Population Pharmacokinetics of Tideglusib in Congenital and Childhood Myotonic Dystrophy Type 1: Influence of Demographic and Clinical Factors on Systemic Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
- Phase I Study in Elderly Healthy Subjects:
- Phase II Study in DM-1 Patients:
2.2. Data and Demographic Characteristics
| Dose Group (mg) | Dosing Regimen | Subjects (n/Female) | Body Weight (kg) | Height (cm) | Age (Years) | |
|---|---|---|---|---|---|---|
| NP03112-07A03 | 300 | b.i.d. | 9/4 | 78.0 (62.5–93.7) | 171 (158–180) | 61 (60–68) |
| 400 | b.i.d. | 9/5 | 77.1 (59.5–98.1) | 166 (157–188) | 68 (62–72) | |
| 600 | q.d. | 9/4 | 74.4 (66.4–88.9) | 169.0 (163–182) | 64 (60–71) | |
| 800 | q.d. | 9/5 | 75.6 (64.2–87.6) | 168 (159–182) | 65 (61–74) | |
| 1000 | q.d. | 9/2 | 72.5 (57.6–87.5) | 170 (156–180) | 63 (60–65) | |
| 1200 | q.d. | 9/4 | 72.7 (50.7–92.7) | 167 (155–181) | 64 (60–70) | |
| AMO-02 MD-2-001 | 400 | q.d. | 8/1 | 60.3 (36.8–81.4) | 168.5 (156–179) | 19.4 (13.8–32.6) |
| 1000 | q.d. | 8/5 | 58.7 (45.1–122.6) | 160.5 (148–178) | 20.8 (17.2–34.9) |
2.3. Bioanalytical Method
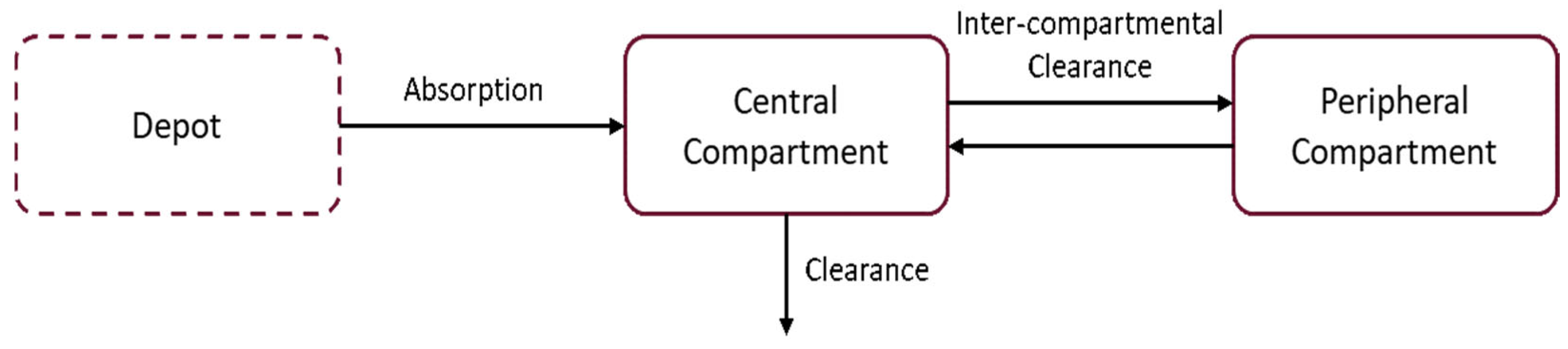
2.4. Population Pharmacokinetic Modelling
2.4.1. Model Development Using Phase I Adult Data
2.4.2. Parameter Estimation in DM-1 Patients
2.4.3. Model Evaluation
2.5. Secondary Pharmacokinetic Parameters
2.6. Exploratory Analysis of the Effect of Food Intake on the Systemic Exposure in DM-1 Patients
2.7. Effect of Body Weight on the Pharmacokinetics of Tideglusib and Dose Rationale for the Paediatric Population
2.8. Software
3. Results
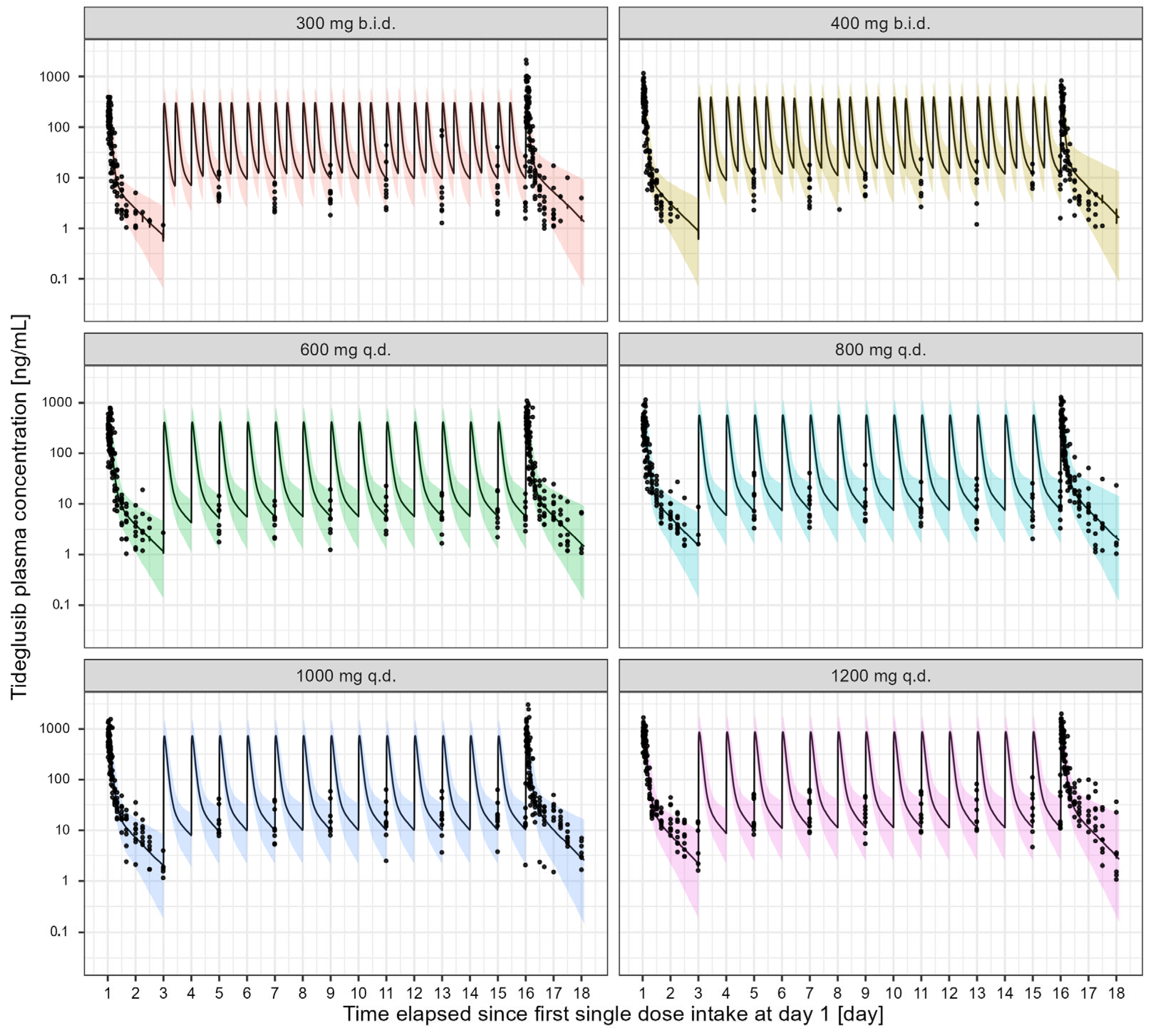
3.1. Tideglusib Pharmacokinetics in Elderly Healthy Subjects and DM-1 Patients
3.2. Secondary PK Parameters
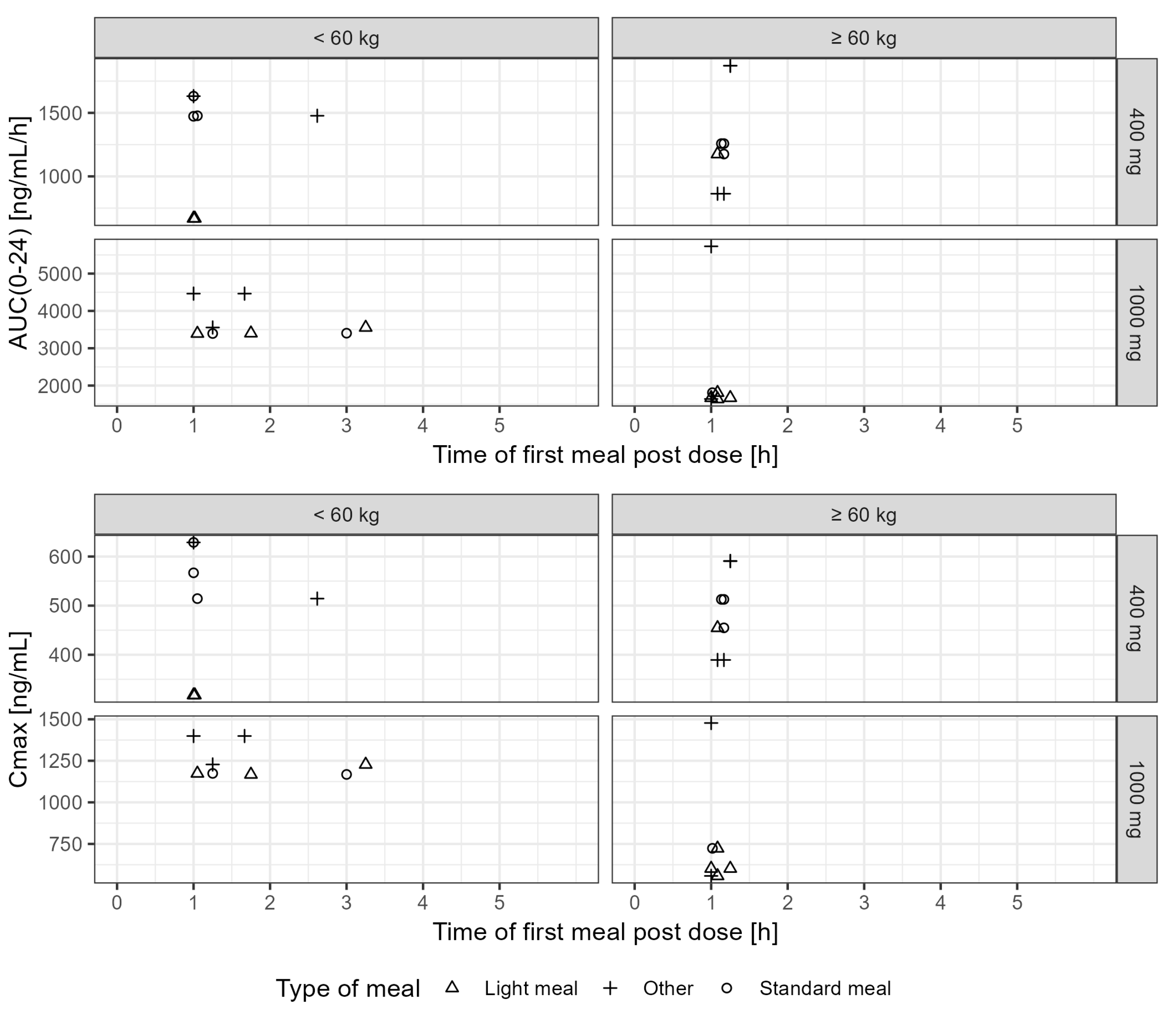
3.3. Influence of Type and Time of Food Intake on the Systemic Exposure to Tideglusib
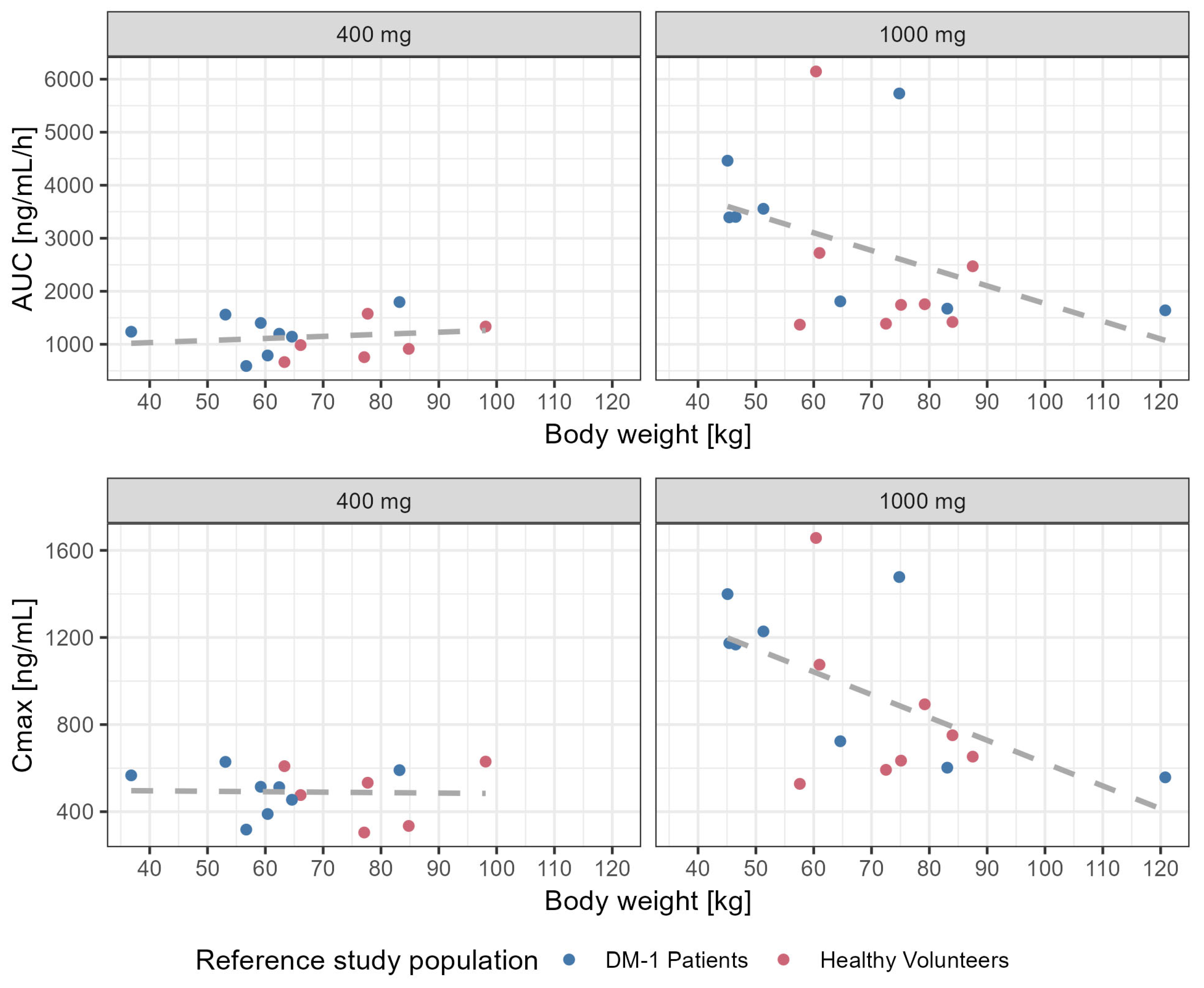
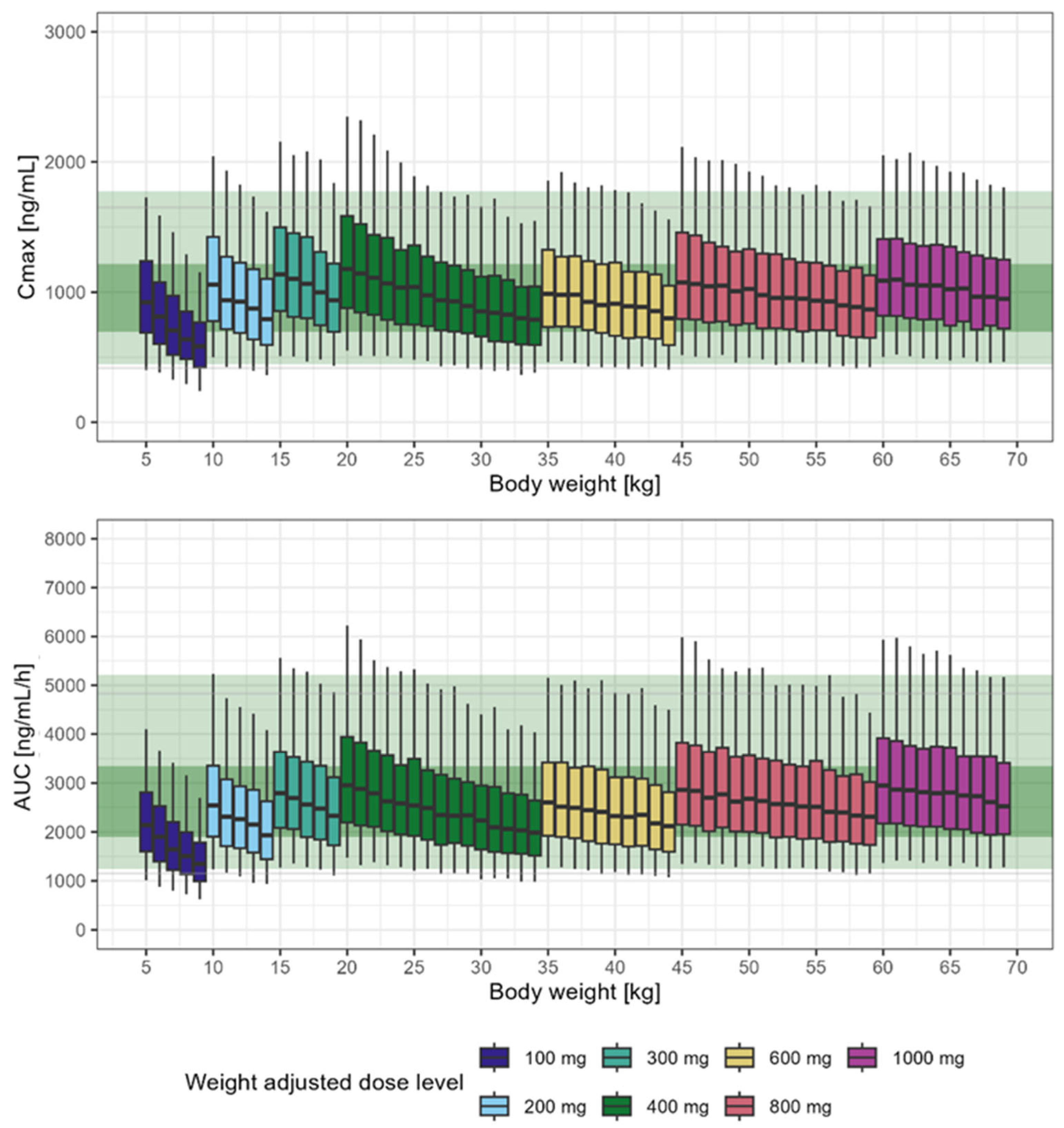
3.4. Influence of Body Weight on Systemic Exposure to Tideglusib
3.5. Dose Rationale for Paediatric Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bird, T.D. Myotonic Dystrophy Type 1. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle WA, USA, 1993. [Google Scholar]
- Prior, T.W. American College of Medical Genetics Laboratory Quality Assurance, Technical Standards and Guidelines for Myotonic Dystrophy Type 1 Testing. Genet. Med. 2009, 11, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Pavicevic, D.S.; Miladinović, J.; Brkušanin, M.; Šviković, S.; Djurica, S.; Brajušković, G.; Romac, S. Molecular genetics and genetic testing in myotonic dystrophy type 1. BioMed Res. Int. 2013, 2013, 391821. [Google Scholar] [CrossRef] [PubMed]
- Udd, B.; Krahe, R. The myotonic dystrophies: Molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012, 11, 891–905. [Google Scholar] [CrossRef]
- Heatwole, C.; Bode, R.; Johnson, N.; Quinn, C.; Martens, W.; McDermott, M.P.; Rothrock, N.; Thornton, C.; Vickrey, B.; Victorson, D.; et al. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1). Neurology 2012, 79, 348–357. [Google Scholar] [CrossRef]
- Kroksmark, A.K.; Stridh, M.L.; Ekstrom, A.B. Long-term follow-up of motor function and muscle strength in the congenital and childhood forms of myotonic dystrophy type 1. Neuromuscul. Disord. 2017, 27, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Petri, H.; Vissing, J.; Witting, N.; Bundgaard, H.; Køber, L. Cardiac manifestations of myotonic dystrophy type 1. Int. J. Cardiol. 2012, 160, 82–88. [Google Scholar] [CrossRef]
- Kaidanovich-Beilin, O.; Woodgett, J.R. GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef]
- King, M.K.; Pardo, M.; Cheng, Y.; Downey, K.; Jope, R.S.; Beurel, E. Glycogen synthase kinase-3 inhibitors: Rescuers of cognitive impairments. Pharmacol. Ther. 2014, 141, 1–12. [Google Scholar] [CrossRef]
- Okkersen, K.; Buskes, M.; Groenewoud, J.; Kessels, R.P.; Knoop, H.; van Engelen, B.; Raaphorst, J. The cognitive profile of myotonic dystrophy type 1: A systematic review and meta-analysis. Cortex 2017, 95, 143–155. [Google Scholar] [CrossRef]
- Horrigan, J.; Gomes, T.B.; Snape, M.; Nikolenko, N.; McMorn, A.; Evans, S.; Yaroshinsky, A.; Della Pasqua, O.; Oosterholt, S.; Lochmüller, H. A Phase 2 Study of AMO-02 (Tideglusib) in Congenital and Childhood-Onset Myotonic Dystrophy Type 1 (DM1). Pediatr. Neurol. 2020, 112, 84–93. [Google Scholar] [CrossRef]
- Bellanti, F.; Del Vecchio, G.C.; Putti, M.C.; Maggio, A.; Filosa, A.; Cosmi, C.; Mangiarini, L.; Spino, M.; Connelly, J.; Ceci, A.; et al. Population pharmacokinetics and dosing recommendations for the use of deferiprone in children younger than 6 years. Br. J. Clin. Pharmacol. 2017, 83, 593–602. [Google Scholar] [CrossRef]
- Borella, E.; Oosterholt, S.; Magni, P.; Della Pasqua, O. Use of prior knowledge and extrapolation in paediatric drug development: A case study with deferasirox. Eur. J. Pharm. Sci. 2019, 136, 104931. [Google Scholar] [CrossRef]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef]
- Oosterholt, S.P.; Della Pasqua, O. Extrapolation and dosing recommendations for raxibacumab in children from birth to age <18 years. Br. J. Clin. Pharmacol. 2021, 87, 4709–4717. [Google Scholar] [CrossRef]
- Hsu, C.H.; Steven Xu, X.; Wang, J.; Zhang, L.; Liu, C.; Wang, Y. Utilization of Adult Data in Designing Pediatric Pharmacokinetic Studies: How Much Are Historical Adult Data Worth? J. Clin. Pharmacol. 2019, 59, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Best, N.; Crawford, J.; Zi, L.; Shelton, C.; Fowler, A. Using Bayesian Dynamic Borrowing to Maximize the Use of Existing Data: A Case-Study. Ther. Innov. Regul. Sci. 2024, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Piana, C.; Zhao, W.; Adkison, K.; Burger, D.; Jacqz-Aigrain, E.; Danhof, M.; Della Pasqua, O. Covariate effects and population pharmacokinetics of lamivudine in HIV-infected children. Br. J. Clin. Pharmacol. 2014, 77, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Chan Kwong, A.H.P.; Calvier, E.A.M.; Fabre, D.; Gattacceca, F.; Khier, S. Prior information for population pharmacokinetic and pharmacokinetic/pharmacodynamic analysis: Overview and guidance with a focus on the NONMEM PRIOR subroutine. J. Pharmacokinet. Pharmacodyn. 2020, 47, 431–446. [Google Scholar] [CrossRef]
- Knebel, W.; Gastonguay, M.R.; Malhotra, B.; El-Tahtawy, A.; Jen, F.; Gandelman, K. Population pharmacokinetics of atorvastatin and its active metabolites in children and adolescents with heterozygous familial hypercholesterolemia: Selective use of informative prior distributions from adults. J. Clin. Pharmacol. 2013, 53, 505–516. [Google Scholar] [CrossRef]
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentré, F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 2006, 23, 2036–2049. [Google Scholar] [CrossRef]
- Yano, Y.; Beal, S.L.; Sheiner, L.B. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J. Pharmacokinet. Pharmacodyn. 2001, 28, 171–192. [Google Scholar] [CrossRef]
- Ette, E.I. Stability and performance of a population pharmacokinetic model. J. Clin. Pharmacol. 1997, 37, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Lindbom, L.; Ribbing, J.; Jonsson, E.N. Perl-speaks-NONMEM (PsN)—A Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 2004, 75, 85–94. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bellanti, F.; Della Pasqua, O. Modelling and simulation as research tools in paediatric drug development. Eur. J. Clin. Pharmacol. 2011, 67 (Suppl. S1), 75–86. [Google Scholar] [CrossRef]
- Basu, C.; Ahmed, M.A.; Kartha, R.V.; Brundage, R.C.; Raymond, G.V.; Cloyd, J.C.; Carlin, B.P. A hierarchical Bayesian approach for combining pharmacokinetic/pharmacodynamic modeling and Phase IIa trial design in orphan drugs: Treating adrenoleukodystrophy with Lorenzo′s oil. J. Biopharm. Stat. 2016, 26, 1025–1039. [Google Scholar] [CrossRef]
- Simeoli, R.; Lava, S.A.G.; Di Deo, A.; Roversi, M.; Cairoli, S.; Tambucci, R.; Rea, F.; Malamisura, M.; Angelino, G.; Biondi, I.; et al. Pharmacokinetic Evaluation of Oral Viscous Budesonide in Paediatric Patients with Eosinophilic Oesophagitis in Repaired Oesophageal Atresia. Pharmaceutics 2024, 16, 872. [Google Scholar] [CrossRef]
- Danhof, M.; Delange, E.; Dellapasqua, O.; Ploeger, B.; Voskuyl, R. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol. Sci. 2008, 29, 186–191. [Google Scholar] [CrossRef]
- Lyauk, Y.K.; Jonker, D.M.; Lund, T.M. Dose Finding in the Clinical Development of 60 US Food and Drug Administration-Approved Drugs Compared With Learning vs. Confirming Recommendations. Clin. Transl. Sci. 2019, 12, 481–489. [Google Scholar] [CrossRef]
- Pang, K.S.; Rowland, M. Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J. Pharmacokinet. Biopharm. 1977, 5, 625–653. [Google Scholar] [CrossRef] [PubMed]
- Jusko, W.J.; Chiang, S.T. Distribution volume related to body weight and protein binding. J. Pharm. Sci. 1982, 71, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Kersting, G.; Willmann, S.; Würthwein, G.; Lippert, J.; Boos, J.; Hempel, G. Physiologically based pharmacokinetic modelling of high- and low-dose etoposide: From adults to children. Cancer Chemother. Pharmacol. 2012, 69, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Piana, C.; Danhof, M.; Della Pasqua, O. Influence of covariate distribution on the predictive performance of pharmacokinetic models in paediatric research. Br. J. Clin. Pharmacol. 2014, 78, 145–157. [Google Scholar] [CrossRef] [PubMed]

| AMO-02-MD-2-001 | NP031112-07A03 | |||||
|---|---|---|---|---|---|---|
| Parameter (Unit) | Population Estimate | RSE (%) | Bootstrap Median (5th–95th Percentiles) | Population Estimate | RSE (%) | Bootstrap Median a (5th–95th Percentiles) |
| Clearance, CL (L/h) | 341 | 12.4 | 336 (268–389) | 327 | 17.0 | 333 (260–408) |
| Volume of distribution central compartment, V2 (L) | 154 | 16.4 | 151 (108–183) | 152 | 27.4 | 156 (93–239) |
| Absorption rate constant, kA (1/h) | 0.767 | 6.8 | 0.760 (0.677–0.828) | 0.78 | 11.3 | 0.78 (0.67–1.09) |
| Absorption rate constant, kA (dose > 400 mg) (1/h) | 0.609 | 1.6 | 0.608 (0.589–0.623) | 0.61 | 6.6 | 0.61 (0.55–0.69) |
| Intercompartmental clearance, Q (L/h) | 66.1 | 22.1 | 64.6 (41.9–82.2) | 66.1 | 26.0 | 67.0 (46.8–94.1) |
| Volume of distribution peripheral compartment, V3 (L) | 986 | 18.3 | 971 (692–1180) | 1010 | 21.1 | 1021 (759–1375) |
| Bioavailability (doses ≤ 400 mg) | 1 | FIXED | FIXED | 1 | FIXED | FIXED |
| Bioavailability (doses > 400 mg) | 0.88 | 13.2 | 0.87 (0.68–1.01) | 0.85 | 18.8 | 0.86 (0.64–1.11) |
| Interindividual Variability | Population Estimate (CV%) b | RSE (%) | Bootstrap Median (5th–95th Percentiles) | Population Estimate (CV%) b | RSE (%) | Bootstrap Median a (5th–95th Percentiles) |
| ηCL variance | 0.186 (43.1) | 22.7 | 0.18 (0.13–0.27) | 0.18 (43.5) | 16.9 | 0.18 (0.12–0.24) |
| ηQ variance | 1.15 (107.2) | 31.6 | 1.09 (0.71–1.83) | 0.96 (98.2) | 23.5 | 0.93 (0.61–1.31) |
| ηV3 variance | 1.01 (100.5) | 28.6 | 0.96 (0.67–1.57) | 0.90 (95.1) | 22.2 | 0.89 (0.59–1.24) |
| ηV2 variance | 0.321 (56.7) | 3.1 | 0.32 (0.31–0.34) | 0.33 (57.8) | 36.5 | 0.32 (0.15–0.58) |
| ηKA variance | 0.069 (26.4) | 2.2 | 0.07 (0.07–0.07) | 0.06 (26.2) | 41.1 | 0.06 (0.02–0.11) |
| Residual Error | Population Estimate | RSE (%) | Bootstrap Median (5th–95th Percentiles) | Population Estimate | RSE (%) | Bootstrap Median a (5th–95th Percentiles) |
| Proportional error (doses ≤ 400 mg) | 0.54 | 1.8 | 0.54 (0.53–0.56) | 0.56 | 7.2 | 0.55 (0.49–0.62) |
| Proportional error (doses > 400 mg) | 0.46 | 0.9 | 0.46 (0.45–0.47) | 0.45 | 4.0 | 0.45 (0.42–0.48) |
| Study | Dose (mg) | Cmax (ng/mL) | Cmin (ng/mL) | CSS (ng/mL) | Tmax (h) | T1/2 (h) | AUC(0–12) (ng/mL∙h) |
|---|---|---|---|---|---|---|---|
| NP03112-07A03 a | 400 b.i.d. | 504.7 (312.0–624.9) | 9.8 (7.2–22.4) | 79.2 (57.3–126.4) | 0.7 (0.4–1.0) | 1.5 (1.0–2.8) | 949.7 (687.9–1516.1) |
| 1000 q.d. | 702.0 (550.3–1453.3) | 18.5 (7.0–37.8) | 78.3 (59.4–219.0 | 0.7 (0.3–1.3) | 4.0 (1.8–6.7) | 1749.8 (1376.7–4947.7) | |
| AMO-02 MD-2-001 b | 400 q.d. | 513.5 (342.9–615.4) | 4.1 (2.1–11.3) | 56.9 (30.7–74.4) | 0.75 (0.3–1.0) | 2.0 (1.3–5.4) | 1218.1 (660.3–1713.8) |
| 1000 q.d. | 1170.9 (573.3–1450.1) | 5.6 (3.4–16.2) | 141.6 (68.8–220.3) | 0.7 (0.6–1.2) | 1.7 (1.0–2.5) | 3145.7 (1571.4–5109.4) |
| Dose (mg) | Weight (kg) | n | Cmax (ng/mL) | AUC(0–12) (ng/mL·h) | AUC(0–24) (ng/mL·h) | |
|---|---|---|---|---|---|---|
| Light Meal | 400 | <60 | 1 | 317.9 | 590.8 | 669.2 |
| ≥60 | 1 | 454.8 | 1143.5 | 1175.8 | ||
| 1000 | <60 | 3 | 1174.1 (1167.8–1227.8) | 3153.4 (3138.1–3462.7) | 3402.9 (3394.4–3555.8) | |
| ≥60 | 3 | 602.1 (557.8–723.6) | 1586.6 (1563.2–1743.7) | 1672.7 (1640.7–1810) | ||
| Standard Meal | 400 | <60 | 3 | 566.8 (514.3–628.7) | 1402.3 (1237.3–1560.0) | 1477.2 (1473.0–1631.5) |
| ≥60 | 2 | 483.8 (454.8–512.7) | 1171.3 (1143.5–1199.1) | 1216.9 (1175.7–1258.1) | ||
| 1000 | <60 | 2 | 1170.9 (1167.8–1174.1) | 3145.8 (3138.1–3153.4) | 3398.7 (3394.5–3402.9) | |
| ≥60 | 1 | 723.6 | 1743.8 | 1810.0 | ||
| Other Meal | 400 | <60 | 2 | 571.5 (514.3–628.7) | 1481.1 (1402.3–1560.0) | 1554.3 (1477.2–1631.5) |
| ≥60 | 2 | 490.1 (389.4–590.8) | 1293.1 (789.4–1796.7) | 1367.3 (863.5–1871.2) | ||
| 1000 | <60 | 2 | 1313.5 (1227.8–1399.2) | 3817.5 (3462.7–4172.4) | 4009.4 (3555.8–4463.0) | |
| ≥60 | 2 | 1017.7 (557.8–1477.6) | 3588.6 (1563.2–5614) | 3685.8 (1640.7–5730.8) |
| Weight-Banded Dosing Regimen (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Weight-band (kg) | ≥5–<10 | ≥10–<15 | ≥15–<20 | ≥20–<35 | ≥35–<45 | ≥45–<60 | 60+ |
| Maintenance Dose (mg) | 100 | 200 | 300 | 400 | 600 | 800 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Deo, A.; Oosterholt, S.; Horrigan, J.; Evans, S.; McMorn, A.; Della Pasqua, O. Population Pharmacokinetics of Tideglusib in Congenital and Childhood Myotonic Dystrophy Type 1: Influence of Demographic and Clinical Factors on Systemic Exposure. Pharmaceutics 2025, 17, 1065. https://doi.org/10.3390/pharmaceutics17081065
Di Deo A, Oosterholt S, Horrigan J, Evans S, McMorn A, Della Pasqua O. Population Pharmacokinetics of Tideglusib in Congenital and Childhood Myotonic Dystrophy Type 1: Influence of Demographic and Clinical Factors on Systemic Exposure. Pharmaceutics. 2025; 17(8):1065. https://doi.org/10.3390/pharmaceutics17081065
Chicago/Turabian StyleDi Deo, Alessandro, Sean Oosterholt, Joseph Horrigan, Stuart Evans, Alison McMorn, and Oscar Della Pasqua. 2025. "Population Pharmacokinetics of Tideglusib in Congenital and Childhood Myotonic Dystrophy Type 1: Influence of Demographic and Clinical Factors on Systemic Exposure" Pharmaceutics 17, no. 8: 1065. https://doi.org/10.3390/pharmaceutics17081065
APA StyleDi Deo, A., Oosterholt, S., Horrigan, J., Evans, S., McMorn, A., & Della Pasqua, O. (2025). Population Pharmacokinetics of Tideglusib in Congenital and Childhood Myotonic Dystrophy Type 1: Influence of Demographic and Clinical Factors on Systemic Exposure. Pharmaceutics, 17(8), 1065. https://doi.org/10.3390/pharmaceutics17081065








