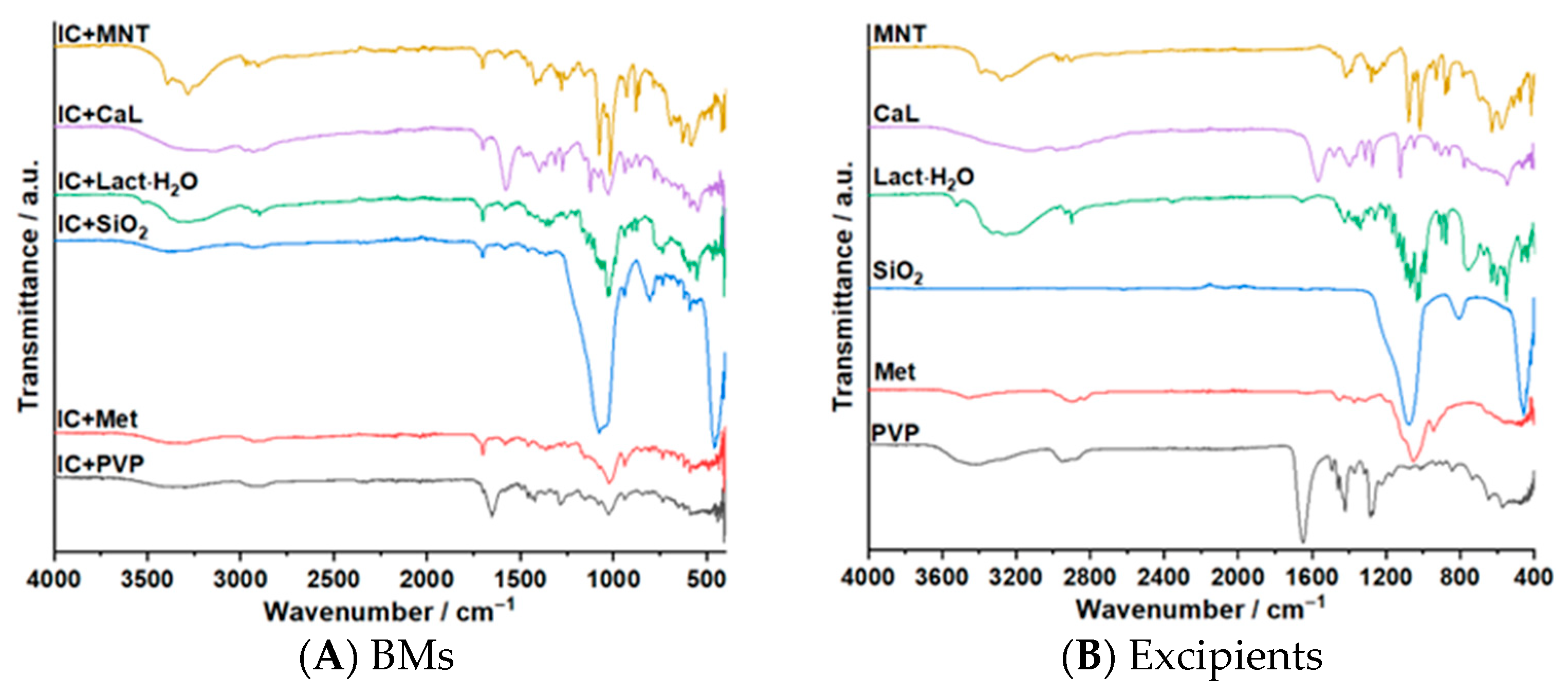
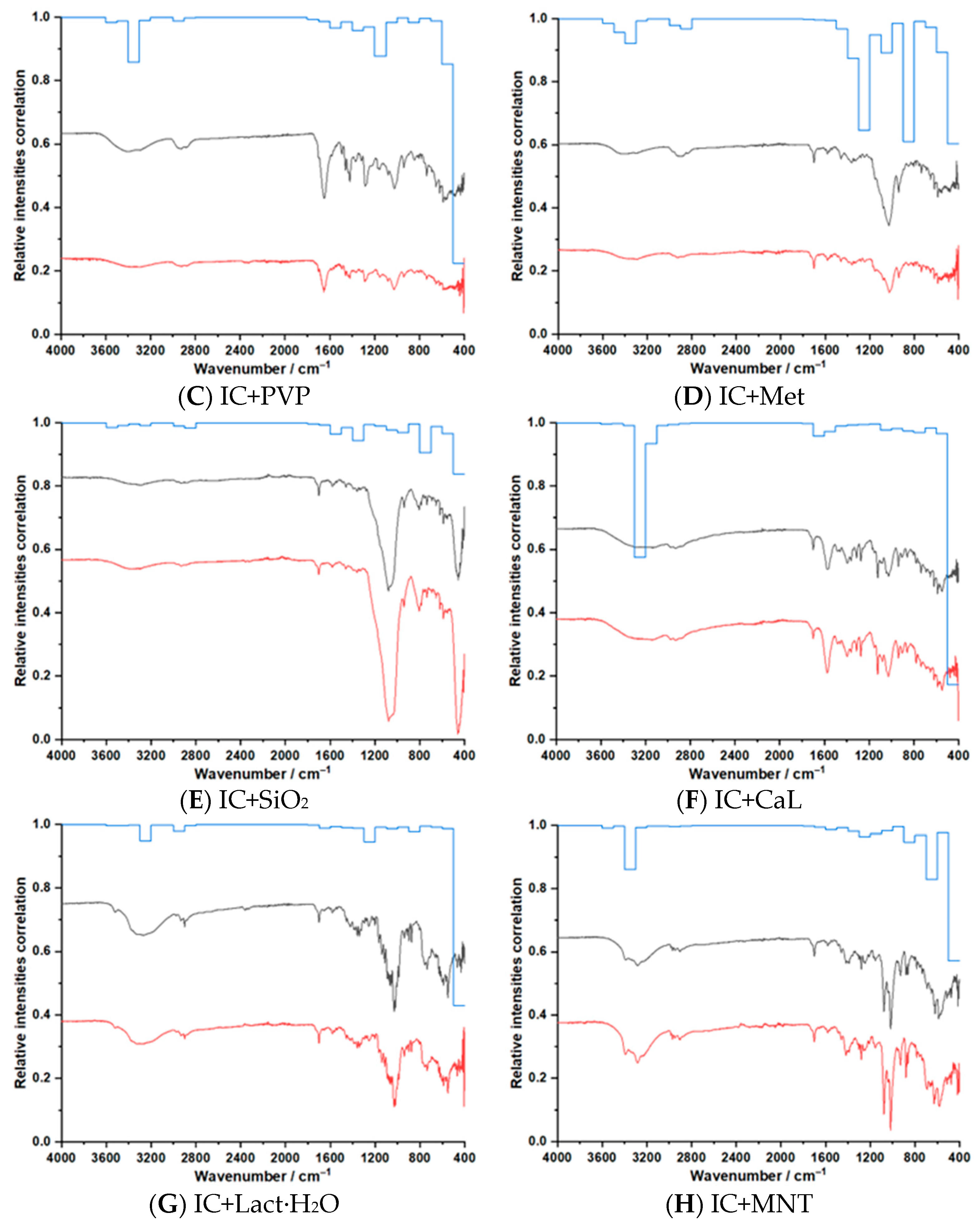
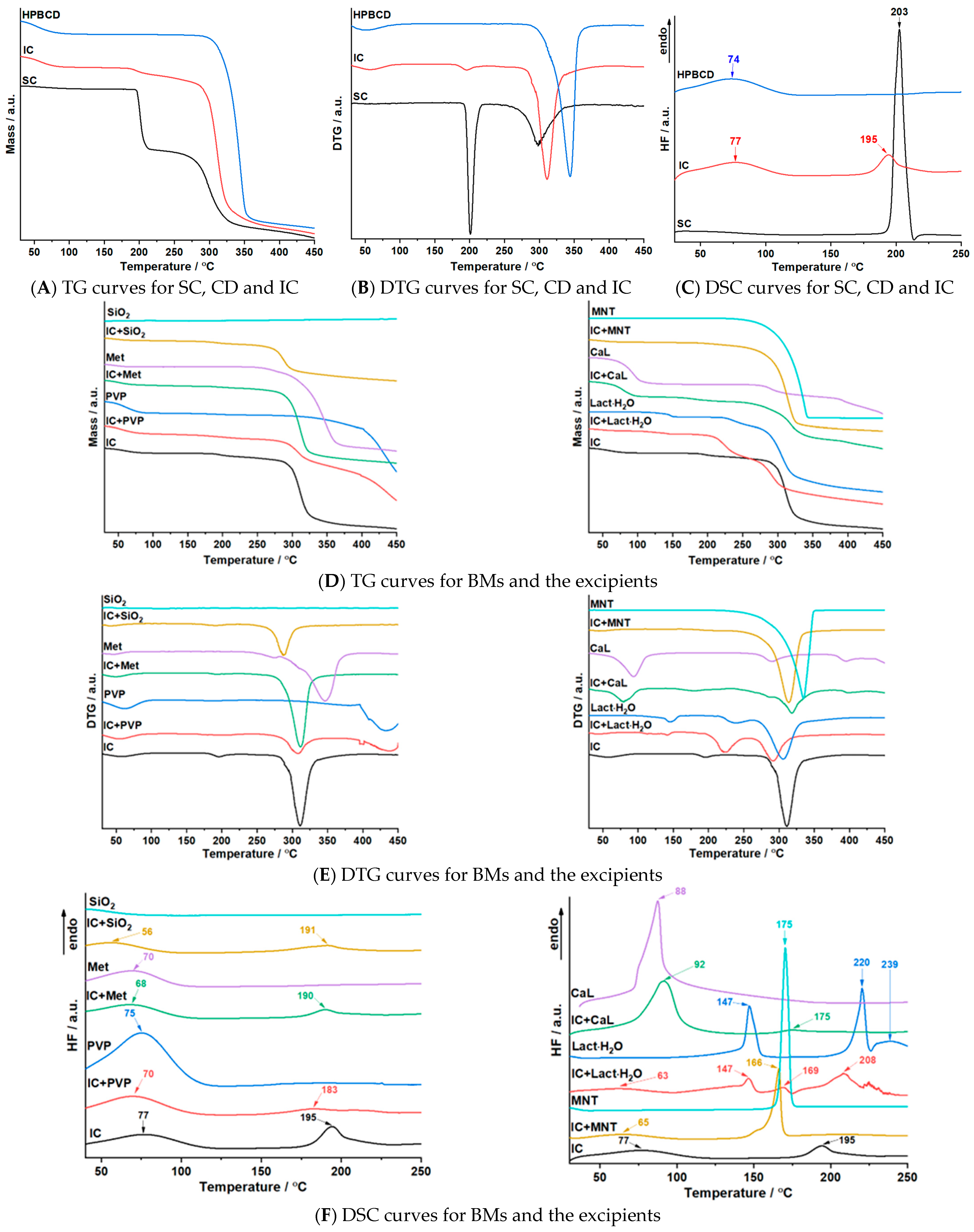
| Sample | Step | Tonset (°C) | Toffset (°C) | Tmax DTG (°C) | Tpeak DSC (°C) | ΔHfus (J g−1) | Δm (%) |
|---|---|---|---|---|---|---|---|
| SC | I | 188 | 220 | 201 | 203 | 380.2 | 27.4 |
| II | 243 | 353 | 298 | 34.1 | |||
| HPBCD | I | 30 | 100 | 51 | 74 | 181.0 | 5.9 |
| II | 280 | 374 | 344 | 84.4 | |||
| SC+HPBCD (IC) | I | 30 | 100 | 57 | 77 195 | 134.0 75.7 | 4.9 |
| II | 173 | 273 | 195 | 6.2 | |||
| III | 273 | 391 | 311 | 65.8 | |||
| IC+ SiO2 | I | 30 | 100 | 42 | 56 191 | 82.1 36.0 | 2.7 |
| II | 162 | 242 | 191 | 3.6 | |||
| III | 242 | 334 | 288 | 29.1 | |||
| SiO2 | - | - | - | - | - | - | - |
| IC+PVP | I | 30 | 100 | 54 | 70 183 | 156.2 42.4 | 7.7 |
| II | 160 | 238 | 199 | 3.8 | |||
| III | 253 | 352 | 309 | 26.4 | |||
| IV | 352 | 450 | 437 | 34.4 | |||
| PVP | I | 30 | 104 | 63 | 75 | 409.2 | 11.6 |
| II | 312 | 450 | 433 | 55.4 | |||
| IC+CaL | I | 30 | 117 | 80 | 92 175 | 499.4 10.5 | 15.3 |
| II | 143 | 228 | 179 | 4.4 | |||
| III | 228 | 373 | 286; 318 | 37.6 | |||
| IV | 379 | 427 | 398 | 5.7 | |||
| CaL | I | 30 | 156 | 94 | 88 | 731.6 | 26.5 |
| II | 244 | 354 | 288 | 12.0 | |||
| III | 354 | 450 | 395 | 17.2 | |||
| IC+Lact·H2O | I | 30 | 100 | 43 | 63 147 169 208 | 54.6 85.8 17.0 109.7 | 2.3 |
| II | 100 | 155 | 142 | 3.0 | |||
| III | 194 | 256 | 224 | 22.5 | |||
| IV | 256 | 344 | 292 | 36.1 | |||
| Lact·H2O | I | 95 | 167 | 144 | 147 220 239 | 150.0 153.3 43.6 | 5.0 |
| II | 213 | 265 | 237 | 9.1 | |||
| III | 265 | 410 | 305 | 62.1 | |||
| IC+MNT | I | 30 | 100 | 58 | 65 166 | 50.8 166.6 | 2.4 |
| II | 240 | 345 | 314 | 79.5 | |||
| MNT | I | 221 | 352 | 312 | 175 | 295.0 | 97.1 |
| IC+Met | I | 30 | 100 | 48 | 68 190 | 118.3 28.6 | 5.0 |
| II | 177 | 222 | 193 | 2.5 | |||
| III | 264 | 358 | 311 | 66.9 | |||
| Met | I | 30 | 100 | 47 | 70 | 92.1 | 2.6 |
| II | 232 | 282 | 275 | 5.3 | |||
| III | 282 | 397 | 346 | 75.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertici, R.A.; Ridichie, A.; Bertici, N.S.; Ledeţi, A.; Ledeţi, I.; Văruţ, R.-M.; Sbârcea, L.; Albu, P.; Rădulescu, M.; Rusu, G.; et al. Compatibility Studies of Sildenafil-HPBCD Inclusion Complex with Pharmaceutical Excipients. Pharmaceutics 2025, 17, 1114. https://doi.org/10.3390/pharmaceutics17091114
Bertici RA, Ridichie A, Bertici NS, Ledeţi A, Ledeţi I, Văruţ R-M, Sbârcea L, Albu P, Rădulescu M, Rusu G, et al. Compatibility Studies of Sildenafil-HPBCD Inclusion Complex with Pharmaceutical Excipients. Pharmaceutics. 2025; 17(9):1114. https://doi.org/10.3390/pharmaceutics17091114
Chicago/Turabian StyleBertici, Răzvan Adrian, Amalia Ridichie, Nicoleta Sorina Bertici, Adriana Ledeţi, Ionuţ Ledeţi, Renata-Maria Văruţ, Laura Sbârcea, Paul Albu, Matilda Rădulescu, Gerlinde Rusu, and et al. 2025. "Compatibility Studies of Sildenafil-HPBCD Inclusion Complex with Pharmaceutical Excipients" Pharmaceutics 17, no. 9: 1114. https://doi.org/10.3390/pharmaceutics17091114
APA StyleBertici, R. A., Ridichie, A., Bertici, N. S., Ledeţi, A., Ledeţi, I., Văruţ, R.-M., Sbârcea, L., Albu, P., Rădulescu, M., Rusu, G., Jianu, D. C., & Fira-Mladinescu, O. (2025). Compatibility Studies of Sildenafil-HPBCD Inclusion Complex with Pharmaceutical Excipients. Pharmaceutics, 17(9), 1114. https://doi.org/10.3390/pharmaceutics17091114












