Transfersome-Based Delivery of Optimized Black Tea Extract for the Prevention of UVB-Induced Skin Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Optimization by Response Surface Methodology
2.3. Process Optimization
2.4. Spectrophotometric Determination of Thearubigins and Theaflavins
2.5. Transfersomes’ Production
2.6. Transfersomes’ Size, Homogeneity, Charge, Entrapment Efficiency and Storage Stability
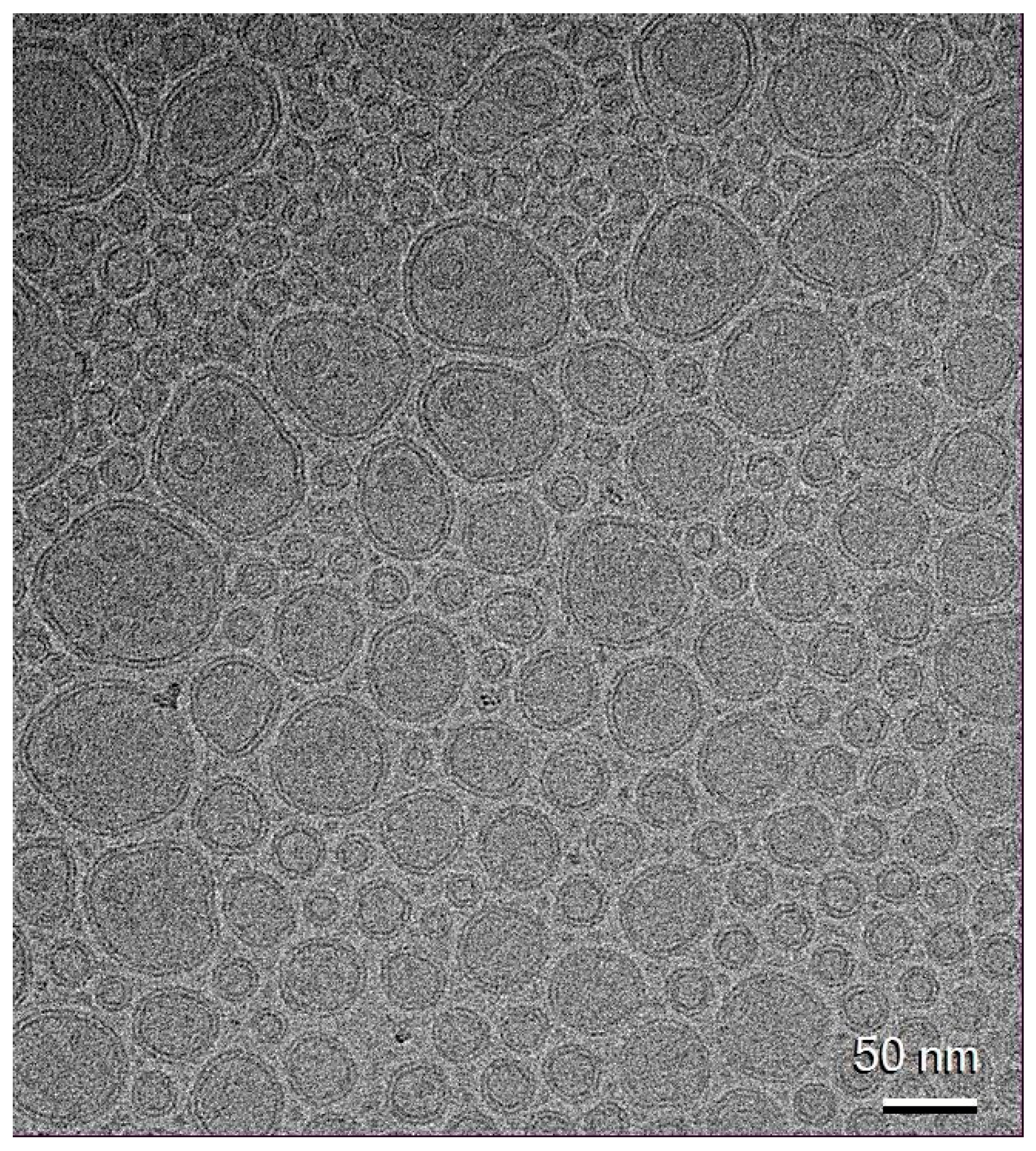
2.7. Transfersomes’ Ultrastructure
2.8. Cell Culture
2.9. UVB Irradiation
2.10. Cell Viability Assay
2.11. Total Collagen Production
2.12. Quantitative Real-Time PCR
2.13. Cellular Uptake of Black Tea Extract
2.14. LC-MS/MS Analysis
2.15. Statistical Analysis
3. Results
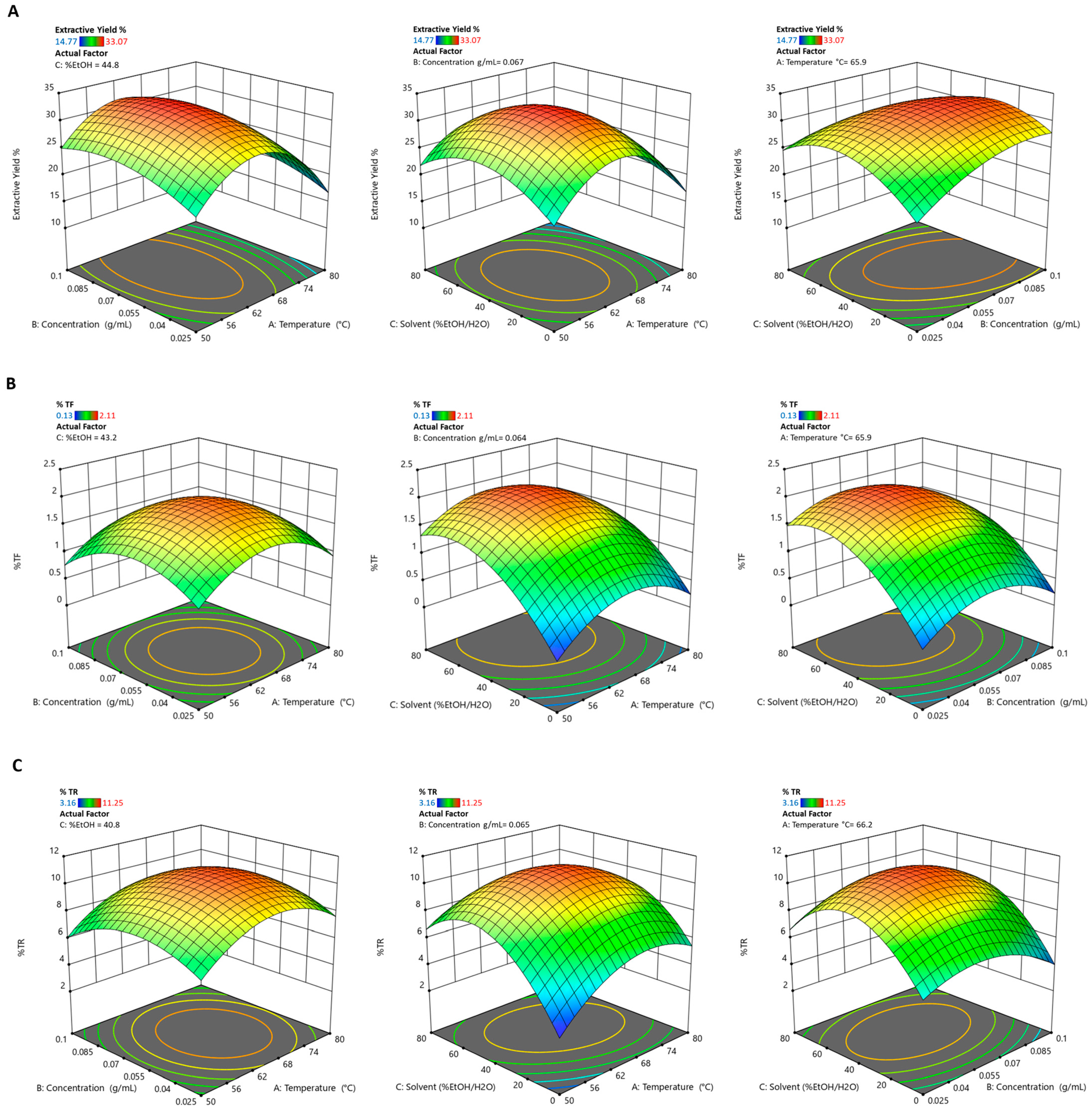
3.1. Response Surface Methodology Model Adequacy
3.2. Effect of Extraction Parameters on Dependent Variables
3.3. Multiple Response Optimization
3.4. Transfersomes’ Production and Characterization
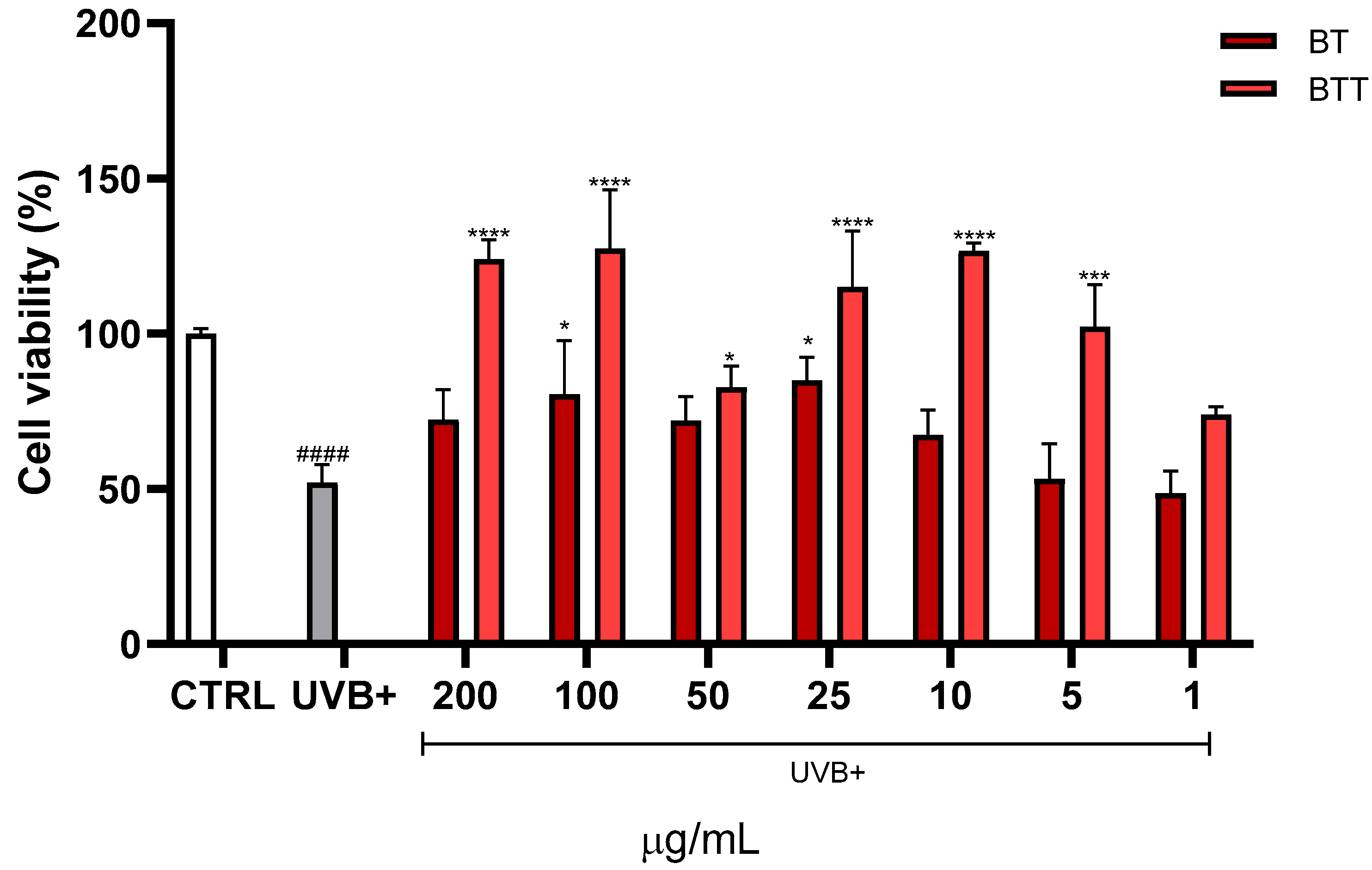
3.5. Cell Viability Assessment
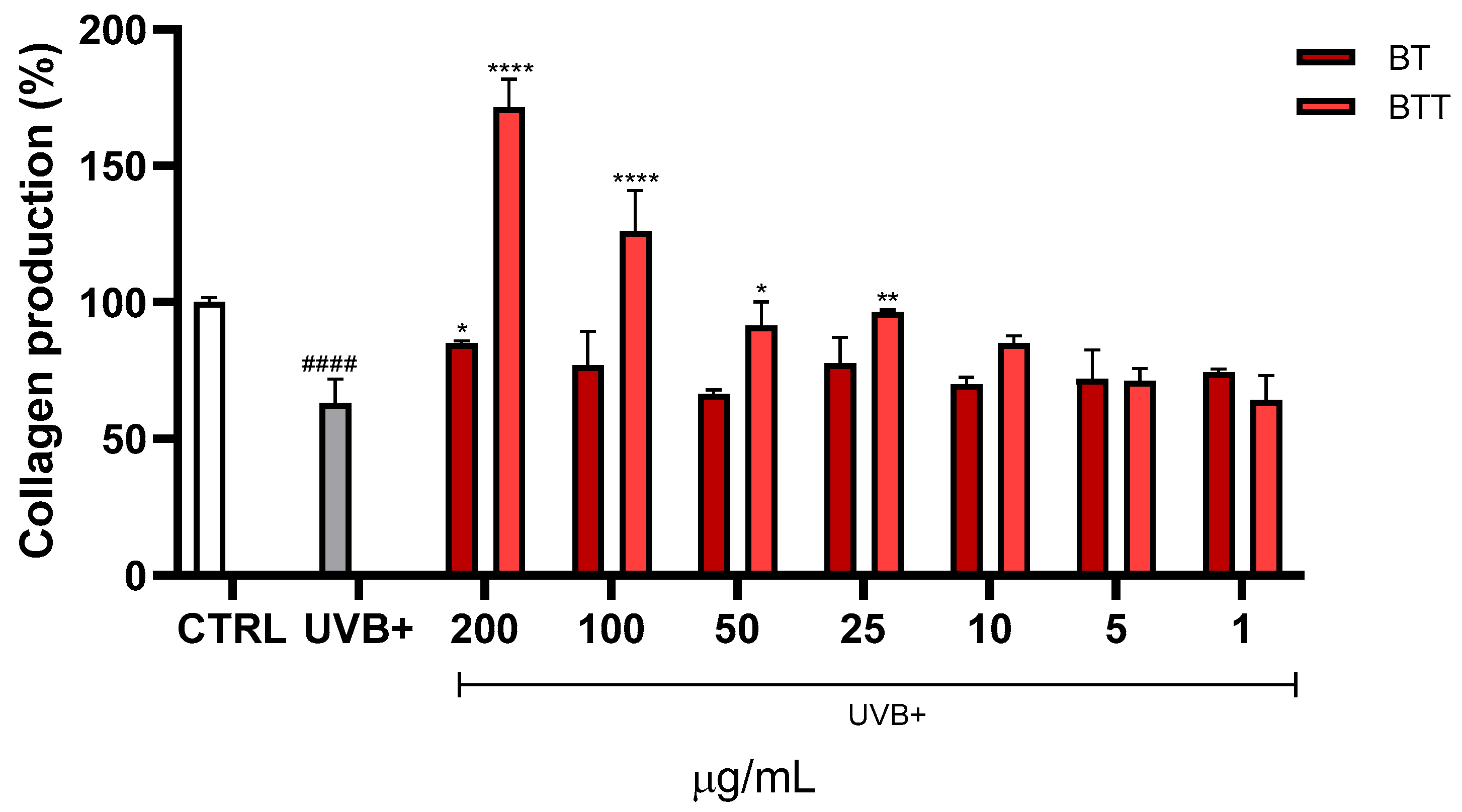
3.6. Collagen Degradation
3.7. Gene Expression Analysis
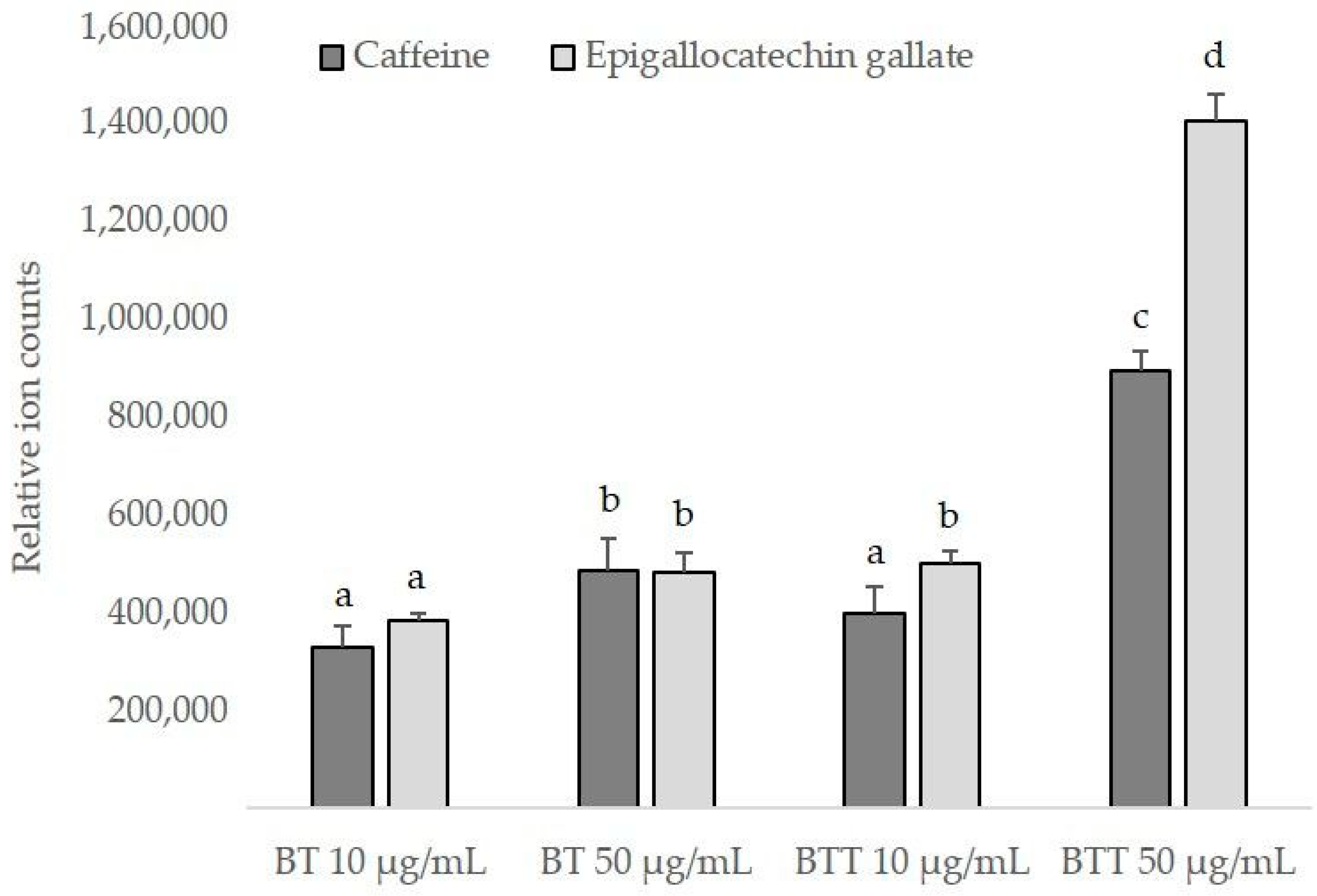
3.8. Intracellular Uptake of Black Tea Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Xie, M.; Liang, X.; Luo, P.; Yang, X.; Zhao, J.; Bian, J.; Sun, B.; Tang, Q.; Du, X.; et al. The preventive effect of theabrownin from Ya’an Tibetan tea against UVB-induced skin photodamage in BALB/c mice via the MAPK/NF-κB and Nrf2 signaling pathways. Foods 2025, 14, 600. [Google Scholar] [CrossRef]
- Sajeeda, A.; Bhat, A.M.; Gorke, S.; Wani, I.A.; Sidiqui, A.; Ahmed, Z.; Sheikh, T.A. Naringenin, a flavanone constituent from Sea buckthorn pulp extract, prevents ultraviolet (UV)-B radiation-induced skin damage via alleviation of impaired mitochondrial dynamics mediated inflammation in human dermal fibroblasts and Balb/c mice models. J. Photochem. Photobiol. B Biol. 2024, 256, 112944. [Google Scholar] [CrossRef]
- Ghazi, S. Do the polyphenolic compounds from natural products can protect the skin from ultraviolet rays? Results Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.-F.; Lam, T.-K.; Leung, K.S.-Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Scinicariello, F.; Buser Melanie, C. Serum testosterone concentrations and urinary bisphenol A, benzophenone-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011–2012. Environ. Health Perspect. 2016, 124, 1898–1904. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Mumford, S.L.; Krall, J.R.; Carmichael, A.E.; Sjaarda, L.A.; Perkins, N.J.; Kannan, K.; Schisterman, E.F. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: A chemical mixture approach. Environ. Int. 2018, 120, 137–144. [Google Scholar] [CrossRef]
- Aker, A.M.; Johns, L.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ. Int. 2018, 113, 341–349. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Lan, Z.; Yu, Y.; Mukherjee, B.; McElrath, T.F.; Meeker, J.D. Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: Assessment of effects independent of phthalates. Environ. Int. 2019, 131, 104903. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, L.; Wang, Y. Theaflavins with health-promoting properties: From extraction, synthesis to medicinal application. Trends Food Sci. Technol. 2025, 155, 104804. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, M.; Wan, J.; Shi, Y.; Xie, X.; Pan, W.; Hu, B.; Wang, Y.; Wen, H.; Wang, K.; et al. Anti-damage effect of theaflavin-3′-gallate from black tea on UVB-irradiated HaCaT cells by photoprotection and maintaining cell homeostasis. J. Photochem. Photobiol. B Biol. 2021, 224, 112304. [Google Scholar] [CrossRef]
- Feng, M.; Zheng, X.; Wan, J.; Pan, W.; Xie, X.; Hu, B.; Wang, Y.; Wen, H.; Cai, S. Research progress on the potential delaying skin aging effect and mechanism of tea for oral and external use. Food Func. 2021, 12, 2814–2828. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular carriers as alternative drug delivery systems: Ethosomes in focus. Expert. Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Nur, S.; Alwani, M.F.N.; Sami, F.J.; Sapra, A.; Aisyah, A.N.; Indrisari, M.; Yusuf, N.A. Transfersome-Based Delivery of Muntingia calabura Fruit Extract for Anti-Aging Applications. Biocatal. Agric. Biotechnol. 2025, 66, 103604. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Choi, S.-H.; Kozukue, E.; Kozukue, N. HPLC analysis of catechins, theaflavins, and alkaloids in commercial teas and green tea dietary supplements: Comparison of water and 80% ethanol/water extracts. J. Food Sci. 2006, 71, C328–C337. [Google Scholar] [CrossRef]
- Mehare, P.; Mullick, A. Process for Extracting Theaflavins from Tea. Chinese Patent CN101808529A, 21 August 2008. [Google Scholar]
- Belhaj, D.; Frikha, D.; Athmouni, K.; Jerbi, B.; Ahmed, M.B.; Bouallagui, Z.; Kallel, M.; Maalej, S.; Zhou, J.; Ayadi, H. Box-Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): Partial characterization, in vitro antioxidant and antimicrobial activities. Int. J. Biol. Macromol. 2017, 105, 1501–1510. [Google Scholar] [CrossRef]
- Roberts, E.A.H.; Smith, R.F. The phenolic substances of manufactured tea. IX.—The spectrophotometric evaluation of tea liquors. J. Sci. Food Agric. 1963, 14, 689–700. [Google Scholar] [CrossRef]
- Lela, L.; Ponticelli, M.; Caddeo, C.; Vassallo, A.; Ostuni, A.; Sinisgalli, C.; Faraone, I.; Santoro, V.; De Tommasi, N.; Milella, L. Nanotechnological exploitation of the antioxidant potential of Humulus lupulus L. extract. Food Chem. 2022, 393, 133401. [Google Scholar] [CrossRef]
- Vassallo, A.; Armentano, M.F.; Miglionico, R.; Caddeo, C.; Chirollo, C.; Gualtieri, M.J.; Ostuni, A.; Bisaccia, F.; Faraone, I.; Milella, L. Hura crepitans L. extract: Phytochemical characterization, antioxidant activity, and nanoformulation. Pharmaceutics 2020, 12, 553. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Benedetto, N.; Mangieri, C.; De Biasio, F.; Carvalho, R.F.; Milella, L.; Russo, D. Malus pumila Mill. cv Annurca apple extract might be therapeutically useful against oxidative stress and patterned hair loss. FEBS Open Bio 2024, 14, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Otang-Mbeng, W.; Sagbo, I.J. Anti-melanogenesis, antioxidant and anti-tyrosinase activities of Scabiosa columbaria L. Processes 2020, 8, 236. [Google Scholar] [CrossRef]
- Armentano, M.F.; Caterino, M.; Miglionico, R.; Ostuni, A.; Pace, M.C.; Cozzolino, F.; Monti, M.; Milella, L.; Carmosino, M.; Pucci, P. New insights on the functional role of URG7 in the cellular response to ER stress. Biol. Cell 2018, 110, 147–158. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, S.H. Extraction behavior of caffeine and EGCG from green and black tea. Biotechnol. Bioprocess. Eng. 2008, 13, 646–649. [Google Scholar] [CrossRef]
- Fernandez, P.; Martin, M.; Gonzalez, A.; Pablos, F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst 2000, 125, 421–425. [Google Scholar] [CrossRef]
- Dalla, E.; Koumentakou, I.; Bikiaris, N.; Balla, E.; Lykidou, S.; Nikolaidis, N. Formulation, characterization and evaluation of innovative O/W emulsions containing curcumin derivatives with enhanced antioxidant properties. Antioxidants 2022, 11, 2271. [Google Scholar] [CrossRef]
- Melnikova, V.O.; Ananthaswamy, H.N. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005, 571, 91–106. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from Jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef]
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Yeh, M.I.; Huang, H.C.; Liaw, J.H.; Huang, M.C.; Huang, K.F.; Hsu, F.L. Dermal delivery by niosomes of black tea extract as a sunscreen agent. Int. J. Dermatol. 2013, 52, 239–245. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Choi, J.-S.; Choi, Y.-J.; Shin, S.-Y.; Kang, S.-W.; Han, S.J.; Kang, Y.-H. (−)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food Chem. Toxicol. 2008, 46, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-S.; Kundu, J.K.; Chun, K.-S.; Na, H.-K.; Surh, Y.-J. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR-and SP1-dependent manner. JCI Insight 2022, 7, e156344. [Google Scholar] [CrossRef]
- Buckman, S.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef]
- Peng, G.; Dixon, D.A.; Muga, S.J.; Smith, T.J.; Wargovich, M.J. Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol. Carcinog. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- Tang, S.-C.; Liao, P.-Y.; Hung, S.-J.; Ge, J.-S.; Chen, S.-M.; Lai, J.-C.; Hsiao, Y.-P.; Yang, J.-H. Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. J. Dermatol. Sci. 2017, 86, 238–248. [Google Scholar] [CrossRef]
- Van Tran, V.; Moon, J.-Y.; Lee, Y.-C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]



| Levels | ||||
|---|---|---|---|---|
| Independent Variables | −1 | 0 | 1 | Dependent Variables |
| A-Temperature °C | 50 | 65 | 80 | Extraction yield (%) |
| B-Concentration g/mL | 0.0250 | 0.0625 | 0.1000 | Thearubigins (%) |
| C-Solvent %EtOH/H2O | 0 | 40 | 80 | Theaflavins (%) |
| Formulation | Lecithin (mg) | Black Tea Extract (mg) | Tween 80 (mL) | H2O (mL) |
|---|---|---|---|---|
| Empty transfersomes (ET) | 140 | 0 | 0.05 | 0.95 |
| Black tea transfersomes (BTT) | 140 | 5 | 0.05 | 0.95 |
| Independent Variables | Dependent Variables | |||||
|---|---|---|---|---|---|---|
| A | B | C | Extraction Yield % | %TF | %TR | |
| Run | Temperature °C | Concentration g/mL | %EtOH/H2O | |||
| 1 | 65 | 0.0625 | 40 | 32.34 ± 1.026 a | 1.90 ± 0.010 b | 10.73 ± 0.260 a |
| 2 | 65 | 0.0250 | 80 | 23.99 ± 2.018 b | 1.59 ± 0.030 c | 7.05 ± 0.178 b,c |
| 3 | 50 | 0.1000 | 40 | 24.16 ± 1.791 b | 0.72 ± 0.010 g | 6.00 ± 0.044 c,d,e |
| 4 | 65 | 0.0625 | 40 | 31.93 ± 2.036 a | 2.08 ± 0.067 a | 10.03 ± 1.006 a |
| 5 | 80 | 0.0625 | 80 | 14.77 ± 0.031 f | 1.09 ± 0.007 e | 5.54 ± 0.087 d,e |
| 6 | 50 | 0.0250 | 40 | 21.29 ± 1.150 b,c,d | 0.75 ± 0.003 g | 6.12 ± 0.028 c,d,e |
| 7 | 65 | 0.0625 | 40 | 32.85 ± 0.691 a | 1.54 ± 0.004 c | 11.25 ± 1.093 a |
| 8 | 65 | 0.0250 | 0 | 20.48 ± 1.362 b,c,d | 0.24 ± 0.002 h | 5.17 ± 0.050 e |
| 9 | 65 | 0.0625 | 40 | 33.07 ± 2.107 a | 1.84 ± 0.081 b | 10.49 ± 0.099 a |
| 10 | 80 | 0.1000 | 40 | 19.57 ± 0.714 c,d,e | 0.83 ± 0.012 f | 7.63 ± 0.081 b |
| 11 | 50 | 0.0625 | 80 | 22.81 ± 1.740 b,c | 1.35 ± 0.004 d | 6.74 ± 0.034 b,c,d |
| 12 | 65 | 0.1000 | 80 | 24.58 ± 0.641 b | 1.15 ± 0.011 e | 6.90 ± 0.055 b,c |
| 13 | 65 | 0.1000 | 0 | 29.00 ± 0.532 a | 0.13 ± 0.001 i | 3.53 ± 0.058 f |
| 14 | 80 | 0.0250 | 40 | 17.75 ± 1.624 d,e,f | 0.90 ± 0.012 f | 7.73 ± 0.092 b |
| 15 | 80 | 0.0625 | 0 | 15.70 ± 0.763 e,f | 0.23 ± 0.000 h | 5.39 ± 0.046 e |
| 16 | 65 | 0.0625 | 40 | 31.76 ± 2.057 a | 2.11 ± 0.015 a | 11.05 ± 0.624 a |
| 17 | 50 | 0.0625 | 0 | 19.71 ± 0.681 c,d,e | 0.16 ± 0.007 h,i | 3.16 ± 0.038 f |
| Response 1: Extraction Yield % | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Quadratic Model | 620.67 | 9 | 68.96 | 70.58 | <0.0001 | significant |
| A-Temperature | 50.94 | 1 | 50.94 | 52.13 | 0.0002 * | |
| B-Concentration | 23.8 | 1 | 23.8 | 24.36 | 0.0017 * | |
| C-Solvent | 0.1977 | 1 | 0.1977 | 0.2023 | 0.6665 | |
| AB | 0.2797 | 1 | 0.2797 | 0.2863 | 0.6092 | |
| AC | 4.07 | 1 | 4.07 | 4.17 | 0.0806 | |
| BC | 15.72 | 1 | 15.72 | 16.09 | 0.0051 | |
| A2 | 339.69 | 1 | 339.69 | 347.66 | <0.0001 * | |
| B2 | 31.09 | 1 | 31.09 | 31.82 | 0.0008 * | |
| C2 | 112.13 | 1 | 112.13 | 114.76 | <0.0001 * | |
| Residual | 6.84 | 7 | 0.9771 | |||
| Lack of Fit | 5.55 | 3 | 1.85 | 5.76 | 0.0619 | not significant |
| Pure Error | 1.29 | 4 | 0.3213 | |||
| Corrected Total | 627.51 | 16 | ||||
| Response 2: %TF | ||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Quadratic Model | 7.11 | 9 | 0.7902 | 20.15 | 0.0003 | significant |
| A-Temperature | 0.0005 | 1 | 0.0005 | 0.0117 | 0.917 | |
| B-Concentration | 0.0504 | 1 | 0.0504 | 1.29 | 0.2942 | |
| C-Solvent | 2.45 | 1 | 2.45 | 62.45 | <0.0001 * | |
| AB | 0.0005 | 1 | 0.0005 | 0.0139 | 0.9095 | |
| AC | 0.0275 | 1 | 0.0275 | 0.7017 | 0.4299 | |
| BC | 0.0266 | 1 | 0.0266 | 0.6773 | 0.4376 | |
| A2 | 1.43 | 1 | 1.43 | 36.4 | 0.0005 * | |
| B2 | 1.11 | 1 | 1.11 | 28.22 | 0.0011 * | |
| C2 | 1.55 | 1 | 1.55 | 39.45 | 0.0004 * | |
| Residual | 0.2745 | 7 | 0.0392 | |||
| Lack of Fit | 0.065 | 3 | 0.0217 | 0.4134 | 0.753 | not significant |
| Pure Error | 0.2095 | 4 | 0.0524 | |||
| Corrected Total | 7.39 | 16 | ||||
| Response 3: %TR | ||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Quadratic Model | 103.45 | 9 | 11.49 | 37.64 | <0.0001 | significant |
| A-Temperature | 2.28 | 1 | 2.28 | 7.45 | 0.0293 * | |
| B-Concentration | 0.5039 | 1 | 0.5039 | 1.65 | 0.2398 | |
| C-Solvent | 10.06 | 1 | 10.06 | 32.95 | 0.0007 * | |
| AB | 0.0001 | 1 | 0.0001 | 0.0003 | 0.9858 | |
| AC | 2.94 | 1 | 2.94 | 9.63 | 0.0173 * | |
| BC | 0.562 | 1 | 0.562 | 1.84 | 0.217 | |
| A2 | 19.42 | 1 | 19.42 | 63.59 | <0.0001 * | |
| B2 | 12.04 | 1 | 12.04 | 39.44 | 0.0004 * | |
| C2 | 47.4 | 1 | 47.4 | 155.22 | <0.0001 * | |
| Residual | 2.14 | 7 | 0.3054 | |||
| Lack of Fit | 1.22 | 3 | 0.4064 | 1.77 | 0.2916 | not significant |
| Pure Error | 0.9184 | 4 | 0.2296 | |||
| Corrected Total | 105.58 | 16 | ||||
| %Extraction Yield | %TF | %TR | |
|---|---|---|---|
| Standard Deviation | 0.9885 | 0.198 | 0.5526 |
| Mean | 24.46 | 1.09 | 7.32 |
| %C.V. | 4.04 | 18.11 | 7.54 |
| R2 | 0.9891 | 0.9628 | 0.9798 |
| Adjusted R2 | 0.9751 | 0.9151 | 0.9537 |
| Predicted R2 | 0.8552 | 0.815 | 0.8016 |
| Adequate Precision | 23.1063 | 12.0636 | 18.9109 |
| Intercept | A | B | C | AB | AC | BC | A2 | B2 | C2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Yield % | 32.39 | −2.52335 | 1.72478 | 0.157193 | −0.26445 | −1.00885 | −1.98259 | −8.98207 | −2.71738 | −5.16058 |
| p-values | 0.0002 * | 0.0017 * | 0.6665 | 0.6092 | 0.0806 | 0.0051 | <0.0001 * | 0.0008 * | <0.0001 * | |
| %TF | 1.89404 | 0.007566 | −0.07939 | 0.553254 | −0.01167 | −0.082941 | −0.08149 | −0.58226 | −0.51262 | −0.60614 |
| p-values | 0.917 | 0.2942 | <0.0001 * | 0.9095 | 0.4299 | 0.4376 | 0.0005 * | 0.0011 * | 0.0004 * | |
| %TR | 10.7099 | 0.533363 | −0.25098 | 1.12156 | 0.00511 | −0.85724 | 0.374823 | −2.14763 | −1.69133 | 3.35529 |
| p-values | 0.0293 * | 0.2398 | 0.0007 * | 0.9858 | 0.0173 * | 0.217 | <0.0001 * | 0.0004 * | <0.0001 * |
| Response Prediction | SE Pred | 95% PI | |
|---|---|---|---|
| Extraction Yield % | 32.297 | 1.08138 | 29.740–34.855 |
| %TF | 1.985 | 0.21663 | 1.473–2.497 |
| %TR | 10.771 | 0.604544 | 9.341–12.200 |
| Formulation | MD nm ± SD | PI | ZP mV ± SD | EE % ± SD |
|---|---|---|---|---|
| Empty transfersomes (ET) | 61 ± 3.1 | 0.23 ± 0.01 | −40 ± 2.5 | -- |
| Black tea transfersomes (BTT) | 63 ± 5.0 | * 0.25 ± 0.02 | −40 ± 3.6 | Caffeine 86 ± 2.3 Epigallocatechin gallate 93 ± 4.8 |
| Black Tea Transfersomes | MD nm ± SD | PI | ZP mV ± SD |
|---|---|---|---|
| t0 | 63 ± 5.0 | 0.25 ± 0.02 | −40 ± 3.6 |
| t15 | 63 ± 5.2 | 0.22 ± 0.01 | −43 ± 1.9 |
| t30 | 63 ± 5.4 | 0.23 ± 0.01 | −43 ± 2.2 |
| t60 | 66 ± 2.7 | 0.26 ± 0.04 | −46 ± 2.2 |
| t90 | 66 ± 1.8 | 0.25 ± 0.01 | −41 ± 5.4 |
| t120 | 66 ± 4.6 | 0.26 ± 0.01 | −42 ± 6.2 |
| t150 | 71 ± 8.7 | 0.30 ± 0.07 | −42 ± 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetto, N.; Ponticelli, M.; Lela, L.; Rosa, E.; Carriero, F.; Faraone, I.; Caddeo, C.; Milella, L.; Vassallo, A. Transfersome-Based Delivery of Optimized Black Tea Extract for the Prevention of UVB-Induced Skin Damage. Pharmaceutics 2025, 17, 952. https://doi.org/10.3390/pharmaceutics17080952
Benedetto N, Ponticelli M, Lela L, Rosa E, Carriero F, Faraone I, Caddeo C, Milella L, Vassallo A. Transfersome-Based Delivery of Optimized Black Tea Extract for the Prevention of UVB-Induced Skin Damage. Pharmaceutics. 2025; 17(8):952. https://doi.org/10.3390/pharmaceutics17080952
Chicago/Turabian StyleBenedetto, Nadia, Maria Ponticelli, Ludovica Lela, Emanuele Rosa, Flavia Carriero, Immacolata Faraone, Carla Caddeo, Luigi Milella, and Antonio Vassallo. 2025. "Transfersome-Based Delivery of Optimized Black Tea Extract for the Prevention of UVB-Induced Skin Damage" Pharmaceutics 17, no. 8: 952. https://doi.org/10.3390/pharmaceutics17080952
APA StyleBenedetto, N., Ponticelli, M., Lela, L., Rosa, E., Carriero, F., Faraone, I., Caddeo, C., Milella, L., & Vassallo, A. (2025). Transfersome-Based Delivery of Optimized Black Tea Extract for the Prevention of UVB-Induced Skin Damage. Pharmaceutics, 17(8), 952. https://doi.org/10.3390/pharmaceutics17080952






