Polymorphic Transformations of Pharmaceutical Materials Induced by Mechanical Milling: A Review
Abstract
1. Introduction
2. Compounds with Polymorphic Transformations Reported in Literature
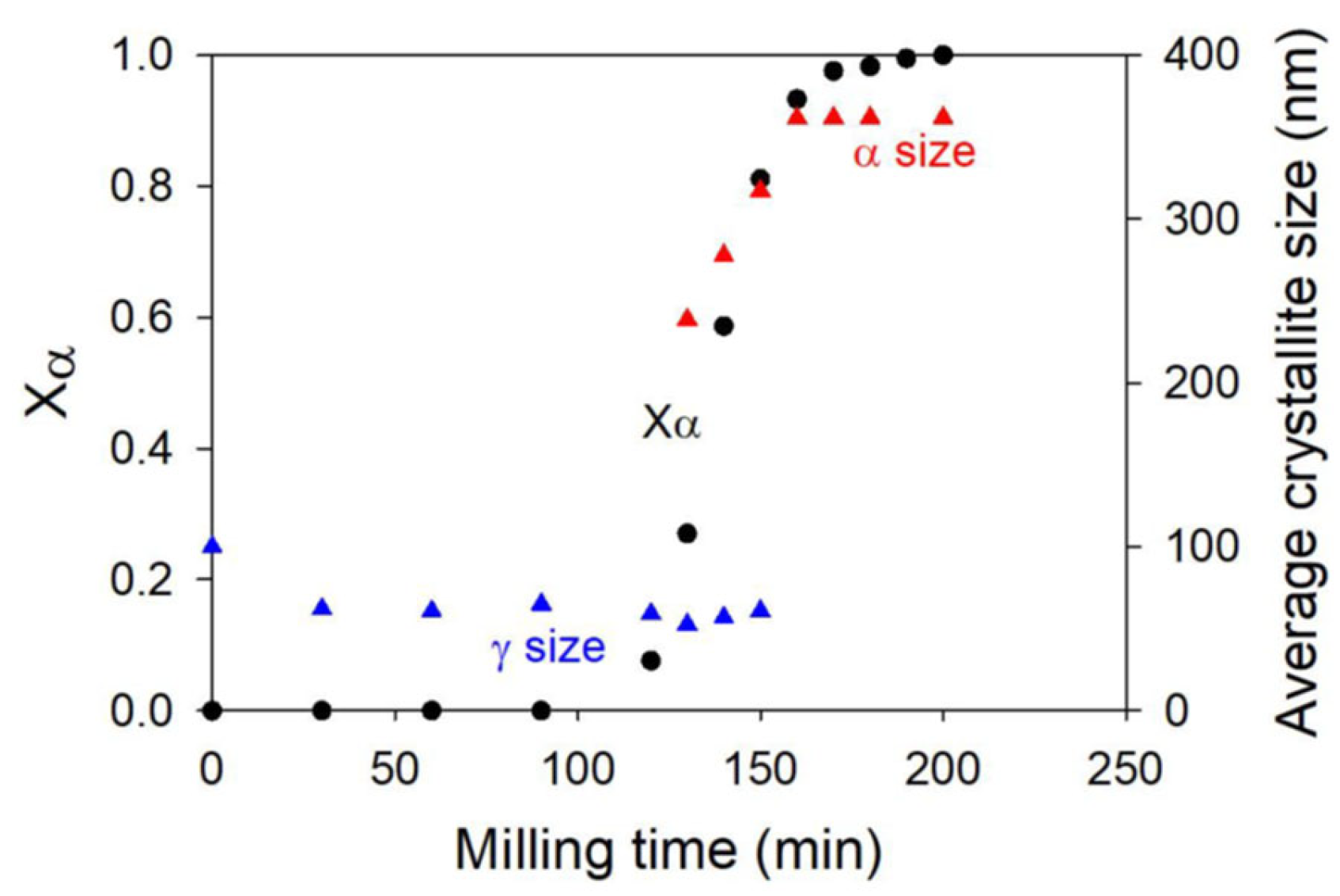
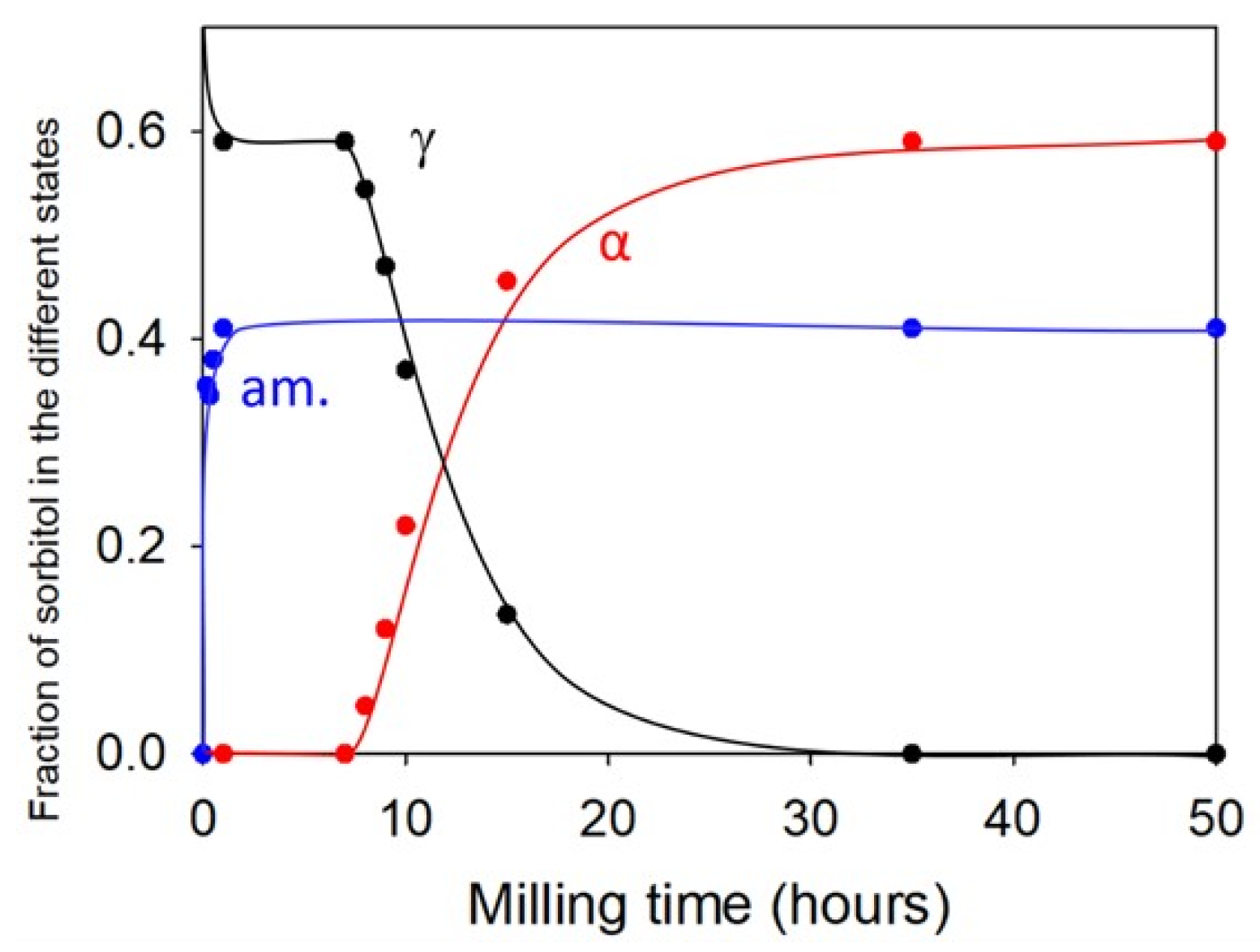
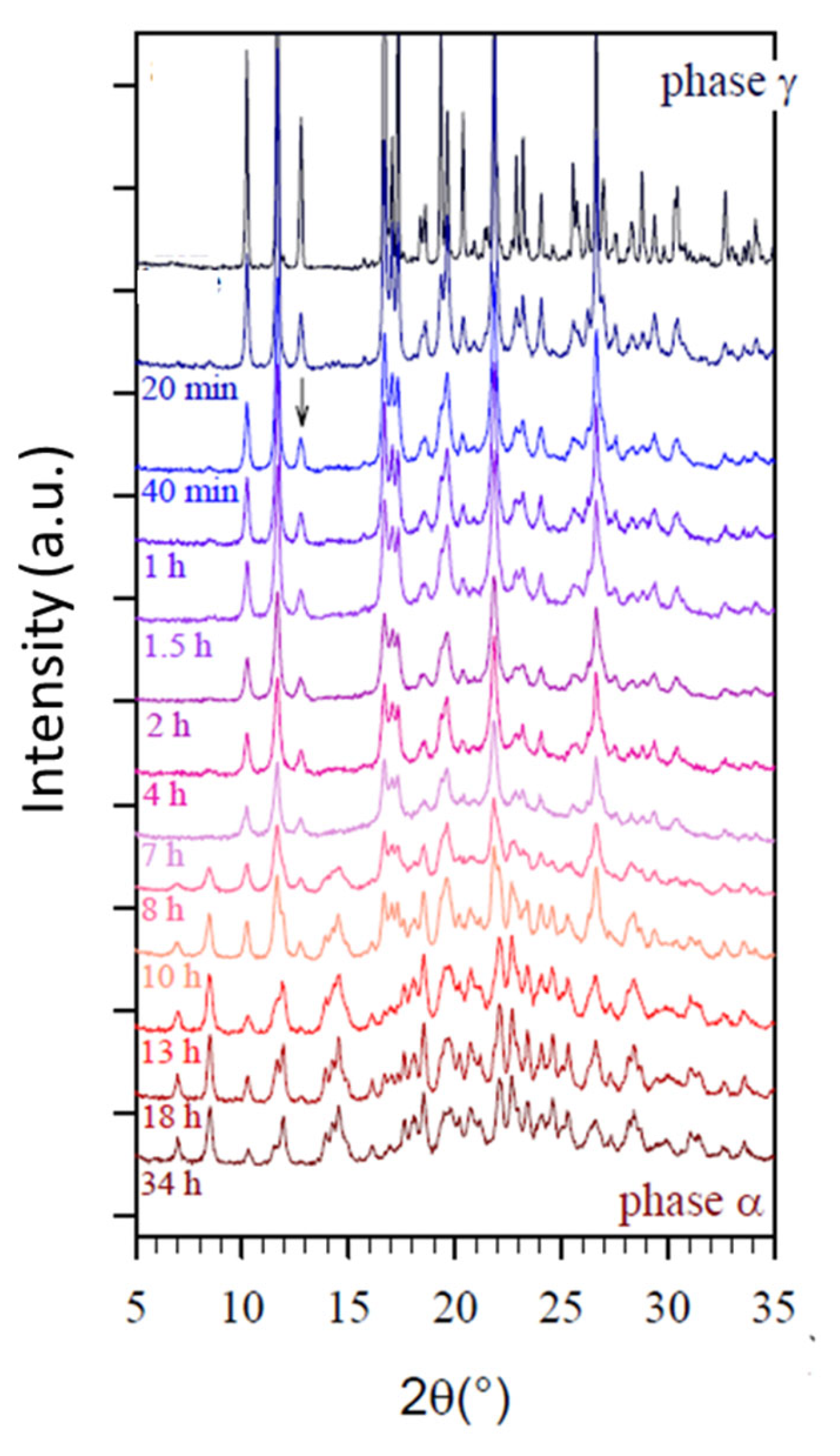
2.1. Sorbitol
2.2. Bezafibrate
2.3. Sulfamerazine
2.4. Mannitol
2.5. Glycine
2.6. Sulfathiazole
2.7. Ranitidine Hydrochloride
2.8. Rivastigmine Hydrogen Tartrate
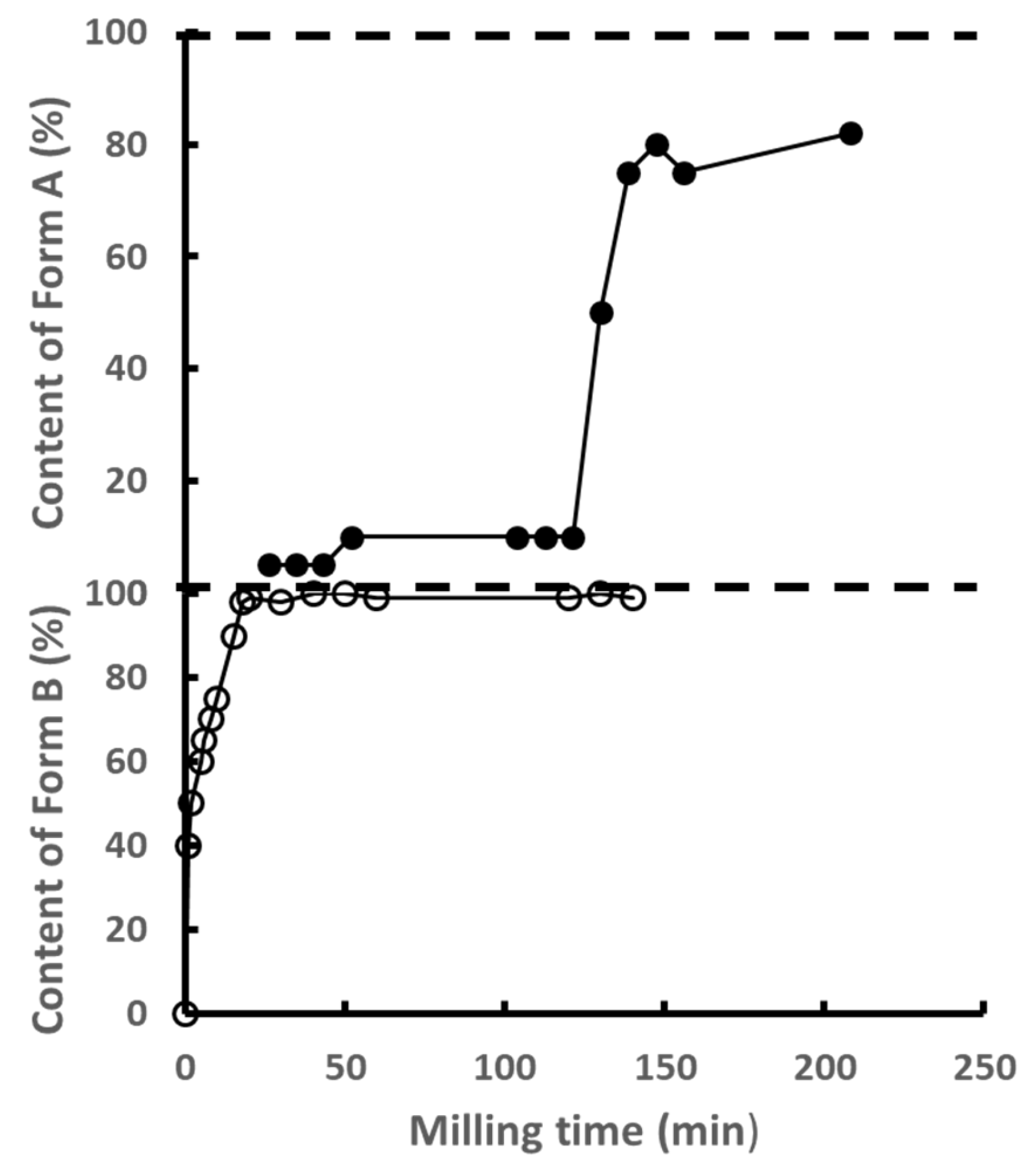
2.9. Famotidine
2.10. Gabapentin
2.11. Indomethacin
2.12. Modafinil
2.13. Fananserin
2.14. Chloramphenicol Palmitate
2.15. Cimetidine
2.16. Phenylbutazone
2.17. Nolomirole Hydrochloride
2.18. Caffeine
3. Analysis of the Mechanism of Polymorphic Transformation
3.1. Kinetics of Polymorphic Transformation Involving Two Monotropic Forms
3.2. Kinetics of Polymorphic Transformation Involving Two Enantiotropic Forms
3.3. Detection of a Transient Amorphous Fraction During Polymorphic Transformations
4. Conclusions
- -
- Total and long-lasting amorphization occurs when the milling is permed below the Tg of the material gives rise to a crystal to glass transformation.
- -
- Transient amorphization is followed by a more or less rapid recrystallization when the milling is performed above Tg. If this recrystallization occurs towards the starting polymorphic form, no apparent structural change is observed. On the other hand, if the recrystallization occurs towards another polymorphic form, an apparent polymorphic transformation is observed. The nature of the form which recrystallizes can hardly be anticipated as one or several metastable phases can develop as predicted by the Ostwald’s rule of stages [87]. However, in practice, the recrystallized form is often identical to that obtained for the recrystallization of the corresponding quenched liquid upon heating.
Funding
Conflicts of Interest
References
- Davey, R.J. Polymorphism in Molecular Crystals Joel Bernstein. Oxford University Press, New York, 2002. ISBN 0198506058. Cryst. Growth Des. 2002, 2, 675–676. [Google Scholar] [CrossRef]
- Caron, V.; Willart, J.-F.; Lefort, R.; Derollez, P.; Danède, F.; Descamps, M. Solid state amorphization kinetic of alpha lactose upon mechanical milling. Carbohydr. Res. 2011, 346, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Tsukushi, I.; Yamamuro, O.; Suga, H. Solid state amorphization and glass transition of tri-O-methyl-β-cyclodextrin. J. Therm. Anal. 1991, 37, 1359–1371. [Google Scholar] [CrossRef]
- Willart, J.-F.; Durand, M.; Briggner, L.-E.; Marx, A.; Danède, F.; Descamps, M. Solid-State Amorphization of Linaprazan by Mechanical Milling and Evidence of Polymorphism. J. Pharm. Sci. 2013, 102, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Tsukushi, I.; Yamamuro, O.; Matsuo, T. Solid state amorphization of organic molecular crystals using a vibrating mill. Solid State Commun. 1995, 94, 1013–1018. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Willart, J.-F.; Siepmann, J.; Siepmann, F.; Descamps, M. Using Milling To Explore Physical States: The Amorphous and Polymorphic Forms of Dexamethasone. Cryst. Growth Des. 2018, 18, 1748–1757. [Google Scholar] [CrossRef]
- Trasi, N.S.; Byrn, S.R. Mechanically Induced Amorphization of Drugs: A Study of the Thermal Behavior of Cryomilled Compounds. AAPS PharmSciTech 2012, 13, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Bordet, P.; Bytchkov, A.; Descamps, M.; Dudognon, E.; Elkaïm, E.; Martinetto, P.; Pagnoux, W.; Poulain, A.; Willart, J.-F. Solid State Amorphization of β-Trehalose: A Structural Investigation Using Synchrotron Powder Diffraction and PDF Analysis. Cryst. Growth Des. 2016, 16, 4547–4558. [Google Scholar] [CrossRef]
- Linol, J.; Morelli, T.; Petit, M.N.; Coquerel, G. Inversion of the relative stability between two polymorphic forms of (±) modafinil under dry high-energy milling: Comparisons with results obtained under wet high-energy milling. Cryst. Growth Des. 2007, 7, 1608–1611. [Google Scholar] [CrossRef]
- Willart, J.-F.; Lefebvre, J.; Danède, F.; Comini, S.; Looten, P.; Descamps, M. Polymorphic transformation of the Γ-form of d-sorbitol upon milling: Structural and nanostructural analyses. Solid State Commun. 2005, 135, 519–524. [Google Scholar] [CrossRef]
- Matsuoka, M.; Hirata, J.; Yoshizawa, S. Kinetics of solid-state polymorphic transition of glycine in mechano-chemical processing. Chem. Eng. Res. Des. 2010, 88, 1169–1173. [Google Scholar] [CrossRef]
- Dupont, A.; Guerain, M.; Danède, F.; Paccou, L.; Guinet, Y.; Hédoux, A.; Willart, J.-F. Kinetics and mechanism of polymorphic transformation of sorbitol under mechanical milling. Int. J. Pharm. 2020, 590, 119902. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.; Guerain, M.; Danède, F.; Willart, J.F. Evidence of transient amorphization during the polymorphic transformation of sorbitol induced by milling. Int. J. Pharm. 2022, 623, 121929. [Google Scholar] [CrossRef] [PubMed]
- Martinetto, P.; Bordet, P.; Descamps, M.; Dudognon, E.; Pagnoux, W.; Willart, J.-F. Structural Transformations of d-Mannitol Induced by in Situ Milling Using Real Time Powder Synchrotron Radiation Diffraction. Cryst. Growth Des. 2017, 17, 6111–6122. [Google Scholar] [CrossRef]
- Willart, J.F.; Descamps, M. Solid State Amorphization of Pharmaceuticals. Mol. Pharm. 2008, 5, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Gusseme, A.D.; Neves, C.; Willart, J.F.; Rameau, A.; Descamps, M. Ordering and disordering of molecular solids upon mechanical milling: The case of fananserine. J. Pharm. Sci. 2008, 97, 5000–5012. [Google Scholar] [CrossRef] [PubMed]
- Nezzal, A.; Aerts, L.; Verspaille, M.; Henderickx, G.; Redl, A. Polymorphism of sorbitol. J. Cryst. Growth 2009, 311, 3863–3870. [Google Scholar] [CrossRef]
- Yu, L. Growth Rings in d-Sorbitol Spherulites: Connection to Concomitant Polymorphs and Growth Kinetics. Cryst. Growth Des. 2003, 3, 967–971. [Google Scholar] [CrossRef]
- Lemmerer, A.; Báthori, N.B.; Esterhuysen, C.; Bourne, S.A.; Caira, M.R. Concomitant Polymorphs of the Antihyperlipoproteinemic Bezafibrate. Cryst. Growth Des. 2009, 9, 2646–2655. [Google Scholar] [CrossRef]
- Dudognon, E.; Danède, F.; Guerain, M. Milling-Induced Phase Transformations, Underlying Mechanisms, and Resulting Physical States in an Enantiotropic System: The Case of Bezafibrate. Cryst. Growth Des. 2022, 22, 363–378. [Google Scholar] [CrossRef]
- Ravindra Acharya, K.; Kuchela, K.N.; Kartha, G. Crystal structure of sulfamerazine. J. Crystallogr. Spectrosc. Res. 1982, 12, 369–376. [Google Scholar] [CrossRef]
- Caria, M.R.; Mohamed, R. Positive indentification of two orthorhombic polymorphs of sulfamerazine (C11H12N4O2S), their thermal analyses and structural comparison. Acta Crystallogr. Sect. B 1992, 48, 492–498. [Google Scholar] [CrossRef]
- Hossain, G.M.G. A new polymorph of sulfamerazine. Acta Crystallogr. Sect. E 2006, 62, o2166–o2167. [Google Scholar] [CrossRef]
- Zhang, G.G.Z.; Gu, C.; Zell, M.T.; Burkhardt, R.T.; Munson, E.J.; Grant, D.J.W. Crystallization and Transitions of Sulfamerazine Polymorphs. J. Pharm. Sci. 2002, 91, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Macfhionnghaile, P.; Hu, Y.; Gniado, K.; Curran, S.; Mcardle, P.; Erxleben, A. Effects of Ball-Milling and Cryomilling on Sulfamerazine Polymorphs: A Quantitative Study. J. Pharm. Sci. 2014, 103, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Henck, J.-O.; Hetz, S.; Rollinger, J.M.; Weissnicht, A.A.; Stöttner, H. Energy/Temperature Diagram and Compression Behavior of the Polymorphs of D-mannitol. J. Pharm. Sci. 2000, 89, 457–468. [Google Scholar] [CrossRef]
- Fronczek, F.R.; Kamel, H.N.; Slattery, M. Three polymorphs (α, β, and δ) of D-mannitol at 100K. Acta Crystallogr. Sect. C 2003, 59, o567–o570. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Willart, J.F.; Dudognon, E.; Caron, V. Transformation of pharmaceutical compounds upon milling and comilling: The role of Tg. J. Pharm. Sci. 2007, 96, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Perlovich, G.L.; Hansen, L.K.; Bauer-Brandl, A. The Polymorphism of Glycine. Thermochemical and structural aspects. J. Therm. Anal. Calorim. 2001, 66, 699–715. [Google Scholar] [CrossRef]
- Chua, Y.Z.; Do, H.T.; Schick, C.; Zaitsau, D.; Held, C. New experimental melting properties as access for predicting amino-acid solubility. RSC Adv. 2018, 8, 6365–6372. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Feng, W. Solubility of Amino Acids in Water and Aqueous Solutions by the Statistical Associating Fluid Theory. Ind. Eng. Chem. Res. 2008, 47, 6275–6279. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Breil, M.P.; Pinho, S.P.; Macedo, E.A.; Mollerup, J.M. Thermodynamic Modeling of Several Aqueous Alkanol Solutions Containing Amino Acids with the Perturbed-Chain Statistical Associated Fluid Theory Equation of State. Ind. Eng. Chem. Res. 2009, 48, 5498–5505. [Google Scholar] [CrossRef]
- Held, C.; Cameretti, L.F.; Sadowski, G. Measuring and Modeling Activity Coefficients in Aqueous Amino-Acid Solutions. Ind. Eng. Chem. Res. 2011, 50, 131–141. [Google Scholar] [CrossRef]
- Gupta, R.B.; Heidemann, R. Solubility models for amino acids and antibiotics. Aiche J. 1990, 36, 333–341. [Google Scholar] [CrossRef]
- Park, B.H.; Yoo, K.-P.; Lee, C.S. Phase equilibria and properties of amino acids + water mixtures by hydrogen-bonding lattice fluid equation of state. Fluid Phase Equilib. 2003, 212, 175–182. [Google Scholar] [CrossRef]
- Suzuki, T.; Franks, F. Solid–liquid phase transitions and amorphous states in ternary sucrose–glycine–water systems. J. Chem. Soc. Faraday Trans. 1993, 89, 3283–3288. [Google Scholar] [CrossRef]
- Anwar, J.; Tarling, S.E.; Barnes, P. Polymorphism of Sulfathiazole. J. Pharm. Sci. 1989, 78, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Apperley, D.C.; Fletton, R.A.; Harris, R.K.; Lancaster, R.W.; Tavener, S.; Threlfall, T.L. Sulfathiazole polymorphism studied by magic-angle spinning NMR. J. Pharm. Sci. 1999, 88, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Shakhtshneider, T.P.; Boldyrev, V. V Phase Transformations in Sulfathiazole During Mechanical Activation. Drug Dev. Ind. Pharm. 1993, 19, 2055–2067. [Google Scholar] [CrossRef]
- Hu, Y.; Erxleben, A.; Hodnett, B.K.; Li, B.; McArdle, P.; Rasmuson, Å.C.; Ryder, A.G. Solid-State Transformations of Sulfathiazole Polymorphs: The Effects of Milling and Humidity. Cryst. Growth Des. 2013, 13, 3404–3413. [Google Scholar] [CrossRef]
- Aji, D.P.B.; Khouri, J.; Johari, G.P. Non-exponential relaxation, fictive temperatures, and dispersive kinetics in the liquid-glass-liquid transition range of acetaminophen, sulfathiazole, and their mixtures. J. Chem. Phys. 2014, 141, 174507. [Google Scholar] [CrossRef] [PubMed]
- Hempel, A.; Camerman, N.; Mastropaolo, D.; Camerman, A. Ranitidine hydrochloride, a polymorphic crystal form. Acta Crystallogr. Sect. C 2000, 56, 1048–1049. [Google Scholar] [CrossRef] [PubMed]
- Huq, A.; Stephens, P.W. Subtleties in Crystal Structure Solution from Powder Diffraction Data Using Simulated Annealing:Ranitidine Hydrochloride. J. Pharm. Sci. 2003, 92, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Madan, T.; Kakkar, A. Preparation and Characterization of Ranitidine-HC1 Crystals. Drug Dev. Ind. Pharm. 1994, 20, 1571–1588. [Google Scholar] [CrossRef]
- Chieng, N.; Zujovic, Z.; Bowmaker, G.; Rades, T.; Saville, D. Effect of milling conditions on the solid-state conversion of ranitidine hydrochloride form 1. Int. J. Pharm. 2006, 327, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.I.; Simon, A.; Cabral, L.M.; de Sousa, V.P.; Healy, A.M. Rivastigmine hydrogen tartrate polymorphs: Solid-state characterisation of transition and polymorphic conversion via milling. Solid State Sci. 2015, 49, 29–36. [Google Scholar] [CrossRef]
- Kaduk, J.A.; Zhong, K.; Gindhart, A.M.; Blanton, T.N. Crystal structure of rivastigmine hydrogen tartrate Form I (Exelon®), C14H23N2O2(C4H5O6). Powder Diffr. 2016, 31, 97–103. [Google Scholar] [CrossRef]
- Golič, L.; Djinović, K.; Florjanič, M. Structure of a new crystalline form of famotidine. Acta Crystallogr. Sect. C 1989, 45, 1381–1384. [Google Scholar] [CrossRef]
- Yanagisawa, I.; Hirata, Y.; Ishii, Y. Studies on histamine H2 receptor antagonists. 2. Synthesis and pharmacological activities of N-sulfamoyl and N-sulfonyl amidine derivatives. J. Med. Chem. 1987, 30, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Cheng, W.-T.; Wang, S.-L. Thermodynamic and kinetic characterization of polymorphic transformation of famotidine during grinding. Int. J. Pharm. 2006, 318, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Mahlin, D.; Bergström, C.A.S. Early drug development predictions of glass-forming ability and physical stability of drugs. Eur. J. Pharm. Sci. 2013, 49, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Hsu, C.-H.; Ke, W.-T. Solid-state transformation of different gabapentin polymorphs upon milling and co-milling. Int. J. Pharm. 2010, 396, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ibers, J.A. Gabapentin and gabapentin monohydrate. Acta Crystallogr. Sect. C 2001, 57, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Reece, H.A.; Levendis, D.C. Polymorphs of gabapentin. Acta Crystallogr. Sect. C 2008, 64, o105–o108. [Google Scholar] [CrossRef] [PubMed]
- Kistenmacher, T.J.; Marsh, R.E. Crystal and molecular structure of an antiinflammatory agent, indomethacin, 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic acid. J. Am. Chem. Soc. 1972, 94, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Morris, K.R.; Griesser, U.J.; Byrn, S.R.; Stowell, J.G. Reactivity Differences of Indomethacin Solid Forms with Ammonia Gas. J. Am. Chem. Soc. 2002, 124, 15012–15019. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Ichikawa, J.; Kaneniwa, N.; Otsuka, M. Effect of Environmental Temperature on the Polymorphic Transformation of Phenylbutazone during Grinding. Chem. Pharm. Bull. 1988, 36, 1074–1085. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsumoto, T.; Kaneniwa, N. Effect of environmental temperature on polymorphic solid-state transformation of indomethacin during grinding. Chem. Pharm. Bull. 1986, 34, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Otsuka, K.; Kaneniwa, N. Relation Between Polymorphic Transformation Pathway During Grinding and the Physicochemical Properties of Bulk Powders for Pharmaceutical Preparations. Drug Dev. Ind. Pharm. 1994, 20, 1649–1660. [Google Scholar] [CrossRef]
- Desprez, S. Transformation de Phases Induites par Broyage Dans un Composé Moléculaire: L’indométhacine. Ph.D. Thesis, of the University of Lille, Villeneuve d’Ascq, France, 2004. [Google Scholar]
- Desprez, S.; Descamps, M. Transformations of glassy indomethacin induced by ball-milling. J. Non. Cryst. Solids 2006, 352, 4480–4485. [Google Scholar] [CrossRef]
- Luisi, B.S.; Medek, A.; Liu, Z.; Mudunuri, P.; Moulton, B. Milling-Induced Disorder of Pharmaceuticals: One-Phase or Two-Phase System? J. Pharm. Sci. 2012, 101, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Sun, L.; Shao, Y.; Li, D. 2-(Benzhydryl sulfinyl) acet amide. Acta Crystallogr. Sect. E 2004, 60, o372–o373. [Google Scholar] [CrossRef]
- Pauchet, M.; Morelli, T.; Coste, S.; Malandain, J.-J.; Coquerel, G. Crystallization of (±)-Modafinil in Gel: Access to Form I, Form III, and Twins. Cryst. Growth Des. 2006, 6, 1881–1889. [Google Scholar] [CrossRef]
- Mahieux, J.; Sanselme, M.; Coquerel, G. Access to Single Crystals of (±)-Form IV of Modafinil by Crystallization in Gels. Comparisons between (±)-Forms I, III, and IV and (−)-Form I. Cryst. Growth Des. 2013, 13, 908–917. [Google Scholar] [CrossRef]
- Giovannini, J.; Ter Minassian, L.; Céolin, R.; Toscani, S.; Perrin, M.-A.; Louër, D.; Leveiller, F. Tetramorphism of fananserine: P, T diagram and stability hierarchy from crystal structure determinations and thermodynamic studies. J. Phys. IV 2001, 11, Pr10-123–Pr10-126. [Google Scholar] [CrossRef]
- Szulzewsky, K.; Kulpe, S.; Schulz, B.; Kunath, D. The structure of the β modification of chloramphenicol palmitate—A redetermination. Acta Crystallogr. Sect. B 1981, 37, 1673–1676. [Google Scholar] [CrossRef]
- Szulzewsky, K.; Kulpe, S.; Schulz, B.; Fichtner Schmittler, H. Crystallographic results on the polymorphism of chloramphenicol palmitate. Acta Pharm. Suec. 1982, 19, 457–470. [Google Scholar]
- Otsuka, M.; Kaneniwa, N. Effect of seed crystals on solid-state transformation of polymorphs of chloramphenicol palmitate during grinding1. J. Pharm. Sci. 1986, 75, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Kaneniwa, N.; Otsuka, M. Effect of Grinding on the Transformations of Polymorphs of Chloramphenicol Palmitate. Chem. Pharm. Bull. 1985, 33, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Cernik, R.J.; Cheetham, A.K.; Prout, C.K.; Watkin, D.J.; Wilkinson, A.P.; Willis, B.T.M. The structure of cimetidine (C10H16N6S) solved from synchrotron-radiation X-ray powder diffraction data. J. Appl. Crystallogr. 1991, 24, 222–226. [Google Scholar] [CrossRef]
- Guzmán-Afonso, C.; Hong, Y.; Colaux, H.; Iijima, H.; Saitow, A.; Fukumura, T.; Aoyama, Y.; Motoki, S.; Oikawa, T.; Yamazaki, T.; et al. Understanding hydrogen-bonding structures of molecular crystals via electron and NMR nanocrystallography. Nat. Commun. 2019, 10, 3537. [Google Scholar] [CrossRef] [PubMed]
- Arakcheeva, A.; Pattison, P.; Bauer-Brandl, A.; Birkedal, H.; Chapuis, G. Cimetidine, C10H16N6S, form C: Crystal structure and modelling of polytypes using the superspace approach. J. Appl. Crystallogr. 2013, 46, 99–107. [Google Scholar] [CrossRef]
- Bauer-Brandl, A. Polymorphic transitions of cimetidine during manufacture of solid dosage forms. Int. J. Pharm. 1996, 140, 195–206. [Google Scholar] [CrossRef]
- Thayyil, M.S. Fragility of Cimetidine Drug Probed by Broadband Dielectric Spectroscopy. Transl. Med. 2014, 4, 138. [Google Scholar] [CrossRef]
- Kaneniwa, N.; Ichikawa, J.-I.; Matsumoto, T. Preparation of Phenylbutazone Polymorphs and Their Transformation in Solution. Chem. Pharm. Bull. 1988, 36, 1063–1073. [Google Scholar] [CrossRef][Green Version]
- Fukuoka, E.; Makita, M.; Yamamura, S. Glassy State of Pharmaceuticals. III.: Thermal Properties and Stability of Glassy Pharmaceuticals and Their Binary Glass Systems. Chem. Pharm. Bull. 1989, 37, 1047–1050. [Google Scholar] [CrossRef]
- Taddei, P.; Torreggiani, A.; Fini, G. Vibrational study of polymorphism of tetralin derivative for treatment of cardiovascular diseases. Biopolymers 2002, 67, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Pirttimäki, J.; Laine, E.; Ketolainen, J.; Paronen, P. Effects of grinding and compression on crystal structure of anhydrous caffeine. Int. J. Pharm. 1993, 95, 93–99. [Google Scholar] [CrossRef]
- Mazel, V.; Delplace, C.; Busignies, V.; Faivre, V.; Tchoreloff, P.; Yagoubi, N. Polymorphic transformation of anhydrous caffeine under compression and grinding: A re-evaluation. Drug Dev. Ind. Pharm. 2011, 37, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Correia, N.T.; Derollez, P.; Danede, F.; Capet, F. Plastic and Glassy Crystal States of Caffeine. J. Phys. Chem. B 2005, 109, 16092–16098. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A. Cinétiques de Transformations de Produits Pharmaceutiques Sous Broyage. Ph.D. Thesis, Université de Lille, Lille, France, 2022. [Google Scholar]
- Kohno, Y.; Hiyoshi, R.I.; Yamaguchi, Y.; Matsumoto, S.; Koseki, A.; Takahashi, O.; Yamasaki, K.; Ueda, K. Molecular Dynamics Studies of the Structural Change in 1,3-Diamino-2,4,6-trinitrobenzene (DATB) in the Crystalline State under High Pressure. J. Phys. Chem. A 2009, 113, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Decroix, A.A. Polymorphism and disorder in caffeine: Dielectric investigation of molecular mobilities. J. Mol. Struct. 2014, 1078, 165–173. [Google Scholar] [CrossRef]
- Okumura, T.; Ishida, M.; Takayama, K.; Otsuka, M. Polymorphic Transformation of Indomethacin Under High Pressures*. J. Pharm. Sci. 2006, 95, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, N.; Willart, J.F.; Dudognon, E.; Danède, F.; Descamps, M. Mechanism of Solid State Amorphization of Glucose upon Milling. J. Phys. Chem. B 2013, 117, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, T. Structural and Thermodynamic Explanations of Ostwald’s Rule. Org. Process Res. Dev. 2003, 7, 1017–1027. [Google Scholar] [CrossRef]
| Pharmaceutical Materials and Molecular Weight | Stating Form and Lattice Parameters (Å) | Final Form and Lattice Parameters (Å) | Relationship | Kinetic Transformation Curve Shape | Information on the Polymorphic Transformation | Tg (°C) | Melting Temperature (°C) | Ref |
|---|---|---|---|---|---|---|---|---|
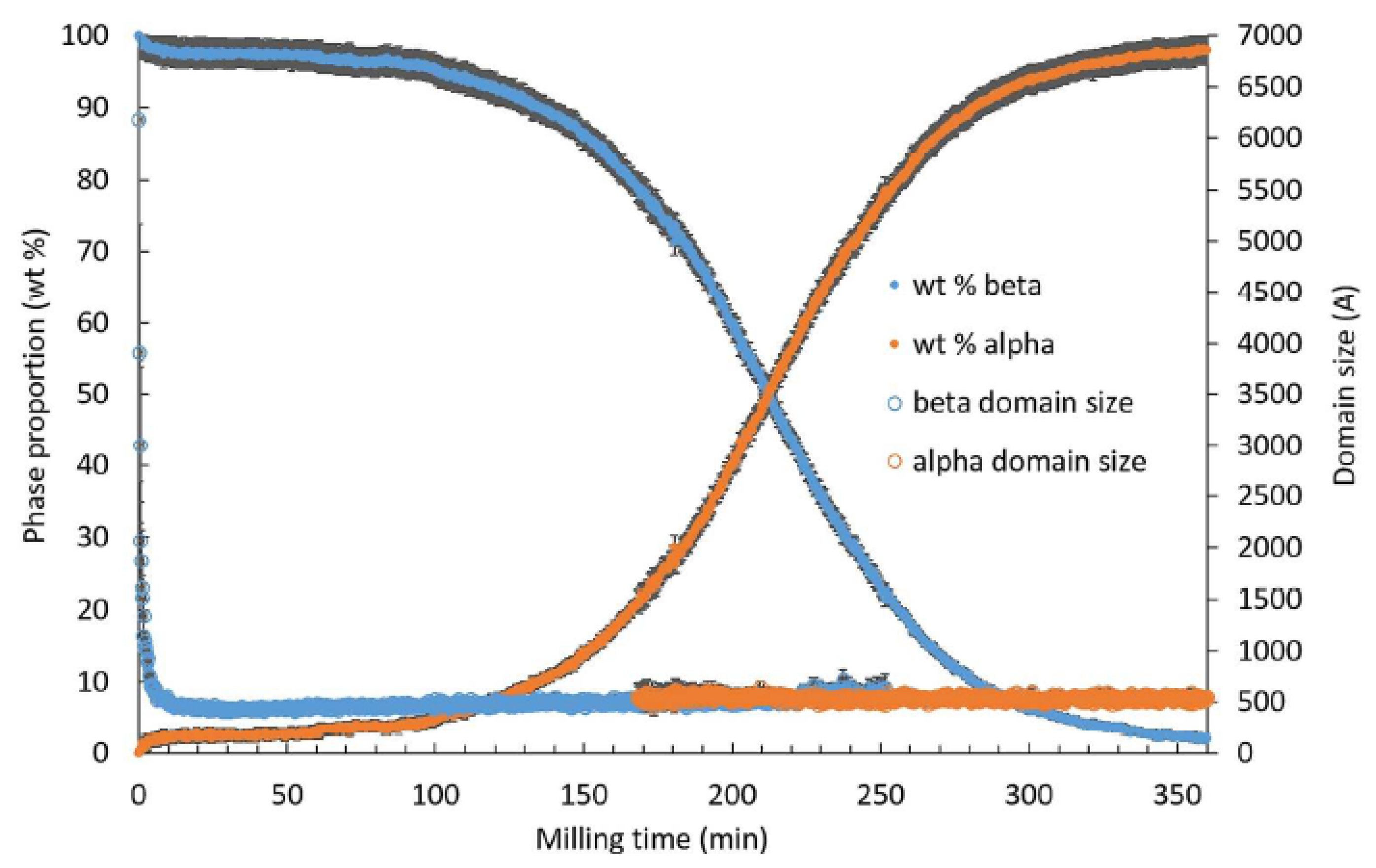
| Sorbitol (C6H14O6) 182.17 g/mol | γ a = 24.301 b = 20.572 c = 4.867 α = β = γ = 90° | α a = 9.048 b = 4.870 c = 18.262 α = β = γ = 90° | Monotropic | Sigmoidal | Transformation from the stable γ form to the metastable α form through a transient amorphous phase | −3 | 95 | [12,13] |
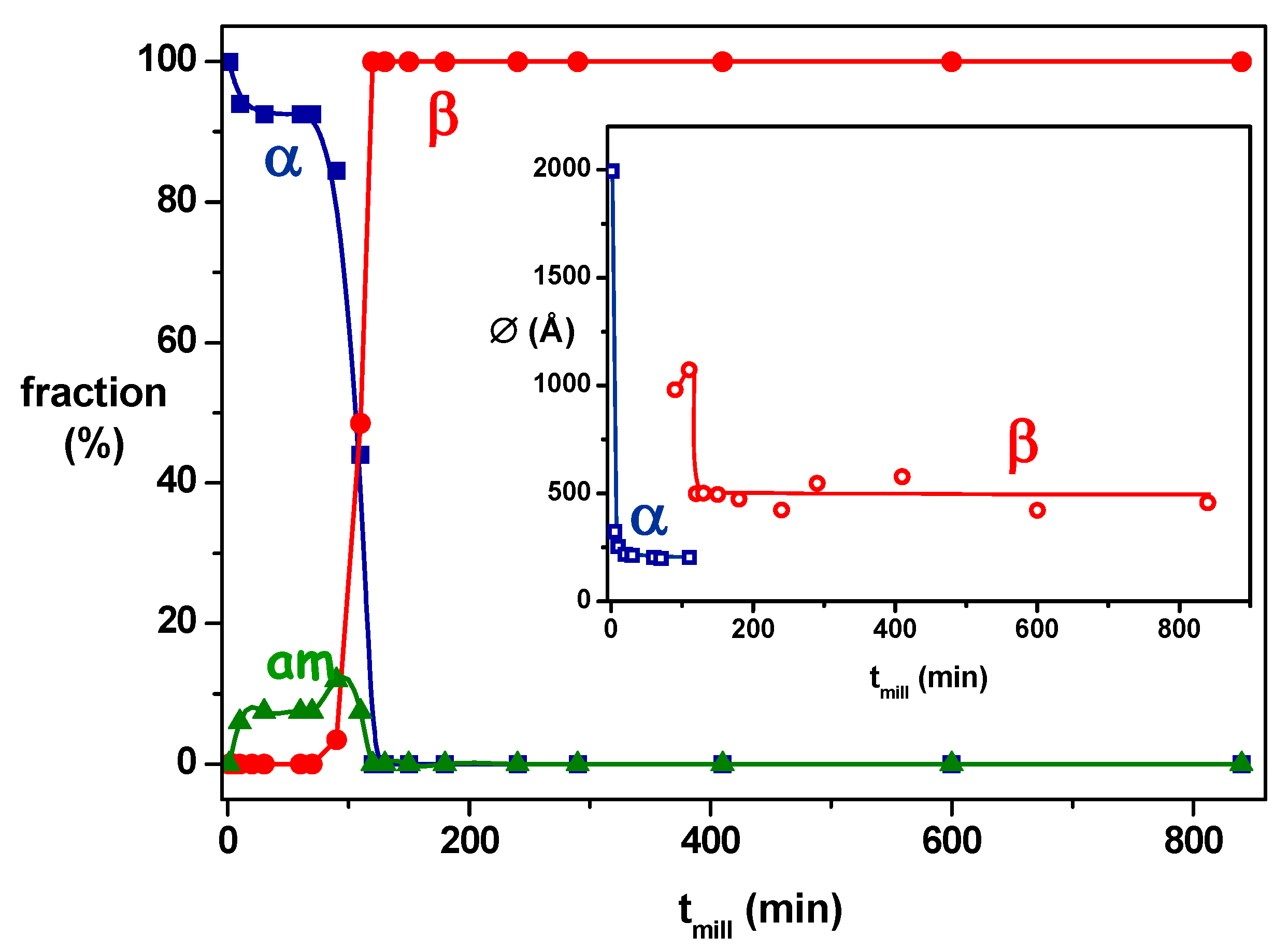
| Bezafibrate (C19H20ClNO4) 361.82 g/mol | α a = 10.3118 b = 17.6601 c = 19.7133 α = β = γ = 90° | β a = 10.7849 b = 15.7886 c = 11.4932 α = γ = 90° β = 115.875° | Enantiotropic | Sigmoidal | Transformation from the α form (stable at high temperature) to the β form (stable at room temperature) through a transient amorphous phase | 40 | 175 | [20] |
| Sulfamerazine (C11H12N4O2S) 264.30 g/mol | I a = 14.474 b = 21.953 c = 8.203 α = β = γ = 90° | II a = 9.145 b = 11.704 c = 22.884 α = β = γ = 90° | Enantiotropic | Unknown | Transformation from form I (stable at high temperature) to a mixture of amorphous phase and form II (stable at room temperature) | 62 | 237 | [25] |
| Mannitol (C6H14O6) 182.17 g/mol | β a = 5.5381 b = 8.580 c = 16.795 α = β = γ = 90° | α a = 4.8653 b = 8.873 c = 18.739 α = β = γ = 90° | Monotropic | Sigmoidal | Transformation from the stable β form to the metastable α form | 13 | 166 | [14] |
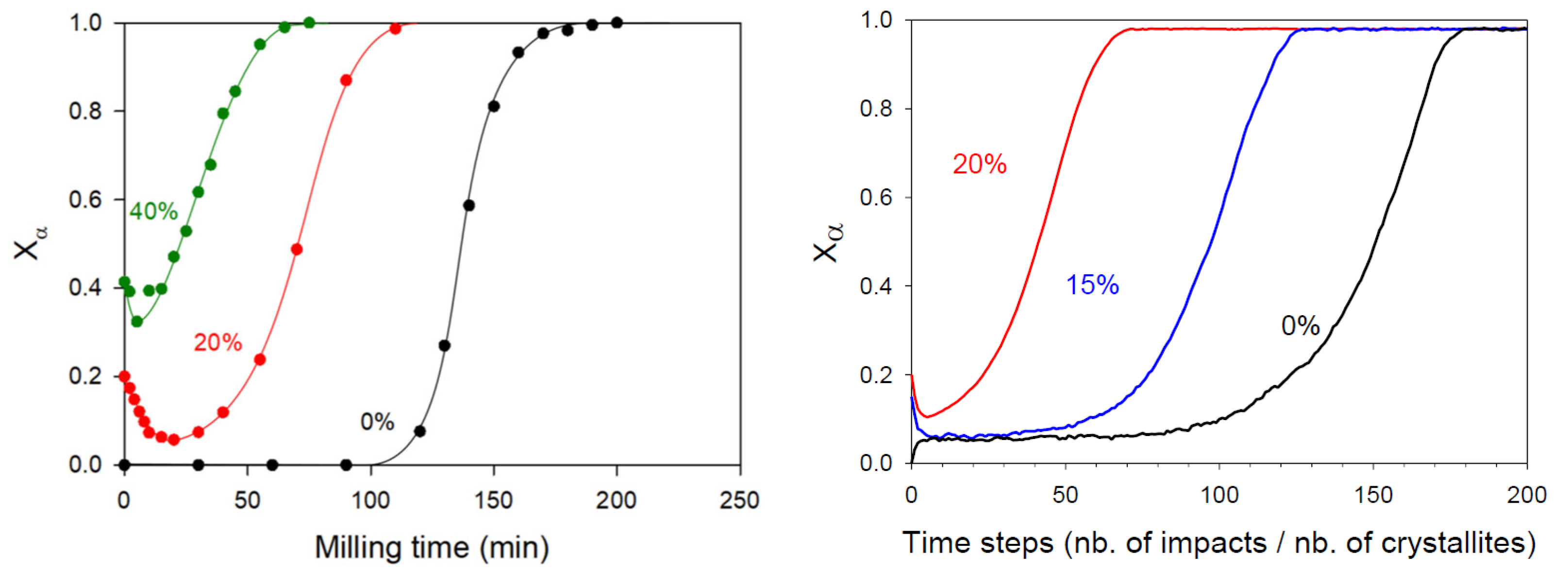
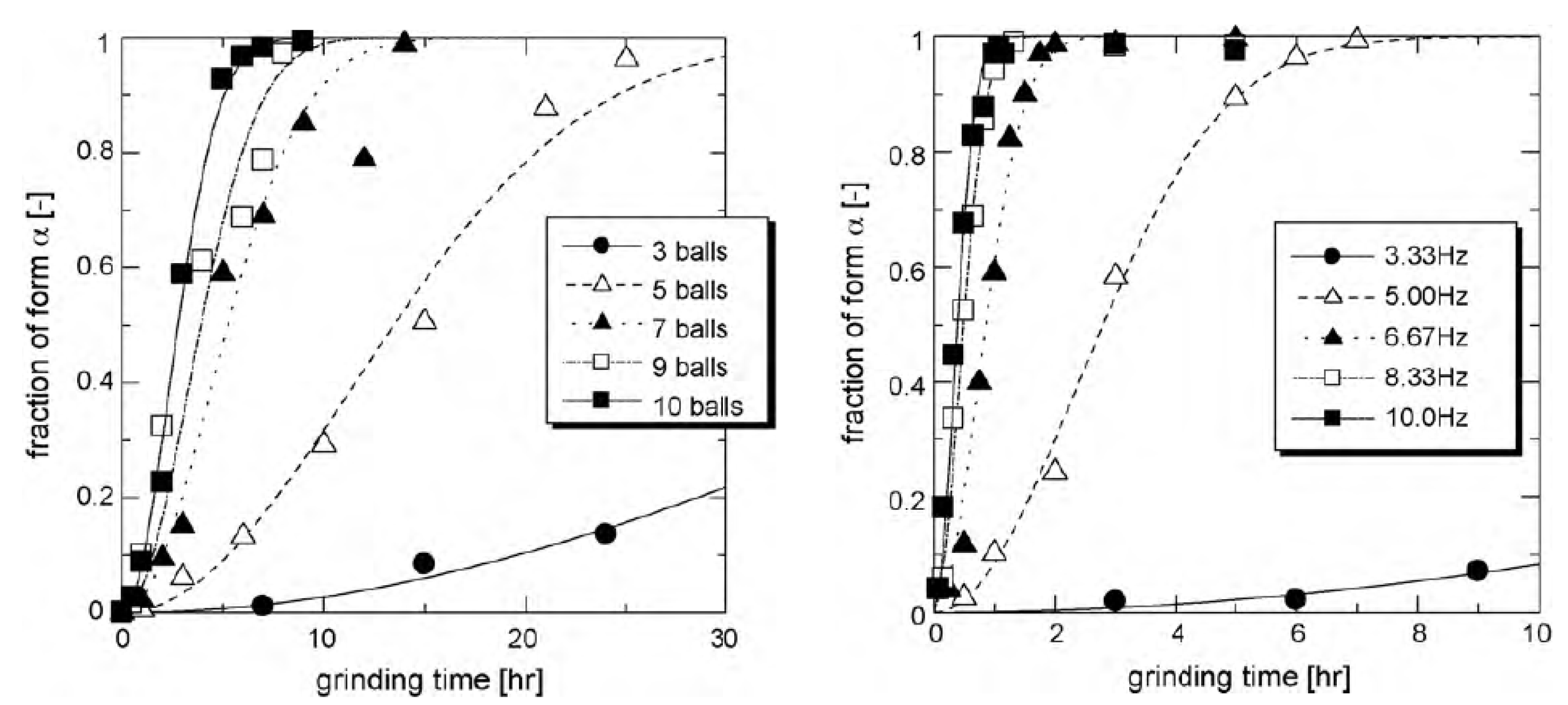
| Glycine (C2H5NO2) 75 g/mol | γ a = b = 7.035 c = 5.481 α = β = 90° γ = 120° | α a = 5.107 b = 12.040 c = 5.460 α = γ = 90° β = 111.82° | Enantiotropic | Depends on the milling intensity | Transformation from the γ form (stable at room temperature) to the α form | Unknown | Unknown | [11,82] |
| Sulfathiazole (C9H9N3O2S2) 255.31 g/mol | V a = 10.399 b = 15.132 c = 14.280 α = γ = 90° β = 1.21° IV a = 10.867 b = 11.456 c = 8.543 α = β = 90° γ = 91.87° III a = 17.570 b = 8.574 c = 15.583 α = γ = 90° β = 112.93° II a = 8.235 b = 8.550 c = 15.558 α = γ = 90° β = 93.67° | I a = 10.554 b = 13.220 c = 17.050 α = γ = 90° β = 108.06° I I I | Unknown | Unknown | Transformation from form II, III, IV, and V to a mixture of amorphous phase and form I | 67 | 200 | [40,41] |
| Ranitidine Hydrochloride (C13H22N4O3S·HCl) 350.86 g/mol | I a = 12.1918 b = 6.5318 c = 22.0382 α = γ = 90° β = 93.985° | II a = 18.798 b = 12.980 c = 7.204 α = γ = 90° β = 95.09° | Monotropic | Sigmoidal | Transformation from form I (metastable) to form II (stable) through a transient amorphous phase | 13–30 | I→134–140 II→140–144 | [44,45] |
| Rivastigmine hydrogen tartrate (C14H22N2O2·C4H4O6) 400.42 g/mol | II a = 17.538 b = 8.326 c = 7.261 α = γ = 90° β = 98.799° | I Unknown | Monotropic | Induction time of 1 h | Transformation from form II (metastable) to form I (stable) through a transient amorphous phase | 38.2 | II→97.4 I→124.5 | [46] |
| Famotidine (C8H15N7O2S3) 337.44 g/mol | B a = 17.057 b = 5.335 c = 17.776 α = γ = 90° β = 116.6° | A a = 11.986 b = 7.200 c = 16.818 α = γ = 90° β = 99.82° | Monotropic | Unknown | Transformation from form B (metastable) to form A (stable) | 50 | 165 | [50,51] |
| Gabapentin (C9H17NO2) 171.24 g/mol | I a = 14.567 b = 9.2153 c = 7.6503 α = γ = 90° β = 93.375° II III | II a = 5.8759 b = 6.9198 c = 22.262 α = γ = 90° β = 90.080° III a = 30.5452 b = 5.9268 c = 10.8841 α = γ = 90° β = 108.316° IV a = 14.537 b = 6.633 c = 9.834 α = γ = 90° β = 105.92° IV | Unknown | Unknown | Transformation from form I to form II Transformation from form II to a mixture of form III and IV Transformation from form III to form IV | Unknown | 166 | [52] |
| Indomethacin (C19H16ClNO4) 357.79 g/mol | γ a = 9.2173 b = 9.6060 c = 10.8436 α = 69.959 β = 87.1970 γ = 69.501 | α a = 5.4616 b = 25.310 c = 18.152 α = γ = 90° β = 94.38° | Monotropic | Sigmoidal | Transformation from the γ form (stable) to the α form (metastable) through a transient amorphous phase | 47 °C | 163 | [59,60,62] |
| Modafinil (C15H15NO2S) 273.35 g/mol | I a = 14.5022 b = 9.6875 c = 20.8445 α = γ = 90° β = 110.17° IV a = 18.172 b = 52.375 c = 5.698 V Unknown VI Unknown | III a = 14.510 b = 9.710 c = 19.569 α = β = γ = 90° III III III | Monotropic | Unknown | Transformation from form I, IV, V, and VI (metastable) to the III (stable) | 43 °C | 165 | [9] |
| Fananserin (C23H24FN3O2S) 425.52 g/mol | III a = 14.625 b = 14.370 c = 720.356 α = γ = 90° β = 92.84° IV a = 8.633 b = 9.714 c = 12.270 α = γ = 90° β = 96.70° | I a = 8.359 b = 17.228 c = 8.089 α = 101.32 β = 110.85 γ = 86.85 I | Monotropic | Induction time of more than 1 h for the IV→I transformation | Transformation from form III (metastable) and IV (stable) to form I (metastable) through a transient amorphous phase | 19 | III→101 IV→99 | [16] |
| Chloramphenicol Palmitate (C27H42Cl2N2O6) 561.54 g/mol | C Unknown B Unknown | B Unknown A a = 7.805 b = 52.503 c = 7.414 α = β = γ = 90° | Enantiotropic Monotropic | Exponential Relaxation Sigmoidal | Transformation from form C (metastable) to form B (metastable) then to form A (stable) | Unknown | C→B à 64.5 A→90.3 B→86.7 | [69] |
| Cimetidine (C10H16N6S) 252.34 g/mol | B a = 55.45 b = 5 c = 18.72 α = γ = 90° β = 100.4° C a = 82.904 b = 4.85 c = 18.760 α = γ = 90° β = 74.34° | A a = 10.7029 b = 18.8262 c = 6.8266 α = γ = 90° β = 111.306° A | Monotropic | Unknown | Transformation from form B and C (both metastable) to form A (stable) through a transient amorphous phase | 43 | A→140–152 B→142–145 C→145–154 | [74,75] |
| Phenylbutazone (C19H20N2O2) 308.37 g/mol | 4 °C Milling α a = 21.415 b = 5.7295 c = 27.782 α = γ = 90° β = 108.4° ß Unknown δ Unknown 35 °C Miling α ß δ | 4 °C Milling ɛ Unknown ɛ ɛ 35 °C Milling δ δ δ | ß/δ Monotropic α/ß Enantiotropic α/δ Enantiotropic | Unknown | At 4 °C: transformation from the α, β, and δ form to the ε form after several hours of milling At 35 °C: transformation from the α and β form to the δ form after several hours of milling | 4 | α→91.2 ß→93.3 δ→101.4 | [76,77,83] |
| Nolomirole Hydrochlorride (C19H28ClNO4) 369.88 g/mol | α Unknown | β Unknown | Unknown | Unknown | Transformation from the α form to the β form | Unknown | Unknown | [78] |
| Caffeine (C8H10N4O2) 194.19 g/mol | I a = 14.9372 b = 14.9372 c = 6.8980 α = β = 90 γ = 120 II | II a = 43.0390 b = 15.06758 c = 6.95314 α = γ = 90° β = 99.0274° I | Enantiotropic | Transformation too fast to observe kinetics | Transformation from form I (metastable) to form II (stable) Transformation from form II (stable) to form I (metastable) | −17 | 227 | [79,81,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerain, M.; Willart, J.-F. Polymorphic Transformations of Pharmaceutical Materials Induced by Mechanical Milling: A Review. Pharmaceutics 2025, 17, 946. https://doi.org/10.3390/pharmaceutics17070946
Guerain M, Willart J-F. Polymorphic Transformations of Pharmaceutical Materials Induced by Mechanical Milling: A Review. Pharmaceutics. 2025; 17(7):946. https://doi.org/10.3390/pharmaceutics17070946
Chicago/Turabian StyleGuerain, Mathieu, and Jean-François Willart. 2025. "Polymorphic Transformations of Pharmaceutical Materials Induced by Mechanical Milling: A Review" Pharmaceutics 17, no. 7: 946. https://doi.org/10.3390/pharmaceutics17070946
APA StyleGuerain, M., & Willart, J.-F. (2025). Polymorphic Transformations of Pharmaceutical Materials Induced by Mechanical Milling: A Review. Pharmaceutics, 17(7), 946. https://doi.org/10.3390/pharmaceutics17070946






