Liposome-Loaded Mesenchymal Stem Cells Enhance Tumor Accumulation and Anti-Tumor Efficacy of Doxorubicin in Mouse Tumor Models of Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animals
2.3. Preparation of DOX-Mag-AL/ATCOL Complexes
2.4. Cellular Association of Mag-AL/ATCOL Complexes in MSCs
2.5. Cytotoxicity and Association of DOX-Mag-AL/ATCOL Complexes with MSCs
2.6. DOX Release from DOX-Lip-MSCs
2.7. In Vitro Migration of DOX-Lip-MSCs
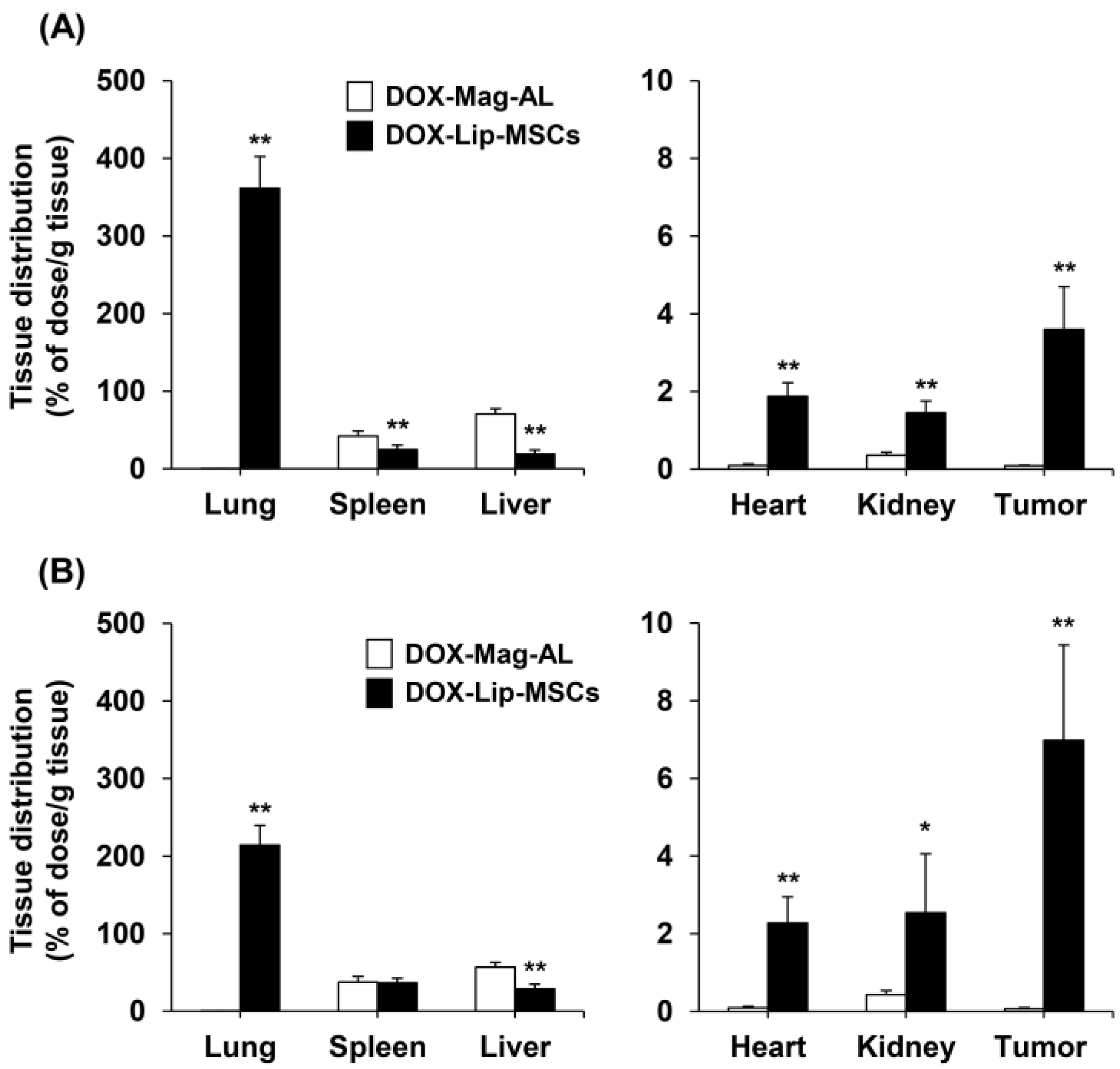
2.8. In Vivo Tissue Distribution of DOX-Lip-MSCs
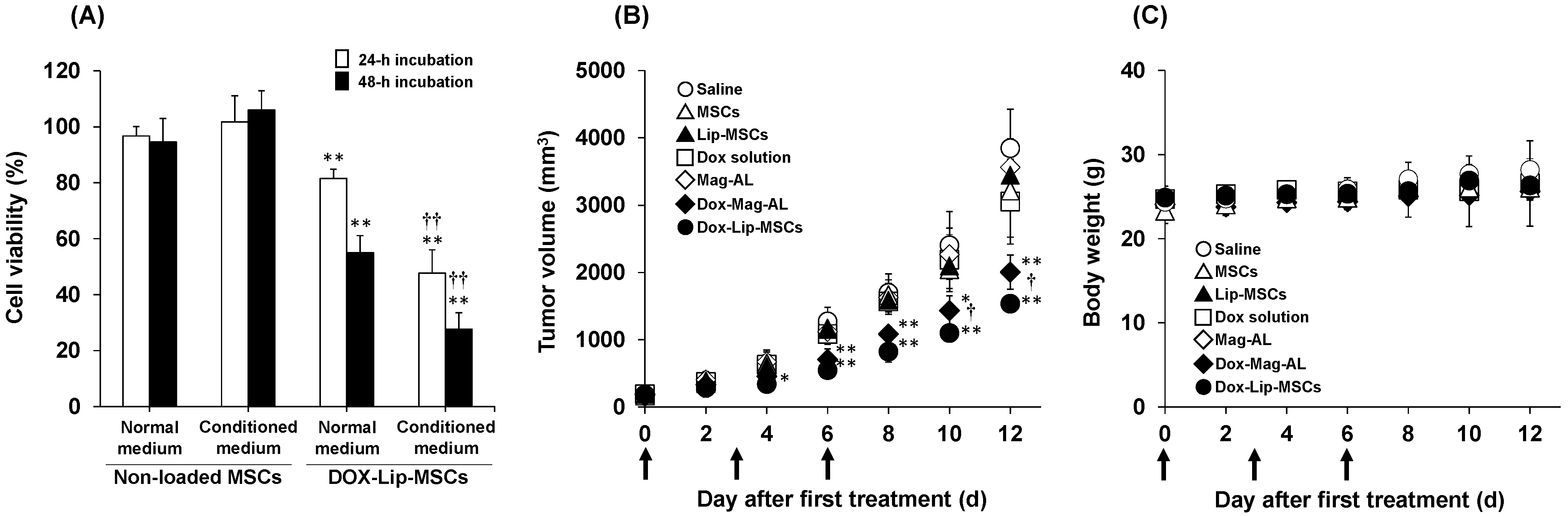
2.9. In Vivo Anti-Tumor Efficacy of DOX-Lip-MSCs
2.10. HPLC Measurement of DOX
2.11. Statistical Analysis
3. Results
3.1. Optimization of the Composition and Particle Size of Mag-AL for Efficient and Stable Remote Loading of DOX
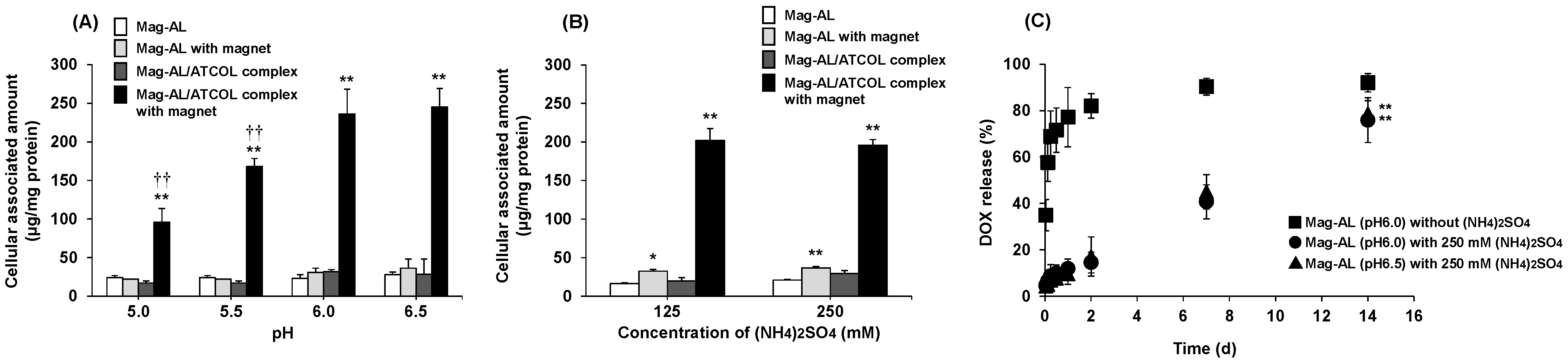
3.1.1. Effect of the Inner Aqueous Phase of Mag-AL on the Cellular Association and DOX Retention of Mag-AL/ATCOL Complexes
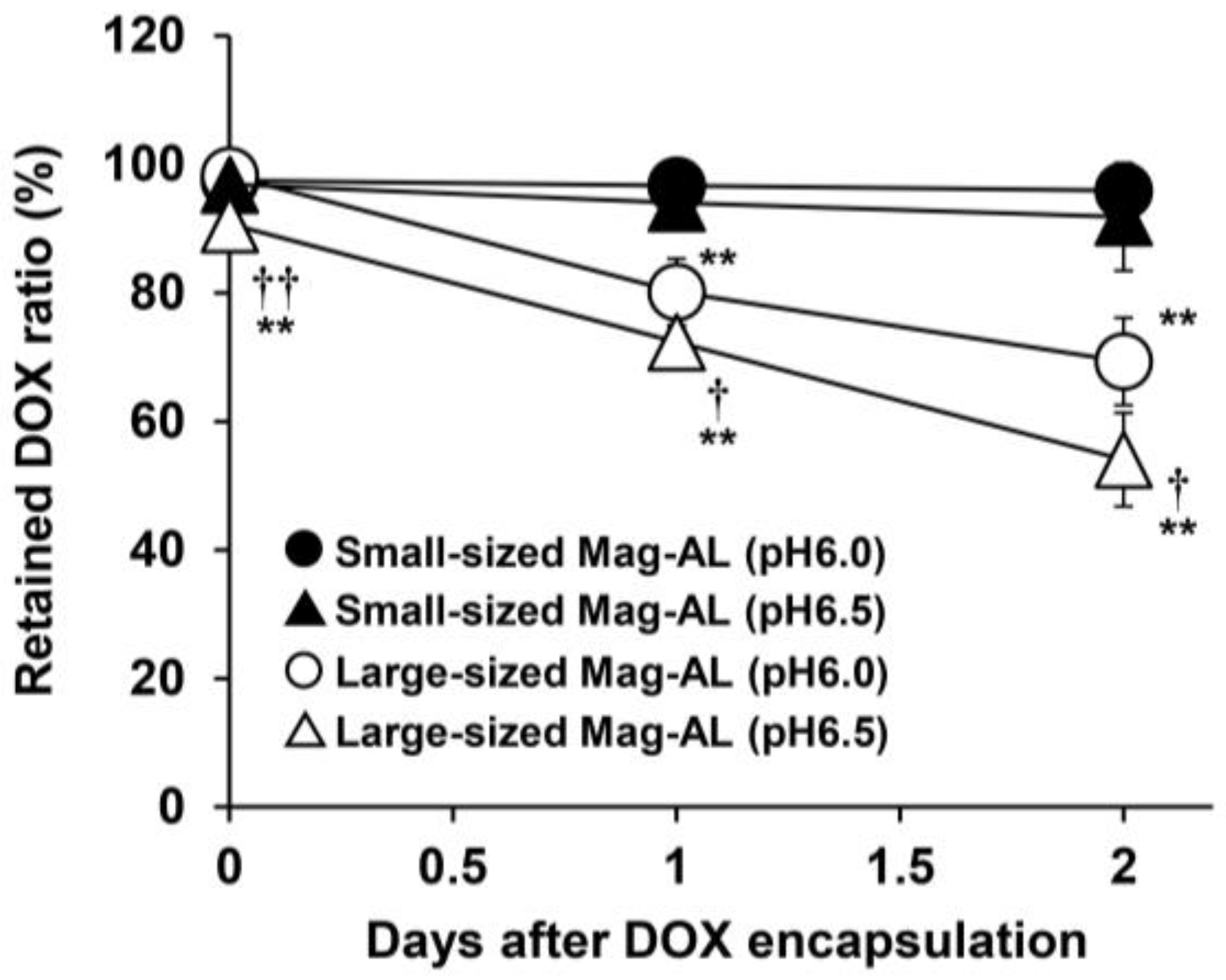
3.1.2. Effect of the Particle Size of Mag-AL on DOX Retention Properties
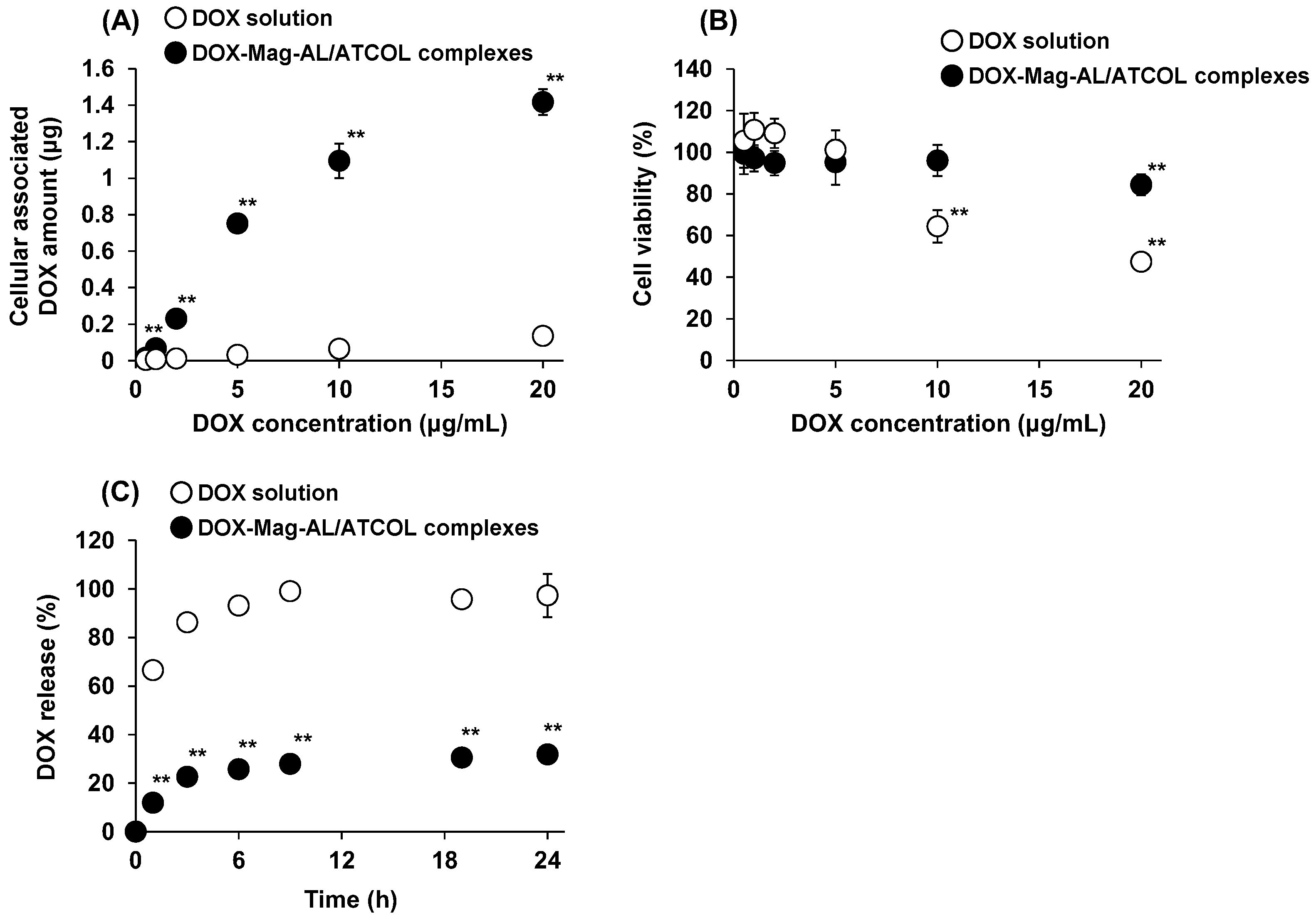
3.2. DOX Loading Amount, Cell Viability, and DOX-Releasing Properties of DOX-Lip-MSCs
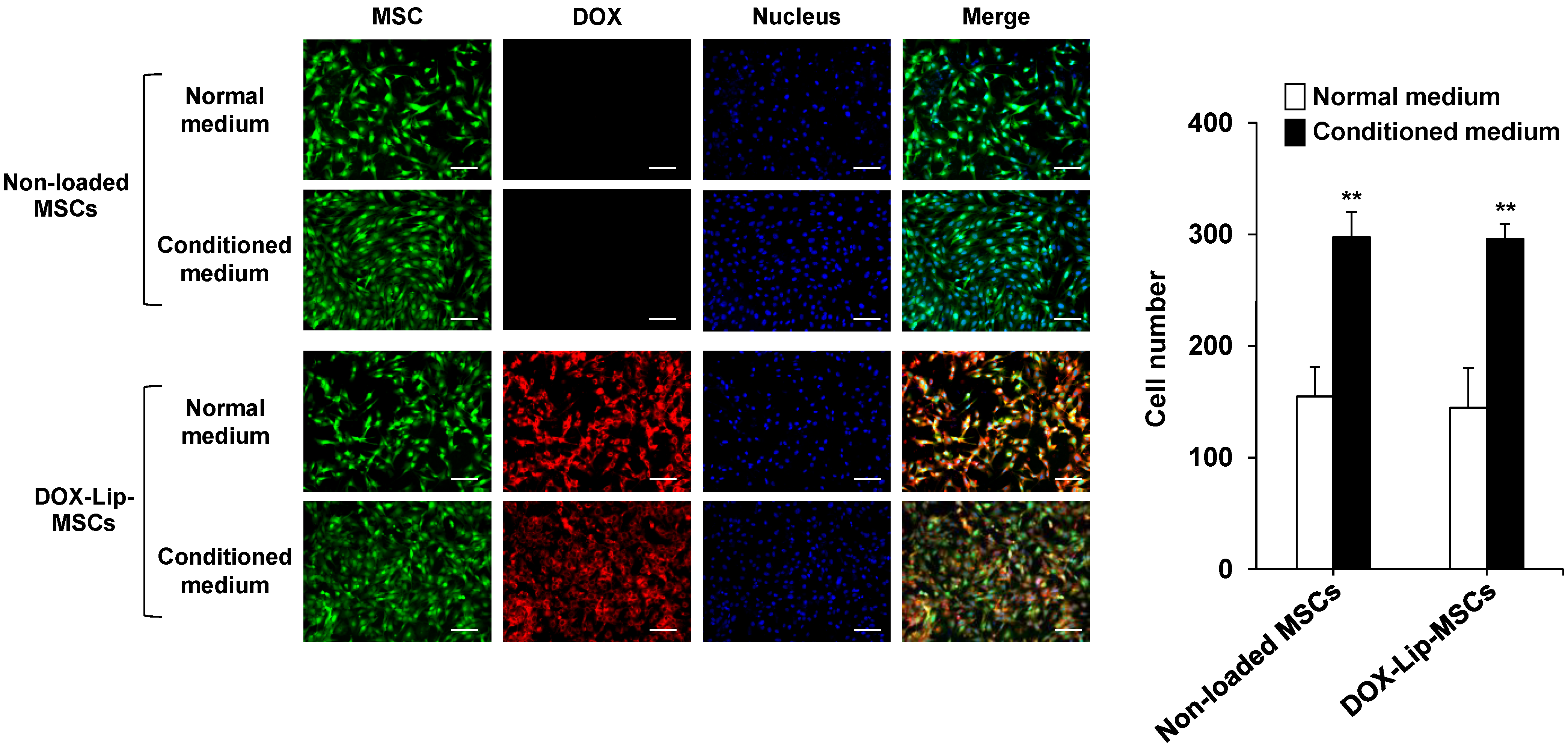
3.3. In Vitro and In Vivo Tumor-Tropic Ability of DOX-Lip-MSCs
3.4. In Vitro and in Vivo Anti-Tumor Efficacy of DOX-Lip-MSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdolmaleki, A.; Asadi, A.; Gurushankar, K.; Shayan, T.K.; Sarvestani, F.A. Importance of nano medicine and new drug therapies for cancer. Adv. Pharm. Bull. 2021, 11, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Bio. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Iwata, C.; Tanaka, H.Y.; Cabral, H.; Morishita, Y.; Miyazono, K.; Kano, M.R. Increased fibrosis and impaired intratumoral accumulation of macromolecules in a murine model of pancreatic cancer co-administered with FGF-2. J. Control. Release 2016, 230, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Tanei, T.; Godin, B.; van de Ven, A.L.; Hanibuchi, M.; Matsunoki, A.; Alexander, J.; Ferrani, M. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer. Lett. 2014, 345, 48–55. [Google Scholar] [CrossRef]
- Ogawara, K.I.; Un, K.; Minato, K.; Tanaka, K.I.; Higaki, K.; Kimura, T. Determinants for in vivo anti-tumor effects of PEG liposomal doxorubicin: Importance of vascular permeability within tumors. Int. J. Pharm. 2008, 359, 234–240. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, S. Cell-mediated nanoparticle delivery systems: Towards precision nanomedicine. Drug. Deliv. Transl. Res. 2024, 14, 3032–3054. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Li, F.; Xu, L.; Sun, Y. Cell-mediated targeting drugs delivery systems. Drug. Deliv. 2020, 27, 1425–1437. [Google Scholar] [CrossRef]
- Tiet, P.; Berlin, J.M. Exploiting homing abilities of cell carriers: Targeted delivery of nanoparticles for cancer therapy. Biochem. Pharmacol. 2017, 145, 18–26. [Google Scholar] [CrossRef]
- Waqar, M.A.; Zaman, M.; Khan, R.; Rahman, M.S.U.; Majeed, I. Navigating the tumor microenvironment: Mesenchymal stem cell-mediated delivery of anticancer agents. J. Drug Target. 2024, 32, 624–634. [Google Scholar] [CrossRef]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent advances on drug-loaded mesenchymal stem cells with anti-neoplastic agents for targeted treatment of cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef]
- Wu, H.H.; Zhou, Y.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef]
- Heris, R.M.; Shirvaliloo, M.; Abbaspour-Aghdam, S.; Hazrati, A.; Shariati, A.; Youshanlouei, H.R.; Niaragh, F.J.; Valizadeh, H.; Ahmadi, M. The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment. Stem Cell Res. Ther. 2022, 13, 371. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal stem cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Lenna, S.; Bellotti, C.; Duchi, S.; Martella, E.; Columbaro, M.; Dozza, B.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Frisoni, T.; et al. Mesenchymal stromal cells mediated delivery of photoactive nanoparticles inhibits osteosarcoma growth in vitro and in a murine in vivo ectopic model. J. Exp. Clin. Cancer Res. 2020, 39, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, J.; Ouyang, X.; Wang, J.; Sun, X.; Lv, Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic- co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, S.; Guo, J.; Alahdal, M.; Cao, S.; Yang, Z.; Zhang, F.; Shen, Y.; Sun, M.; Mo, R.; et al. Targeted delivery of doxorubicin by nano-loaded mesenchymal stem cells for lung melanoma metastasis therapy. Sci. Rep. 2017, 7, 44758. [Google Scholar] [CrossRef]
- Wróblewska, A.; Szczygieł, A.; Szermer-Olearnik, B.; Pajtasz-Piasecka, E. Macrophages as promising carriers for nanoparticle delivery in anticancer therapy. Int. J. Nanomed. 2023, 18, 4521–4539. [Google Scholar] [CrossRef]
- Hosseinalizadeh, H.; Mahmoodpour, M.; Bahabadi, Z.R.; Hamblin, M.R.; Mirzaei, H. Neutrophil mediated drug delivery for targeted glioblastoma therapy: A comprehensive review. Biomed. Pharmacother. 2022, 156, 113841. [Google Scholar] [CrossRef]
- Takayama, Y.; Kusamori, K.; Tsukimori, C.; Shimizu, Y.; Hayashi, M.; Kiyama, I.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Nishikawa, M. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J. Control. Release 2021, 329, 1090–1101. [Google Scholar] [CrossRef]
- Xu, M.; Asghar, S.; Dai, S.; Wang, Y.; Feng, S.; Jin, L.; Shao, F.; Xiao, Y. Mesenchymal stem cells-curcumin loaded chitosan nanoparticles hybrid vectors for tumor-tropic therapy. Int. J. Bio. Macromol. 2019, 134, 1002–1012. [Google Scholar] [CrossRef]
- Li, L.; Guan, Y.; Liu, H.; Hao, N.; Liu, T.; Meng, X.; Fu, C.; Li, Y.; Qu, Q.; Zhang, Y.; et al. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano 2011, 5, 7462–7470. [Google Scholar] [CrossRef]
- Kono, Y.; Kamino, R.; Hirabayashi, S.; Kishimoto, T.; Kanbara, H.; Danjo, S.; Hosokawa, M.; Ogawara, K.I. Efficient liposome loading onto surface of mesenchymal stem cells via electrostatic interactions for tumor-targeted drug delivery. Biomedicines 2023, 11, 558. [Google Scholar] [CrossRef]
- Kono, Y.; Takegaki, J.; Ohba, T.; Matsuda, K.; Negoro, R.; Fujita, S.; Fujita, T. Magnetization of mesenchymal stem cells using magnetic liposomes enhances their retention and immunomodulatory efficacy in mouse inflamed skeletal muscle. Int. J. Pharm. 2021, 596, 120298. [Google Scholar] [CrossRef]
- Kono, Y.; Nakai, T.; Taguchi, H.; Fujita, T. Development of magnetic anionic liposome/atelocollagen complexes for efficient magnetic drug targeting. Drug Deliv. 2017, 24, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Onishi, K.; Kitamura, H.; Ogawara, K.I. Development of liposome-loaded macrophages as tumor-targeted drug delivery carriers: Impact of macrophage phenotype on their liposome-loading capacity and tumor-homing potential. J. Drug Deliv. Sci. Technol. 2025, 108, 106866. [Google Scholar] [CrossRef]
- Makwana, V.; Karanjia, J.; Haselhorst, T.; Anoopkumar-Dukie, S.; Rudrawar, S. Lipsoma; doxorubicin as targeted delivery platform: Current trends in surface functionalization. Int. J. Pharm. 2021, 593, 120117. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®--the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Lee, P.Y.; Tsai, B.C.K.; Sitorus, M.A.; Lin, P.Y.; Lin, S.Z.; Shih, C.Y.; Lu, S.Y.; Lin, Y.M.; Ho, T.J.; Huang, C.Y. Ohwia caudata aqueous extract attenuates doxorubicin-induced mitochondrial dysfunction in Wharton’s jelly-derived mesenchymal stem cells. Environ. Toxicol. 2023, 38, 2450–2461. [Google Scholar] [CrossRef]
- Dezfouli, A.B.; Salar-Amoli, J.; Pourfathollar, A.A.; Yazdi, M.; Nikougoftar-Zarif, M.; Khosravi, M.; Hassan, J. Doxorubicin-indued senescence through NF-κB affected by the age of mouse mesenchymal stem cells. J. Cell Physiol. 2020, 235, 2336–2349. [Google Scholar] [CrossRef]
- Kozhukharova, I.; Zemelko, V.; Kovaleva, Z.; Alekseenko, L.; Lyublinskaya, O.; Nikolsky, N. Therapeutic doses of doxorubicin induce premature senescence of human mesenchymal stem cells derived from menstrual blood, bone marrow and adipose tissue. Int. J. Hematol. 2018, 107, 286–296. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, S.; Liu, C.; Jiang, Y. Tumor tropic delivery of doxorubicin-polymer conjugates using mesenchymal stem cells for glioma therapy. Biomaterials 2015, 39, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Sadhukha, T.; O’Brien, T.D.; Prabha, S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control. Release 2014, 196, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, A.J.; Lee, S.J.; Suh, H.S.; Shin, I.H. Inhibition of P-glycoprotein facilitated glycosaminoglycan accumulation during chondrogenesis of human bone marrow mesenchymal stem cells. Int. J. Rheum. Dis. 2011, 14, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, X.; Wang, W.; Qiu, N.; Wen, J.; Duan, X.; Li, X.; Chen, X.; Yang, L.; Qian, Z.; et al. Peptide ligand and PEG-modified long-circulating liposome targeted to FGFR overexpressing tumor in vivo. Int. J. Nanomed. 2012, 7, 4499–4510. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Kono, Y.; Ogawara, K.I.; Kimura, T.; Higaki, K. PEG liposomalization of paclitaxel improved its in vivo disposition and anti-tumor efficacy. Int. J. Pharm. 2011, 412, 132–141. [Google Scholar] [CrossRef]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise review: Mesenchymal stem cell-based drug delivery: The good, the bad, the ugly, and the promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improved myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Takayama, Y.; Kusamori, K.; Katsurada, Y.; Obana, S.; Itakura, S.; Nishikawa, M. Efficient delivery of mesenchymal stem/stromal cells to injured liver by surface PEGylation. Stem Cell Res. Ther. 2023, 14, 216. [Google Scholar] [CrossRef]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem cell transplantation: The lung barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef]
| pH | (NH4)2SO4 Concentration (mM) | Particle Size (nm) | ζ-Potential (mV) | Polydispersity Index (PDI) | DOX Encapsulation | |
|---|---|---|---|---|---|---|
| Small-sized | 5.0 | 250 | 111.4 ± 6.4 | −37.2 ± 1.7 | 0.14 ± 0.05 | NE |
| 5.5 | 250 | 107.6 ± 2.3 | −36.4 ± 2.3 | 0.17 ± 0.03 | NE | |
| 6.0 | 125 | 117.0 ± 5.5 | −34.4 ± 4.1 | 0.11 ± 0.02 | 96.2 ± 2.0 | |
| 6.0 | 250 | 111.6 ± 9.3 | −38.3 ± 1.7 | 0.15 ± 0.04 | 97.5 ± 1.3 | |
| 6.5 | 250 | 106.0 ± 4.2 | −33.1 ± 2.5 | 0.19 ± 0.04 | 96.9 ± 1.3 | |
| Large-sized | 6.0 | 250 | 634.4 ± 15.0 | −36.6 ± 3.1 | 0.32 ± 0.03 | 98.3 ± 0.8 |
| 6.5 | 250 | 618.0 ± 17.2 | −36.8 ± 0.9 | 0.31 ± 0.06 | 90.7 ± 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kono, Y.; Kanbara, H.; Danjo, S.; Yoshikawa, A.; Iwayama, Y.; Ogawara, K.-i. Liposome-Loaded Mesenchymal Stem Cells Enhance Tumor Accumulation and Anti-Tumor Efficacy of Doxorubicin in Mouse Tumor Models of Melanoma. Pharmaceutics 2025, 17, 947. https://doi.org/10.3390/pharmaceutics17080947
Kono Y, Kanbara H, Danjo S, Yoshikawa A, Iwayama Y, Ogawara K-i. Liposome-Loaded Mesenchymal Stem Cells Enhance Tumor Accumulation and Anti-Tumor Efficacy of Doxorubicin in Mouse Tumor Models of Melanoma. Pharmaceutics. 2025; 17(8):947. https://doi.org/10.3390/pharmaceutics17080947
Chicago/Turabian StyleKono, Yusuke, Himi Kanbara, Saki Danjo, Aiga Yoshikawa, Yoshihiro Iwayama, and Ken-ichi Ogawara. 2025. "Liposome-Loaded Mesenchymal Stem Cells Enhance Tumor Accumulation and Anti-Tumor Efficacy of Doxorubicin in Mouse Tumor Models of Melanoma" Pharmaceutics 17, no. 8: 947. https://doi.org/10.3390/pharmaceutics17080947
APA StyleKono, Y., Kanbara, H., Danjo, S., Yoshikawa, A., Iwayama, Y., & Ogawara, K.-i. (2025). Liposome-Loaded Mesenchymal Stem Cells Enhance Tumor Accumulation and Anti-Tumor Efficacy of Doxorubicin in Mouse Tumor Models of Melanoma. Pharmaceutics, 17(8), 947. https://doi.org/10.3390/pharmaceutics17080947






