Aptamers Targeting Immune Checkpoints for Tumor Immunotherapy
Abstract
1. Introduction
1.1. Aptamer Binding Targets in Cancer Immunotherapy
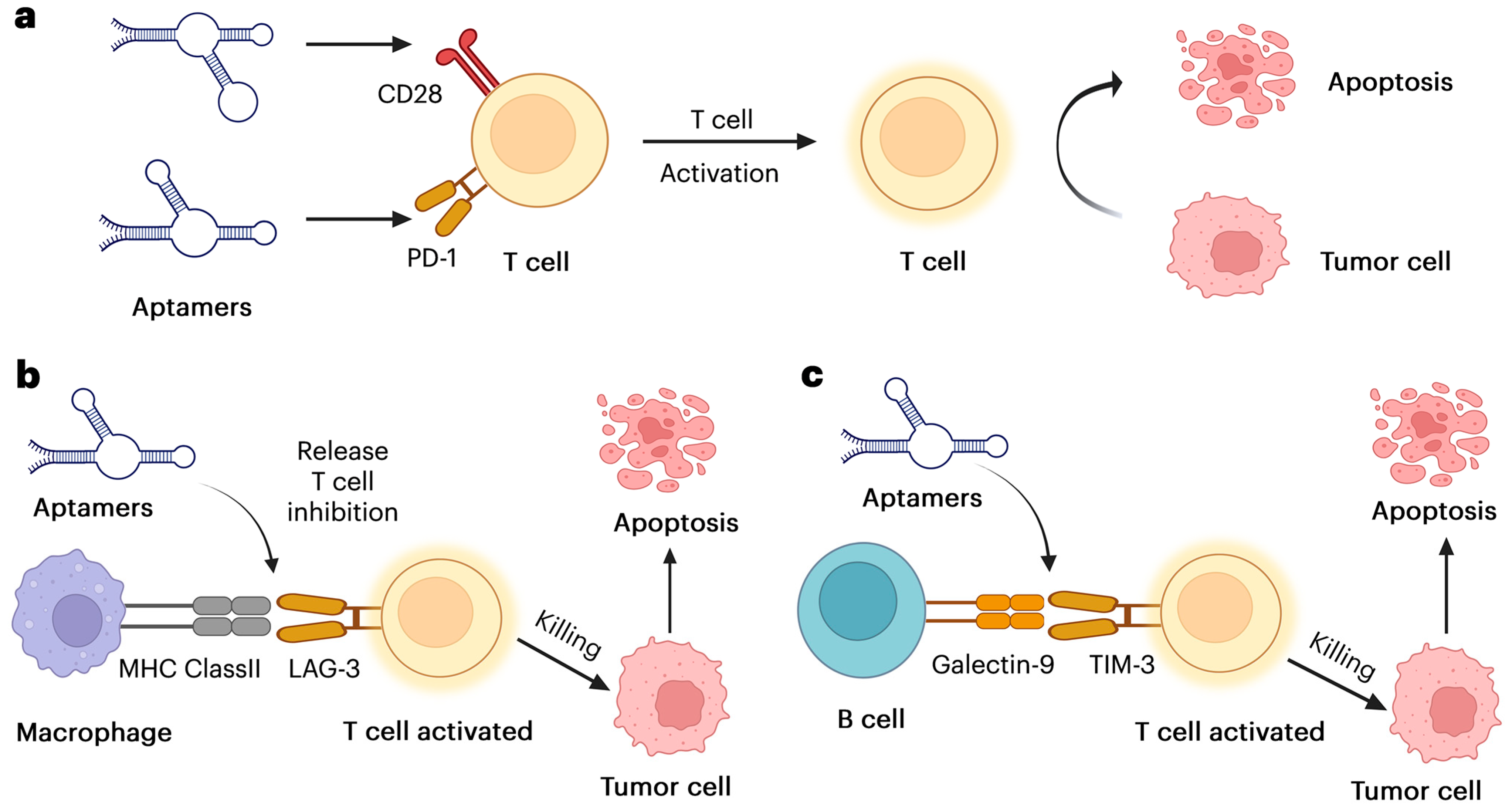
1.2. The Mechanisms of Aptamer Enhancing Immune Responses
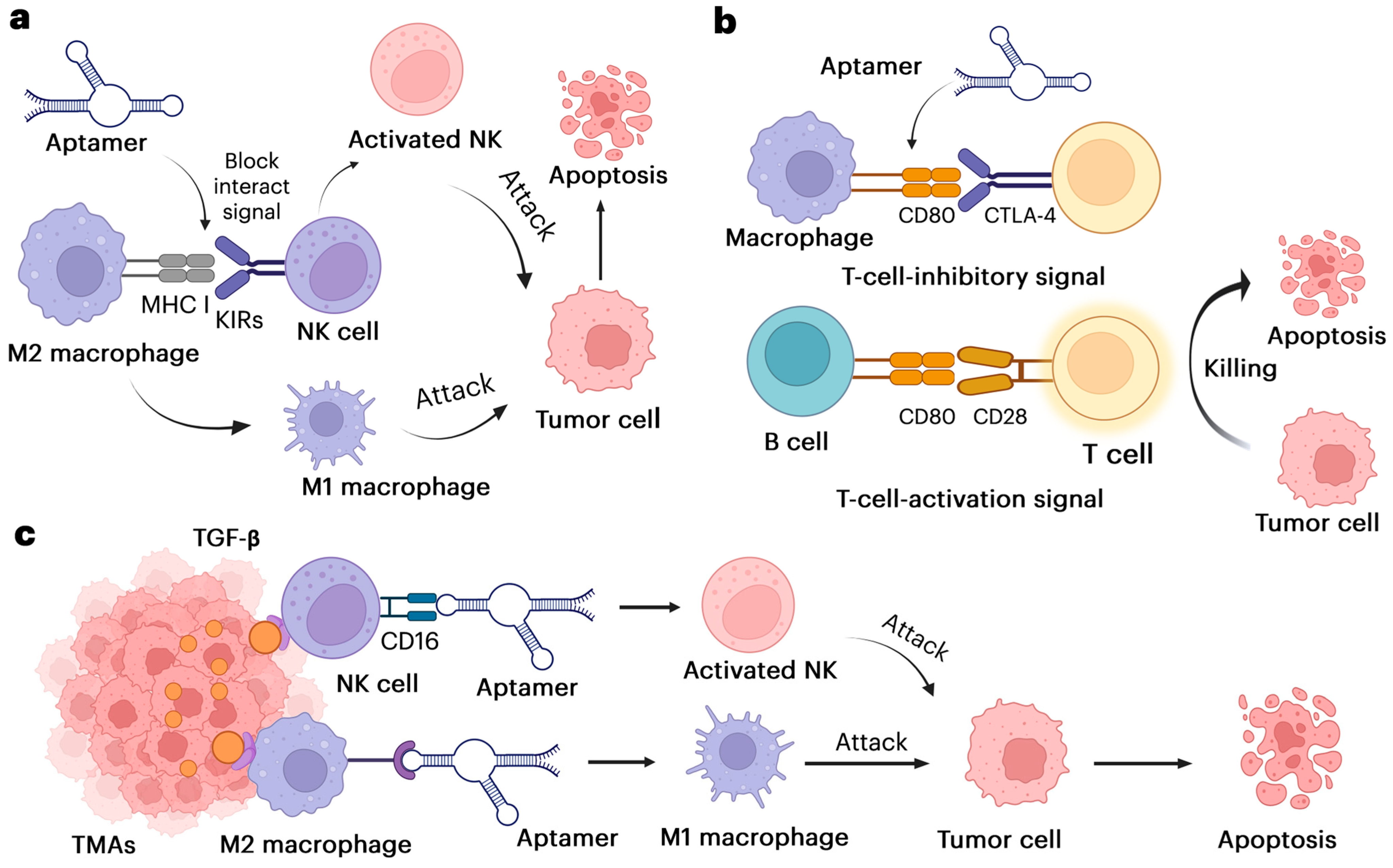
1.2.1. Aptamer Direct NK Cells and Macrophages to the Tumor
1.2.2. Aptamer Alters Immune Cells and Delivers Immune-Modulating Agents
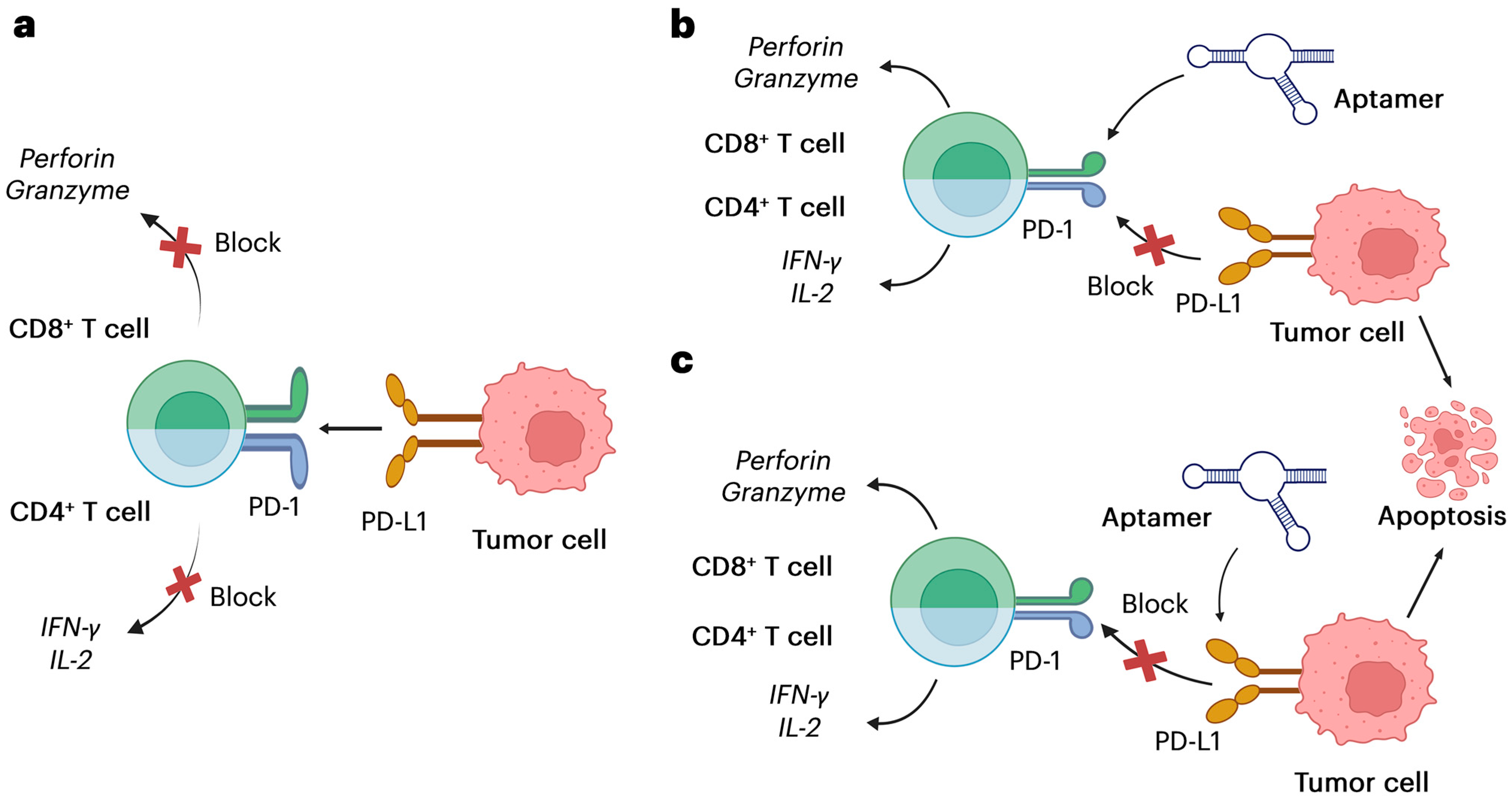
2. PD-1/PD-L1 Pathway and Aptamer Development
2.1. The PD-1/PD-L1 Immune Checkpoint Axis
2.2. Aptamers Targeting PD-1/PD-L1
2.3. Comparison of PD-1/PD-L1 Aptamers with Monoclonal Antibodies
2.3.1. Therapeutic Efficacy
2.3.2. Safety Profiles
2.3.3. Cost Implications
2.3.4. Clinical Applications
3. Other Immune Checkpoints and Associated Aptamers
3.1. Immune Checkpoints and Tumor Immune Evasion
3.2. CTLA-4 Aptamers
3.3. TIM-3 and LAG-3 Aptamers
3.4. Immune Checkpoints Aptamer Clinical Progress
3.5. Emerging Targets in Tumor Immunotherapy
4. Multifunctional Aptamers in Tumor Immunotherapy
4.1. Bispecific and Multivalent Aptamers
4.2. Aptamer–Drug Conjugates (ApDCs)
4.3. Aptamer–Nanoparticle Conjugates
5. Challenges and Limitations in Aptamer Development
6. Future Directions and Advancements in Aptamer Engineering
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Aden, D.; Zaheer, S.; Sureka, N.; Trisal, M.; Chaurasia, J.K.; Zaheer, S. Exploring immune checkpoint inhibitors: Focus on PD-1/PD-L1 axis and beyond. Pathol. Res. Pract. 2025, 269, 155864. [Google Scholar] [CrossRef] [PubMed]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer immunotherapy: Challenges and limitations. Pathol. Res. Pract. 2022, 229, 153723. [Google Scholar] [CrossRef] [PubMed]
- Kejamurthy, P.; Devi, K.T.R. Immune checkpoint inhibitors and cancer immunotherapy by aptamers: An overview. Med. Oncol. 2023, 41, 40. [Google Scholar] [CrossRef] [PubMed]
- Ayass, M.A.; Tripathi, T.; Griko, N.; Okyay, T.; Ramankutty Nair, R.; Zhang, J.; Zhu, K.; Melendez, K.; Pashkov, V.; Abi-Mosleh, L. Dual Checkpoint Aptamer Immunotherapy: Unveiling Tailored Cancer Treatment Targeting CTLA-4 and NKG2A. Cancers 2024, 16, 1041. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P. Aptamers Targeting the PD-1/PD-L1 Axis: A Perspective. J. Med. Chem. 2023, 66, 10878–10888. [Google Scholar] [CrossRef] [PubMed]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.P.T. LAG3, TIM3 and TIGIT: New Targets for Immunotherapy and Potential Associations with Radiotherapy. Curr. Oncol. 2025, 32, 230. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.J.; Weber, J.S.; et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA 2016, 315, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; He, M.X.; Bakouny, Z.; Kanodia, A.; Napolitano, S.; Wu, J.; Grimaldi, G.; Braun, D.A.; Cuoco, M.S.; Mayorga, A.; et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell 2021, 39, 649–661.e5. [Google Scholar] [CrossRef] [PubMed]
- García Melián, M.F.; Moreno, M.; Cerecetto, H.; Calzada, V. Aptamer-Based Immunotheranostic Strategies. Cancer Biother. Radiopharm. 2023, 38, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, J. Emerging roles of CAR-NK cell therapies in tumor immunotherapy: Current status and future directions. Cell Death Discov. 2024, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Li, A.W.; Briones, J.D.; Lu, J.; Walker, Q.; Martinez, R.; Hiraragi, H.; Boldajipour, B.A.; Sundar, P.; Potluri, S.; Lee, G.; et al. Engineering potent chimeric antigen receptor T cells by programming signaling during T-cell activation. Sci. Rep. 2024, 14, 21331. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Granata, I.; Collina, F.; Leonetti, F.; Cantile, M.; Botti, G.; Fedele, M.; Guarracino, M.R.; Cerchia, L. Novel Aptamers Selected on Living Cells for Specific Recognition of Triple-Negative Breast Cancer. iScience 2020, 23, 100979. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, Y.; Peng, F.; Wang, T.; Cheng, Z.; Chen, Q.; Li, M.; Xu, L.; Man, Y.; Zhang, Z.; et al. Aptamer-controlled stimuli-responsive drug release. Int. J. Biol. Macromol. 2024, 279, 135353. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zu, Y. Aptamers and their applications in nanomedicine. Small 2015, 11, 2352–2364. [Google Scholar] [CrossRef] [PubMed]
- Ruckman, J.; Green, L.S.; Beeson, J.; Waugh, S.; Gillette, W.L.; Henninger, D.D.; Claesson-Welsh, L.; Janjić, N. 2’-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 1998, 273, 20556–20567. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Mao, Z.; Li, W.; Pei, R. Anti-PD-L1 DNA aptamer antagonizes the interaction of PD-1/PD-L1 with antitumor effect. J. Mater. Chem. B 2021, 9, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-C.; Chang, Y.-C.; Lai, W.-Y. Antagonistic PD-1 Aptamer and Its Applications in Cancer Therapy Related Applications. US Patent US10329570B2, 25 June 2019. [Google Scholar]
- Santulli-Marotto, S.; Nair, S.K.; Rusconi, C.; Sullenger, B.; Gilboa, E. Multivalent RNA aptamers that inhibit CTLA-4 and enhance tumor immunity. Cancer Res. 2003, 63, 7483–7489. [Google Scholar] [PubMed]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef] [PubMed]
- Gefen, T.; Castro, I.; Muharemagic, D.; Puplampu-Dove, Y.; Patel, S.; Gilboa, E. A TIM-3 Oligonucleotide Aptamer Enhances T Cell Functions and Potentiates Tumor Immunity in Mice. Mol. Ther. 2017, 25, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Soldevilla, M.M.; Hervas, S.; Villanueva, H.; Lozano, T.; Rabal, O.; Oyarzabal, J.; Lasarte, J.J.; Bendandi, M.; Inoges, S.; López-Díaz de Cerio, A.; et al. Identification of LAG3 high affinity aptamers by HT-SELEX and Conserved Motif Accumulation (CMA). PLoS ONE 2017, 12, e0185169. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.L.; Sattler, M.; Azab, A.K.; Eulberg, D.; Kruschinski, A.; Manley, P.W.; Stone, R.; Griffin, J.D. Inhibition of SDF-1-induced migration of oncogene-driven myeloid leukemia by the L-RNA aptamer (Spiegelmer), NOX-A12, and potentiation of tyrosine kinase inhibition. Oncotarget 2017, 8, 109973–109984. [Google Scholar] [CrossRef] [PubMed]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Sabet, M.; Hayashi, T.; Tawatao, R.; Fierer, J.; Carson, D.A.; Guiney, D.G.; Corr, M. In vivo efficacy of a phosphodiester TLR-9 aptamer and its beneficial effect in a pulmonary anthrax infection model. Cell Immunol. 2008, 251, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Orava, E.W.; Jarvik, N.; Shek, Y.L.; Sidhu, S.S.; Gariépy, J. A short DNA aptamer that recognizes TNFα and blocks its activity in vitro. ACS Chem. Biol. 2013, 8, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hirota, M.; Waugh, S.M.; Murakami, I.; Suzuki, T.; Muraguchi, M.; Shibamori, M.; Ishikawa, Y.; Jarvis, T.C.; Carter, J.D.; et al. Chemically modified DNA aptamers bind interleukin-6 with high affinity and inhibit signaling by blocking its interaction with interleukin-6 receptor. J. Biol. Chem. 2014, 289, 8706–8719. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Hu, Y.; Duan, J.; Ma, J.; Xu, D.; Yang, X.D. Selection of a novel DNA aptamer for assay of intracellular interferon-gamma. PLoS ONE 2014, 9, e98214. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Ekker, S.C. Zebrafish as a genomics research model. Curr. Pharm. Biotechnol. 2004, 5, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Yao, H.; Wang, L.; Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes. Int. J. Mol. Sci. 2017, 18, 1683. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Krieg, A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Prodeus, A.; Abdul-Wahid, A.; Fischer, N.W.; Huang, E.H.; Cydzik, M.; Gariépy, J. Targeting the PD-1/PD-L1 Immune Evasion Axis With DNA Aptamers as a Novel Therapeutic Strategy for the Treatment of Disseminated Cancers. Mol. Ther. Nucleic Acids 2015, 4, e237. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Forzan, M.; Sproat, B.; Pantophlet, R.; McGowan, I.; Burton, D.; James, W. An aptamer that neutralizes R5 strains of HIV-1 binds to core residues of gp120 in the CCR5 binding site. Virology 2008, 381, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.; Shima, D.T.; Calias, P.; Cunningham, E.T., Jr.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Hayashi, T.; Takabayashi, K.; Sabet, M.; Smee, D.F.; Guiney, D.D.; Cottam, H.B.; Carson, D.A. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc. Natl. Acad. Sci. USA 2007, 104, 3990–3995. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Curigliano, G. Targeting Immune Checkpoint. Breast Cancer: Innovations in Research and Management; Springer: Berlin/Heidelberg, Germany, 2017; pp. 781–785. [Google Scholar] [CrossRef]
- Sun, H.; Zu, Y. A Highlight of Recent Advances in Aptamer Technology and Its Application. Molecules 2015, 20, 11959–11980. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Castanares, M.; Mukherjee, A.; Lupold, S.E. Nucleic acid aptamers: Clinical applications and promising new horizons. Curr. Med. Chem. 2011, 18, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.C.; Hoogenboezem, E.N.; Sorets, A.G.; Duvall, C.L.; Lippmann, E.S. Albumin-Binding Aptamer Chimeras for Improved siRNA Bioavailability. Cell Mol. Bioeng. 2022, 15, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, H.P.; Arya, S.P.; Tan, X. Biotechnological Advances Utilizing Aptamers and Peptides Refining PD-L1 Targeting. Front. Biosci. (Elite Ed.) 2024, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Malonia, S.K. Advancing cellular immunotherapy with macrophages. Life Sci. 2023, 328, 121857. [Google Scholar] [CrossRef] [PubMed]
- Ugai, T.; Yao, Q.; Ugai, S.; Ogino, S. Advancing precision oncology: Insights into the tumor microenvironment and immunotherapy outcomes. Innovation 2024, 5, 100656. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fang, J.; Peng, J.; Wang, X.; Xing, P.; Jia, K.; Hu, J.; Wang, D.; Ding, Y.; Wang, X.; et al. PD-1/PD-L1 immune checkpoint blockade in breast cancer: Research insights and sensitization strategies. Mol. Cancer 2024, 23, 266. [Google Scholar] [CrossRef] [PubMed]
- Trojaniello, C.; Luke, J.J.; Ascierto, P.A. Therapeutic Advancements Across Clinical Stages in Melanoma, with a Focus on Targeted Immunotherapy. Front. Oncol. 2021, 11, 670726. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Passariello, M.; Agnello, L.; Esposito, S.; Collina, F.; Cantile, M.; Di Bonito, M.; Ulasov, I.V.; Fedele, M.; Zannetti, A.; et al. Aptamer targeted therapy potentiates immune checkpoint blockade in triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2020, 39, 180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Xu, H.; Zhou, Y.; Xie, W.; Guo, Y.; Liao, Z.; Jiang, X.; Liu, J.; Ren, C. Aptamers combined with immune checkpoints for cancer detection and targeted therapy: A review. Int. J. Biol. Macromol. 2024, 262, 130032. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Solomon, D.; Avis, F.P.; Chang, A.E.; Freerksen, D.L.; Linehan, W.M.; Lotze, M.T.; Robertson, C.N.; Seipp, C.A.; Simon, P.; et al. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: A pilot study. J. Clin. Oncol. 1988, 6, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; Rusconi, C.P.; Sullenger, B.A. Aptamers: An emerging class of therapeutics. Annu. Rev. Med. 2005, 56, 555–583. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Shigdar, S. Future of PD-1/PD-L1 axis modulation for the treatment of triple-negative breast cancer. Pharmacol. Res. 2022, 175, 106019. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Schoenfelt, K.Q.; Hoffman, A.; Blank, A.; McBeth, B.; Pulliam, N.; Reardon, C.A.; Kulkarni, S.A.; et al. A lysosome-targeted DNA nanodevice selectively targets macrophages to attenuate tumours. Nat. Nanotechnol. 2021, 16, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Wiecken, M.; Machiraju, D.; Chakraborty, S.; Mayr, E.M.; Lenoir, B.; Eurich, R.; Richter, J.; Pfarr, N.; Halama, N.; Hassel, J.C. The immune checkpoint LAG-3 is expressed by melanoma cells and correlates with clinical progression of the melanoma. Oncoimmunology 2025, 14, 2430066. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yao, F.; An, Y.; Lai, X.; Li, X.; Yu, Z.; Yang, X.-D. Novel nanotherapeutics for cancer immunotherapy by albumin nanoparticles functionalized with PD-1 and PD-L1 aptamers. Cancer Nanotechnol. 2024, 15, 3. [Google Scholar] [CrossRef]
- Lee, M.; Lee, M.; Song, Y.; Kim, S.; Park, N. Recent Advances and Prospects of Nucleic Acid Therapeutics for Anti-Cancer Therapy. Molecules 2024, 29, 4737. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yao, F.; An, Y.; Li, X.; Wang, W.; Yang, X.-D. Novel bispecific aptamer targeting PD-1 and nucleolin for cancer immunotherapy. Cancer Nanotechnol. 2023, 14, 27. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, G.; Liu, S.; Chen, M.; Yang, C.; Song, Y. Aptamer-based Immune Checkpoint Inhibition for Cancer Immunotherapy. ChemBioChem 2025, 26, e202400599. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Yu, X.; Wang, Q.; Jiang, Z.; Li, X.; Chen, W.; Song, C. The immune checkpoint TIGIT/CD155 promotes the exhaustion of CD8 + T cells in TNBC through glucose metabolic reprogramming mediated by PI3K/AKT/mTOR signaling. Cell Commun. Signal 2024, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- ElTanbouly, M.A.; Croteau, W.; Noelle, R.J.; Lines, J.L. VISTA: A novel immunotherapy target for normalizing innate and adaptive immunity. Semin. Immunol. 2019, 42, 101308. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.J.; Porciani, D.; Burke, D.H. Cancer immunomodulation using bispecific aptamers. Mol. Ther. Nucleic Acids 2022, 27, 894–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, H.; Sun, J.; Liu, Y.; Li, J.; Li, J.; Li, J.; Yang, H. Bispecific Aptamer Induced Artificial Protein-Pairing: A Strategy for Selective Inhibition of Receptor Function. J. Am. Chem. Soc. 2019, 141, 12673–12681. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Wei, D.; Zhou, H.; Zhu, J.; Yu, Q.; Luo, L.; Dai, X.; Jiang, Y.; Yu, L.; et al. Polyvalent Aptamer Nanodrug Conjugates Enable Efficient Tumor Cuproptosis Therapy Through Copper Overload and Glutathione Depletion. J. Am. Chem. Soc. 2024, 146, 30033–30045. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, S.; Wei, X.; Wan, S.; Huang, M.; Song, T.; Lu, Y.; Weng, X.; Lin, Z.; Chen, H.; et al. Aptamer Blocking Strategy Inhibits SARS-CoV-2 Virus Infection. Angew. Chem. Int. Ed. Engl. 2021, 60, 10266–10272. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, Y.; Xie, Y.; Pu, J. Aptamer-based applications for cardiovascular disease. Front. Bioeng. Biotechnol. 2022, 10, 1002285. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.O.; Kolonias, D.; Pastor, F.; Mittler, R.S.; Chen, L.; Giangrande, P.H.; Sullenger, B.; Gilboa, E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Investig. 2008, 118, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Shangguan, D.; Liu, H.; Phillips, J.A.; Zhang, X.; Chen, Y.; Tan, W. Molecular assembly of an aptamer-drug conjugate for targeted drug delivery to tumor cells. ChemBioChem 2009, 10, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; Cui, C.; Cansiz, S.; Liang, H.; Wu, C.; Teng, I.T.; Chen, W.; Liu, Y.; Hou, W.; et al. Enhanced Targeted Gene Transduction: AAV2 Vectors Conjugated to Multiple Aptamers via Reducible Disulfide Linkages. J. Am. Chem. Soc. 2018, 140, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular α-particle therapy of cancer. J. Nucl. Med. 2014, 55, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kruspe, S.; Meyer, C.; Hahn, U. Chlorin e6 Conjugated Interleukin-6 Receptor Aptamers Selectively Kill Target Cells Upon Irradiation. Mol. Ther. Nucleic Acids 2014, 3, e143. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Ding, P.; Luo, Y.; Gao, T.; Zhang, Y.; Pei, R. Aptamer-integrated α-Gal liposomes as bispecific agents to trigger immune response for killing tumor cells. J. Biomed. Mater. Res. A 2019, 107, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Hou, X.; Yang, W.; Shi, W.; Yang, X.; Duan, S.; Mo, F.; Liu, A.; Wang, W.; Lu, X. Endoglin-Aptamer-Functionalized Liposome-Equipped PD-1-Silenced T Cells Enhance Antitumoral Immunotherapeutic Effects. Int. J. Nanomed. 2021, 16, 6017–6034. [Google Scholar] [CrossRef] [PubMed]
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W.; Langer, R.; Farokhzad, O.C. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007, 7, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, J.; Wu, X.; Zhao, J.; Yang, Q.; Lou, X. A highly specific aptamer probe targeting PD-L1 in tumor tissue sections: Mutation favors specificity. Anal. Chim. Acta 2021, 1185, 339066. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X.; Xu, J.; Cui, C.; Safari Yazd, H.; Pan, X.; Zhu, Y.; Chen, X.; Li, X.; Li, J.; et al. Circular Bispecific Aptamer-Mediated Artificial Intercellular Recognition for Targeted T Cell Immunotherapy. ACS Nano 2020, 14, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, N.; Trajanoski, Z. Advancing cancer immunotherapy: A vision for the field. Genome Med. 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Dow, S. A Role for Dogs in Advancing Cancer Immunotherapy Research. Front. Immunol. 2019, 10, 2935. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, Y.; An, Y.; Duan, J.; Li, X.; Yang, X.D. Novel Bispecific Aptamer Enhances Immune Cytotoxicity Against MUC1-Positive Tumor Cells by MUC1-CD16 Dual Targeting. Molecules 2019, 24, 478. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mo, L.; Hu, X.; Yu, D.; Xie, S.; Li, J.; Zhao, Z.; Fang, X.; Ye, M.; Qiu, L.; et al. Bispecific Aptamer-Based Recognition-then-Conjugation Strategy for PD1/PDL1 Axis Blockade and Enhanced Immunotherapy. ACS Nano 2022, 16, 21129–21138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Zhang, S.; Jiang, H.; Zhu, J.; Jiang, G.; Liu, Y.; Zhu, Y.; Li, J. Bispecific Nanobody-Aptamer Conjugates for Enhanced Cancer Therapy in Solid Tumors. Small 2024, 20, e2308265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Abudureheman, T.; Zheng, W.W.; Yang, L.T.; Zhu, J.M.; Liang, A.B.; Duan, C.W.; Chen, K. CAR-Aptamers Enable Traceless Enrichment and Monitoring of CAR-Positive Cells and Overcome Tumor Immune Escape. Adv. Sci. 2024, 11, e2305566. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Dunn, Z.S.; Yu, Y.; Li, M.; Wang, P.; Yang, L. Advancing cell-based cancer immunotherapy through stem cell engineering. Cell Stem Cell 2023, 30, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Y.; Huang, B.T.; Wang, J.W.; Lin, P.Y.; Yang, P.C. A Novel PD-L1-targeting Antagonistic DNA Aptamer With Antitumor Effects. Mol. Ther. Nucleic Acids 2016, 5, e397. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, X.; Yao, F.; Duan, J.; Yang, X.D. Novel Complex of PD-L1 Aptamer and Albumin Enhances Antitumor Efficacy In Vivo. Molecules 2022, 27, 1482. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Yang, G.; Bai, X.; Yuan, D.; Li, L.; Wang, D.; Lu, X.; Cheng, Y.; Wang, Y.; Song, X.; et al. Anti-tumor effect of PD-L1-targeting antagonistic aptamer-ASO delivery system with dual inhibitory function in immunotherapy. Cell Chem. Biol. 2023, 30, 1390–1401.e1396. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Pei, R. Isolation of DNA Aptamer Targeting PD-1 with an Antitumor Immunotherapy Effect. ACS Appl. Bio Mater. 2020, 3, 7080–7086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Satheesan, S.; Li, H.; Weinberg, M.S.; Morris, K.V.; Burnett, J.C.; Rossi, J.J. Cell-specific RNA aptamer against human CCR5 specifically targets HIV-1 susceptible cells and inhibits HIV-1 infectivity. Chem. Biol. 2015, 22, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Su, M.A. AIRE expands: New roles in immune tolerance and beyond. Nat. Rev. Immunol. 2016, 16, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, T.; Wu, Y.; Tan, Y.; Jiang, T.; Li, K.; Lou, B.; Chen, L.; Liu, Y.; Liu, Z. Aptamer based probes for living cell intracellular molecules detection. Biosens. Bioelectron. 2022, 208, 114231. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Liu, Y.; Shi, M.; Chen, J.; Li, K.; Tan, Y.; Chen, L.; Wu, Y.; Wang, T.; Liu, X.; et al. Aptamer-based biosensors for virus protein detection. Trends Analyt. Chem. 2022, 157, 116738. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Yang, G.; Qu, F. Research advances in non-immobilized aptamer screening techniques for small-molecule targets. Chin. J. Chromatogr. 2025, 43, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, P.; Kurniawan, H.; Byrne, M.E.; Wower, J. Therapeutic RNA aptamers in clinical trials. Eur. J. Pharm. Sci. 2013, 48, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.M.; Lewis, S.D.; Kurz, M.; Boomer, R.M.; Thompson, K.M.; Wilson, C.; McCauley, T.G. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm. Res. 2004, 21, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.O., 2nd; Andrechek, E.R.; Wang, Y.; Viles, K.D.; Rempel, R.E.; Gilboa, E.; Sullenger, B.A.; Giangrande, P.H. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006, 24, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.I.; Herrera, A.; Rossi, J.J.; Zhou, J. Current Advances in Aptamers for Cancer Diagnosis and Therapy. Cancers 2018, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A. Gene Regulation with Polyvalent Oligonucleotide Nanoparticle Conjugates; Northwestern University: Evaston, IL, USA, 2009. [Google Scholar]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef] [PubMed]
- Nolte, A.; Klussmann, S.; Bald, R.; Erdmann, V.A.; Fürste, J.P. Mirror-design of L-oligonucleotide ligands binding to L-arginine. Nat. Biotechnol. 1996, 14, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Hoinka, J.; Zotenko, E.; Friedman, A.; Sauna, Z.E.; Przytycka, T.M. Identification of sequence-structure RNA binding motifs for SELEX-derived aptamers. Bioinformatics 2012, 28, i215–i223. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Lavaee, P.; Ramezani, M.; Abnous, K. Reversible targeting and controlled release delivery of daunorubicin to cancer cells by aptamer-wrapped carbon nanotubes. Eur. J. Pharm. Biopharm. 2011, 77, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Havaei, S.A.; Sedighian, H.; Kachuei, R.; Fooladi, A.A.I. Prediction of aptamer affinity using an artificial intelligence approach. J. Mater. Chem. B 2024, 12, 8825–8842. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sun, M.; Zhang, J.; Lin, X.; Zhang, Y.; Lin, F.; Zhang, P.; Yang, C.; Song, J. Computational tools for aptamer identification and optimization. TrAC Trends Anal. Chem. 2022, 157, 116767. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.-Y.; Xia, J.; Li, X.; Yang, T.; Wang, J.-H. Ratiometric 3D DNA Machine Combined with Machine Learning Algorithm for Ultrasensitive and High-Precision Screening of Early Urinary Diseases. ACS Nano 2021, 15, 19522–19534. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.; Ferdousi, R. AptaNet as a deep learning approach for aptamer–protein interaction prediction. Sci. Rep. 2021, 11, 6074. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jia, C.; Li, T. Prediction of aptamer-protein interacting pairs based on sparse autoencoder feature extraction and an ensemble classifier. Math. Biosci. 2019, 311, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zheng, Y.; Huang, M.; Wu, L.; Wang, W.; Zhu, Z.; Song, Y.; Yang, C. A Sequential Multidimensional Analysis Algorithm for Aptamer Identification based on Structure Analysis and Machine Learning. Anal. Chem. 2020, 92, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Quazi, S. The potential implementation of biosensors for the diagnosis of biomarkers of various cancer. Med. Pharmacol.-Oncol. Oncog. 2022. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, X.; Zhang, Y.; Zhou, G.; Ke, X.; Wang, H.; Tinnefeld, P.; He, Z. One-pot synthesized aptamer-functionalized CdTe:Zn2+ quantum dots for tumor-targeted fluorescence imaging in vitro and in vivo. Anal. Chem. 2013, 85, 5843–5849. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dai, A.; Sun, J.; Li, X.; Gao, F.; Wu, L.; Fang, Y.; Yang, H.; An, L.; Wu, H.; et al. Aptamer-conjugated Mn3O4@SiO2 core-shell nanoprobes for targeted magnetic resonance imaging. Nanoscale 2013, 5, 10447–10454. [Google Scholar] [CrossRef] [PubMed]
- Geleta, G.S. A colorimetric aptasensor based on gold nanoparticles for detection of microbial toxins: An alternative approach to conventional methods. Anal. Bioanal. Chem. 2022, 414, 7103–7122. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Chen, T.; Kamath, R.; Xiong, X.; Tan, W.; Fan, Z.H. Aptamer-enabled efficient isolation of cancer cells from whole blood using a microfluidic device. Anal. Chem. 2012, 84, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shang, J.; Jia, Y.; Pei, R.; Stojanovic, M.; Lin, Q. Spatially selective release of aptamer-captured cells by temperature mediation. IET Nanobiotechnol. 2014, 8, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
| Targets | Target Cells | Aptamers | Binding Affinities | Functions | Aptamer Sequences | References |
|---|---|---|---|---|---|---|
| PD-L1 | Tumor cell | AptPD-L1 | ~5–50 nM | PD-L1/PD-1 interaction inhibitors | 5′-ATACCAGCTTATTCAATTGTAGAGTATAAAAAGAGTGATGATCTTTTGTAGGTTTTTTAGATAGTAAGTGCAATCT-3′ | [21] |
| PD-1 | T-cells | PD-1 Apt1 | ~10–100 nM | PD-L1/PD-1 interaction inhibitors | 5′-TCCCTACGGCGCTAACCCTCCCCTAGTATATATTGTCCTCGTCTATGCCACCGTGCTA CAAC-3′ | [22] |
| CTLA-4 | T-cells | CTLA-4 Apt | ~10–100 nM | Regulate T-cell activation by outcompeting CD28 for B7 binding | 5′-GGGAGAGAGGAAGAGGGATAGGCACCGGAAGGGCTACACTCCTATATCCCCTGCCcAGCCCGCCATAACCCAGAGGTCGATAGTACYGGATCCCCCC-3′ | [23] |
| CTLA-4/NKG2A dual receptors | T-cells/NK cells | AYA22T-R2-13 | ~1–50 nM | Enhancing CD8+ T-cells and NK cells effector functions | 5′-ACACdUdUdUdUCCCCCACCdUGAdUCCdUCAGdUdUCCGGAAAAGdUGdU-3′ | [5] |
| Nucleolin | Tumor cells | AS1411 | ~1–10 nM | Inhibiting cancer cell proliferation | 5′- CCAGCCATCCAAAACTCTGTGGTGGTGGTGGTTGTGGTGGTGGTGGTAACTATCCTTGCCCGAACG-3′ | [24] |
| PTK7 | Tumor cells | Sgc8 | ~0.2–2 nM | Inhibit EGFR signaling | 5′-ATACCAGCTTATTCAATTAAAGNTAATCGCCGTAGAAAAGCATGTCAAAGCCGGAACCNCAGATAGTAAGTGCAATCT-3′ | [25] |
| TIM-3 | T cells | S3.1 | ~10–20 nM | Block TIM-3/galectin- 9 interaction | 5′-GGGGGAATTCTAATACGACTCACTATAGGGAGGACGATGCGGGGGAUGCUCAUUCAACGUUCCAGAUAUCAGGGCAUCCCCAGACGACTCGCTGAGGATCC-3′ | [26] |
| LAG-3 | T cells | SL15 | ~20–200 nM | Block LAG-3/MHC-II interaction | 5′-GGGGAATTCTAATACGACTCACTATAGGGAGAGAGATATAAGGGAGAGAATTTGGTAATGGGCCCTTATATCTCTCTCCCATTACCAAATTCTCTCCC-3′ | [27] |
| SDF-1 | Tumor cells | NOX-A12 (Spiegelmer) | 0.2–0.5 nM | Binds and neutralizes SDF-1 thereby blocking its interaction with CXCR4 and CXCR7 | 5′-GGCGACAUUGGUGGCUUUCUACUGCUUGUGAGUAUUUCGUACAGCUGCUAUAGUGAGUA-3′ | [28] |
| CXCL12 | Tumor cells | CXCL12 Apt (Spiegelmer) | ~0.4–1.5 nM | Inhibits CXCL12-mediated chemotaxis and inhibits tumor metastasis | 5′-ATGAACGCCAAGGTCGTGGTCTGGCTGTTGTGCTTACTTGTTT-3′ | [29] |
| VEGF | Vascular endothelial cells | Pegaptanib (Macugen®) | ~0.1–2 nM | Block VEGF interaction with VEGFR2, reducing endothelial cell proliferation | 5′-TCGGGCGAGTCGTCTGTAATACGACTCACTATAGGGAGGACGATGCGG(N30or40)CAGACGACTCGCCCGATAATACGACTCACTATAGGGAGGACGATGCGG-3′ | [19] |
| TLR9 | B cells | CpG7909 | ~1–100 nM | Active innate immunity and promotes Th1 responses | 5′-CCAGTCGTACAGGAAACATGCGTTCTAGATGTTCGGGGC-3′ | [30] |
| TNF-α | Macrophages | VR11 | ~1–10 nM | Inhibit TNFα signaling | 5′-TGGTGGATGGCGCAGTCGGCGACAA-3′ | [31] |
| IL-6 | Macrophages | SL1025 | ~0.4–9.6 nM | Block IL-6/IL-6R interaction | 5′-GATGTGAGTGTGTGACGAGN40CACAGAGAAGAAACAAGACC-3′ | [32] |
| IFN-γ | Macrophages | ARC225 | ~0.26–10 nM | Activate macrophages | 5′-TGCCCGTGTCCCGAGGAGGTGCCCTATTTTGCTTGATTATCTCTAAGGGATTTGGGCGG-3′ | [33] |
| Parameter | Monoclonal Antibodies (mAbs) | PD-1/PD-L1 Aptamers |
|---|---|---|
| Therapeutic efficacy | Proven efficacy in blocking PD-1/PD-L1 interaction, causes sustained anti-tumor reactions in cancers like melanoma and NSCLC [6] | Comparable blocking of PD-1/PD-L1 interaction, with enhanced tumor penetration [5] |
| Safety profile | Associated with immune-related adverse events (irAEs), including colitis and pneumonitis, due to broad immune activation [8] | Minimal irAEs and off-target effects; lower risk of systemic immune activation and reduced likelihood of allergic reactions [10] |
| Immunogenicity | High immunogenicity, as antibodies are protein-based, leading to potential allergic reactions and anti-drug antibody responses [6] | Low immunogenicity due to their non-proteinaceous, synthetic nature, making them safer for repeated use [9] |
| Production cost | High cost due to complex bioprocessing in mammalian cell cultures, requiring stringent quality control [9] | Significantly lower cost due to entirely chemical synthesis, with easier scalability and lower material costs [5] |
| Development timeline | Longer timelines are driven by the complexities of antibody discovery, optimization, and cell-based production [8] | Synthetic design and high-throughput screening techniques enable faster timelines [10] |
| Stability and handling | Requires refrigeration and controlled conditions to maintain bioactivity, with limited stability outside cold-chain logistics [6] | Chemically modifiable for enhanced stability; can withstand harsher conditions and longer storage periods [9] |
| Cost to patients | Expensive, with treatment costs ranging between $100,000–$150,000 per patient annually, limiting accessibility [5] | Lower cost per treatment cycle, making them more affordable and accessible, especially in low-income settings [10] |
| Clinical accessibility | Widely approved and available for various cancers, forming the backbone of current immunotherapy protocols [6] | Preclinical and early clinical stages; promising results suggest potential for future approvals [5] |
| Tissue penetration | Limited penetration in dense tumor microenvironments due to large molecular size [8] | Superior penetration in solid tumors due to smaller size and enhanced molecular flexibility [10] |
| Environmental impact | High environmental impact due to reliance on biologics manufacturing facilities and extensive resource consumption [9] | Low environmental impact due to pure chemical synthesis processes and reduced reliance on animal-based production systems [5] |
| Focus | Key Findings/Contributions | Relevance | References |
|---|---|---|---|
| Dual checkpoint aptamer immunotherapy targeting CTLA-4 and NKG2A | Demonstrated enhanced efficacy in tailored cancer treatment using aptamer-based dual checkpoint targeting | Advances in tailored cancer immunotherapy strategies | [5] |
| A highly specific aptamer probe targeting PD-L1 in tumor tissues | High PD-L1 specificity of aptamer probe mutations in the aptamer sequence can increase its specificity | Making aptamer-targeted therapies may aid personalized medicine approaches | [87] |
| Tumor and immune reprogramming in advanced renal cell carcinoma | Identified molecular and immune changes during immunotherapy | Insights into the mechanisms of immunotherapy responses | [9] |
| Aptamer therapy in triple-negative breast cancer | Found that aptamer-targeted therapy enhances immune checkpoint blockade | Supports aptamer use in potentiating immunotherapy | [52] |
| Circular bispecific aptamer-mediated artificial intercellular recognition for targeted T cell | T cell accumulation and activation improved with reduced complexity and time | Create a new T cell “recognition activation” strategy without ex vivo engineering | [88] |
| Advancing cancer immunotherapy | Summarizes recent progress and future directions in cancer immunotherapy | Visionary insights for the immunotherapy field | [89] |
| Dogs in cancer immunotherapy research | Proposed dogs as model organisms for translational cancer immunotherapy | Broadens the scope of animal models in cancer research | [90] |
| Bispecific aptamer targeting PD-1 and nucleolin | Demonstrated anti-tumor efficacy and immune modulation in vitro and in vivo | Highlights bispecific aptamers as innovative cancer therapies | [69] |
| Bispecific aptamer enhances immune cytotoxicity against MUC1-positive tumors | A novel approach to targeting antitumor immune reactions against MUC1 | Develop a bispecific aptamer targeting MUC1 (tumor marker) and CD16 (on immune cells | [91] |
| Bispecific aptamer-based recognition-then-conjugation strategy for PD1/PDL1 axis blockade and enhanced immunotherapy | The recognition-then-conjugation strategy may boost tumor immunity | Develop a bispecific aptamer-based PD1/PDL1 axis blocker to improve immunotherapy | [92] |
| Bispecific nanobody-aptamer conjugates for enhanced cancer therapy | Strong steric hindrance, high affinity, and specificity for tumor cells expressing both targets | Target two distinct antigens or receptors on cancer cells simultaneously | [93] |
| CAR-aptamers enable traceless enrichment and monitoring of CAR-positive cells | Enriching CAR-T cells efficiently and cheaply, monitoring expansion in vivo, and overcoming tumor escape | Design bispecific circular aptamers to retarget CAR-T cells to tumors after antigen loss | [94] |
| Nanotherapeutics functionalized with PD-1/PD-L1 aptamers | Developed functionalized nanoparticles for cancer immunotherapy. | Advances in nanotechnology in immunotherapy applications | [67] |
| Immune checkpoint inhibitors via aptamers | Highlights recent progress in aptamer-based checkpoint inhibitors | Explores alternative checkpoint inhibitors | [4] |
| Targeting the PD-1/PD-L1 immune evasion axis with DNA aptamers as therapeutics | PD-1 antagonistic aptamers may be a better alternative to antibody-based therapies | Produce synthetic DNA aptamers that bind specifically to the murine extracellular domain of PD-1 and inhibit interaction | [38] |
| Cell-based cancer immunotherapy via stem cell engineering | Developed engineered stem cells for enhanced immunotherapy | Advances in cellular approaches in cancer therapy | [95] |
| A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects | Increased lymphocyte proliferation in vitro and inhibited tumor growth | Develop DNA aptamer blocks PD-1/PD-L1 interaction and targets PD-L1. | [96] |
| Novel complex of PD-L1 aptamer and albumin enhances antitumor efficacy | A promising strategy to improve aptamer in vivo functionality and which may be useful in immunotherapy | Test PD-L1 aptamer albumin complex could improve antitumor efficacy in vivo | [97] |
| Dual inhibitory aptamer-ASO delivery system | Anti-tumor efficacy of a dual-functional delivery system | Combining aptamers and ASOs in therapy | [98] |
| Isolation of DNA aptamer targeting PD-1 with an antitumor immunotherapy effect | Highest affinity and restored T cell function suppressed by PD-1/PD-L1 | Identify PD-1-targeted DNA aptamers for cancer immunotherapy | [99] |
| Modification Type | Added Agents | Contributions | References |
|---|---|---|---|
| Chemical modifications | 2′-O-Methyl(2′-OMe), 2′-Fluoro(2′-F) and 2′-Amino (2′-NH2) | Significantly promote nuclease resistance of aptamer without sacrificing binding affinity. | [16] |
| PEGylation | Increase aptamer stability and prolong circulation time in the bloodstream. | [107] | |
| Molecule conjugation | Protein (e.g., albumin) | Enhance aptamer pharmacokinetic properties. | [108] |
| Lipid | Enhance aptamer bioavailability and cellular absorption. | [109] | |
| Nanoparticle encapsulation | AuNPs | Improve aptamer stability and cellular uptake. | [110] |
| Polymeric and liposomes | Protect aptamer from nuclease degradation and promote delivery to target tissues. | [111] | |
| Spiegelmers | L-enantiomers | Enhanced resistance of aptamer to nuclease degradation. | [112] |
| Optimization sequence | Computational tools | Aptamer with optimal stability and binding properties. | [113] |
| Drug conjugation | Therapeutic substances (e.g., Dox and Dau) | Improve aptamer stability and targeted effectiveness. | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdu, A.M.A.; Liu, Y.; Abduljabbar, R.; Man, Y.; Chen, Q.; Liu, Z. Aptamers Targeting Immune Checkpoints for Tumor Immunotherapy. Pharmaceutics 2025, 17, 948. https://doi.org/10.3390/pharmaceutics17080948
Abdu AMA, Liu Y, Abduljabbar R, Man Y, Chen Q, Liu Z. Aptamers Targeting Immune Checkpoints for Tumor Immunotherapy. Pharmaceutics. 2025; 17(8):948. https://doi.org/10.3390/pharmaceutics17080948
Chicago/Turabian StyleAbdu, Amir Mohammed Abker, Yanfei Liu, Rami Abduljabbar, Yunqi Man, Qiwen Chen, and Zhenbao Liu. 2025. "Aptamers Targeting Immune Checkpoints for Tumor Immunotherapy" Pharmaceutics 17, no. 8: 948. https://doi.org/10.3390/pharmaceutics17080948
APA StyleAbdu, A. M. A., Liu, Y., Abduljabbar, R., Man, Y., Chen, Q., & Liu, Z. (2025). Aptamers Targeting Immune Checkpoints for Tumor Immunotherapy. Pharmaceutics, 17(8), 948. https://doi.org/10.3390/pharmaceutics17080948








