The Potential of Amphiphilic Cyclodextrins as Carriers for Therapeutic Purposes: A Short Overview
Abstract
1. Introduction
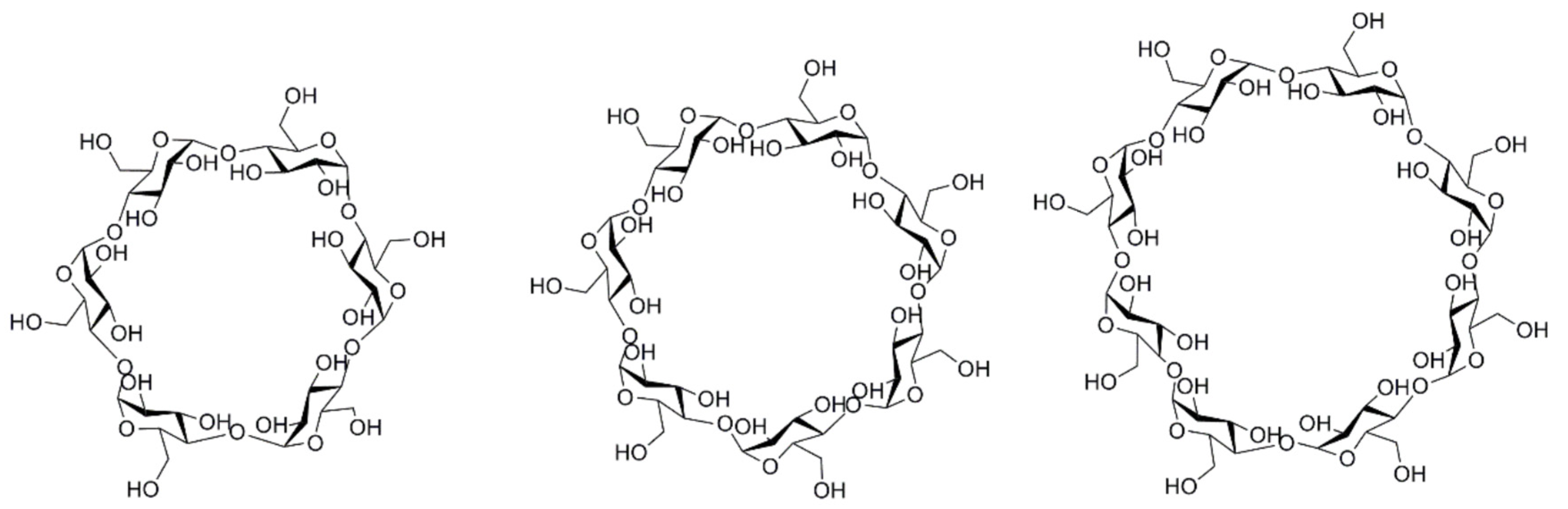
1.1. General Overview of Cyclodextrins
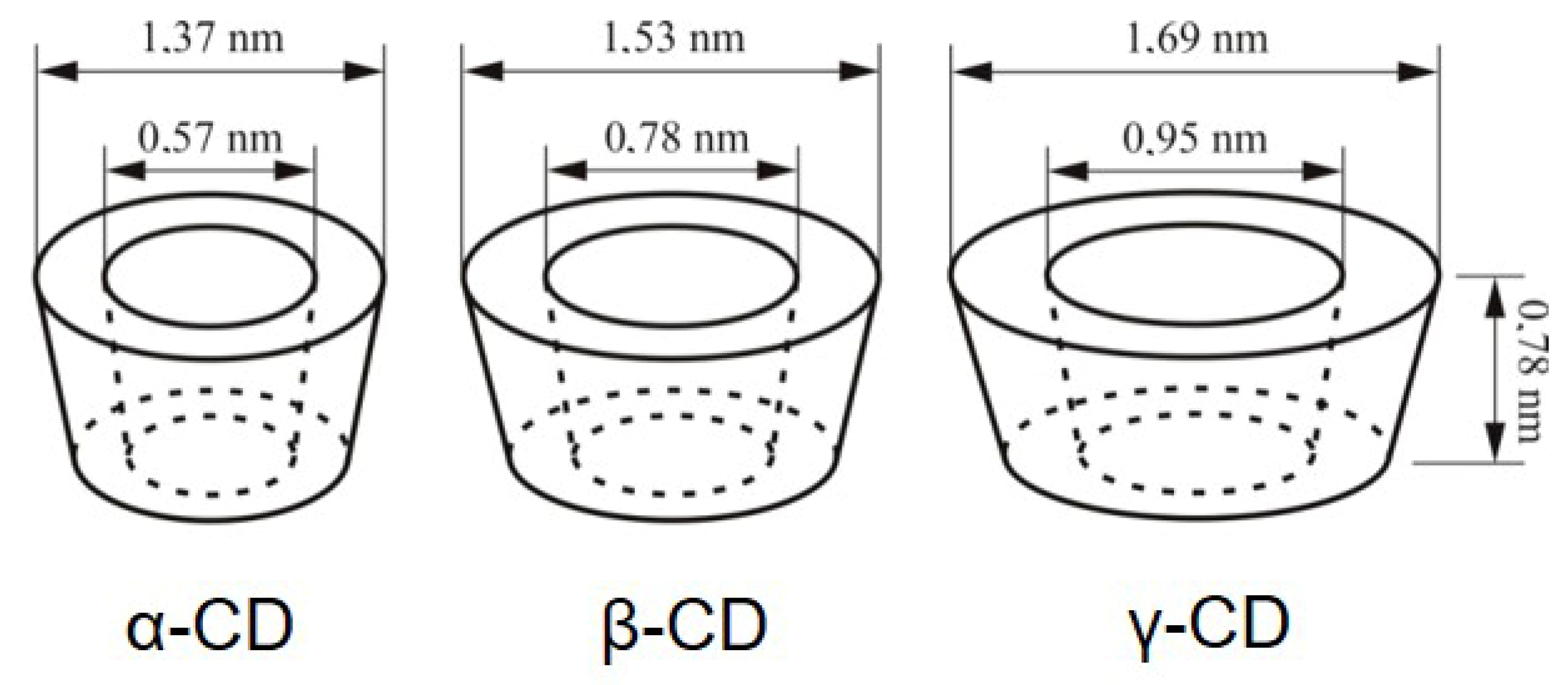
1.2. Properties of Natural Cyclodextrins and Their Derivatives
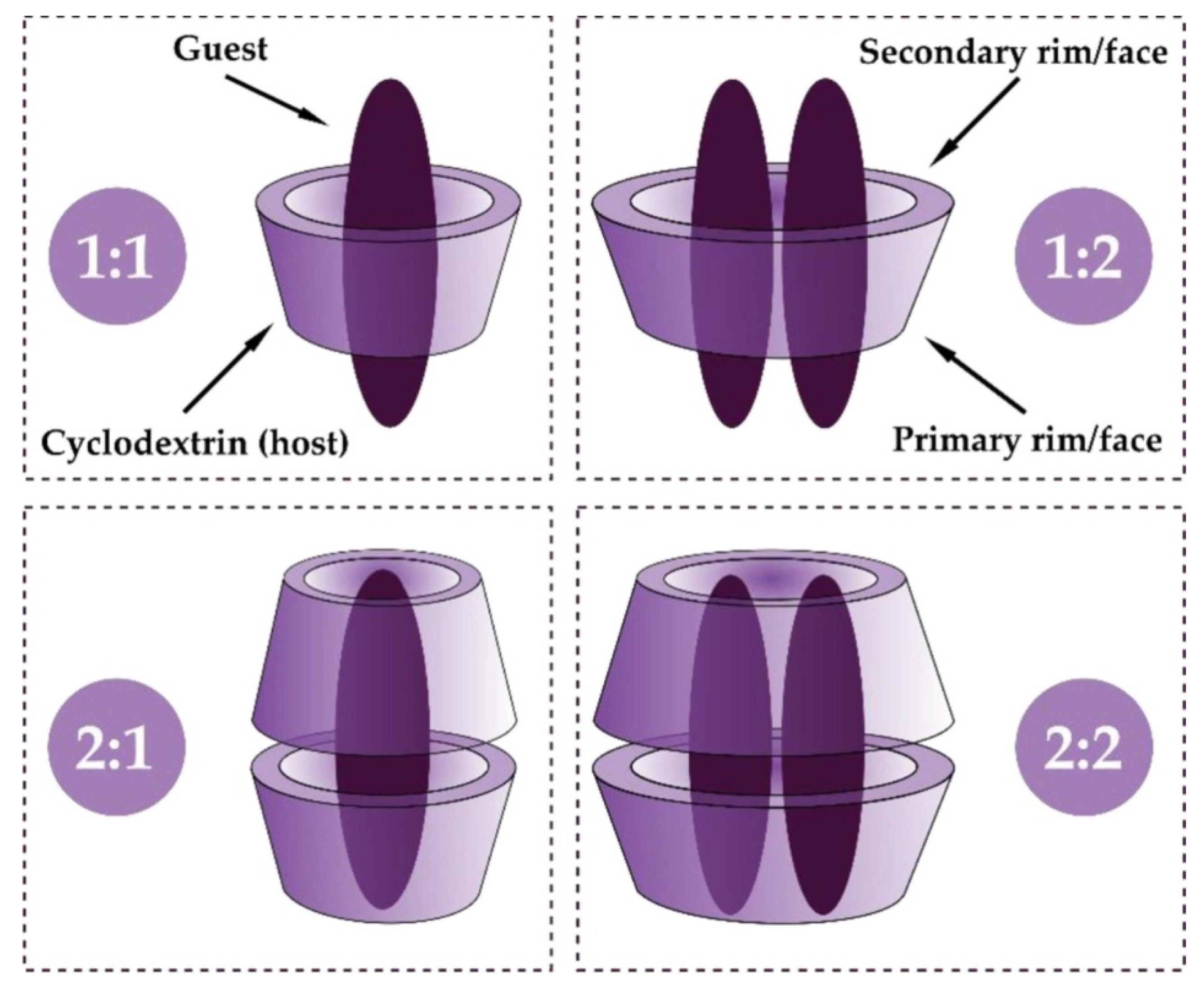
1.3. Cyclodextrin Inclusion Complex Formation
1.4. Toxicology Aspects
2. Amphiphilic Cyclodextrin Derivatives
2.1. Classification
2.2. Synthesis
- 2.2.1
- Medusa-like CDs can be obtained using monosubstitution by grafting of single hydrophobic moieties, such as alkyl chains, fluorinated chains, cholesterol, or more complex moieties (peptidolipidyl, phospholipidyl substituents).
- 2.2.2
- The skirt-shaped structures can be obtained by esterification of the secondary hydroxyl groups (C2 and C3), using acyl donors with variable chain lengths (C4-C16).
- 2.2.3
- Bouquet-shaped structure can be obtained by grafting hydrophobic and hydrophilic substituents on the primary and secondary faces, which leads to libraries of new compounds. For example, phospholipidyl-CDs, fluorinated-CDs, and octadecylperylene-CDs modified on both faces, with the secondary face being substituted by methyl groups, were obtained [4].
2.3. Advantages
2.4. Toxicology Aspects of Amphiphilic CD Derivatives
3. Amphiphilic Cyclodextrin-Based Nanoparticles
Preparation of Amphiphilic Cyclodextrin-Based Nanoparticles
4. The Role of Amphiphilic Cyclodextrins in Enhancing the Therapeutic Efficacy of Drugs
4.1. Amphiphilic Cyclodextrins in Anticancer Drug Delivery
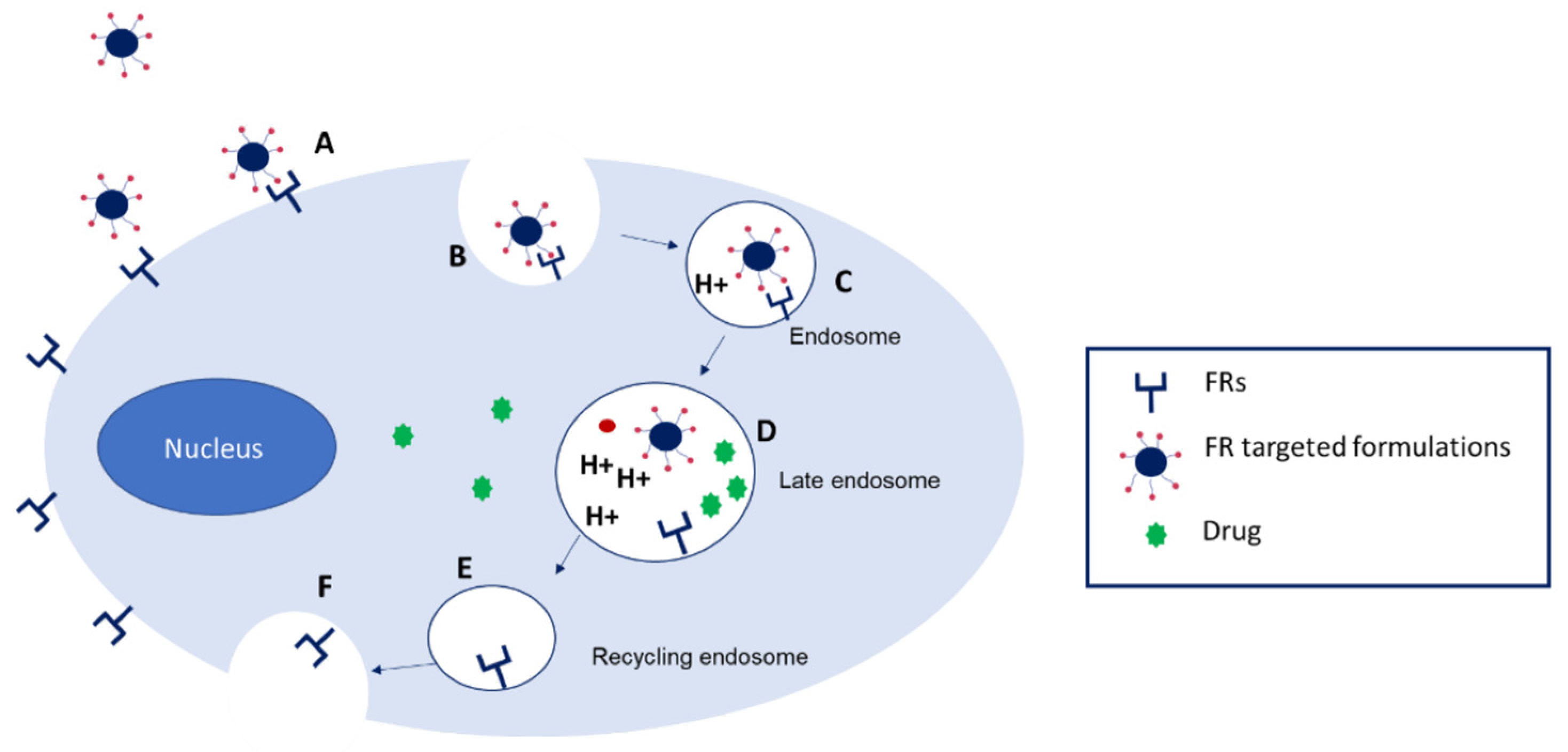
4.2. Folate-Targeted Amphiphilic Cyclodextrin Nanosystems
4.3. Cholesterol-Targeted Amphiphilic Cyclodextrin Nanosystems
4.4. Amphiphilic Cyclodextrins for Gene Delivery
4.4.1. Amphiphilic Cationic CDs in Gene Delivery
4.4.2. Charge Modulation of the Surface of Amphiphilic CD Derivatives to Improve the Delivery of Genetic Material
4.4.3. Glyco-Coating of Amphiphilic CDs to Enhance Gene Delivery
5. Conclusions and Future Perspectives
5.1. Conclusions
5.2. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| CD | cyclodextrin |
| CNS | central nervous system |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| DNA | deoxyribonucleic acid |
| pDNA | plasmid deoxyribonucleic acid |
| DSPE-PEG | 1,2-Distearoyl-sn-glycero-3-phosphoethanol-amine-poly (ethylene glycol) |
| HeLa | cervical cancer cells |
| HepG2 | hepatocellular carcinoma cell line |
| HP-β-CD | hydroxypropyl-beta-cyclodextrin |
| HD | Huntington disease |
| HTT | huntingtin gene |
| EPR | enhanced permeability and retention effect |
| FA | folic acid |
| FR | folic acid receptor |
| IC | inclusion complex |
| IC50 | half-maximal inhibitory concentration |
| IL | interleukine |
| L929 | mouse fibroblast cell line |
| MAPK | mitogen-activated protein kinase |
| MCF-7 | Michigan Cancer Foundation-7 human breast adenocarcinoma cell line |
| NP | nanoparticle |
| MB49 | murine bladder carcinoma cell line |
| PC | prostate cancer |
| PCL | poly-ε-caprolactone |
| PDI | polydispersity index |
| PEG | polyethylene glycol |
| PLGA | poly(lactide-co-glycolide) |
| RES | reticuloendothelial system |
| Rheb | Ras homologue enriched in brain |
| RNA | ribonucleic acid |
| RNAi | ribonucleic acid interference |
| mRNA | messenger ribonucleic acid |
| siRNA | small interfering ribonucleic acid |
| RVG | rabies virus glycoprotein |
| TNF | tumour necrosis factor |
References
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Esteso, M.A.; Romero, C.M. Cyclodextrins: Properties and Applications. Int. J. Mol. Sci. 2024, 25, 4547. [Google Scholar] [CrossRef]
- Zerkoune, L.; Angelova, A.; Lesieur, S. Nano-assemblies of modified cyclodextrins and their complexes with guest molecules: Incorporation in naostructured membranes and amphiphile nanoarchitectonics design. Nanomaterials 2014, 4, 741–765. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Benito, J.M.; Mellet, C.O.; Bilensoy, E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein J. Nanotechnol. 2017, 8, 1457–1468. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Erdoğar, N.; Hıncal, A.; Bilensoy, E. Amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2017, 531, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Vukomanovic, M.; Gazvoda, L.; Kurtjak, M.; Hrescak, J.; Jaklic, B.; Moya-Andérico, L.; Cendra, M.D.M.; Torrents, E. Development of a ternary cyclodextrin-arginine-ciprofloxacin antimicrobial complex with enhanced stability. Commun. Biol. 2022, 5, 1234. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Alonso Nuñez, G.; Espinoza-Gómez, H.; Flores-López, L.Z. A comparative study of the effect of α-, β-, and γ-cyclodextrins as stabilizing agents in the synthesis of silver nanoparticles using a green chemistry method. Mater. Sci. Eng. C 2014, 43, 21–26. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Niculescu, A.-G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An Updated Overview of Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefánsson, E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int. J. Pharm. 2017, 531, 413–423. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Păduraru, L.; Panainte, A.-D.; Peptu, C.-A.; Apostu, M.; Vieriu, M.; Bibire, T.; Sava, A.; Bibire, N. Smart drug delivery systems based on cyclodextrins and chitosan for cancer therapy. Pharmaceuticals 2025, 18, 564. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kyun, M.L.; Lee, Y.J.; Shim, H.E.; Huh, K.M.; Kang, S.W. Cyclodextrins as multifunctional tools for advanced biomaterials in tissue repair and regeneration. Bioact. Mater. 2025, 49, 627–651. [Google Scholar] [CrossRef] [PubMed]
- Frőmming, K.-H.; Szejtli, J. Cyclodextrins in Pharmacy. Chapter 1. Cyclodextrins, Chapter 2. Cyclodextrin Derivatives, and Chapter 4. Cyclodextrin Inclusion Complexes. In Topics in Inclusion Science; Davies, J.E.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; Volume 5, pp. 1–17, 19–32, 45–79. [Google Scholar]
- Qiu, N.; Li, X.; Liu, J. Application of cyclodextrins in cancer treatment. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 229–246. [Google Scholar] [CrossRef]
- Dodziuk, H. Chapter 1. Molecules with Holes—Cyclodextrins. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley Online Library: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. γ-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef]
- Di Cagno, M.P. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2017, 22, 1. [Google Scholar] [CrossRef]
- Figueiras, A.; Sarraguça, J.M.; Pais, A.A.; Carvalho, R.A.; Veiga, J.F. The role of L-arginine in inclusion complexes of omeprazole with cyclodextrins. AAPS PharmSciTech 2010, 11, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, W.A.; Alanazi, M.M.; Imam, S.S.; Alshehri, S.; Hussain, A.; Altamimi, M.A.; Alhudaithi, S.S. Formulation of multicomponent inclusion complex of cyclodextrin-amino acid with Chrysin: Physicochemical characterization, cell viability and apoptosis assessment in human primary glioblastoma cell line. Int. J. Pharm. X 2023, 6, 100211. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M.d.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin inclusion complexes for improved drug bioavailability and activity: Synthetic and analytical aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef] [PubMed]
- Trajano, V.C.D.C.; Brasileiro, C.B.; Henriques, J.A.S.; Cota, L.M.; Lanza, C.R.; Cortés, M.E. Doxycycline encapsulated in β-cyclodextrin for periodontitis: A clinical trial. Braz. Oral Res. 2020, 33, e112. [Google Scholar] [CrossRef]
- Torres-Alvarez, C.; Castillo, S.; Sánchez-García, E.; Aguilera González, C.; Galindo-Rodríguez, S.A.; Gabaldón-Hernández, J.A.; Báez-González, J.G. Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations. Molecules 2020, 25, 5109. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, M.; Lu, C.; Ren, D.; Fan, G.; Liu, C.; Liu, M.; Shu, G.; Peng, G.; Yuan, Z.; et al. The hydroxypropyl-β-cyclodextrin complexation of toltrazuril for enhancing bioavailability. Drug Des. Dev. Ther. 2018, 12, 583–589. [Google Scholar] [CrossRef]
- Messner, M.; Kurkov, S.V.; Jansook, P.; Loftsson, T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010, 387, 199–208. [Google Scholar] [CrossRef]
- Cîrcioban, D.; Ledeţi, A.; Vlase, G.; Ledeţi, I.; Vlase, T.; Văruț, R.; Pavel, I.Z.; Dehelean, C. Preparation, physicochemical characterization and biological activity evaluation of some inclusion complexes containing artesunate. J. Therm. Anal. Calorim. 2020, 141, 1041–1051. [Google Scholar] [CrossRef]
- He, Y.; Fu, P.; Shen, X.; Gao, H. Cyclodextrin-based aggregates and characterization by microscopy. Micron 2008, 39, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Ramzy, A.; Saleh, B.M.; El-Said Azzazy, H.M. Stimuli-Responsive Amphiphilic Pillar[n]arene Nanovesicles for Targeted Delivery of Cancer Drugs. ACS Omega 2021, 6, 25876–25883. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Loftsson, T.; Sigurdsson, H.H.; Jansook, P. Anomalous properties of cyclodextrins and their complexes in aqueous solutions. Materials 2023, 16, 2223. [Google Scholar] [CrossRef] [PubMed]
- Şuta, L.-M.; Ridichie, A.; Ledeţi, A.; Temereancă, C.; Ledeţi, I.; Muntean, D.; Rădulescu, M.; Văruţ, R.-M.; Watz, C.; Crăineanu, F.; et al. Host-Guest Complexation of Itraconazole with Cyclodextrins for Bioavailability Enhancement. Pharmaceutics 2024, 16, 560. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.S.; Frank, K.J.; Fricker, G.; Brandl, M. Biopharmaceutical classification of poorly soluble drugs with respect to “enabling formulations”. Eur. J. Pharm. Sci. 2013, 50, 8–16. [Google Scholar] [CrossRef]
- Sbârcea, L.; Tănase, I.-M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.-M.; Trandafirescu, C.; Ledeți, I. Encapsulation of Risperidone by Methylated β-Cyclodextrins: Physicochemical and Molecular Modeling Studies. Molecules 2020, 25, 5694. [Google Scholar] [CrossRef]
- Hnin, H.M.; Tun, T.; Loftsson, T.; Jansook, P. A recent update of water-soluble polymers in cyclodextrin-based formulations for mucosal drug delivery. Carbohydr. Polym. 2025, 358, 123539. [Google Scholar] [CrossRef]
- Neaz, S.; Alam, M.; Bin Imran, A. Advancements in cyclodextrin-based controlled drug delivery: Insights into pharmacokinetic and pharmacodynamic profiles. Heliyon 2024, 10, e39917. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, L.; Qiao, C.; Liu, Y.; Wang, Y.; Feng, R.; Zhang, H.; Zhang, Y. Cyclodextrin-based delivery systems for chemical and genetic drugs: Current status and future. Carbohydr. Polym. 2025, 352, 123174. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in chemistry, toxicology, and multifaceted applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Memisoglu-Bilensoy, E.; Vural, I.; Bochot, A.; Renoir, J.M.; Duchene, D.; Hincal, A.A. Tamoxifen citrate loaded amphiphilic beta-cyclodextrin nanoparticles: In vitro characterization and cytotoxicity. J. Control. Release 2005, 104, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E.; Gürkaynak, O.; Doğan, A.L.; Hincal, A.A. Safety and efficacy of amphiphilic beta-cyclodextrin nanoparticles for paclitaxel delivery. Int. J. Pharm. 2008, 347, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2022, 124, 100869. [Google Scholar] [CrossRef]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug Deliv. Transl. Res. 2020, 10, 661–677. [Google Scholar] [CrossRef]
- Karthic, A.; Roy, A.; Lakkakula, J.; Alghamdi, S.; Shakoori, A.; Babalghith, A.O.; Emran, T.B.; Sharma, R.; Lima, C.M.G.; Kim, B.; et al. Cyclodextrin nanoparticles for diagnosis and potential cancer therapy: A systematic review. Front. Cell Dev. Biol. 2022, 10, 984311. [Google Scholar] [CrossRef]
- Duchene, D.; Wouessidjewe, D. Amphiphilic Cyclodextrins and Targeting of Drugs. In Proceedings of the Eighth International Symposium on Cyclodextrins, Budapest, Hungary, 31 March–2 April 1996; Kluwer Academic Publishers: New York, NY, USA, 1996; pp. 423–430. [Google Scholar] [CrossRef]
- Bonnet, V.; Gervaise, C.; Djedaïni-Pilard, F.; Furlan, A.; Sarazin, C. Cyclodextrin nanoassemblies: A promising tool for drug delivery. Drug Discov. Today 2015, 20, 1120–1126. [Google Scholar] [CrossRef]
- Cui, Y.; Mao, Y.; Mao, J.; Zhang, Y. Smart regioselectivity towards mono 6-hydroxyl α-cyclodextrin amphiphilic derivatives. RSC Adv. 2020, 10, 10695–10702. [Google Scholar] [CrossRef]
- Baâzaoui, M.; Béjaoui, I.; Kalfat, R.; Amdouni, N.; Hbaieb, S.; Chevalier, Y. Preparation and characterization of nanoparticles made fromamphiphilic mono and per-aminoalkyl-β-cyclodextrins. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 365–376. [Google Scholar] [CrossRef]
- Patel, M.R.; Lamprou, D.A.; Vavia, P.R. Synthesis, Characterization, and Drug Delivery Application of Self-assembling Amphiphilic Cyclodextrin. AAPS PharmSciTech 2019, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Erdoğar, N.; Esendağlı, G.; Nielsen, T.T.; Şen, M.; Öner, L.; Bilensoy, E. Design and optimization of novel paclitaxel-loaded folate-conjugated amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2016, 509, 375–390. [Google Scholar] [CrossRef]
- Fernández, M.A.; Silva, O.F.; Vico, R.V.; de Rossi, R.H. Complex systems that incorporate cyclodextrins to get materials for some specific applications. Carbohydr. Res. 2019, 480, 12–34. [Google Scholar] [CrossRef]
- Adeoye, O.; Cabral-Marques, H. Cyclodextrin nanosystems in oral drug delivery: A mini review. Int. J. Pharm. 2017, 531, 521–531. [Google Scholar] [CrossRef]
- Trapani, M.; Scala, A.; Mineo, P.G.; Pistone, A.; Díaz-Moscoso, A.; Fragoso, A.; Monsù Scolaro, L.; Mazzaglia, A. Thiolated amphiphilic β-cyclodextrin-decorated gold colloids: Synthesis, supramolecular nanoassemblies and controlled release of dopamine. J. Mol. Liq. 2021, 336, 116880. [Google Scholar] [CrossRef]
- De Gaetano, F.; Scala, A.; Celesti, C.; Lambertsen Larsen, K.; Genovese, F.; Bongiorno, C.; Leggio, L.; Iraci, N.; Iraci, N.; Mazzaglia, A.; et al. Amphiphilic Cyclodextrin Nanoparticles as Delivery System for Idebenone: A Preformulation Study. Molecules 2023, 28, 3023. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Valle, F.; Tortorella, S.; Scala, A.; Cordaro, A.; Barbalinardo, M.; Biscarini, F.; Mazzaglia, A. Amphiphilic cationic cyclodextrin nanovesicles: A versatile cue for guiding cell adhesion. Nanoscale Adv. 2020, 2, 5897–5904. [Google Scholar] [CrossRef]
- Cho, E.; Yun, D.; Jeong, D.; Im, J.; Kim, H.; Dindulkar, S.D.; Choi, Y.; Jung, S. Regioselective self-acylating cyclodextrins in organic solvent. Sci. Rep. 2016, 6, 23740. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.C.; Huang, Q.; Xu, Z.; Wang, R.; Guo, D.S. Gene delivery based on macrocyclic amphiphiles. Theranostics 2019, 9, 3094–3106. [Google Scholar] [CrossRef]
- Ghera, B.B.; Perret, F.; Chevalier, Y.; Parrot-Lopez, H. Novel nanoparticles made from amphiphilic perfluoroalkyl alpha-cyclodextrin derivatives: Preparation, characterization and application to the transport of acyclovir. Int. J. Pharm. 2009, 375, 155–162. [Google Scholar] [CrossRef]
- Perret, F.; Duffour, M.; Chevalier, Y.; Parrot-Lopez, H. Design, synthesis, and in vitro evaluation of new amphiphilic cyclodextrin-based nanoparticles for the incorporation and controlled release of acyclovir. Eur. J. Pharm. Biopharm. 2013, 83, 25–32. [Google Scholar] [CrossRef]
- Perret, F.; Marminon, C.; Zeinyeh, W.; Nebois, P.; Bollacke, A.; Jose, J.; Parrot-Lopez, H.; Le Borgne, M. Preparation and characterization of CK2 inhibitor-loaded cyclodextrin nanoparticles for drug delivery. Int. J. Pharm. 2013, 441, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef]
- Spiridon, I.; Anghel, N. Cyclodextrins as multifunctional platforms in drug delivery and beyond: Structural features, functional applications, and future trends. Molecules 2025, 30, 3044. [Google Scholar] [CrossRef]
- Çirpanli, Y.; Bilensoy, E.; Lale Doğan, A.; Caliş, S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin delivery. Eur. J. Pharm. Biopharm. 2009, 73, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, Y.; Yenice, I.; Bilensoy, E.; Hıncal, A.A. Amphiphilic cyclodextrins as enabling excipients for drug delivery and for decades of scientific collaboration: Tribute to a distinguished scientist, French representative and friend—A historical perspective. J. Drug Deliv. Sci. Technol. 2015, 30, 261–265. [Google Scholar] [CrossRef]
- Conte, C.; Scala, A.; Siracusano, G.; Sortino, G.; Pennisi, R.; Piperno, A.; Miro, A.; Ungaro, F.; Sciortino, M.T.; Quaglia, F.; et al. Nanoassemblies based on non-ionic amphiphilic cyclodextrin hosting Zn(II)-phthalocyanine and docetaxel: Design, physicochemical properties and intracellular effects. Colloids Surf. B Biointerfaces 2016, 146, 590–597. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Ahlawat, J.; Henriquez, G.; Narayan, M. Enhancing the Delivery of Chemotherapeutics: Role of Biodegradable Polymeric Nanoparticles. Molecules 2018, 23, 2157. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. (Ed.) Handbook of Particulate Drug Delivery; American Scientific Publishers: Stevenson Ranch, CA, USA, 2007; Volume 2, ISBN 978-1-58883-123-X. [Google Scholar]
- Choisnard, L.; Géze, A.; Bigan, M.; Putaux, J.L.; Wouessidjewe, D. Efficient size control of amphiphilic cyclodextrin nanoparticles through a statistical mixture design methodology. J. Pharm. Pharm. Sci. 2005, 8, 593–601. [Google Scholar]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical nanotechnology: From the bench to the market. Future J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef]
- Brettner, F.E.B.; Gier, S.; Haessler, A.; Schreiner, J.; Vogel-Kindgen, S.; Windbergs, M. Anti-inflammatory effects of cyclodextrin nanoparticles enable macrophage repolarization and reduce inflammation. Discov. Nano 2024, 19, 211. [Google Scholar] [CrossRef]
- Li, P.Y.; Chen, Y.; Chen, C.H.; Liu, Y. Amphiphilic multi-charged cyclodextrins and vitamin K co-assembly as a synergistic coagulant. Chem. Commun. 2019, 55, 11790–11793. [Google Scholar] [CrossRef]
- Stancanelli, R.; Guardo, M.; Cannavà, C.; Guglielmo, G.; Ficarra, P.; Villari, V.; Micali, N.; Mazzaglia, A. Amphiphilic cyclodextrins as nanocarriers of genistein: A spectroscopic investigation pointing out the structural properties of the host/drug complex system. J. Pharm. Sci. 2010, 99, 3141–3149. [Google Scholar] [CrossRef]
- Allahyari, S.; Zahednezhad, F.; Khatami, M.; Hashemzadeh, N.; Zakeri-Milani, P.; Trotta, F. Cyclodextrin nanosponges as potential anticancer drug delivery systems to be introduced into the market, compared with liposomes. J. Drug Deliv. Sci. Technol. 2022, 67, 102931. [Google Scholar] [CrossRef]
- Li, L.M.; Xie, Y.P.; Qin, Y.R.; Chu, H.P.; Xie, H.; Zang, D.J.; Liu, T. Tumor microenvironment-responsive drug self-delivery systems to treat cancer and overcome MDR. Rare Met. 2025, 44, 1–33. [Google Scholar] [CrossRef]
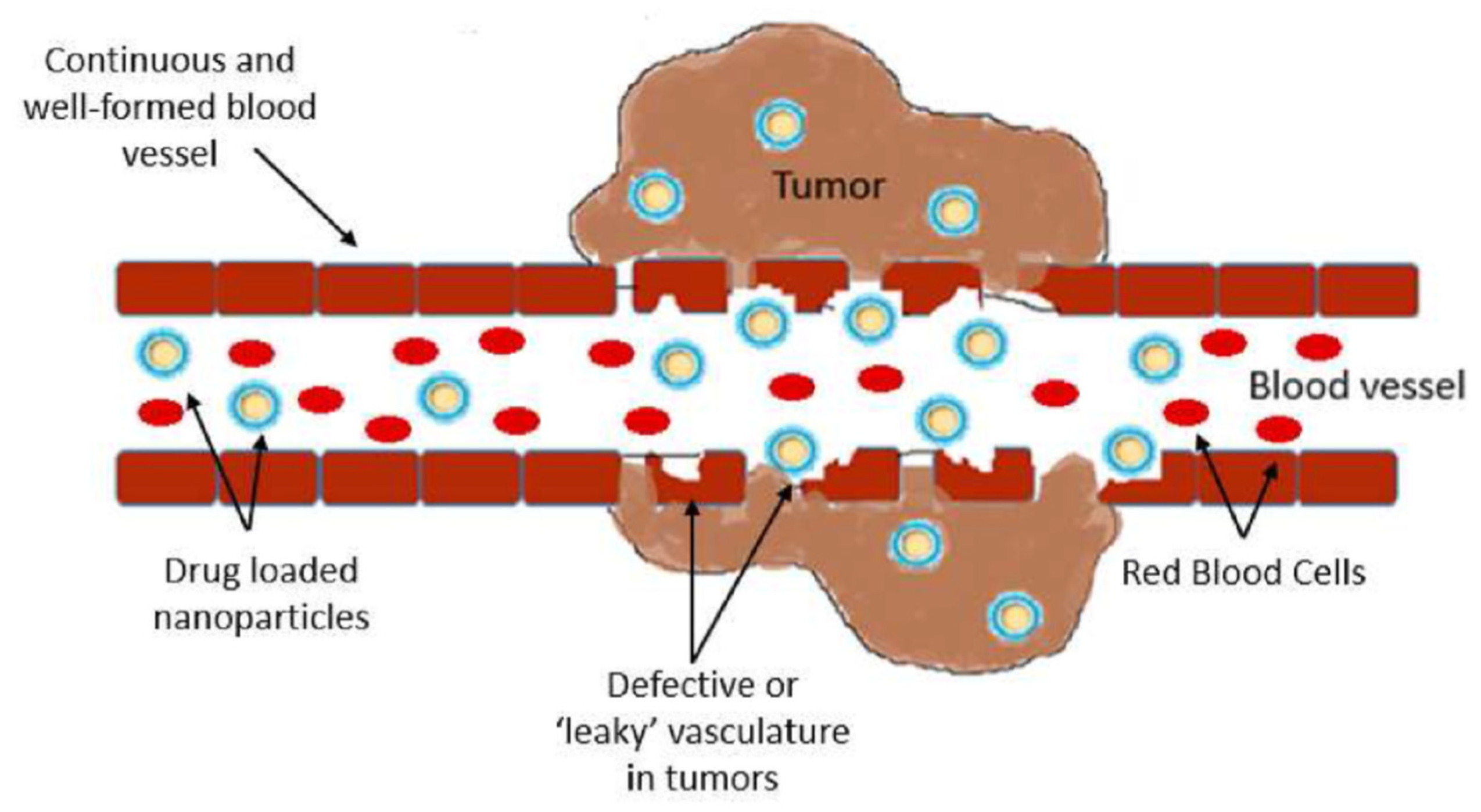
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Shi, K.; Qu, Y.; Chu, B.; Qian, Z. Engineering Nanoparticles for Targeted Delivery of Nucleic Acid Therapeutics in Tumor. Mol. Ther. Methods Clin. Dev. 2018, 12, 1–18. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Ajith, S.; Almomani, F.; Elhissi, A.; Husseini, G.A. Nanoparticle-based materials in anticancer drug delivery: Current and future prospects. Heliyon 2023, 9, e21227. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Rheima, A.M.; Kadhim, M.M.; Ahmed, N.N.; Mohammed, S.H.; Abbas, F.H.; Abed, Z.T.; Mahdi, Z.M.; Abbas, Z.S.; Hachim, S.K.; et al. An overview of nanoparticles in drug delivery: Properties and applications. S. Afr. J. Chem. Eng. 2023, 46, 233–270. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alshubrumi, A.S.; Younis, M.A. Targeted Nanoparticles: The Smart Way for the Treatment of Colorectal Cancer. AAPS PharmSciTech 2024, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Cirpanli, Y.; Allard, E.; Passirani, C.; Bilensoy, E.; Lemaire, L.; Calış, S.; Benoit, J.P. Antitumoral activity of camptothecin-loaded nanoparticles in 9L rat glioma model. Int. J. Pharm. 2011, 403, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Vural, I.; Memisoglu-Bilensoy, E.; Renoir, J.M.; Bochot, A.; Duchêne, D.; Hincal, A.A. Transcription efficiency of tamoxifen citrate-loaded β-cyclodextrin nanoparticles. J. Drug Deliv. Sci. Technol. 2005, 15, 339–342. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Ercan, A.; Çelebier, M.; Oncul, S.; Varan, G.; Kocak, E.; Benito, J.M.; Bilensoy, E. Polycationic cyclodextrin nanoparticles induce apoptosis and affect antitumoral activity in HepG2 cell line: An evaluation at the molecular level. Int. J. Pharm. 2021, 598, 120379. [Google Scholar] [CrossRef]
- Conte, C.; Scala, A.; Siracusano, G.; Leone, N.; Patanè, S.; Ungaro, F.; Miro, A.; Sciortino, M.T.; Quaglia, F.; Mazzaglia, A. Nanoassembly of an Amphiphilic Cyclodextrin and Zn(II)-Phthalocyanine with the Potential for Photodynamic Therapy of Cancer. RCS Adv. 2013, 83, 43903–43911. [Google Scholar] [CrossRef]
- Martín-Sabroso, C.; Torres-Suárez, A.I.; Alonso-González, M.; Fernández-Carballido, A.; Fraguas-Sánchez, A.I. Active Targeted Nanoformulations via Folate Receptors: State of the Art and Future Perspectives. Pharmaceutics 2021, 14, 14. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Goksen, S.; Varan, G.; Bilensoy, E.; Esendagli, G. Folate Receptor β (FRβ) Expression on Myeloid Cells and the Impact of Reticuloendothelial System on Folate-Functionalized Nanoparticles’ Biodistribution in Cancer. Mol. Pharm. 2024, 21, 4688–4699. [Google Scholar] [CrossRef]
- Aranda, C.; Urbiola, K.; Méndez Ardoy, A.; García Fernández, J.M.; Ortiz Mellet, C.; de Ilarduya, C.T. Targeted gene delivery by new folate-polycationic amphiphilic cyclodextrin-DNA nanocomplexes in vitro and in vivo. Eur. J. Pharm. Biopharm. 2013, 85, 390–397. [Google Scholar] [CrossRef]
- Zagami, R.; Rapozzi, V.; Piperno, A.; Scala, A.; Triolo, C.; Trapani, M.; Xodo, L.E.; Monsù Scolaro, L.; Mazzaglia, A. Folate-Decorated Amphiphilic Cyclodextrins as Cell-Targeted Nanophototherapeutics. Biomacromolecules 2019, 20, 2530–2544. [Google Scholar] [CrossRef]
- Guo, J.; Fisher, K.A.; Darcy, R.; Cryan, J.F.; O’Driscoll, C. Therapeutic targeting in the silent era: Advances in non-viral siRNA delivery. Mol. Biosyst. 2010, 6, 1143–1161. [Google Scholar] [CrossRef]
- Anjum, S.; Ishaque, S.; Fatima, H.; Farooq, W.; Hano, C.; Abbasi, B.H.; Anjum, I. Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals 2021, 14, 707. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef]
- de la Torre, C.; Játiva, P.; Posadas, I.; Manzanares, D.; Blanco, J.L.J.; Mellet, C.O.; Fernández, J.M.G.; Ceña, V. A β-Cyclodextrin-Based Nanoparticle with Very High Transfection Efficiency Unveils siRNA-Activated TLR3 Responses in Human Prostate Cancer Cells. Pharmaceutics 2022, 14, 2424. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J. Control. Release 2021, 330, 1046–1070. [Google Scholar] [CrossRef]
- Evans, J.C.; Malhotra, M.; Sweeney, K.; Darcy, R.; Nelson, C.C.; Hollier, B.G.; O’Driscoll, C.M. Folate-targeted amphiphilic cyclodextrin nanoparticles incorporating a fusogenic peptide deliver therapeutic siRNA and inhibit the invasive capacity of 3D prostate cancer tumours. Int. J. Pharm. 2017, 532, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Li, J.; Xu, H.; Zhao, Y.; Yu, X.; Shi, S. Functional significance of cholesterol metabolism in cancer: From threat to treatment. Exp. Mol. Med. 2023, 55, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, M.; Zhang, W.; Zhan, Q. Dysregulation of cholesterol metabolism in cancer progression. Oncogene 2023, 42, 3289–3302. [Google Scholar] [CrossRef]
- Akkın, S.; Varan, G.; Aksüt, D.; Malanga, M.; Ercan, A.; Şen, M.; Bilensoy, E. A different approach to immunochemotherapy for colon Cancer: Development of nanoplexes of cyclodextrins and Interleukin-2 loaded with 5-FU. Int. J. Pharm. 2022, 623, 121940. [Google Scholar] [CrossRef]
- Kovacs, T.; Nagy, P.; Panyi, G.; Szente, L.; Varga, Z.; Zakany, F. Cyclodextrins: Only Pharmaceutical Excipients or Full-Fledged Drug Candidates? Pharmaceutics 2022, 14, 2559. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Kont, A.; Rodriguez Aburto, M.; Cryan, J.F.; O’Driscoll, C.M. Advances in the Design of (Nano) Formulations for Delivery of Antisense Oligonucleotides and Small Interfering RNA: Focus on the Central Nervous System. Mol. Pharm. 2021, 18, 1491–1506. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F. Cyclodextrins in non-viral gene delivery. Biomaterials 2014, 35, 401–411. [Google Scholar] [CrossRef]
- Díaz-Moscoso, A.; Guilloteau, N.; Bienvenu, C.; Méndez-Ardoy, A.; Blanco, J.L.; Benito, J.M.; Le Gourriérec, L.; Di Giorgio, C.; Vierling, P.; Defaye, J.; et al. Mannosyl-coated nanocomplexes from amphiphilic cyclodextrins and pDNA for site-specific gene delivery. Biomaterials 2011, 32, 7263–7273. [Google Scholar] [CrossRef] [PubMed]
- Kont, A.; Mendonça, M.C.P.; Malanga, M.; Felegyi, K.; Lindsay, A.; Cronin, M.F.; Cahill, M.R.; O’Driscoll, C.M. Structure-activity relationship of modified amphiphilic cationic cyclodextrins for enhanced siRNA delivery. Int. J. Pharm. 2025, 670, 125107. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Neill, M.J.; Bourre, L.; Walsh, D.; Quinlan, A.; Hurley, G.; Ogier, J.; Shanahan, F.; Melgar, S.; Darcy, R.; et al. Gene silencing of TNF-alpha in a murine model of acute colitis using a modified cyclodextrin delivery system. J. Control. Release 2013, 168, 28–34. [Google Scholar] [CrossRef]
- Cryan, S.A.; Holohan, A.; Donohue, R.; Darcy, R.; O’Driscoll, C.M. Cell transfection with polycationic cyclodextrin vectors. Eur. J. Pharm. Sci. 2004, 21, 625–633. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Ganguly, K.; Kulkarni, A.R.; Kulkarni, V.H.; Nadagouda, M.N.; Rudzinski, W.E.; Aminabhavi, T.M. Cyclodextrin-based siRNA delivery nanocarriers: A state-of-the-art review. Expert Opin. Drug Deliv. 2011, 8, 1455–1468. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Godinho, B.M.; Ogier, J.; Devocelle, M.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Click-modified cyclodextrins as nonviral vectors for neuronal siRNA delivery. ACS Chem. Neurosci. 2012, 3, 744–752. [Google Scholar] [CrossRef]
- Sun, Y.; Cronin, M.F.; Mendonça, M.C.P.; Guo, J.; O’Driscoll, C.M. M2pep-Modified Cyclodextrin-siRNA Nanoparticles Modulate the Immunosuppressive Tumor Microenvironment for Prostate Cancer Therapy. Mol. Pharm. 2023, 20, 5921–5936. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-Guided Brain Drug Delivery: Expanding the Therapeutic Approach to Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Kont, A.; Mendonça, M.C.P.; Cronin, M.F.; Cahill, M.R.; O’Driscoll, C.M. Co-Formulation of Amphiphilic Cationic and Anionic Cyclodextrins Forming Nanoparticles for siRNA Delivery in the Treatment of Acute Myeloid Leukaemia. Int. J. Mol. Sci. 2022, 23, 9791. [Google Scholar] [CrossRef]
- Gooding, M.; Malhotra, M.; McCarthy, D.J.; Godinho, B.M.; Cryan, J.F.; Darcy, R.; O’Driscoll, C.M. Synthesis and characterization of rabies virus glycoprotein-tagged amphiphilic cyclodextrins for siRNA delivery in human glioblastoma cells: In vitro analysis. Eur. J. Pharm. Sci. 2015, 71, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ogier, J.R.; Desgranges, S.; Darcy, R.; O’Driscoll, C. Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials 2012, 33, 7775–7784. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Ogier, J.; Desgranges, S.; Cryan, J.F.; Darcy, R.; O’Driscoll, C.M. A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: Co-formulation opportunities for siRNA delivery. Org. Biomol. Chem. 2012, 25, 4954–4960. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Cronin, M.F.; Cryan, J.F.; O’Driscoll, C.M. Modified cyclodextrin-based nanoparticles mediated delivery of siRNA for huntingtin gene silencing across an in vitro BBB model. Eur. J. Pharm. Biopharm. 2021, 169, 309–318. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Sun, X.L. Recent developments in carbohydrate-decorated targeted drug/gene delivery. Med. Res. Rev. 2010, 30, 270–289. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | α-CD | β-CD | γ-CD |
|---|---|---|---|
| Number of glucose units | 6 | 7 | 8 |
| Molecular weight | 972 | 1134 | 1296 |
| Number of OH groups/molecule | 18 | 21 | 24 |
| Cavity diametre (Å) | 4.7–5.3 | 6–6.5 | 7.5–8.3 |
| Torus height (Å) | 7.9 ± 0.1 | 7.9 ± 0.1 | 7.9 ± 0.1 |
| External diametre (Å) | 14.6 ± 0.4 | 15.4 ± 0.4 | 17.5 ± 0.4 |
| Water solubility (g/100 mL) at room temperature | 14.5 | 1.85 | 23.2 |
| [α]D 25 °C | 150 ± 0.5 | 162 ± 0.5 | 177.4 ± 0.5 |
| Melting temperature range (°C) | 255–260 | 255–265 | 240–245 |
| Water molecules in the cavity | 6 | 11 | 17 |
| Amphiphilic CD Derivative | Toxicological Data | References |
|---|---|---|
| β-CDC6 and 6-O-Capro-β-CD (amphiphilic β-CD derivatives modified on the secondary face, and, respectively, on the primary face with 6C aliphatic chains) | Non-toxic on mouse fibroblast L929 cells at 1:128 dilution | [68] |
| Folate-conjugated amphiphilic β-CD derivatives | Cell viability on L929 mouse fibroblast cell line in range of 80–100% at any concentration, through 24 or 48 h incubation | [54] |
| Amphiphilic β-CD derivative random substituted with C12 alkyl chains | Well-tolerated in acute toxicity studies in mice, by i.v. administration, at a dose > 2000 mg/kg | [53] |
| 6-O-Capro-β-CD (β-CD derivative modified on the primary face with 6C aliphatic ester) nanospheres/ nanocapsules | Concentration-dependent haemolysis (on human blood samples); Haemolysis of 40–60% (for nanospheres, at maximum concentration of 50 mM, used in the experiment) and 60–80% (in case of nanocapsules) | [45] |
| % of cell viability on L929 mouse fibroblast cell line, upon dilution, was in the range of 40–70% |
| Compound | Drug Delivery System | Key Functionalities | Experimental Conclusions | Ref. | |
|---|---|---|---|---|---|
| 1 | Tamoxifen citrate | Nanospheres and nanocapsules of amphiphilic β-CD modified on the secondary face with 6C aliphatic esters | Pre-loaded NPs were prepared directly using pre-formed tamoxifen citrate: β-CDC6 complex (1:1) with no further drug being added to the system during preparation; High-loaded NPs were prepared directly using pre-formed tamoxifen citrate: β-CDC6 complex (1:1) with further drug being added to the system during preparation; Conventional-loaded NPs were prepared by addition of drug solution to organic phase during preparation; For the preparation of nanocapsules, the organic phase was Miglyol 812® (a neutral oil composed of triglycerides of the fractionated vegetable fatty acids C8 and C10) dissolved in acetone, and for the preparation of nanospheres, the organic phase was acetone. | Particle size and zeta potential values indicated that tamoxifen citrate was adsorbed to the nanospheres’ surface and entangled in aliphatic chains aligning the surface of the NP, while in the case of nanocapsules, the drug was mainly encapsulated within the oily core, with only a small amount on the surface aligned with free –OH groups of the β-CDC6 molecule; The importance of using pre-formed inclusion complexes: (a) increased entrapment efficiency—about 2-fold in case of nanospheres, and 3-fold in case of nanocapsules, as compared to the conventional-loaded NPs, (b) the delay of drug release—(i) a complete release of drug within 15 min for conventionally loaded nanospheres, while for pre-loaded nanospheres and high-loaded ones, the complete release was achieved within 6 h and 2 h, respectively, (ii) the same phenomenon was observed for nanocapsules, with the observation that both pre-loaded and high-loaded systems showed a release profile extended to a period of 6 h; No toxicity was observed for unloaded NPs against MCF-7 breast cancer cells, while loaded NPs exerted an equally efficient cytotoxic activity as tamoxifen citrate (solution in acetone). | [44] |
| 2 | Paclitaxel | Nanospheres and nanocapsules of amphiphilic 6-O-Capro-β-CD | High-loaded NPs were prepared directly using pre-formed paclitaxel: 6-O-Capro-β-CD complex (1:1 molar ratio) with further drug solution being added to the system during preparation; Conventional-loaded NPs were prepared by addition of drug solution (200 μL) in organic phase during preparation; To prepare nanocapsules of amphiphilic CD, Miglyol 812® (50 μL) in organic phase (acetone) was added; The highest possible concentration of NPs and mixture of commercial vehicle cremophor/ethanol were used in order to represent the most exaggerated conditions. | Stable nanospheres and nanocapsules of 150 nm diametre and 500 nm diametre, respectively, with up to 65% entrapment efficiency for paclitaxel were obtained; The NPs were characterised by controlled release profiles of biphasic nature with complete in vitro release in 24 h; The blank NPs showed significantly less haemolytic effect than the blank cremophor/ethanol mixture; nanocapsules were slightly more haemolytic than nanospheres, attributed to the presence, in the case of nanocapsules, of the Miglyol 812 as oil phase; Blank NPs showed a favourable toxicological profile against L929 mouse fibroblast cell line, as compared to cremophor/ethanol mixture, underlying the usefulness of CD-based carrier in avoiding the self-toxicity of the vehicle (carrier); The amphiphilic CD-based nanoparticle system was able to maintain the physical stability of paclitaxel, avoiding the recrystallisation process and precipitation in aqueous medium, a phenomenon that is possible at parenteral administration, resulting in severe necrosis at the injection site and impairment of clinical efficacy of the drug. | [45] |
| 3 | - | Polycationic amphiphilic β-cyclodextrin (β-CDC6) nanoparticles | A belt of 7 primary amino groups on the CD primary face (protonated at physiological pH) for increased cellular binding and uptake by negative cell membrane; A cluster of 14 hexanoyl chains on the CD secondary face. | Spherical-shaped NPs, 75 nm in diametre, +61 mV surface charge (aqueous medium), conferring suitable characteristics for hepatic targeting and improved cellular interaction; Dose-dependent and selective cytotoxicity on HepG2 cell culture and no cytotoxic effect on L929 fibroblast cells; Activation of apoptosis correlated with Caspase-3/7 activation, inhibition of Survivin (an inhibitor of apoptosis in cancer cells) in HepG2 cells; Depletion of cholesterol in HepG2 cells (which was correlated with the suppression of Survivin expression), changes in cell morphology and viability; Overcoming drug resistance in HepG2 cells due to alleviation of P-gp transporter protein. | [91] |
| 4 | Camptothecin | Nanospheres of amphiphilic 6-O-Capro-β-CD Nanospheres of amphiphilic β-CDC6 | 6-O-Capro-β-CD amphiphilic derivative modified on the primary face with substitution of C6 linear alkyl chains and hydrophobic ester bond; β-CDC6 amphiphilic derivative modified on the secondary face with 6C aliphatic esters; high-loaded nanospheres were prepared using pre-formed inclusion complexes of camptothecin and amphiphilic CD derivative, and by further dissolving an additional amount of drug in organic phase. | Amphiphilic 6-O-Capro-β-CD had a higher loading capacity than amphiphilic β-CDC6 derivative, suggesting that leaving the secondary face unsubstituted reduces the steric hindrance and enables drug loading efficiency; Releasing of the drug in its stable and biologically active lactone form from both types of amphiphilic CD NPs for more than 12 days for 6-O-Capro-β-CD NPs and 6 days for β-CDC6 NPs, with a more vigorous burst effect in the case of β-CDC6 NPs, resulting in 50% cumulative release of drug after 5 h in contrast to 24 h in the case of 6-O-Capro-β-CD NPs; Anticancer efficiency of amphiphilic CD NPs determined on MCF-7 human breast adenocarcinoma cells revealed that camptothecin was more effective when encapsulated as compared to camptothecin solution in DMSO, with better results observed in the case of 6-O-Capro-β-CD; Blank amphiphilic CD NPs showed a favourable toxicological profile against L929 mouse fibroblast cells. | [68] |
| 5 | Paclitaxel | Paclitaxel-loaded folate-targeted nanoparticles based on amphiphilic cyclodextrin derivatives for intravenous route | Folate-conjugated amphiphilic CD derivatives modified on the secondary side/primary side (FCD-1/FCD-2) with substitution of C6 linear alkyl chains by esterification, and carrying the folate residue on the substituted face at the end of C6 linker chain; The folate residue enables active targeting folate-positive breast tumour cells; Paclitaxel-loaded nanospheres were prepared using nanoprecipitation technique in which folate-CD and paclitaxel solution were added in the organic phase; Blank nanospheres were also prepared using nanoprecipitation technique. | Blank and paclitaxel-loaded folate-conjugated amphiphilic NPs were characterised by regular spherical shape with a narrow and unimodal size distribution; Encapsulation efficiency was 60.4% for FCD-1 NPs (120.8 μg drug/mg CD) and 35.2% FCD-2 NPs (70.4 μg drug/mg CD); In vitro release studies in pH 7.4 phosphate-buffer saline solution with 0.1% Tween 80 as medium, revealed for FCD-1 NPs, an initial burst effect, with 44% of amount of paclitaxel being released during the first hour, followed by a liberation of 90% of total amount of paclitaxel, in a steady manner, in the next 24 while for FCD-2 NPs, an initial burst release of 72% of paclitaxel was observed during the first 1 h, followed by a steady release for the next 6 h (95% of loaded paclitaxel was released at the end of the experiment); FCD-1 and FCD-2 blank NPs exerted no toxicity on L929 mouse fibroblast cell line, through 24 or 48 h (cell viability range 80–100%); FCD-1 and FCD-2 blank NPs applied onto T-47D and ZR-75-1 human breast cancer cells (FR-α being expressed by these types of cells) exhibited a more efficient cellular uptake in the case of FCD-1 than that of FCD-2, in both cell lines; On the T-47D cell line, both paclitaxel-loaded FCD-1 and FDC-2 NPs exerted comparative anticancer efficacy as compared to that of paclitaxel solution alone, while in the case of ZR-75-1 cells, both types of paclitaxel-loaded NPs showed significantly increased anticancer effect; the effect was considerably clearer in the case of low concentrations of FCD-1 NPs. | [54] |
| 6 | Tamoxifen citrate | Nanovesicles based on amphiphilic β-CDC12 | Substitution of primary and secondary rims of β-CD with C12 hydrophobic alkyl chains, from lauric acid, via ester bonds; Amphiphilic cyclodextrin contains approximately 7 alkyl chains on both the primary and the secondary rim of β-CD (lauric acid: β-CD molar ratio was 15:1); Surfactant was used in order to optimise the particle size and stability of nanovesicles. | Synthesised amphiphilic CDs had the capability of self-assembling to form spherical nanovesicles in aqueous medium; The surfactant Kolliphor P407 (0.5% w/v) (Poloxamer P407, Pluronic F-127) optimised the particle size and the stability of nanovesicles, as compared to those prepared without surfactant; A stable complex was obtained between amphiphilic β-CD and tamoxifen citrate, helping to retain tamoxifen in nanovesicles and overcoming the disadvantage of precipitation of the drug (which is a common problem for liposomal formulations); The encapsulation efficiency of tamoxifen citrate was 94%; Amphiphilic CDs helped in the reduction in burst release of tamoxifen citrate and maintained the prolonged release, approximately 75% of the drug being released over a period of 48 h in both dissolution media (pH 6.5—intratumoural pH, and pH 7.4—blood plasma pH); In vitro haemolysis study showed that amphiphilic CDs revealed no haemolytic effect, as compared to the parent β-CD, probably due to the self-assembling property, and no genotoxicity; In vitro cell cytotoxicity studies on MCF-7 cells showed that blank amphiphilic CD nanovesicles were highly biocompatible, and tamoxifen citrate-loaded nanovesicles showed a significantly enhanced growth inhibitory effect than the freely diffusible tamoxifen solution at lower concentrations; In vitro cell uptake studies revealed a caveolae-dependent cell internalisation pathway of amphiphilic β-CD nanovesicles in MCF-7 cells; Acute toxicity studies in mice demonstrated that amphiphilic β-CD was well-tolerated by intravenous route, at doses as high as 2000 mg/kg; The pharmacokinetic profile of tamoxifen citrate encapsulated in nanovesicles was improved, and a 3-fold higher AUC was observed; The pharmacokinetic properties suggested that the amphiphilic β-CD nanovesicles assured a longer retention of the drug in plasma, and, therefore, for entering tumor tissues through enhanced permeation-retention effect. | [53] |
| 7 | Docetaxel and Zinc (II)–phthalocyanine | Nanovesicles based on heptakis (2-oligo(ethylenoxyde)-6-hexadecylthio-)-β-CD (SC16OH) | NPs containing docetaxel and Zinc (II)–phthalocyanine with theoretical loading of 5% and 0.2%, respectively, were prepared by the emulsion-solvent evaporation technique; all three components were co-dissolved in organic medium (dichloromethane/tetrahydrofuran 9:1 v/v), and the mixture was added to water and then sonicated. The organic solvent was eliminated by mechanical stirring, NPs were re-dispersed in water, freeze-dried, and kept at 4 °C. | The NPs showed a modal size distribution, a hydrodynamic diametre of approx. 200 nm, and a negative zeta potential, and a satisfactory entrapment efficiency of both drugs at a specific mass ratio; The docetaxel was entrapped in the hydrophobic portion of CD, preferring the space occupied by interdigitated hydrophobic chains of two adjacent CDs, while Zinc (II)–phthalocyanine interaction with CDs involved both oligo-ethylenglycol moieties and thioalkyl moieties; Zinc (II)–phthalocyanine was entrapped as a monomer (which is a fundamental prerequisite to show photodynamic effect) in the carrier, thus maintaining a fairly high propensity to photogenerate oxygen singlet; The dissolution profile of NPs allowed a sustained and modulated release of entrapped drugs; A very low haemolysis was observed at the highest investigated concentrations of NPs; In HeLa cells, photodamage due to Zinc(II)–phthalocyanine enhanced the cytotoxic effects of docetaxel. | [70] |
| 8 | Short interfering RNA (siRNA) | Folate-targeted cationic amphiphilic cyclodextrin-based NPs | Cationic amphiphilic cyclodextrin complexed with siRNA; The amphiphilic SC12CDclickpropylamine cyclodextrin (cationic groups on the secondary face and C12 on the primary face) with great therapeutic potential for siRNA delivery; DSPE-PEG5000–folate and GALA peptide were post-inserted into pre-formed CD-siRNA complexes; Incorporation of PEG of various lengths may increase the stability of cyclodextrin-based NPs and may increase the t1/2of siRNA in vivo; DSPE-PEG5000 post-insertion into pre-formed amphiphilic CD-siRNA NPs may result in a more spherical structure; GALA may improve endosomal escape of cationic NPs, thus improving siRNA delivery into cells; DSPE-PEG5000-folate incorporated into pre-formed CD-siRNA complex for improving pharmacokinetic profile of siRNA. | NPs showed favourable physicochemical properties (spherical, with approx. 200 nm in size), and capable of resisting aggregation for up to 24 h in salt-containing medium; NPs efficiently bound siRNA; NPs protected siRNA from serum nucleases due to the presence of cyclodextrin plus PEG; NPs displayed folate-mediated uptake in prostate cancer cell lines PC3 and LNCaP cells, highlighting the benefit of targeting NPs with folate for the treatment of prostate cancer; NPs showed a low cytotoxic profile in LNCaP and PC3 cells compared with untreated controls; NPs complexed with luciferase siRNA showed a specific knockdown of the luciferase reporter gene in vitro due to addition of GALA to the formulation; NPs highlighted a superior silencing ability to targeted genes (NRP1 and ZEB1) in metastatic prostate cancer PC3 cells as compared to control, and reduced infiltration; (ZEB1 is a marker of epithelial to mesenchymal transition, a process responsible for metastasis of different carcinoma types; NRP1 is a therapeutic target responsible for migration of cancer cells). | [103] |
| 9 | Short interfering RNA (siRNA) | PEGylated cationic amphiphilic cyclodextrin-based NP containing siRNA, tagged with a central nervous system-targeting peptide derived from the rabies virus glycoprotein (RVG), at a molar ratio of 1:1.5:0.5 (cationic cyclodextrin/PEGylated cyclodextrin/RVG-tagged PEGylated cyclodextrin) | A low molecular weight PEG with high density was incorporated in co-formulation to reduce the cationic charge; The rabies virus glycoprotein (RVG) was chosen as a ligand to reinstate cellular uptake while maintaining the reduced cationic nature of the co-formulation; RVG specifically binds to nicotinic acetylcholine receptors on neuronal cells, thus increasing cellular uptake and providing the ability to cross the blood–brain barrier. | Incorporation of PEG in CD-based siRNA nanocomplex reduced surface charge, stabilised the NPs, and lowered the zeta potential. The nanocomplex protected siRNA against nucleases degradation for more than 4 h. An optimal amount of RVG-tagged PEGylated cyclodextrin is required to achieve a maximum cellular uptake. The surface density and smaller molecular weight of PEG500 and the residual cationic charge were favourable for achieving the maximum cellular uptake in U87 human glioblastoma cells. CD-based siRNA nanocomplex showed a favourable toxicological profile in U87 human glioblastoma cells. The RVG-specific acetylcholine receptors predominantly facilitated the cellular uptake in U87 human glioblastoma cells, and highlighted the receptor-mediated endocytosis process. An enhanced gene-silencing effect was achieved by CD-based siRNA nanocomplex, compared to the corresponding untargeted PEGylated formulations. | [121] |
| 10 | Short interfering RNA (siRNA) | Co-formulation of cationic CD:siRNA:anionic CD with a mass ratio of 8.5:1:5.3 The anionic amphiphile is a β-CD derivative, with lipid chains (SC12) grafted on the secondary side and a negative moiety on the primary side The cationic amphiphile is a β-CD derivative with lipid chains (SC12) on the primary side and a primary amine function on the secondary side | NPs were obtained using pre-formed cationic CD:siRNA complex and anionic CD. | The co-formulation exhibited a surface charge of 24 ± 6 mV, size of 161 ± 14 nm, and PDI 0.16 ± 0.04, and 75% encapsulation efficiency, with promising in vitro uptake, capacity to escape the reticulo-endothelial system (RES), and uniform particles; The addition of amphiphilic anionic CD to the formulation positively impacted the surface charge of the NP, by reducing the pKa to 7 ± 0.35 (it has been reported that materials with pKa values around 6.5 are optimal for non-viral delivery of nucleic acids); There was no displacement of siRNA on addition of the anionic CD at the mass ratio studied; The examination of size and morphology indicated a structural arrangement with the integration of both lipidic functionalities, forming a bilayer-like structure; The co-formulation was able to protect siRNA from enzymatic degradation in the serum; The siRNA internalisation in HL-60 cells (isolated from the peripheral blood of an acute myeloid leukaemia patient, with overexpression of the epigenetic modulator KAT2a) was confirmed with confocal microscopy; Endo-lysosomal escape appeared at the time of 24 h and may be attributed to a lower electrostatic interaction with endogenous anionic components of the endosomal membrane, due to pKa value of co-formulation, of approx. 7; CD-NP co-formulation significantly reduced mRNA levels relative to free KAT2a by 21 ± 2%, in the absence of toxicity in HL-60 cells (previously transfected with KAT2a siRNA. | [120] |
| 11 | Short interfering RNA (siRNA) targeting mRNA encoding mitogen-activated protein kinase (p42-MAPK) or Ras homologue enriched in brain (Rheb) | CDplexes of polycationic amphiphilic β-CD derivative | Multi-head/multi-tail Janus-type β-CD derivative that combines seven tetraethyleneimine branches attached to the primary rim, through thioureidocysteaminyl connectors, and fourteen hexanoyl tails at the secondary face. | CDplexes were formed having a round shape, an average hydrodynamic diametre of 100 nm, and positive ζ-potential values of 25.4 mV. Hydrophobic interactions involving the lipid domains favoured a lamellar internal order, consisting of successive layers of β-CD derivative bound together by siRNA molecules. The CDplexes protected siRNA from degradation by RNAses. No toxicity was found in the case of β-CD derivative up to 5 μM when used on different cell types, namely rat (C6), mouse (GL261), and human (U87) glioblastoma cell lines, primary rat astrocytes. The β-CD derivative was able to transport siRNA to the interior of the cells, and the complex was able to escape from endosomes into the cytoplasm. The CDplexes caused a significant decrease in both p42-MAPK and Rheb protein levels in C6 rat, GL261 mouse, and in human U87 glioblastoma cell lines, and in p42-MAPK or Rheb protein levels in human prostate cancer LnCaP or PC3; Moreover, a 65% decrease in p42-MAPK protein levels and a 75% decrease in Rheb protein levels were observed in rat astrocytes, a primary culture usually exceedingly difficult to transfect. In human LnCaP cells, a model for the early stages of androgen-dependent prostate cancer, the CDplexes were able to knock down the p42-MAPK and Rheb proteins and to enhance the efficacy of docetaxel. This was not the case for human PC3 cells, a model for more advanced, castration-resistant prostate cancer, attributed to the fact that this type of cell lines are more resistant to taxanes compared to LNCaP cells. The siRNA induced toll-like receptor 3 activation, leading to β-interferon production and caspase activation, in LNCaP cells. | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pârvănescu, R.D.; Păpurică, M.; Alexa, I.O.; Dehelean, C.A.; Șoica, C.; Moacă, E.A.; Ledeți, A.; Voicu, M.; Coricovac, D.; Trandafirescu, C. The Potential of Amphiphilic Cyclodextrins as Carriers for Therapeutic Purposes: A Short Overview. Pharmaceutics 2025, 17, 1086. https://doi.org/10.3390/pharmaceutics17081086
Pârvănescu RD, Păpurică M, Alexa IO, Dehelean CA, Șoica C, Moacă EA, Ledeți A, Voicu M, Coricovac D, Trandafirescu C. The Potential of Amphiphilic Cyclodextrins as Carriers for Therapeutic Purposes: A Short Overview. Pharmaceutics. 2025; 17(8):1086. https://doi.org/10.3390/pharmaceutics17081086
Chicago/Turabian StylePârvănescu, Ramona Daniela, Marius Păpurică, Ionica Oana Alexa, Cristina Adriana Dehelean, Codruța Șoica, Elena Alina Moacă, Adriana Ledeți, Mirela Voicu, Dorina Coricovac, and Cristina Trandafirescu. 2025. "The Potential of Amphiphilic Cyclodextrins as Carriers for Therapeutic Purposes: A Short Overview" Pharmaceutics 17, no. 8: 1086. https://doi.org/10.3390/pharmaceutics17081086
APA StylePârvănescu, R. D., Păpurică, M., Alexa, I. O., Dehelean, C. A., Șoica, C., Moacă, E. A., Ledeți, A., Voicu, M., Coricovac, D., & Trandafirescu, C. (2025). The Potential of Amphiphilic Cyclodextrins as Carriers for Therapeutic Purposes: A Short Overview. Pharmaceutics, 17(8), 1086. https://doi.org/10.3390/pharmaceutics17081086