Cyclosporine Dissolution Test from a Lipid Dosage Form: Next Step Towards the Establishment of Release Method for Solid Lipid Microparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SLM Dispersions
2.3. Characteristics of the Microspheres’ Properties
2.3.1. Distribution of Cs in SLM Dispersions
2.3.2. Particle Size Analysis
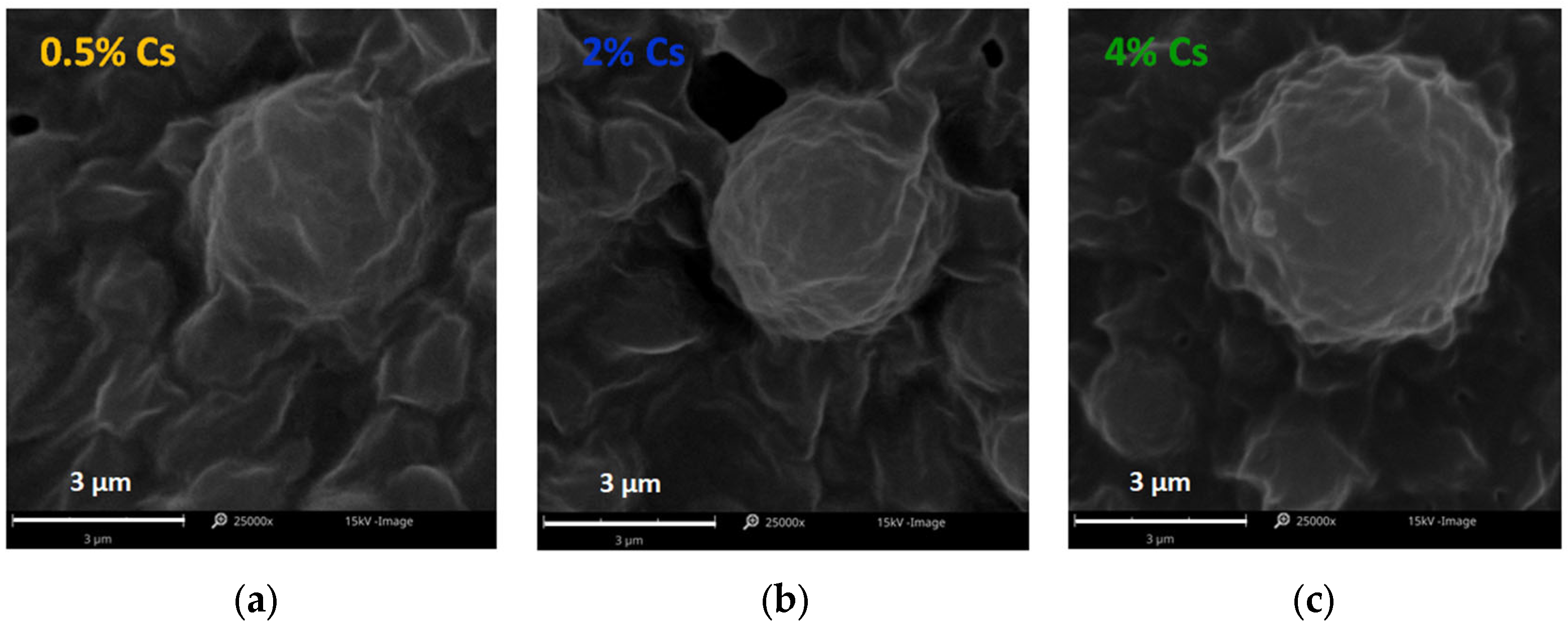
2.3.3. Scanning Electron Microscopy
2.4. Drug Release Study
2.4.1. Dialysis Bag Diffusion Technique (DBDT)
2.4.2. Diffusion Chamber (DCh)
2.4.3. Membrane-Free System (MFS)
2.5. Composition of Fluids Used in Release Studies
2.6. Solubility Studies of Cs
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Lipid Microparticles
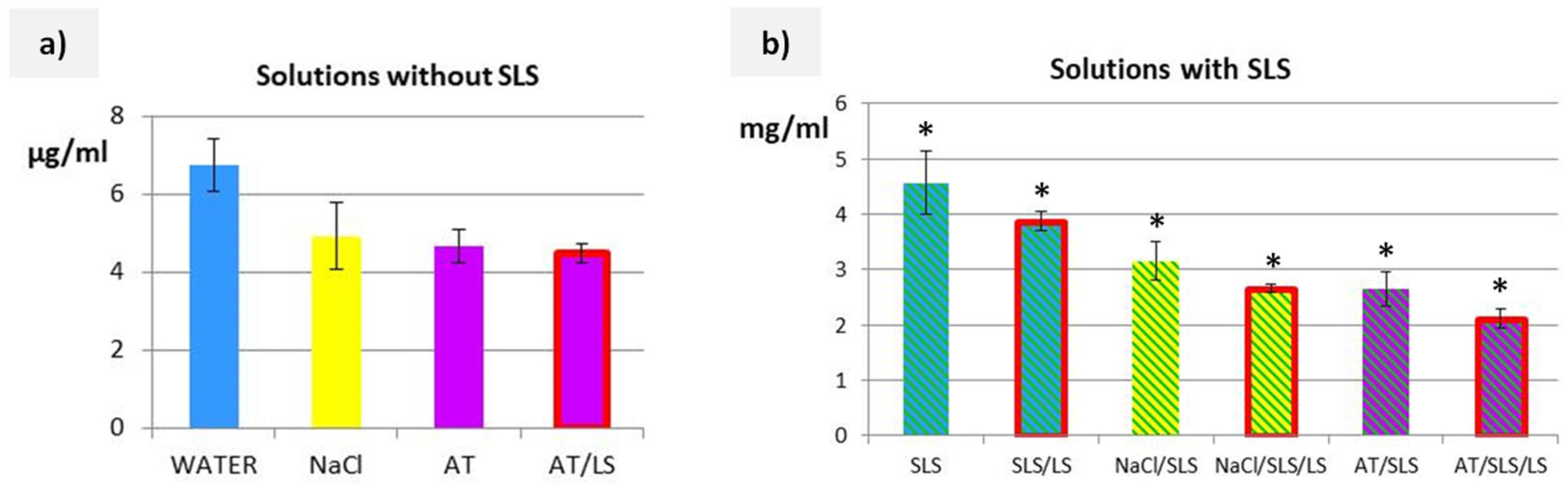
3.2. Solubility Studies of Cs
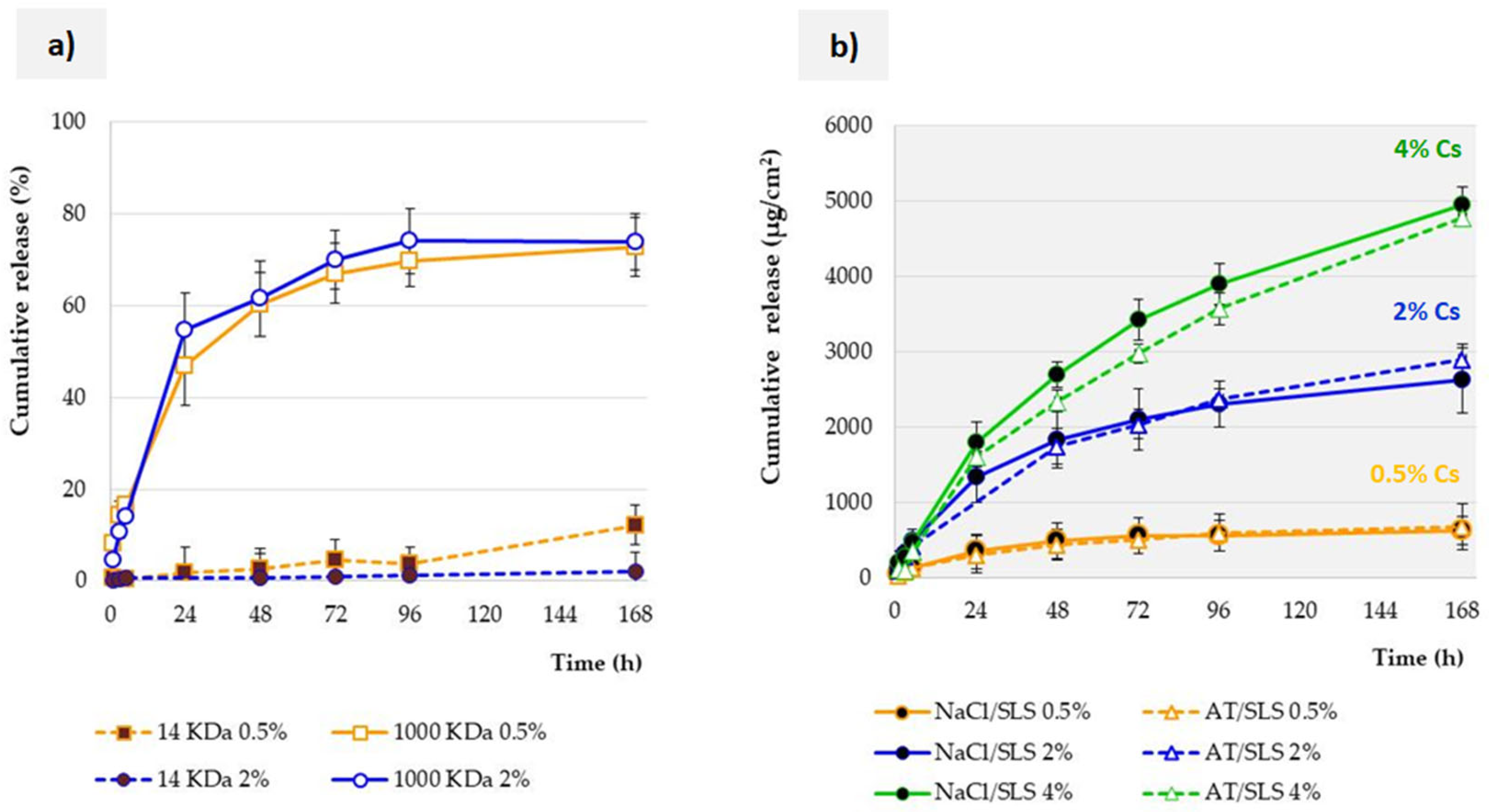
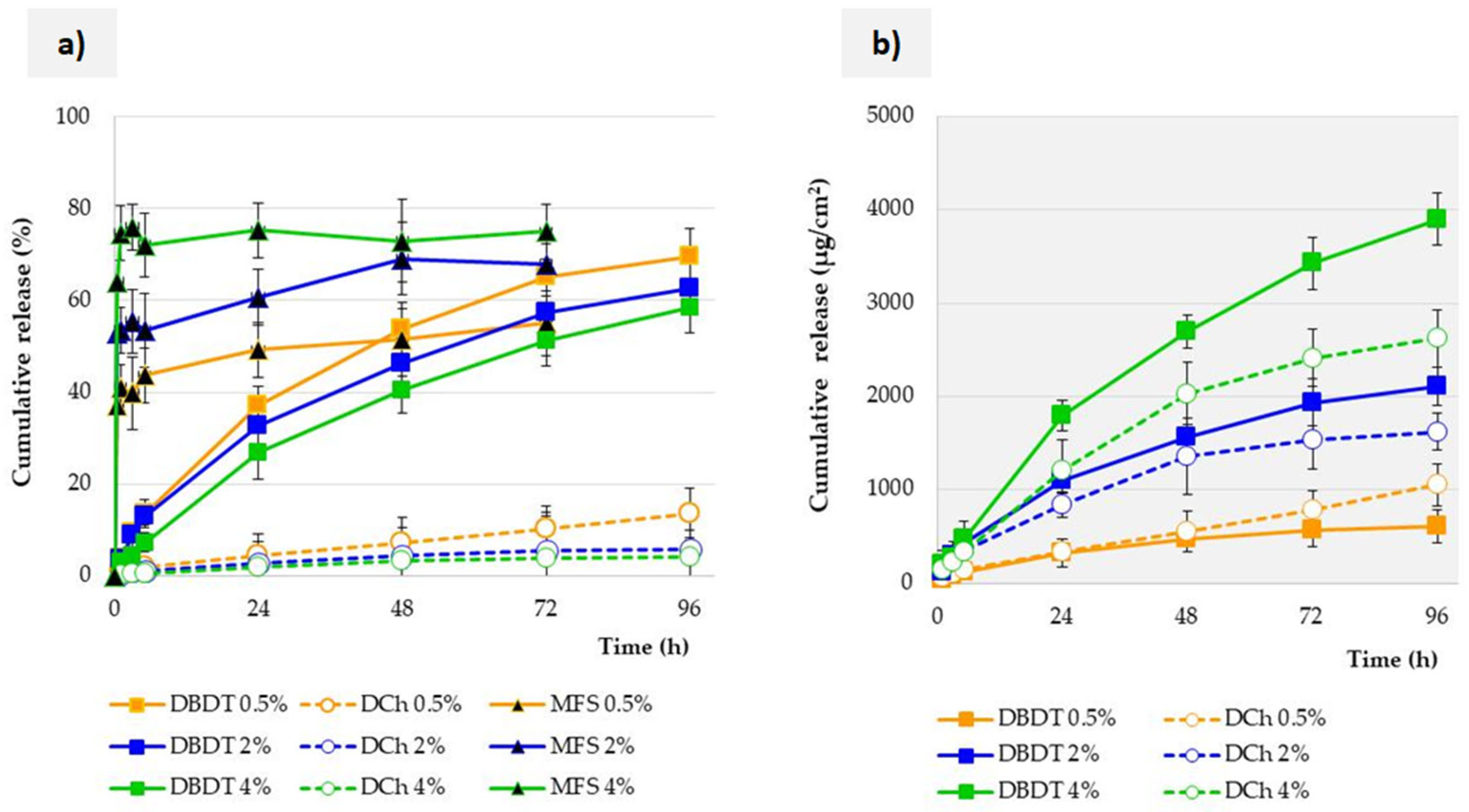
3.3. Influence of Membrane Properties on the Release Profile
3.4. Influence of Acceptor Fluid Composition on the Release Profile
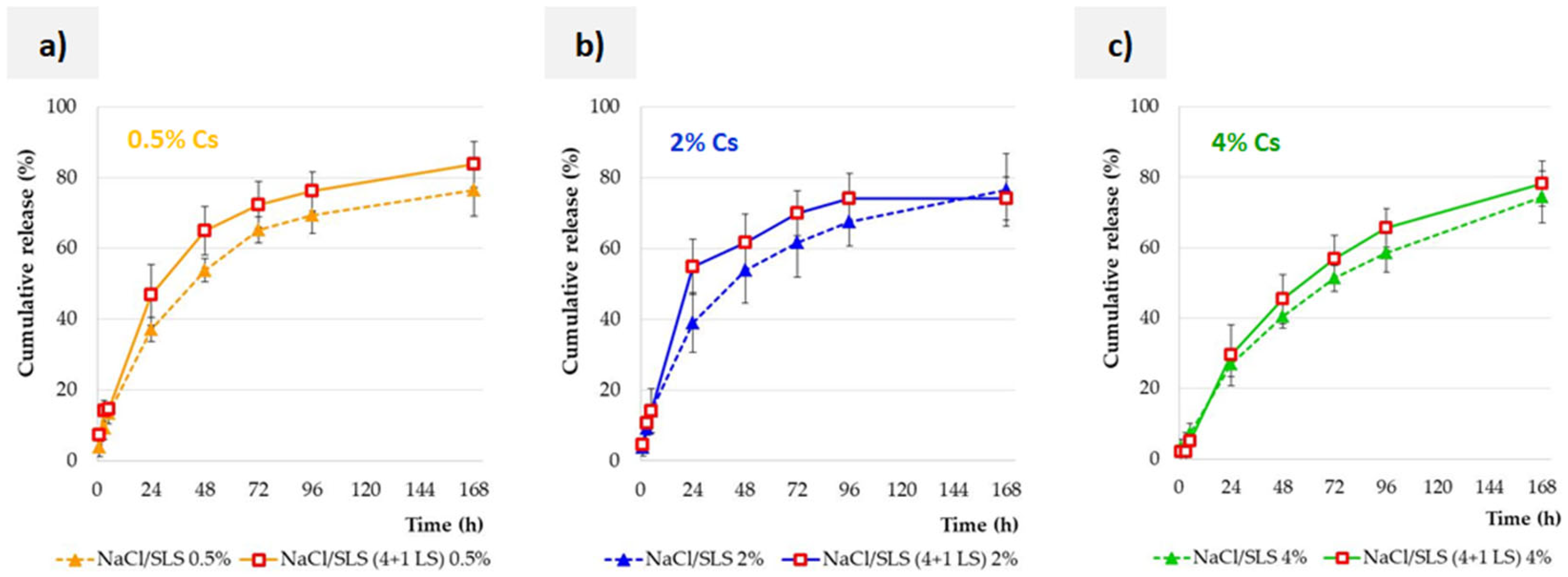
3.5. Effect of the Lysozyme Enzyme on the Release Profile
3.6. Influence of the Test Method on the Release Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Jamal, W.T.; Kostarelos, K. Construction of nanoscale multicompartment liposomes for combinatory drug delivery. Int. J. Pharm. 2007, 331, 182–185. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen Thuy, V.; Van, T.V.; Dao, A.H.; Lee, B.J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano 2022, 8, 100064. [Google Scholar] [CrossRef]
- Saini, V.; Mata Espinosa, D.; Pandey, A.; Dighe, V.; Barrios Payán, J.; Prasad Myneedu, V.; Valdez Zarate, I.; Rajani, D.P.; Anande, L.D.; Hernandez Pando, R.; et al. Antimycobacterial Activity of Solid Lipid Microparticles Loaded with Ursolic Acid and Oleanolic Acid: In Vitro, In Vivo, and Toxicity Assessments. Microorganisms 2024, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Krzeminska, K.; Sznitowska, M.; Wroblewska, M.; Wolska, E.; Winnicka, K. Suspensions of antibiotics in self-emulsifying oils as a novel approach to formulate eye drops with substances which undergo hydrolysis in aqueous environment. Drug Deliv. 2024, 31, 2372279. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef]
- Mohite, P.; Singh, S.; Pawar, A.; Sangale, A.; Prajapati, B.G. Lipid-based oral formulation in capsules to improve the delivery of poorly water-soluble drugs. Front. Drug Deliv. 2023, 3, 1232012. [Google Scholar] [CrossRef]
- Jamroży, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Krzan, M. Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. Int. J. Mol. Sci. 2024, 25, 786. [Google Scholar] [CrossRef]
- Wolska, E.; Sadowska, K. Drug release from lipid microparticles—Insights into drug incorporation and the influence of physiological factors. Pharmaceutics 2024, 16, 545. [Google Scholar] [CrossRef]
- Wolska, E.; Sznitowska, M. Modeling the Analysis Process of a Lipid-Based, Multi-Compartment Drug Delivery System. Processes 2025, 13, 460. [Google Scholar] [CrossRef]
- Bertoni, S.; Tedesco, D.; Bartolini, M.; Prata, C.; Passerini, N.; Albertini, B. Solid Lipid Microparticles for Oral Delivery of Catalase: Focus on the Protein Structural Integrity and Gastric Protection. Mol. Pharm. 2020, 17, 3609–3621. [Google Scholar] [CrossRef]
- Wolska, E.; Sznitowska, M.; Chorążewicz, J.; Szerkus, O.; Radwańska, A.; Markuszewski, M.J.; Kaliszan, R.; Raczyńska, K. Ocular irritation and cyclosporine A distribution in the eye tissues after administration of Solid Lipid Microparticles in the rabbit model. Eur. J. Pharm. Sci. 2018, 121, 95–105. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Kuca, K.; Wu, W. Cyclosporine A: Chemistry and Toxicity—A Review. Curr. Med. Chem. 2021, 28, 3925–3934. [Google Scholar] [CrossRef]
- Guada, M.; Beloqui, A.; Ravi Kumar, M.N.V.; Préat, V.; Dios-Viéitez, M.; Blanco-Prieto, M.J. Reformulating cyclosporine A (CsA): More than just a life cycle management strategy. J. Control. Release 2016, 225, 269–282. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. Chapter 15—In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In Lipid Nanocarriers for Drug Targeting; William Andrew: Norwich, NY, USA, 2018; pp. 563–627. [Google Scholar]
- Liu, P.; De Wulf, O.; Laru, J.; Heikkilä, T.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Peltonen, L.; Laaksonen, T. Dissolution studies of poorly soluble drug nanosuspensions in non-sink conditions. AAPS PharmSciTech 2013, 14, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Brach, M. Distribution of Drug Substances in Solid Lipid Microparticles (SLM)—Methods of Analysis and Interpretation. Pharmaceutics 2022, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Alcoholado, C.; Cifuentes, M.; Merayo-Lloves, J.; Andrades, J.A.; Becerra, J. Regenerative therapies in dry eye disease: From growth factors to cell therapy. Int. J. Mol. Sci. 2017, 18, 2264. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.C.; Chen, C.C.; Hsu, S.M.; Chuang, H.S. Contact-Lens Biosensors. Sensors 2018, 18, 2651. [Google Scholar] [CrossRef]
- Alexeev, V.; Das, S.; Finegold, D.; Asher, S. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 2005, 50, 2353–2360. [Google Scholar] [CrossRef]
- Vo, A.; Feng, X.; Patel, D.; Mohammad, A.; Patel, M.; Zheng, J.; Kozak, D.; Choi, S.; Ashraf, M.; Xu, X. In vitro physicochemical characterization and dissolution of brinzolamide ophthalmic suspensions with similar composition. Int. J. Pharm. 2020, 588, 119761. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Guillarme, D.; El Sanharawi, M.; Behar-Cohen, F.; Veuthey, J.-L.; Gurny, G. In vivo distribution and ex vivo permeation of cyclosporine A prodrug aqueous formulations for ocular application. J. Control. Release 2013, 170, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Szymańska, M. Comparison of the in vitro drug release methods for the selection of test conditions to characterize Solid Lipid Microparticles. Pharmaceutics 2023, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Berton, P.; Mishra, M.K.; Choudhary, H.; Myerson, A.S.; Rogers, R.D. Solubility Studies of Cyclosporine Using Ionic Liquids. ACS Omega 2019, 4, 7938–7943. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E.J.; Kim, T.W.; Na, D.H. Recent Progress in Drug Release Testing Methods of Biopolymeric Particulate System. Pharmaceutics 2021, 13, 1313. [Google Scholar] [CrossRef]
- Available online: https://www.membrane-solutions.com/News_1333.htm (accessed on 25 June 2025).
- Available online: https://www.thermofisher.com/pl/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/dialysis-methods-protein-research.html (accessed on 25 June 2025).
- Wiącek, A.E.; Jurak, M.; Ładniak, A.; Przykaza, K.; Szafran, K. Cyclosporine CsA—The Physicochemical Characterization of Liposomal and Colloidal Systems. Colloids Interfaces 2020, 4, 46. [Google Scholar] [CrossRef]
- Corbett, K.M.; Ford, L.; Warren, D.B.; Pouton, C.W.; Chalmers, D.K. Cyclosporin Structure and Permeability: From A to Z and Beyond. J. Med. Chem. 2021, 64, 13131–13151. [Google Scholar] [CrossRef]
- Vicente, R.; de Andrade, C.J.; de Oliveira, D.; Ambrosi, A. A prospection on membrane-based strategies for downstream processing of surfactin. Chem. Eng. J. 2021, 415, 129067. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Sink conditions do not guarantee the absence of saturation effects. Int. J. Pharm. 2020, 577, 119009. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry, Dissolution Testing of Immediate Release Solid Oral Dosage Forms; U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 1997; pp. 1–17. [Google Scholar]
- Committee for Medicinal Products for Human use (CHMP); Committee for Medicinal Products for Veterinary use (CVMP); Quality Working Party (QWP). Reflection Paper on the Dissolution Specification for Generic Solid Oral Immediate Release Products with Systemic Action; European Medicines Agency: Amsterdam, The Netherlands, 2017; pp. 1–10. [Google Scholar]
- Lim, S.Y.; Steiner, J.M.; Cridge, H. Lipases: It’s not just pancreatic lipase. Am. J. Vet. Res. 2022, 83, ajvr.22.03.0048. [Google Scholar] [CrossRef]
- Torrent-Burgués, J. Lysozyme Influence on Monolayers of Individual and Mixed Lipids. Colloids Interfaces 2022, 6, 15. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. In Vitro Dissolution Testing Strategies for Nanoparticulate Drug Delivery Systems: Recent Developments and Challenges. Drug Deliv. Transl. Res. 2013, 3, 409–415. [Google Scholar] [CrossRef]
| Formulation | Particle Size (µm) | API Distribution (%) | ||||
|---|---|---|---|---|---|---|
| d0.5 | d0.9 | Mean | Aqueous Phase | Interphase | Lipid Matrix | |
| SLM-Cs 0.5% | 1.59 | 6.66 | 2.86 | 0.23 | 48.8 | 51.0 |
| SLM-Cs 2.0% | 1.89 | 5.59 | 2.66 | 0.35 | 66.7 | 32.9 |
| SLM-Cs 4.0% | 1.90 | 10.41 | 3.84 | 0.28 | 63.9 | 35.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, E.; Dudek, P.; Sznitowska, M. Cyclosporine Dissolution Test from a Lipid Dosage Form: Next Step Towards the Establishment of Release Method for Solid Lipid Microparticles. Pharmaceutics 2025, 17, 1030. https://doi.org/10.3390/pharmaceutics17081030
Wolska E, Dudek P, Sznitowska M. Cyclosporine Dissolution Test from a Lipid Dosage Form: Next Step Towards the Establishment of Release Method for Solid Lipid Microparticles. Pharmaceutics. 2025; 17(8):1030. https://doi.org/10.3390/pharmaceutics17081030
Chicago/Turabian StyleWolska, Eliza, Patrycja Dudek, and Małgorzata Sznitowska. 2025. "Cyclosporine Dissolution Test from a Lipid Dosage Form: Next Step Towards the Establishment of Release Method for Solid Lipid Microparticles" Pharmaceutics 17, no. 8: 1030. https://doi.org/10.3390/pharmaceutics17081030
APA StyleWolska, E., Dudek, P., & Sznitowska, M. (2025). Cyclosporine Dissolution Test from a Lipid Dosage Form: Next Step Towards the Establishment of Release Method for Solid Lipid Microparticles. Pharmaceutics, 17(8), 1030. https://doi.org/10.3390/pharmaceutics17081030






