Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics
Abstract
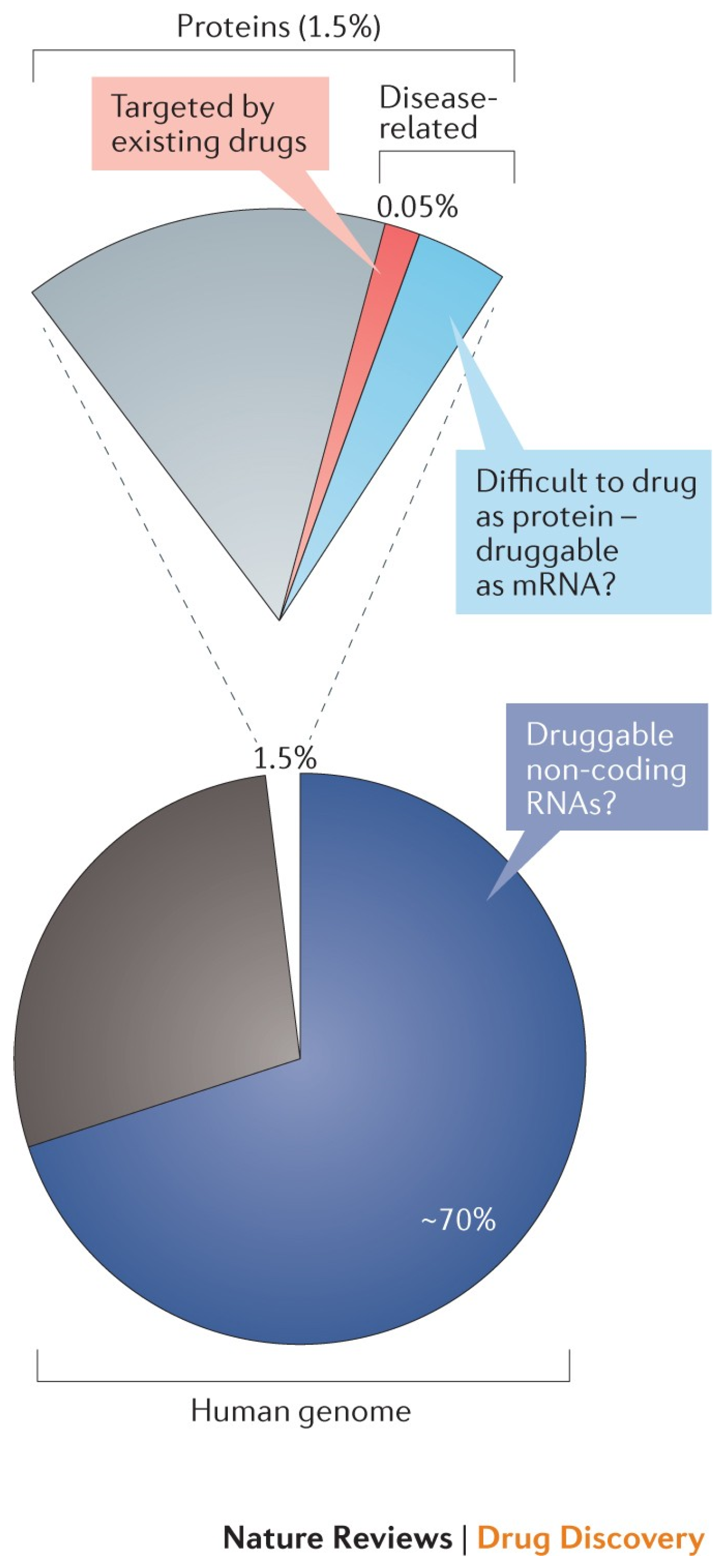
1. Introduction
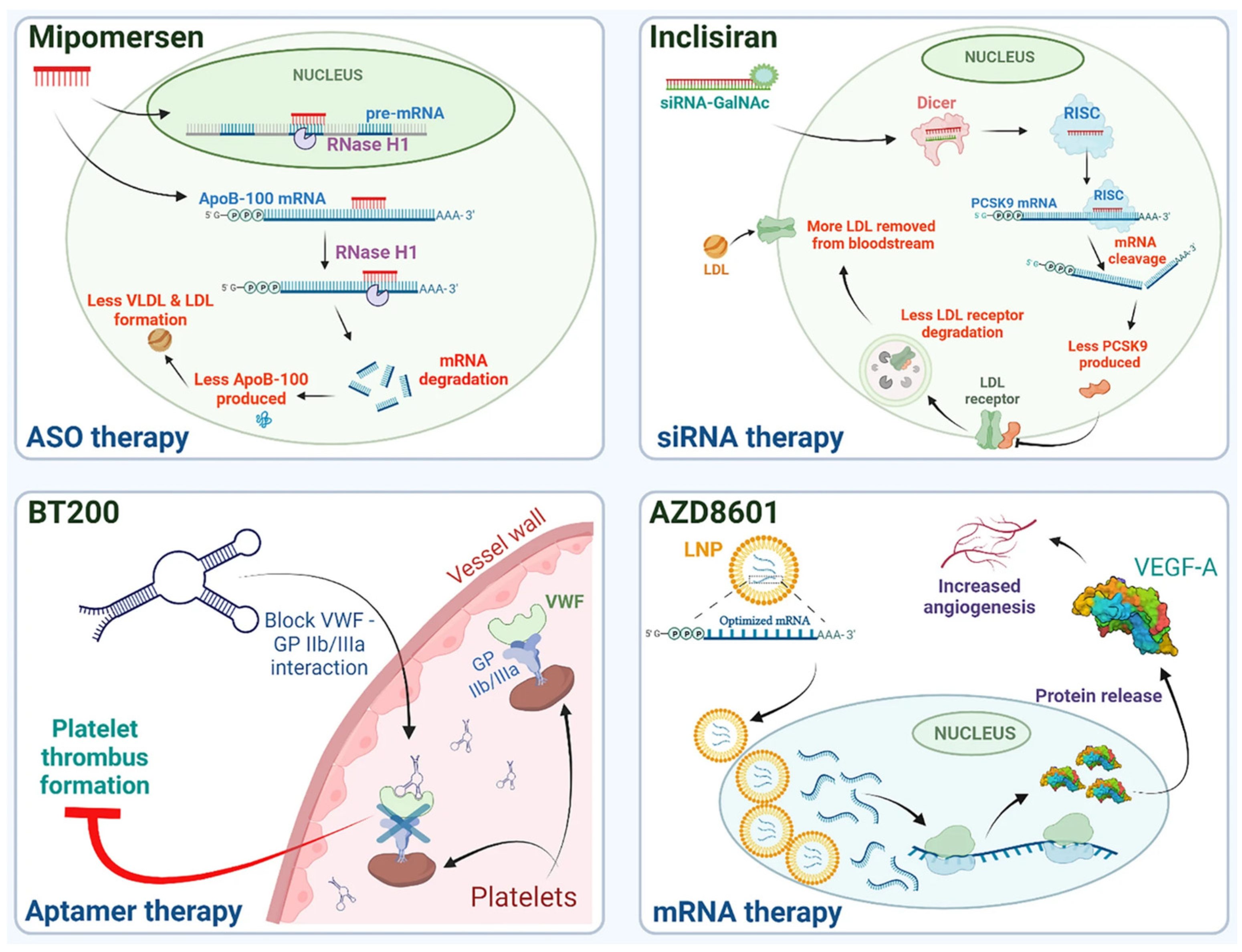
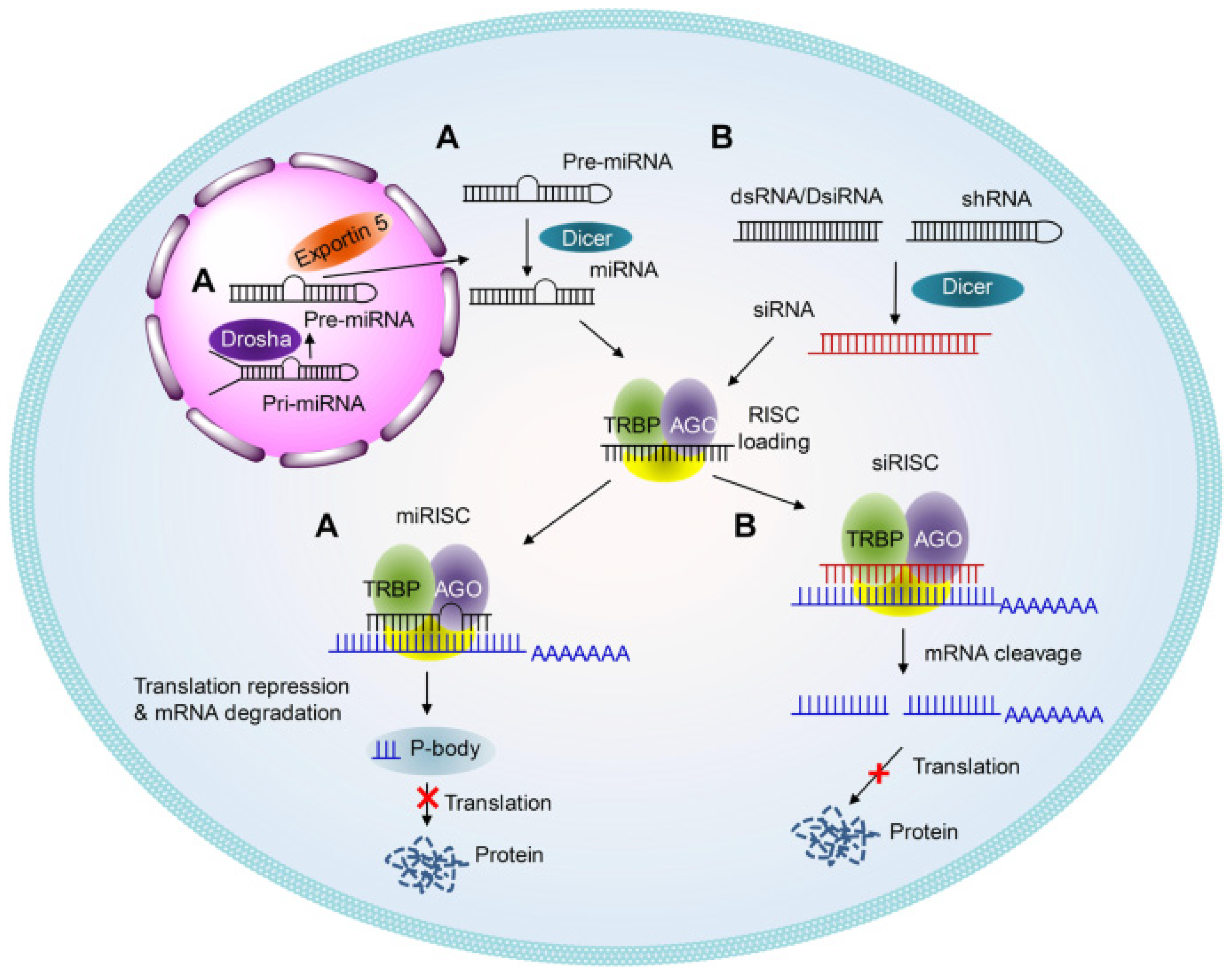
2. Antisense Oligonucleotides and siRNA
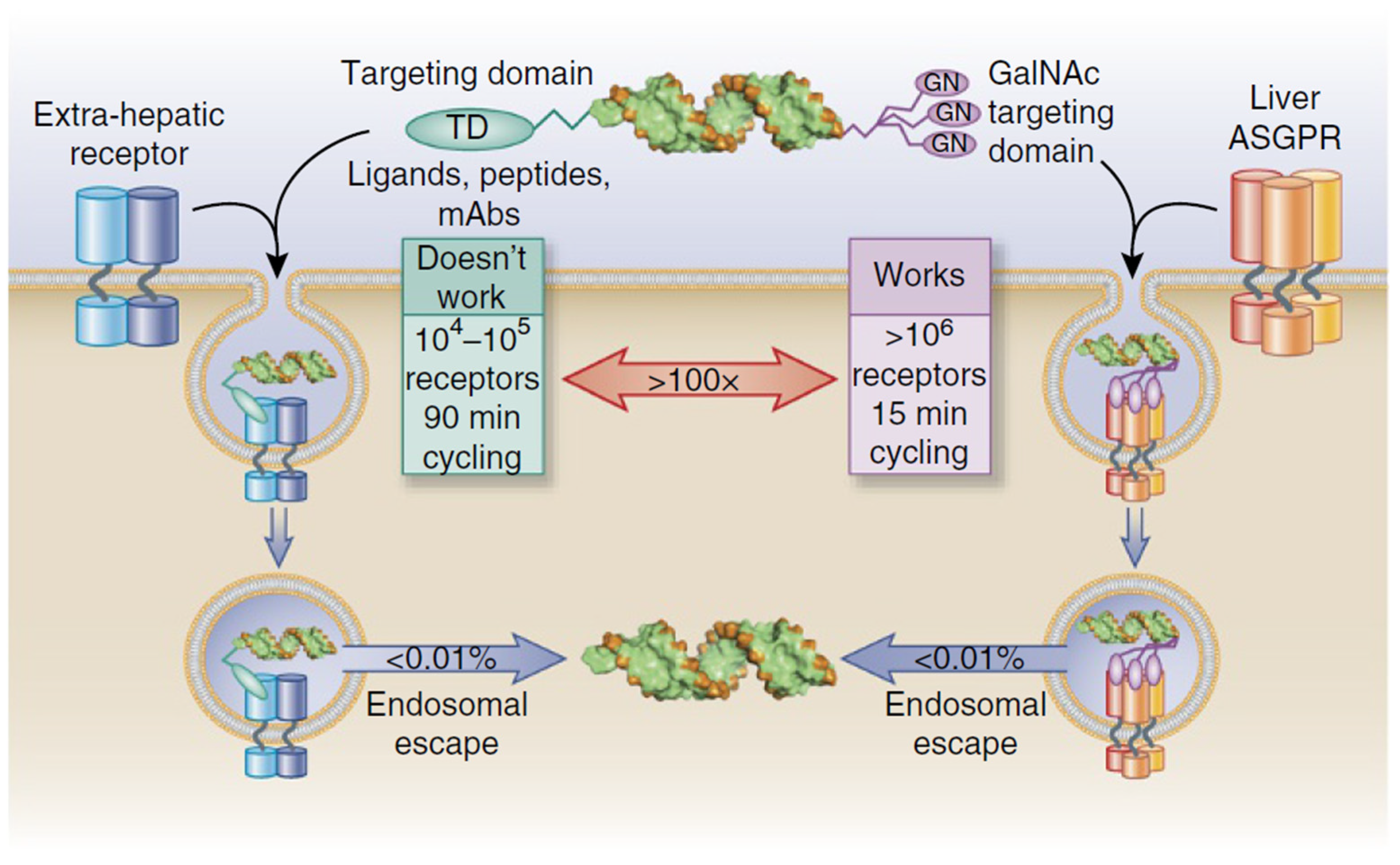
2.1. Tissue Targeting and Drug Delivery Systems
2.1.1. Approved Products
2.1.2. Late Clinical-Phase Products
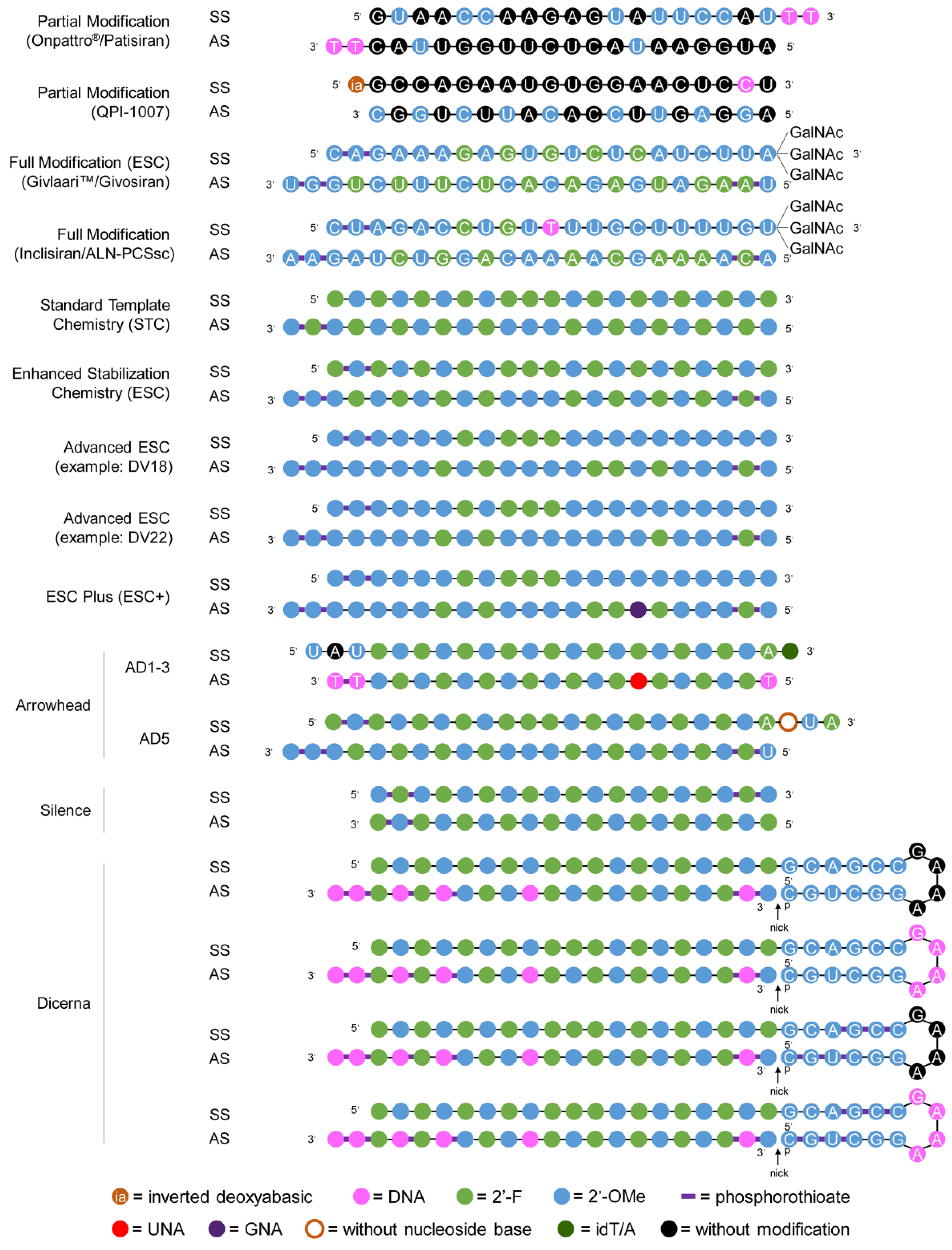
2.2. Structure-Based Approach to Delivery
2.2.1. Approved Products
2.2.2. Late Clinical-Phase Products
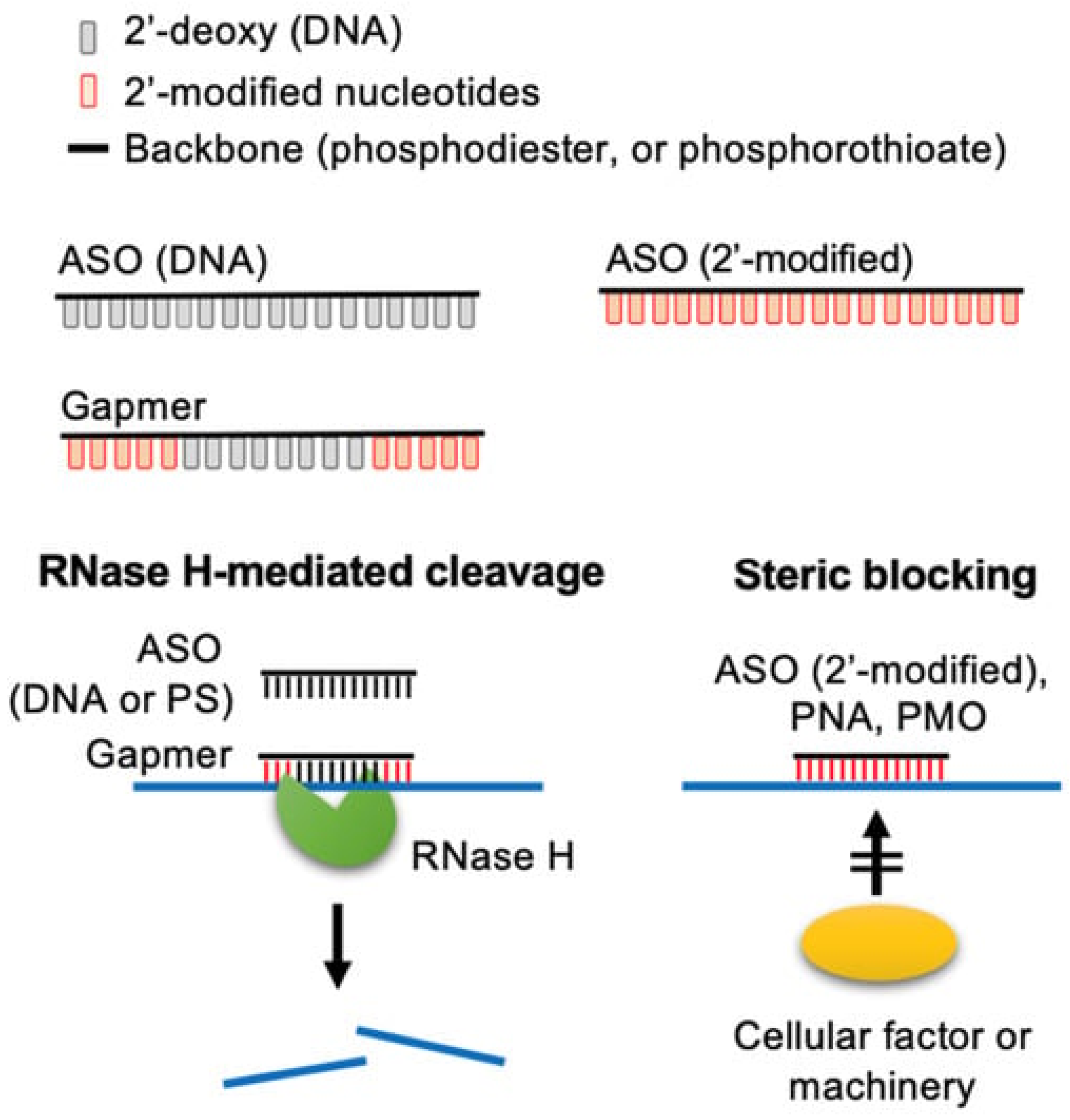
2.3. Case Study: Non-RNase H-Dependent ASOs
2.4. Conclusion and Perspective on Antisense Oligonucleotide and siRNAs Delivery
3. mRNA
3.1. Challenges Compared to Other Nucleic Acids
3.2. mRNA Delivery
3.3. Conclusion and Perspective on mRNA Delivery
4. Aptamers
4.1. Structure-Based Approach to Delivery
4.2. Delivery of Aptamers
4.3. Conclusion and Perspective on Aptamer Delivery
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| ASO | Antisense oligonucleotide |
| ASGPR | Asialoglycoprotein receptor |
| circRNA | Circular RNA |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DDS | Drug delivery system |
| DMD | Duchenne muscular dystrophy |
| DNA | Deoxyribonucleic acid |
| EMA | European Medicines Agency |
| EV | Extracellular vesicle |
| FDA | Food and Drug Administration |
| GalNac | N-acetylgalactosamine |
| LNP | Lipid nanoparticle |
| RNA | Ribonucleic acid |
| mRNA | Messenger RNA |
| miRNA | Micro-RNA |
| ORF | Open Reading Frame |
| PMO | Phosphorodiamidate morpholino-oligomer |
| PS | Phosphorothioate |
| RISC | RNA-induced silencing complex |
| saRNA | Self-amplifying RNA |
| siRNA | Small interfering RNA |
| sgRNA | Single guide RNA |
| UTR | Untranslated region |
| 2′-F | 2′-Fluororibonucleotides |
| 2′-OMe | 2′-O-methyl-ribonucleotides |
| 2′-O-MOE | 2‘-O-(2-Methoxyethyl)-ribonucleotides |
Appendix A
| Modality | Drug Names | Clinical Trial Status | Deliv. Route | Nucleic Acid Modifications | Target Site (Target) | Deliv. System | Disease Indication |
|---|---|---|---|---|---|---|---|
| Oligonucleotide | Lefitolimod/GS-1703/MGN1703 [207,208] | Ongoing | SC | Fully natural | Undisclosed (TLR9 agonist) | Undisclosed | Acquired immunodeficiency syndrome (AIDS); advanced solid tumors; extensive-stage (ES) small cell lung cancer (SCLC); HIV infection; melanoma; metastatic colorectal cancer; metastatic solid tumors; neuroendocrine tumors of pancreatic origin; peritoneal cancer; small cell lung cancer (SCLC) |
| Cobitolimod [209] | Ongoing | Rectal | PS | Undisclosed (TLR9 agonist) | Undisclosed | Severe ulcerative colitis; ulcerative colitis | |
| Imetelstat/GRN163L/Imetelstat sodium [210] | Ongoing | IV | Thiophosphoramidate (NPS), lipid conjugate (for permeability) | Undisclosed (telomerase inhibitor) | Undisclosed | Acute myeloid leukemia; adenocarcinoma of the breast; adult giant cell glioblastoma; advanced non-small cell lung cancer (NSCLC); anaplastic astrocytoma; anaplastic ependymoma; anaplastic oligodendroglioma; astrocytoma; bone marrow metastatic disease; brain cancer; brain tumor; brainstem glioma; brainstem tumor; breast adenocarcinoma; breast neoplasm; central nervous system tumors; cytopenia; diffuse intrinsic pontine glioma; ependymoma; essential thrombocythemia; Ewing’s sarcoma; glioblastoma multiforme; gliosarcoma; hepatoblastoma; HER2-positive breast cancer; high-grade glioma; liver cancer; lymphoma; lymphoproliferative disease; malignant solid tumor; metastatic breast cancer; metastatic non-small cell lung cancer; myelodysplastic syndrome; myelofibrosis; myeloid malignancies; myeloma/multiple myeloma/Kahler’s disease/myelomatosis; myeloproliferative neoplasm and myelodysplastic syndrome; neuroblastoma; non-secretory myeloma; non-small cell lung cancer (NSCLC); oligodendroglioma; osteosarcoma; peripheral primitive neuroectodermal tumor; polycythemia vera; post-essential thrombocythemia (Post-ET) myelofibrosis; post-polycythemia vera myelofibrosis; post-transplant lymphoproliferative disorders (PTLD); primary myelofibrosis; rhabdomyosarcoma; cecondary myelofibrosis; secretory multiple myeloma; small intestine lymphoma; solid tumors | |
| gRNA | Exa-cel/CTX001/Exagamglogene autotemcel [211] | Ongoing | IV | Undisclosed | Hematopietic stem cells (BCL11A) | Ex vivo—electroporation | Anemia; beta thalassemia; genetic-related diseases; hematological disease; hemoglobinopathy; sickle cell anemia; sickle cell diseases; thalassemia; transfusion-dependent β-thalassemia |
| LBP-EC01/crPhageTM [212,213] | Ongoing | Intraurethral/IV | Undisclosed | Vesical E. coli | In vivo—bacteriophage | Urinary tract infection | |
| saRNA | ARCT-154 | Ongoing | IM | Undisclosed | Undisclosed | Undisclosed | SARS-CoV-2 (COVID-19) infection |
| GRANITE-001/GRT-C901 (Prime)/GRT-R902 (Boost) [214,215] | Ongoing | IM | Undisclosed | Undisclosed (neoantigen) | ChAd68 LNP; Replicon RNA (samRNA) (adenovirus) | Colorectal cancer; colorectal neoplasms; gastroesophageal adenocarcinoma; metastatic cancer; metastatic colorectal cancer; metastatic gastroesophageal adenocarcinoma; metastatic microsatellite-stable colorectal cancer; metastatic non-small cell lung cancer; metastatic urothelial carcinoma; microsatellite-stable colorectal cancer; non-small cell lung cancer (NSCLC); solid tumors; urothelial cancer | |
| shRNA | Vigil/FANG Immunotherapy/Gemogenovatucel-T/VigilTM Vaccine [216] | Clinically active | ID | Undisclosed | Undisclosed (neoantigen) | Undisclosed | Advanced breast cancer; advanced cervical cancer; advanced endometrial cancer; advanced fallopian tube cancer; advanced gynecologic cancer; advanced melanoma; advanced non-small cell lung cancer (NSCLC); advanced ovarian cancer; advanced peritoneal cancer; advanced solid tumors; advanced uterine cancer; bone tissue neoplasm; breast cancer; cervical cancer; clear cell epithelial ovarian cancer; colorectal carcinoma; connective tissue neoplasm; endometrial cancer; endometrioid epithelial ovarian cancer; endometrioid ovarian cancer; Ewing’s sarcoma; Ewing’s tumor; fallopian tube cancer; high-grade serous ovarian cancer; large bowel (colon) cancer; liver cancer; lung cancer; melanoma; metastatic breast cancer; metastatic cervical cancer; metastatic endometrial cancer; metastatic Ewing sarcoma; metastatic Ewing’s tumor; metastatic fallopian tube cancer; metastatic melanoma; metastatic non-small cell lung cancer; metastatic ovarian cancer; metastatic peritoneal cancer; metastatic solid tumor; metastatic uterine cancer; neoplasms; non-small cell lung cancer (NSCLC); ovarian cancer; ovarian clear cell carcinoma; ovarian neoplasm; papillary ovarian cancer; peritoneal cancer; peritoneal neoplasms; primary peritoneal carcinoma; rare tumors; sarcoma; serous epithelial ovarian cancer; serous ovarian cancer; soft tissue neoplasms; solid tumors; uterine cancer; uterine papillary serous carcinoma |
References
- Kim, Y.-K. RNA Therapy: Rich History, Various Applications and Unlimited Future Prospects. Exp. Mol. Med. 2022, 54, 455–465. [Google Scholar] [CrossRef]
- Newey, S. ‘No Limits’ to Possibilities of MRNA, Says Mastermind behind Technology. The Telegraph, 29 December 2022. [Google Scholar]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lopez-Berestein, G.; Calin, G.A.; Sood, A.K. RNAi Therapies: Drugging the Undruggable. Sci. Transl. Med. 2014, 6, 240ps7. [Google Scholar] [CrossRef] [PubMed]
- Warner, K.D.; Hajdin, C.E.; Weeks, K.M. Principles for Targeting RNA with Drug-like Small Molecules. Nat. Rev. Drug Discov. 2018, 17, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Rodon, J. Taking Aim at the Undruggable. In American Society of Clinical Oncology Educational Book; ASCO Publications: Alexandria, VA, USA, 2021; pp. e145–e152. [Google Scholar] [CrossRef]
- Bejar, N.; Tat, T.T.; Kiss, D.L. RNA Therapeutics: The Next Generation of Drugs for Cardiovascular Diseases. Curr. Atheroscler. Rep. 2022, 24, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.G. Gene Transfer Approaches for Cardiovascular Disease. In Molecular Basis of Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2001; pp. 195–216. ISBN 978-0-7216-9428-3. [Google Scholar]
- Kumar, M.; DeVaux, R.S.; Herschkowitz, J.I. Chapter Thirteen-Molecular and Cellular Changes in Breast Cancer and New Roles of LncRNAs in Breast Cancer Initiation and Progression. In Progress in Molecular Biology and Translational Science; Pruitt, K., Ed.; Molecular and Cellular Changes in the Cancer Cell; Academic Press: Cambridge, MA, USA, 2016; Volume 144, pp. 563–586. [Google Scholar]
- Foti, R.S. 1.28-ADME of Biologicals and New Therapeutic Modalities. In Comprehensive Pharmacology; Kenakin, T., Ed.; Elsevier: Oxford, UK, 2022; pp. 716–742. ISBN 978-0-12-820876-2. [Google Scholar]
- DeVos, S.L.; Miller, T.M. Antisense Oligonucleotides: Treating Neurodegeneration at the Level of RNA. Neurotherapeutics 2013, 10, 486–497. [Google Scholar] [CrossRef]
- Kurreck, J. SiRNA Efficiency: Structure or Sequence—That Is the Question. J. Biomed. Biotechnol. 2006, 2006, 83757. [Google Scholar] [CrossRef]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.A.; Awidi, A. SiRNA: Mechanism of Action, Challenges, and Therapeutic Approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Natural and Artificially-Engineered Nucleic Acid Aptamers: Design, Characterization and Technological Applications|Frontiers Research Topic. Available online: https://www.frontiersin.org/research-topics/4117/natural-and-artificially-engineered-nucleic-acid-aptamers-design-characterization-and-technological (accessed on 13 January 2023).
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Ismail, S.; Fattal, E. Functionalizing Liposomes with Anti-CD44 Aptamer for Selective Targeting of Cancer Cells. Bioconjugate Chem. 2015, 26, 1307–1313. [Google Scholar] [CrossRef]
- Cell Biology by the Numbers. Which Is Bigger, MRNA or the Protein It Codes For? Available online: http://book.bionumbers.org/which-is-bigger-mrna-or-the-protein-it-codes-for/ (accessed on 13 January 2023).
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. MRNA Structure Regulates Protein Expression through Changes in Functional Half-Life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef] [PubMed]
- Feher, J. 2.2-DNA and Protein Synthesis. In Quantitative Human Physiology, 2nd ed.; Feher, J., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 120–129. ISBN 978-0-12-800883-6. [Google Scholar]
- Chen, R.; Wang, S.K.; Belk, J.A.; Amaya, L.; Li, Z.; Cardenas, A.; Abe, B.T.; Chen, C.-K.; Wender, P.A.; Chang, H.Y. Engineering Circular RNA for Enhanced Protein Production. Nat. Biotechnol. 2022, 41, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 MRNA Vaccine Development. Sig. Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Drug Discovery Perspectives of Antisense Oligonucleotides. Biomol. Ther 2023, 31, 241–252. [Google Scholar] [CrossRef]
- Mandal, A. RNA Discovery. Available online: https://www.news-medical.net/life-sciences/RNA-Discovery.aspx (accessed on 22 April 2025).
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic Liposome-Mediated RNA Transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef]
- Agrawal, G.; Hermann, R.; Møller, M.; Poetes, R.; Steinmann, M. Fast-Forward: Will the Speed of COVID-19 Vaccine Development Reset Industry Norms?|McKinsey. Available online: https://www.mckinsey.com/industries/life-sciences/our-insights/fast-forward-will-the-speed-of-covid-19-vaccine-development-reset-industry-norms (accessed on 22 April 2025).
- Padda, I.S.; Mahtani, A.U.; Patel, P.; Parmar, M. Small Interfering RNA (SiRNA) Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jones, C.H.; Androsavich, J.R.; So, N.; Jenkins, M.P.; MacCormack, D.; Prigodich, A.; Welch, V.; True, J.M.; Dolsten, M. Breaking the Mold with RNA—A “RNAissance” of Life Science. npj Genom. Med. 2024, 9, 2. [Google Scholar] [CrossRef]
- Kleinman, M.E.; Yamada, K.; Takeda, A.; Chandrasekaran, V.; Nozaki, M.; Baffi, J.Z.; Albuquerque, R.J.C.; Yamasaki, S.; Itaya, M.; Pan, Y.; et al. Sequence- and Target-Independent Angiogenesis Suppression by SiRNA via TLR3. Nature 2008, 452, 591–597. [Google Scholar] [CrossRef]
- Welch, A.R. Defining the RNA Therapeutics Industry in 2023. Available online: https://www.cellandgenecollaborative.com/doc/defining-the-rna-therapeutics-industry-in-0002 (accessed on 22 April 2025).
- Dolgin, E. The Tangled History of MRNA Vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic SiRNA: State of the Art. Sig. Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. SiRNA Versus MiRNA as Therapeutics for Gene Silencing. Mol. Ther.—Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Tarn, W.-Y.; Cheng, Y.; Ko, S.-H.; Huang, L.-M. Antisense Oligonucleotide-Based Therapy of Viral Infections. Pharmaceutics 2021, 13, 2015. [Google Scholar] [CrossRef]
- Majeed, C.N.; Ma, C.D.; Xiao, T.; Rudnick, S.; Bonkovsky, H.L. Spotlight on Givosiran as a Treatment Option for Adults with Acute Hepatic Porphyria: Design, Development, and Place in Therapy. Drug Des. Dev. Ther. 2022, 16, 1827–1845. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X. The Growth of SiRNA-Based Therapeutics: Updated Clinical Studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef] [PubMed]
- New Drug Approvals. Lumasiran. Available online: https://newdrugapprovals.org/2020/12/15/lumasiran/ (accessed on 5 April 2023).
- DailyMed. AMVUTTRA-Vutrisiran Injection. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8db0facb-81b6-4006-9239-27dc6409c5d3 (accessed on 14 April 2023).
- Alnylam Learn More About AMVUTTRA® (Vutrisiran). Available online: https://www.amvuttra.com/node (accessed on 5 April 2023).
- EMA. Amvuttra. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/amvuttra (accessed on 5 April 2023).
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, Biodistribution and Cell Uptake of Antisense Oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of MRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; McEver, R.P. C-Type Lectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; ISBN 978-0-87969-770-9. [Google Scholar]
- Sliedregt, L.A.J.M.; Rensen, P.C.N.; Rump, E.T.; van Santbrink, P.J.; Bijsterbosch, M.K.; Valentijn, A.R.P.M.; van der Marel, G.A.; van Boom, J.H.; van Berkel, T.J.C.; Biessen, E.A.L. Design and Synthesis of Novel Amphiphilic Dendritic Galactosides for Selective Targeting of Liposomes to the Hepatic Asialoglycoprotein Receptor. J. Med. Chem. 1999, 42, 609–618. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming Cellular Barriers for RNA Therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Key for Effective Application of a New Revolutionary Cholesterol Lowering Drug Resides in a 30 Year Old Leiden Patent-Leiden University. Available online: https://www.staff.universiteitleiden.nl/news/2021/03/key-for-effective-application-of-a-new-revolutionary-cholesterol-lowering-drug-resides-in-a-30-year-old-leiden-patent (accessed on 10 June 2025).
- Debacker, A.J.; Voutila, J.; Catley, M.; Blakey, D.; Habib, N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 2020, 28, 1759–1771. [Google Scholar] [CrossRef]
- Biessen, E.A.; Vietsch, H.; Rump, E.T.; Fluiter, K.; Kuiper, J.; Bijsterbosch, M.K.; van Berkel, T.J. Targeted Delivery of Oligodeoxynucleotides to Parenchymal Liver Cells in Vivo. Biochem. J. 1999, 340, 783–792. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted Delivery of RNAi Therapeutics with Endogenous and Exogenous Ligand-Based Mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Endosomal Escape of RNA Therapeutics: How Do We Solve This Rate-Limiting Problem? RNA 2023, 29, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.D.; Dowdy, S.F. GalNAc-SiRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef] [PubMed]
- jpbd.nihs JAN: Japanese Accepted Names for Pharmaceuticals-Tofersen. Available online: https://jpdb.nihs.go.jp/jan/DetailList_en?submit=all_alp%20Search&keyword=Tofersen (accessed on 11 April 2023).
- Clinicaltrials.gov. An Open-Label Extension Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of RO7234292 (ISIS 443139) in Huntington’s Disease Patients Who Participated in Prior Investigational Studies of RO7234292 (ISIS 443139). Available online: https://clinicaltrials.gov/ct2/show/NCT03342053 (accessed on 9 April 2023).
- Ionis. Explore Our Pipeline-Zilganersen. Available online: https://ionis.com/science-and-innovation/pipeline (accessed on 11 April 2023).
- Russell, S.R.; Drack, A.V.; Cideciyan, A.V.; Jacobson, S.G.; Leroy, B.P.; Van Cauwenbergh, C.; Ho, A.C.; Dumitrescu, A.V.; Han, I.C.; Martin, M.; et al. Intravitreal Antisense Oligonucleotide Sepofarsen in Leber Congenital Amaurosis Type 10: A Phase 1b/2 Trial. Nat. Med. 2022, 28, 1014–1021. [Google Scholar] [CrossRef]
- Xiong, H.; Veedu, R.N.; Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22, 3295. [Google Scholar] [CrossRef]
- Flanigan, K.M.; Voit, T.; Rosales, X.Q.; Servais, L.; Kraus, J.E.; Wardell, C.; Morgan, A.; Dorricott, S.; Nakielny, J.; Quarcoo, N.; et al. Pharmacokinetics and Safety of Single Doses of Drisapersen in Non-Ambulant Subjects with Duchenne Muscular Dystrophy: Results of a Double-Blind Randomized Clinical Trial. Neuromuscul. Disord. 2014, 24, 16–24. [Google Scholar] [CrossRef]
- McDonald, C.M.; Wong, B.; Flanigan, K.M.; Wilson, R.; de Kimpe, S.; Lourbakos, A.; Lin, Z.; Campion, G.; The DEMAND V Study Group. Placebo-Controlled Phase 2 Trial of Drisapersen for Duchenne Muscular Dystrophy. Ann. Clin. Transl. Neurol. 2018, 5, 913–926. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A First-in-Human Study to Evaluate the Safety and Tolerability of QR-421a in Subjects with Retinitis Pigmentosa (RP) Due to Mutations in Exon 13 of the USH2A Gene. Available online: https://clinicaltrials.gov/ct2/show/NCT03780257 (accessed on 9 April 2023).
- MCE. Ultevursen (QR-421a)|Usherin Synthesis Promotor|MedChemExpress. Available online: https://www.medchemexpress.com/ultevursen.html (accessed on 23 April 2025).
- Yuen, M.-F.; Heo, J.; Jang, J.-W.; Yoon, J.-H.; Kweon, Y.-O.; Park, S.-J.; Tami, Y.; You, S.; Yates, P.; Tao, Y.; et al. Safety, Tolerability and Antiviral Activity of the Antisense Oligonucleotide Bepirovirsen in Patients with Chronic Hepatitis B: A Phase 2 Randomized Controlled Trial. Nat. Med. 2021, 27, 1725–1734. [Google Scholar] [CrossRef]
- Monteleone, G.; Neurath, M.F.; Ardizzone, S.; Di Sabatino, A.; Fantini, M.C.; Castiglione, F.; Scribano, M.L.; Armuzzi, A.; Caprioli, F.; Sturniolo, G.C.; et al. Mongersen, an Oral SMAD7 Antisense Oligonucleotide, and Crohn’s Disease. N. Engl. J. Med. 2015, 372, 1104–1113. [Google Scholar] [CrossRef]
- Fijen, L.M.; Riedl, M.A.; Bordone, L.; Bernstein, J.A.; Raasch, J.; Tachdjian, R.; Craig, T.; Lumry, W.R.; Manning, M.E.; Alexander, V.J.; et al. Inhibition of Prekallikrein for Hereditary Angioedema. N. Engl. J. Med. 2022, 386, 1026–1033. [Google Scholar] [CrossRef]
- Longhurst, H.J.; Ameratunga, R. An Antisense Oligonucleotide for Hereditary Angioedema. N. Engl. J. Med. 2022, 386, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Ando, Y.; Benson, M.D.; Berk, J.L.; Waddington-Cruz, M.; Dyck, P.J.; Gillmore, J.D.; Khella, S.L.; Litchy, W.J.; Obici, L.; et al. Design and Rationale of the Global Phase 3 NEURO-TTRansform Study of Antisense Oligonucleotide AKCEA-TTR-LRx (ION-682884-CS3) in Hereditary Transthyretin-Mediated Amyloid Polyneuropathy. Neurol. Ther. 2021, 10, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Diep, J.K.; Yu, R.Z.; Viney, N.J.; Schneider, E.; Guo, S.; Henry, S.; Monia, B.; Geary, R.; Wang, Y. Population Pharmacokinetic/Pharmacodynamic Modelling of Eplontersen, an Antisense Oligonucleotide in Development for Transthyretin Amyloidosis. Br. J. Clin. Pharmacol. 2022, 88, 5389–5398. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pizarro, A.; Desviat, L.R. RNA Solutions to Treat Inborn Errors of Metabolism. Mol. Genet. Metab. 2022, 136, 289–295. [Google Scholar] [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Laskin, J.J.; Nicholas, G.; Lee, C.; Gitlitz, B.; Vincent, M.; Cormier, Y.; Stephenson, J.; Ung, Y.; Sanborn, R.; Pressnail, B.; et al. Phase I/II Trial of Custirsen (OGX-011), an Inhibitor of Clusterin, in Combination with a Gemcitabine and Platinum Regimen in Patients with Previously Untreated Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 579–586. [Google Scholar] [CrossRef]
- Sosnowska, B.; Surma, S.; Banach, M. Targeted Treatment against Lipoprotein (a): The Coming Breakthrough in Lipid Lowering Therapy. Pharmaceuticals 2022, 15, 1573. [Google Scholar] [CrossRef]
- Wexler, M. Wave Life Sciences Discontinues Development of Suvodirsen for DMD. Available online: https://musculardystrophynews.com/news/wave-life-sciences-discontinues-suvodirsen-development-for-dmd/ (accessed on 11 April 2023).
- Clinicaltrials.gov. Prospective, Randomised, Placebo-Controlled, Double-Masked, Three-Armed Multi-Centre Trial of Aganirsen Versus Vehicle in Patients After Ischaemic Central Retinal Vein Occlusion with a High Risk to Develop Neovascular Glaucoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02947867 (accessed on 10 April 2023).
- Sharma, V.K.; Sharma, R.K.; Singh, S.K. Antisense Oligonucleotides: Modifications and Clinical Trials. Med. Chem. Commun. 2014, 5, 1454–1471. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Sitaraman, S. Alicaforsen. Isis Pharmaceuticals. Curr. Opin. Investig. Drugs 2001, 2, 1401–1406. [Google Scholar]
- Moumné, L.; Marie, A.-C.; Crouvezier, N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics 2022, 14, 260. [Google Scholar] [CrossRef]
- Olezarsen. Available online: https://web.archive.org/web/20240224163536/https://www.ionispharma.com/medicines/akcea-apociii-l/ (accessed on 24 February 2024).
- Prakash, T.P.; Seth, P.P.; Swayze, E.E.; Graham, M.J. Compositions and Methods for Modulating Apolipoprotein C-Iii Expression. Singapore Patent SG11201508800WA, 27 November 2015. [Google Scholar]
- Chi, X.; Gatti, P.; Papoian, T. Safety of Antisense Oligonucleotide and SiRNA-Based Therapeutics. Drug Discov. Today 2017, 22, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, M.; Corteville, D.; Szabo, G.; Swaminathan, M.; Lamy, A.; Lehner, L.J.; Brown, C.D.; Noiseux, N.; Atta, M.G.; Squiers, E.C.; et al. Teprasiran, a Small Interfering RNA, for the Prevention of Acute Kidney Injury in High-Risk Patients Undergoing Cardiac Surgery. Circulation 2021, 144, 1133–1144. [Google Scholar] [CrossRef]
- Moreno-Montañés, J.; Bleau, A.-M.; Jimenez, A.I. Tivanisiran, a Novel SiRNA for the Treatment of Dry Eye Disease. Expert Opin. Investig. Drugs 2018, 27, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhao, J.; Shah, M.; Migliorati, J.M.; Tawfik, S.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X. Nedosiran, a Candidate SiRNA Drug for the Treatment of Primary Hyperoxaluria: Design, Development, and Clinical Studies. ACS Pharmacol. Transl. Sci. 2022, 5, 1007–1016. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, X.; Li, S.; Wang, P.; Fang, J. Liver-Targeted Delivery of Oligonucleotides with N-Acetylgalactosamine Conjugation. ACS Omega 2021, 6, 16259–16265. [Google Scholar] [CrossRef] [PubMed]
- Gareri, C.; Polimeni, A.; Giordano, S.; Tammè, L.; Curcio, A.; Indolfi, C. Antisense Oligonucleotides and Small Interfering RNA for the Treatment of Dyslipidemias. J. Clin. Med. 2022, 11, 3884. [Google Scholar] [CrossRef]
- Alnylam. Alnylam Reports Positive Topline Results from Phase 2 Study of Investigational Cemdisiran for the Treatment of IgA Nephropathy. Available online: https://investors.alnylam.com/press-release?id=26771 (accessed on 23 April 2025).
- Badri, P.; Jiang, X.; Borodovsky, A.; Najafian, N.; Kim, J.; Clausen, V.A.; Goel, V.; Habtemariam, B.; Robbie, G.J. Pharmacokinetic and Pharmacodynamic Properties of Cemdisiran, an RNAi Therapeutic Targeting Complement Component 5, in Healthy Subjects and Patients with Paroxysmal Nocturnal Hemoglobinuria. Clin. Pharmacokinet. 2021, 60, 365–378. [Google Scholar] [CrossRef]
- Maraganore, J. Reflections on Alnylam. Nat. Biotechnol. 2022, 40, 641–650. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Gore, K.R. Advances in SiRNA Therapeutics and Synergistic Effect on SiRNA Activity Using Emerging Dual Ribose Modifications. RNA Biol. 2022, 19, 452–467. [Google Scholar] [CrossRef]
- NBDF. Sanofi Provides Fitusiran Trial Updates at ISTH. Available online: https://www.hemophilia.org/news/sanofi-provides-fitusiran-trial-updates-at-isth (accessed on 23 April 2025).
- Park, J.; Park, J.; Pei, Y.; Xu, J.; Yeo, Y. Pharmacokinetics and Biodistribution of Recently-Developed SiRNA Nanomedicines. Adv. Drug Deliv. Rev. 2016, 104, 93–109. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS Infection and Immune Privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Bevivino, G.; Monteleone, G. Mongersen, an Oral Smad7 Antisense Oligonucleotide, in Patients with Active Crohn’s Disease. Ther. Adv. Gastroenterol. 2016, 9, 527–532. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal PH of the Human Gastrointestinal Tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar] [PubMed]
- Wang, B. An ASO Modification That Enhances Nuclease Resistance, Lowers Toxicity, and Increases Binding Affinity. Available online: https://eu.idtdna.com/pages/education/decoded/article/an-aso-modification-that-enhances-nuclease-resistance-lowers-toxicity-and-increases-binding-affinity (accessed on 22 April 2025).
- Pollak, A.J.; Zhao, L.; Vickers, T.A.; Huggins, I.J.; Liang, X.-H.; Crooke, S.T. Insights into Innate Immune Activation via PS-ASO–Protein–TLR9 Interactions. Nucleic Acids Res. 2022, 50, 8107–8126. [Google Scholar] [CrossRef]
- Mansoor, M.; Melendez, A. Advances in Antisense Oligonucleotide Development for Target Identification, Validation, and as Novel Therapeutics. Gene Regul. Syst. Biol. 2008, 2, 275–295. [Google Scholar] [CrossRef]
- Alharbi, A.S.; Garcin, A.J.; Lennox, K.A.; Pradeloux, S.; Wong, C.; Straub, S.; Valentin, R.; Pépin, G.; Li, H.-M.; Nold, M.F.; et al. Rational Design of Antisense Oligonucleotides Modulating the Activity of TLR7/8 Agonists. Nucleic Acids Res. 2020, 48, 7052–7065. [Google Scholar] [CrossRef]
- Lim, K.H.; Han, Z.; Jeon, H.Y.; Kach, J.; Jing, E.; Weyn-Vanhentenryck, S.; Downs, M.; Corrionero, A.; Oh, R.; Scharner, J.; et al. Antisense Oligonucleotide Modulation of Non-Productive Alternative Splicing Upregulates Gene Expression. Nat. Commun. 2020, 11, 3501. [Google Scholar] [CrossRef]
- Bege, M.; Borbás, A. The Medicinal Chemistry of Artificial Nucleic Acids and Therapeutic Oligonucleotides. Pharmaceuticals 2022, 15, 909. [Google Scholar] [CrossRef]
- Shukla, S.; Sumaria, C.S.; Pradeepkumar, P.I. Exploring Chemical Modifications for SiRNA Therapeutics: A Structural and Functional Outlook. ChemMedChem 2010, 5, 328–349. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Kato, S.; Yeseom Cho, K.; Tiwari, R.K. Overcoming Barriers for SiRNA Therapeutics: From Bench to Bedside. Pharmaceuticals 2020, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Dowler, T.; Bergeron, D.; Tedeschi, A.-L.; Paquet, L.; Ferrari, N.; Damha, M.J. Improvements in SiRNA Properties Mediated by 2′-Deoxy-2′-Fluoro-β- d -Arabinonucleic Acid (FANA). Nucleic Acids Res. 2006, 34, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Chae, S.U.; Lee, C.B.; Bae, S.K. Clinical Pharmacokinetics of Approved RNA Therapeutics. Int. J. Mol. Sci. 2023, 24, 746. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; Moufawad El Achkar, C.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef]
- DailyMed. VILTEPSO- Viltolarsen Injection, Solution. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1ffff9a8-6d6a-4dcb-8493-1b6cc3a5d123 (accessed on 5 April 2023).
- Crooke, S.; Baker, B.; Xia, S.; Yu, R.; Viney, N.; Wang, Y.; Tsimikas, S.; Geary, R. Integrated Assessment of the Clinical Performance of GalNAc 3 -Conjugated 2′-O-Methoxyethyl Chimeric Antisense Oligonucleotides: I. Human Volunteer Experience. Nucleic Acid Ther. 2018, 29, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X. Cellular Uptake and Trafficking of Antisense Oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Nan, Y.; Zhang, Y.-J. Antisense Phosphorodiamidate Morpholino Oligomers as Novel Antiviral Compounds. Front. Microbiol. 2018, 9, 750. [Google Scholar] [CrossRef]
- Shokrzadeh, N.; Winkler, A.-M.; Dirin, M.; Winkler, J. Oligonucleotides Conjugated with Short Chemically Defined Polyethylene Glycol Chains Are Efficient Antisense Agents. Bioorg. Med. Chem. Lett. 2014, 24, 5758–5761. [Google Scholar] [CrossRef]
- Hagiwara, K.; Kurihara, K.; Honma, M.; Yamamoto, J.; Shinohara, F. PEG-Modification on the Endo-Position of an Antisense Oligonucleotide Increases Tumor Accumulation via the EPR Effect. J. Biomater. Sci. Polym. Ed. 2018, 29, 448–459. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nagasaki, Y. Impacts of PEGylation on the Gene and Oligonucleotide Delivery System. J. Appl. Polym. Sci. 2014, 131, 40293. [Google Scholar] [CrossRef]
- Li, C.; Callahan, A.J.; Simon, M.D.; Totaro, K.A.; Mijalis, A.J.; Phadke, K.-S.; Zhang, G.; Hartrampf, N.; Schissel, C.K.; Zhou, M.; et al. Fully Automated Fast-Flow Synthesis of Antisense Phosphorodiamidate Morpholino Oligomers. Nat. Commun. 2021, 12, 4396. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Allerson, C.R.; Dande, P.; Vickers, T.A.; Sioufi, N.; Jarres, R.; Baker, B.F.; Swayze, E.E.; Griffey, R.H.; Bhat, B. Positional Effect of Chemical Modifications on Short Interference RNA Activity in Mammalian Cells. J. Med. Chem. 2005, 48, 4247–4253. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Ma, Y.; Liang, Z.; Yang, Z.; Cao, H. Site-Specific Modification Using the 2′-Methoxyethyl Group Improves the Specificity and Activity of SiRNAs. Mol. Ther. Nucleic Acids 2017, 9, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Minikel, E.V.; Pulst, S.M. Antisense Oligonucleotides. Neurol. Genet. 2019, 5, e323. [Google Scholar] [CrossRef] [PubMed]
- Drolet, D.W.; Green, L.S.; Gold, L.; Janjic, N. Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 2016, 26, 127–146. [Google Scholar] [CrossRef]
- Zhou, R.; Caspi, R.R. Ocular Immune Privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef]
- Shah, S.; Gnanasegaran, G.; Sundberg-Cohon, J.; Buscombe, J. The Heart: Anatomy, Physiology and Exercise Physiology. In Integrating Cardiology for Nuclear Medicine Physicians: A Guide to Nuclear Medicine Physicians; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–22. ISBN 978-3-540-78673-3. [Google Scholar]
- Shadid, M.; Badawi, M.; Abulrob, A. Antisense Oligonucleotides: Absorption, Distribution, Metabolism, and Excretion. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1281–1292. [Google Scholar] [CrossRef]
- MDA. FDA Issues Response to BioMarin’s DMD Treatment. Available online: https://www.mda.org/press-releases/fda-issues-response-biomarin%E2%80%99s-dmd-treatment (accessed on 17 March 2023).
- WLS. Wave Life Sciences Announces Discontinuation of Suvodirsen Development for Duchenne Muscular Dystrophy-Wave Life Sciences. Available online: https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-announces-discontinuation-suvodirsen (accessed on 22 April 2025).
- Sheng, L.; Rigo, F.; Bennett, C.F.; Krainer, A.R.; Hua, Y. Comparison of the Efficacy of MOE and PMO Modifications of Systemic Antisense Oligonucleotides in a Severe SMA Mouse Model. Nucleic Acids Res. 2020, 48, 2853–2865. [Google Scholar] [CrossRef]
- EMA. Exondys. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/exondys (accessed on 22 April 2025).
- Markati, T.; De Waele, L.; Schara-Schmidt, U.; Servais, L. Lessons Learned from Discontinued Clinical Developments in Duchenne Muscular Dystrophy. Front. Pharmacol. 2021, 12, 735912. [Google Scholar] [CrossRef]
- Sands, B.E.; Feagan, B.G.; Sandborn, W.J.; Schreiber, S.; Peyrin-Biroulet, L.; Frédéric Colombel, J.; Rossiter, G.; Usiskin, K.; Ather, S.; Zhan, X.; et al. Mongersen (GED-0301) for Active Crohn’s Disease: Results of a Phase 3 Study. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, 738. [Google Scholar] [CrossRef]
- Karaki, S.; Paris, C.; Rocchi, P.; Karaki, S.; Paris, C.; Rocchi, P. Antisense Oligonucleotides, A Novel Developing Targeting Therapy; IntechOpen: London, UK, 2019; ISBN 978-1-78984-533-4. [Google Scholar]
- Salahpour Anarjan, F. Active Targeting Drug Delivery Nanocarriers: Ligands. Nano Struct. Nano Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- Attia, M.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T. An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, Active and Endogenous Organ-Targeted Lipid and Polymer Nanoparticles for Delivery of Genetic Drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 MRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Thaplyal, P.; Bevilacqua, P.C. Experimental Approaches for Measuring PKa’s in RNA and DNA. Methods Enzym. 2014, 549, 189–219. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. MRNA-Based Therapeutics—Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D. What MRNA Is Good For, And What It Maybe Isn’t. Available online: https://www.science.org/content/blog-post/what-mrna-good-and-what-it-maybe-isn-t (accessed on 22 April 2025).
- Elkhalifa, D.; Rayan, M.; Negmeldin, A.T.; Elhissi, A.; Khalil, A. Chemically Modified MRNA beyond COVID-19: Potential Preventive and Therapeutic Applications for Targeting Chronic Diseases. Biomed. Pharmacother. 2022, 145, 112385. [Google Scholar] [CrossRef]
- Hoyer, S.; Gerer, K.F.; Pfeiffer, I.A.; Prommersberger, S.; Höfflin, S.; Jaitly, T.; Beltrame, L.; Cavalieri, D.; Schuler, G.; Vera, J.; et al. Electroporated Antigen-Encoding MRNA Is Not a Danger Signal to Human Mature Monocyte-Derived Dendritic Cells. J. Immunol. Res. 2015, 2015, 952184. [Google Scholar] [CrossRef]
- Cella, M.; Salio, M.; Sakakibara, Y.; Langen, H.; Julkunen, I.; Lanzavecchia, A. Maturation, Activation, and Protection of Dendritic Cells Induced by Double-Stranded RNA. J. Exp. Med. 1999, 189, 821–829. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, L.; Liu, T. Development and Delivery Systems of MRNA Vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar] [CrossRef]
- Kawaguchi, D.; Kodama, A.; Abe, N.; Takebuchi, K.; Hashiya, F.; Tomoike, F.; Nakamoto, K.; Kimura, Y.; Shimizu, Y.; Abe, H. Phosphorothioate Modification of MRNA Accelerates the Rate of Translation Initiation to Provide More Efficient Protein Synthesis. Angew. Chem. 2020, 132, 17556–17560. [Google Scholar] [CrossRef]
- Keedy, H.E.; Thomas, E.N.; Zaher, H.S. Decoding on the Ribosome Depends on the Structure of the MRNA Phosphodiester Backbone. Proc. Natl. Acad. Sci. USA 2018, 115, E6731–E6740. [Google Scholar] [CrossRef] [PubMed]
- GSRS. ELASOMERAN. Available online: https://gsrs.ncats.nih.gov/ginas/app/beta/substances/EPK39PL4R4 (accessed on 23 April 2025).
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of MRNA Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Morais, P.; Adachi, H.; Yu, Y.-T. The Critical Contribution of Pseudouridine to MRNA COVID-19 Vaccines. Front. Cell Dev. Biol. 2021, 9, 789427. [Google Scholar] [CrossRef]
- Marks, P.; Sudhakar, A.; Joseph, K.; Mark, C.; Rachel, Z.; Ross, P.; Ye, Y.; Alena, D.; Keith, P.; Li, S.; et al. Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum; FDA: Silver Spring, MD, USA, 2022. [Google Scholar]
- Alsoussi, W.B.; Malladi, S.K.; Zhou, J.Q.; Liu, Z.; Ying, B.; Kim, W.; Schmitz, A.J.; Lei, T.; Horvath, S.C.; Sturtz, A.J.; et al. SARS-CoV-2 Omicron Boosting Induces de Novo B Cell Response in Humans. bioRxiv 2022, bioRxiv:2022.09.22.509040. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Phase 2/3 Study to Evaluate the Immunogenicity and Safety of MRNA Vaccine Boosters for SARS-CoV-2 Variants. Available online: https://clinicaltrials.gov/ct2/show/NCT04927065 (accessed on 10 April 2023).
- Tan, S.; Zhao, J.; Hu, X.; Li, Y.; Wu, Z.; Lu, G.; Yu, Z.; Du, B.; Liu, Y.; Li, L.; et al. Preclinical Evaluation of RQ3013, a Broad-Spectrum MRNA Vaccine against SARS-CoV-2 Variants. Sci. Bull. 2023, 68, 3192–3206. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Phase I Clinical Trial to Evaluate the Safety, Tolerability and Preliminary Immunogenicity of SARS-CoV-2 MRNA Vaccine (LVRNA009) in Chinese People Aged 18 Years and Over. Available online: https://clinicaltrials.gov/ct2/show/NCT05364047 (accessed on 10 April 2023).
- CAN Newswire. Phase I Clinical Trial of AIM MRNA COVID-19 Vaccine (LVRNA009) Won the Praise of the Industry for High Safety and Well Tolerance. Available online: https://www.acnnewswire.com/press-release/english/72367/phase-i-clinical-trial-of-aim-mrna-covid-19-vaccine-(lvrna009)-won-the-praise-of-the-industry-for-high-safety-and-well-tolerance (accessed on 23 April 2025).
- Li, J.; Liu, Q.; Liu, J.; Fang, Z.; Luo, L.; Li, S.; Lei, Y.; Li, Z.; Jin, J.; Xie, R.; et al. Development of Bivalent MRNA Vaccines against SARS-CoV-2 Variants. Vaccines 2022, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic Vaccination against Autologous Cancer Stem Cells with MRNA-Transfected Dendritic Cells in Patients with Glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef]
- Pathak, Y. Gene Delivery Systems: Development and Applications; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-058031-0. [Google Scholar]
- Clinicaltrials.gov. A Randomized, Double-Blind, Placebo-Controlled Clinical Study to Evaluate the Efficacy, Safety, and Immunogenicity of SARS-CoV-2 Variant (BA.4/5) MRNA Vaccine (ABO1020) in Healthy Subjects Aged 18 Years and Older Who Have Completed the Full Vaccination. Available online: https://clinicaltrials.gov/ct2/show/NCT05636319 (accessed on 10 April 2023).
- Figlin, R.A.; Tannir, N.M.; Uzzo, R.G.; Tykodi, S.S.; Chen, D.Y.T.; Master, V.; Kapoor, A.; Vaena, D.; Lowrance, W.; Bratslavsky, G.; et al. Results of the ADAPT Phase 3 Study of Rocapuldencel-T in Combination with Sunitinib as First-Line Therapy in Patients with Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2020, 26, 2327–2336. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Phase 1/2 Randomized Study to Evaluate the Safety, Tolerability, and Immunogenicity of a Modified RNA Vaccine against Influenza in Healthy Individuals. Available online: https://clinicaltrials.gov/ct2/show/NCT05052697 (accessed on 10 April 2023).
- BioNTech. Technologies for Customized Treatment Approaches. Available online: https://www.biontech.com/int/en/home/pipeline-and-products/pipeline.html (accessed on 11 April 2023).
- Pharmaceutical Technology. BNT-161 by BioNTech for Influenzavirus B Infections: Likelihood of Approval. Available online: https://www.pharmaceutical-technology.com/data-insights/bnt-161-biontech-influenzavirus-b-infections-likelihood-of-approval/ (accessed on 11 April 2023).
- Clinicaltrials.gov. A Phase 3, Randomized, Observer-Blinded Study to Evaluate the Efficacy, Safety, Tolerability, and Immunogenicity of a Modified RNA Vaccine against Influenza Compared to Licensed Inactivated Influenza Vaccine in Healthy Adults 18 Years of Age or Older. Available online: https://clinicaltrials.gov/ct2/show/study/NCT05540522 (accessed on 10 April 2023).
- Branche, A.R.; Rouphael, N.G.; Diemert, D.J.; Falsey, A.R.; Losada, C.; Baden, L.R.; Frey, S.E.; Whitaker, J.A.; Little, S.J.; Anderson, E.J.; et al. SARS-CoV-2 Variant Vaccine Boosters Trial: Preliminary Analyses. medRxiv 2022. preprint. [Google Scholar]
- EMC. Spikevax Bivalent-Summary of Product Characteristics (SmPC)-(Emc). Available online: https://web.archive.org/web/20231205084548/https://www.medicines.org.uk/emc/product/13983/smpc#gref (accessed on 5 December 2023).
- Walvax. Products List. Available online: https://en.walvax.com/products/products-list (accessed on 11 April 2023).
- Qiu, X.; Xu, S.; Lu, Y.; Luo, Z.; Yan, Y.; Wang, C.; Ji, J. Development of MRNA Vaccines against Respiratory Syncytial Virus (RSV). Cytokine Growth Factor Rev. 2022, 68, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, N.; Vale, N.; Mihic, A.; Amor, T.; Reiter, L.; Arita, Y.; Samson, R.; Hu, Q.; Gingras, A.-C.; Sorenson, B.T.; et al. Phase I Randomized, Observer-Blinded, Placebo-Controlled Study of a SARS-CoV-2 MRNA Vaccine PTX-COVID19-B. Sci. Rep. 2023, 13, 8557. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Deng, Y.; Huang, B.; Huang, L.; Lin, A.; Li, Y.; Wang, W.; Liu, J.; Lu, S.; Zhan, Z.; et al. A Core-Shell Structured COVID-19 MRNA Vaccine with Favorable Biodistribution Pattern and Promising Immunity. Signal Transduct. Target. Ther. 2021, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Technology. COVID-19 Vaccination Tracker: Daily Rates, Statistics & Updates. Available online: https://www.pharmaceutical-technology.com/covid-19-vaccination-tracker/ (accessed on 23 April 2025).
- Clinicaltrials.gov. A Multi-National, Randomized, Observer-Blinded, Parallel Controlled Trial to Evaluate the Immunogenicity and Safety of New Generation COVID-19 MRNA Booster Vaccination Against Emerging Variants of Concern (VOC). Available online: https://clinicaltrials.gov/ct2/show/NCT05580159 (accessed on 10 April 2023).
- Clinicaltrials.gov. A Randomized, Single-Blind, Parallel Controlled Trial to Evaluate the Immunogenicity, Safety, Efficacy of A Heterologous Booster Dose with SW-BIC-213, in Previously Vaccinated Subjects Against COVID-19 with Two Inactivated COVID-19 Vaccine Doses Compared to a Booster Dose with Pfizer of Sinopharmin Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT05686161 (accessed on 10 April 2023).
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. MRNA-Based SARS-CoV-2 Vaccine Candidate CVnCoV Induces High Levels of Virus-Neutralising Antibodies and Mediates Protection in Rodents. npj Vaccines 2021, 6, 57. [Google Scholar] [CrossRef]
- Xu, K.; Lei, W.; Kang, B.; Yang, H.; Wang, Y.; Lu, Y.; Lv, L.; Sun, Y.; Zhang, J.; Wang, X.; et al. A Novel MRNA Vaccine, SYS6006, against SARS-CoV-2. Front. Immunol. 2023, 13, 1051576. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Randomized, Observer-Blinded, Placebo-Controlled Phase 2 Clinical Study to Evaluate the Immunogenicity and Safety of a SARS-CoV-2 MRNA Vaccine (SYS6006) in Healthy Participants Aged 18 Years or More. Available online: https://clinicaltrials.gov/ct2/show/NCT05439824 (accessed on 10 April 2023).
- Zuckerman, J.N. The Importance of Injecting Vaccines into Muscle. BMJ 2000, 321, 1237–1238. [Google Scholar] [CrossRef]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified MRNA in LNPs Constitutes a Competitive Technology for Prophylactic Vaccines. npj Vaccines 2017, 2, 29. [Google Scholar] [CrossRef]
- Lowe, D. MRNA Vaccines: What Happens. Available online: https://www.science.org/content/blog-post/mrna-vaccines-what-happens (accessed on 28 February 2023).
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid Nanoparticles Enhance the Efficacy of MRNA and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Yang, L.; Gong, L.; Wang, P.; Zhao, X.; Zhao, F.; Zhang, Z.; Li, Y.; Huang, W. Recent Advances in Lipid Nanoparticles for Delivery of MRNA. Pharmaceutics 2022, 14, 2682. [Google Scholar] [CrossRef]
- EMA. Ends Rolling Review of CVnCoV COVID-19 Vaccine Following Withdrawal by CureVac AG|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/news/ema-ends-rolling-review-cvncov-covid-19-vaccine-following-withdrawal-curevac-ag (accessed on 17 June 2025).
- Lenart, K.; Hellgren, F.; Ols, S.; Yan, X.; Cagigi, A.; Cerveira, R.A.; Winge, I.; Hanczak, J.; Mueller, S.O.; Jasny, E.; et al. A Third Dose of the Unmodified COVID-19 MRNA Vaccine CVnCoV Enhances Quality and Quantity of Immune Responses. Mol. Ther. Methods Clin. Dev. 2022, 27, 309–323. [Google Scholar] [CrossRef]
- Pharmaceutical Technology. Mapping the RNA Therapeutics R&D Landscape in 2022. Available online: https://www.pharmaceutical-technology.com/features/mapping-the-rna-therapeutics-rd-landscape-in-2022/ (accessed on 3 March 2023).
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The Promise of MRNA Vaccines: A Biotech and Industrial Perspective. npj Vaccines 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Buyanova, M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjugate Chem. 2019, 30, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Vavilis, T.; Stamoula, E.; Ainatzoglou, A.; Sachinidis, A.; Lamprinou, M.; Dardalas, I.; Vizirianakis, I.S. MRNA in the Context of Protein Replacement Therapy. Pharmaceutics 2023, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, R.N.; Crist, R.M.; Stern, S.T. The Future of Tissue-Targeted Lipid Nanoparticle-Mediated Nucleic Acid Delivery. Pharmaceuticals 2022, 15, 897. [Google Scholar] [CrossRef]
- Cui, S.; Wang, Y.; Gong, Y.; Lin, X.; Zhao, Y.; Zhi, D.; Zhou, Q.; Zhang, S. Correlation of the Cytotoxic Effects of Cationic Lipids with Their Headgroups. Toxicol. Res. 2018, 7, 473–479. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.O.; Rowntree, L.C.; et al. Anti-PEG Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle MRNA Vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar] [CrossRef]
- Ju, Y.; Carreño, J.M.; Simon, V.; Dawson, K.; Krammer, F.; Kent, S.J. Impact of Anti-PEG Antibodies Induced by SARS-CoV-2 MRNA Vaccines. Nat. Rev. Immunol. 2023, 23, 135–136. [Google Scholar] [CrossRef]
- Bathula, N.V.; Friesen, J.J.; Casmil, I.C.; Wayne, C.J.; Liao, S.; Soriano, S.K.V.; Ho, C.H.; Strumpel, A.; Blakney, A.K. Delivery Vehicle and Route of Administration Influences Self-Amplifying RNA Biodistribution, Expression Kinetics, and Reactogenicity. J. Control. Release 2024, 374, 28–38. [Google Scholar] [CrossRef]
- Gómez-Aguado, I.; Rodríguez-Castejón, J.; Vicente-Pascual, M.; Rodríguez-Gascón, A.; Solinís, M.Á.; del Pozo-Rodríguez, A. Nanomedicines to Deliver MRNA: State of the Art and Future Perspectives. Nanomaterials 2020, 10, 364. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide Aptamers: New Tools for Targeted Cancer Therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zheng, C.; Zhou, S.-F.; Qiao, S.; Tran, P.H.-L.; Pu, C.; Li, Y.; Kong, L.; Kouzani, A.Z.; Lin, J.; et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics 2015, 5, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-Based Biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef]
- DailyMed. DEFITELIO-Defibrotide Sodium Injection, Solution. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c3db989-d7ad-41ed-9ebf-698dcf6c24ec (accessed on 14 April 2023).
- Ozer, I.; Pitoc, G.A.; Layzer, J.M.; Moreno, A.; Olson, L.B.; Layzer, K.D.; Hucknall, A.M.; Sullenger, B.A.; Chilkoti, A. PEG-Like Brush Polymer Conjugate of RNA Aptamer That Shows Reversible Anticoagulant Activity and Minimal Immune Response. Adv. Mater. 2022, 34, 2107852. [Google Scholar] [CrossRef]
- Takahashi, M.; Han, S.-p.; Scherer, L.J.; Yoon, S.; Rossi, J.J. 6.12-Current Progress and Future Prospects in Nucleic Acid Based Therapeutics. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Oxford, UK, 2017; pp. 280–313. ISBN 978-0-12-803201-5. [Google Scholar]
- Maier, K.E.; Levy, M. From Selection Hits to Clinical Leads: Progress in Aptamer Discovery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16014. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Phase 3 Multicenter, Randomized, Double Masked, Sham- Controlled Clinical Trial to Assess the Safety and Efficacy of Intravitreal Administration of Zimura (Complement C5 Inhibitor) in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT04435366 (accessed on 10 April 2023).
- Ni, S.; Yao, H.; Wang, L.; Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Chemical Modifications of Nucleic Acid Aptamers for Therapeutic Purposes. Int. J. Mol. Sci. 2017, 18, 1683. [Google Scholar] [CrossRef]
- PubChem. Pegaptanib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/56603655 (accessed on 22 April 2025).
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three Decades of Nucleic Acid Aptamer Technologies: Lessons Learned, Progress and Opportunities on Aptamer Development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- Floege, J.; Ostendorf, T.; Janssen, U.; Burg, M.; Radeke, H.H.; Vargeese, C.; Gill, S.C.; Green, L.S.; Janjic, N. Novel Approach to Specific Growth Factor Inhibition in Vivo. Am. J. Pathol. 1999, 154, 169–179. [Google Scholar] [CrossRef]
- Pescador, R.; Porta, R.; Ferro, L. An Integrated View of the Activities of Defibrotide. Semin. Thromb. Hemost. 1996, 22 (Suppl. 1), 71–75. [Google Scholar]
- Dimitrova, D.G.; Teysset, L.; Carré, C. RNA 2′-O-Methylation (Nm) Modification in Human Diseases. Genes 2019, 10, 117. [Google Scholar] [CrossRef]
- Boo, S.H.; Kim, Y.K. The Emerging Role of RNA Modifications in the Regulation of MRNA Stability. Exp. Mol. Med. 2020, 52, 400–408. [Google Scholar] [CrossRef] [PubMed]
- EMA. Defitelio. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/defitelio (accessed on 22 April 2025).
- Kovacevic, K.D.; Gilbert, J.C.; Jilma, B. Pharmacokinetics, Pharmacodynamics and Safety of Aptamers. Adv. Drug Deliv. Rev. 2018, 134, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Rossi, J.J. Aptamers: Uptake Mechanisms and Intracellular Applications. Adv. Drug Deliv. Rev. 2018, 134, 22–35. [Google Scholar] [CrossRef]
- Wittig, B.; Schmidt, M.; Scheithauer, W.; Schmoll, H.-J. MGN1703, an Immunomodulator and Toll-like Receptor 9 (TLR-9) Agonist: From Bench to Bedside. Crit. Rev. Oncol. Hematol. 2015, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Combining a TLR9 Agonist with Broadly Neutralizing Antibodies for Reservoir Reduction and Immunological Control of HIV Infection: An Investigator-Initiated Randomized, Placebo-Controlled, Phase IIa Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT03837756 (accessed on 10 April 2023).
- New Drug Approvals. COBITOLIMOD. Available online: https://newdrugapprovals.org/2022/06/08/cobitolimod/ (accessed on 22 April 2025).
- Mascarenhas, J.; Harrison, C.N.; Kiladjian, J.-J.; Komrokji, R.S.; Koschmieder, S.; Vannucchi, A.M.; Berry, T.; Redding, D.; Sherman, L.; Dougherty, S.; et al. Imetelstat in Intermediate-2 or High-Risk Myelofibrosis Refractory to JAK Inhibitor: IMpactMF Phase III Study Design. Future Oncol. 2022, 18, 2393–2402. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Jacobsen, R.K. Interview: World’s-First Clinical Trial for a CRISPR-Cas3 Phage Therapy. Interview: Joseph Nixon, Locus Biosciences. Available online: https://crisprmedicinenews.com/news/worlds-first-clinical-trial-for-a-crispr-cas3-phage-therapy-interview-joseph-nixon-locus-bioscie/ (accessed on 22 April 2025).
- Clinicaltrials.gov. A Phase 2/3, Double-Blind, Randomized, Active-Controlled Evaluation of the Safety, Tolerability, Pharmacokinetics and Efficacy of LBP-EC01 in the Treatment of Acute Uncomplicated Urinary Tract Infection Caused by Multidrug Resistant E. Coli. Available online: https://clinicaltrials.gov/ct2/show/NCT05488340 (accessed on 10 April 2023).
- Gritstone. Our Pipeline. Available online: https://web.archive.org/web/20241210154107/https://gritstonebio.com/our-pipeline/ (accessed on 10 December 2024).
- Clinicaltrials.gov. Gritstone Begins Treatment in GRANITE-001’s Phase I/II Trial. Available online: https://www.clinicaltrialsarena.com/news/gritstone-granite-001-trial/ (accessed on 22 April 2025).
- Rocconi, R.P.; Grosen, E.A.; Ghamande, S.A.; Chan, J.K.; Barve, M.A.; Oh, J.; Tewari, D.; Morris, P.C.; Stevens, E.E.; Bottsford-Miller, J.N.; et al. Gemogenovatucel-T (Vigil) Immunotherapy as Maintenance in Frontline Stage III/IV Ovarian Cancer (VITAL): A Randomised, Double-Blind, Placebo-Controlled, Phase 2b Trial. Lancet Oncol. 2020, 21, 1661–1672. [Google Scholar] [CrossRef]
| INN Name | Approval(s) | Target Site (Target) | Disease | Posology | Delivery Route | Delivery System | Conjugation/Termination |
|---|---|---|---|---|---|---|---|
| Casimersen (Amondys 45) | FDA 2021 | Muscle fibers (DMD exon 45) | Duchenne muscular dystrophy (DMD) | 30 mg/kg q.w. | Intravenous (IV) | Naked in PBS | Short PEG tail |
| Eteplirsen (Exondys 51) | FDA 2016 | Muscle fibers (DMD exon 51) | Duchenne muscular dystrophy | 30 mg/kg q.w. | IV | Naked in ~PBS | Short PEG tail |
| Golodirsen (Vyondys 53) | FDA 2019 | Muscle fibers (DMD exon 53) | Duchenne muscular dystrophy | 30 mg/kg q.w. | IV | Naked in PBS | Short PEG tail |
| Milasen117 | FDA 2017 | SNC (CLN7) | Batten’s disease (patient-customized) | 42 mg quarterly after induction phase | Intrathecal (ITh) | Undisclosed | NA |
| Nusinersen (Spinraza) | FDA 2016 EMA 2017 | SNC (SMN2 pre-mRNA) | Spinal muscular atrophy | 12 mg every four months after induction phase | ITh | Naked in ~PBS | NA |
| Viltolarsen118 (Viltepso) | FDA 2020 | Muscle fibers (DMD exon 53) | Duchenne muscular dystrophy | 80 mg/kg q.w. | IV | Naked in pH-adjusted saline | NA |
| Fomivirsen (Vitravene) | FDA 1998 EMA 1999 | Eye (CMV mRNA) | Cytomegalovirus retinitis | 0.165/0.330 mg/eye q.2w after induction phase | Intravitreal (IVt) | Naked in bicarbonate buffer, NaCl | NA |
| Inotersen (Tegsedi) | FDA 2018 EMA 2018 | Liver (TTR mRNA) | Hereditary transthyretin mediated amyloidosis | 285 mg q.w. | Sub- cutaneous (SC) | Naked in pH-adjusted water | NA |
| Mipomersen (Kynamro) | FDA 2013 | Liver/Intestine (apo-B-100 mRNA) | Familial hypercholesterolemia | 200 mg q.w. | SC | Naked in pH-adjusted water | NA |
| Volanesorsen (Waylivra) | EMA 2019 | Liver/Intestine (apoC-III mRNA) | Familial chylomicronemia | 285 mg q.2w after induction phase | SC | Naked in pH-adjusted water | NA |
| INN Name | Approval(s) | Target Site (Target) | Disease | Posology | Delivery Route | Delivery System | Conjugation/Termination |
|---|---|---|---|---|---|---|---|
| Givosiran (Givlaari) [35] | FDA 2019 EMA 2020 | Liver/hepato-cytes (ALS1 mRNA) | Acute hepatic porphyria | 2.5 mg/kg q.m. | SC | Naked in pH-adjusted water | GalNAc-conjugate |
| Inclisiran (Leqvio) [32,36] | EMA 2020 | Liver/hepatocytes (PCSK9 mRNA) | Primary hypercholesterolemia | 284 mg every six months after induction phase | SC | Naked in pH-adjusted water | GalNAc-conjugate |
| Lumasiran (Oxlumo) [37] | FDA 2020 EMA 2020 | Liver/hepatocytes (HAO1 mRNA) | Primary hyperoxaluria | 3 to 6 mg/kg quarterly after induction phase | SC | Naked in pH-adjusted water | GalNAc-conjugate |
| Vutrisiran (Amvuttra) [38,39,40] | FDA 2022 EMA 2022 | Liver/hepato-cytes (TTR mRNA) | hATTR amyloidosis | 25 mg quarterly | SC | Naked in phosphate buffer + NaCl | GalNAc-conjugate |
| Patisiran (Onpattro) | FDA 2018 EMA 2018 | Liver (TTR mRNA) | hATTR amyloidosis | 0.3 mg/kg every three weeks | IV | Lipid nanoparticles (LNPs) | NA |
| Drug Names | Clinical Trial Status | Target Site (Target) | Disease Indication | Deliv. Route | Deliv. System | Conjugation/Termination |
|---|---|---|---|---|---|---|
| Tofersen */BIIB067/IONIS-SOD1Rx/ISIS 333611/BIIB067/IONIS-SOD1Rx/ISIS 333611 [52] | Ongoing | CNS (SOD1 mRNA) | Amyotrophic lateral sclerosis (ALS); superoxide dismutase 1-amyotropic lateral sclerosis (SOD1-ALS) | ITh | Naked RNA | NA |
| Tominersen/IONIS-HTTRx/ISIS 443139/RG6042/RO7234292 [52,53] | Ongoing | CNS (HTT mRNA) | Huntington’s disease | ITh | Naked RNA | NA |
| Zilganersen/ION-1166998/ION373 [54] | Ongoing | CNS (GFAP mRNA) | Alexander disease | ITh | Undisclosed | NA |
| Sepofarsen/ QR-110 [55] | Ongoing | Eye (CEP290 mRNA) | Blindness; congenital eye disorders; eye disorders; Leber congenital amaurosis (LCA); Leber congenital amaurosis 10 (LCA10); neurological disorders; retinal degeneration; retinal disease; retinal dystrophy; sensation disorders; vision disorders | Intraocular (IO) | Naked RNA | NA |
| OT-101/AP 12009/AP-12009/ATB-301/TASO-001/TGF-β2 targeting anti-sense oligonucleotide/Trabedersen [56] | Ongoing | Tumor (TGF-B2 mRNA) | Advanced malignant pleural mesothelioma; advanced solid tumors; cancer indications; colorectal cancer; diffuse intrinsic pontine glioma; glioma; melanoma; metastatic solid tumors; pancreatic cancer; pleural malignant mesothelioma; progressive myopia; SARS-CoV-2 (COVID-19) infection; solid tumors | Intratumoral (IT) | Undisclosed | NA |
| Drisapersen/GSK2402968/Kyndrisa/PRO051 [57,58] | Stopped | Muscles (DMD exon 51) | Duchenne muscular dystrophy (DMD); muscular dystrophies | SC | Undisclosed | NA |
| Ultevursen/QR-421a [59,60] | Ongoing | Eye (USH2A exon 13) | Blindness; congenital eye disorders; deafness; eye disorders; hereditary conditions; retinal disease; retinitis pigmentosa; Usher syndrome type 2; Usher syndrome type 2a; vision disorders | IO | Undisclosed | NA |
| Bepirovirsen/GSK3228836/IONIS-HBVRx/ISIS 505358/ISIS-GSK3Rx/ISIS-HBVRx [61] | Ongoing | Liver (HBsAg mRNA) | Hepatic impairment; hepatitis B; liver cirrhosis; liver disorders | SC | Undisclosed | NA |
| Mongersen/GED-0301 [62] | Ongoing | GI epithelium (SMAD7 mRNA) | Crohn’s disease; ulcerative colitis | Oral | Delayed release tablet | NA |
| IONIS-PKK-LRx/Donidalorsen/ISIS 721744 [63,64] | Ongoing | Liver (KLKB1 mRNA) | Cardiovascular diseases; hereditary angioedema; SARS-CoV-2 (COVID-19) infection | SC | Undisclosed | Ligand-conjugated antisense (LICA) (GalNac) |
| Eplontersen */AKCEA-TTR-LRx/ION-682884/IONIS-TTR-LRx/ISIS-TTR(Rx) [65,66] | Ongoing | Liver (TTR mRNA) | Amyloidosis; familial amyloid polyneuropathy (hereditary transthyretin-mediated amyloid polyneuropathy); transthyretin amyloid cardiomyopathy | SC | Undisclosed | Ligand-conjugated antisense (LICA) (GalNac) |
| ION363/Jacifusen [67] | Ongoing | CNS (FUS mRNA) | Amyotrophic lateral sclerosis (ALS) | ITh | AAV (Adeno Associated Virus) | NA |
| Custirsen/OGX-011/TV-1011 [68,69] | Not active (clinical) | Tumor (sCLU mRNA) | Adenocarcinoma of the prostate; advanced non-small cell lung cancer (NSCLC); cancer indications; cardiac conduction and repolarization; castrate prostate cancer; metastatic castrate prostate cancer; metastatic non-small cell lung cancer; prostate cancer; solid tumors | IV | Undisclosed | NA |
| Pelacarsen/AKCEA-APO(a)-LRx/ASO 144367/IONIS-APO(a)-LRx/ISIS 681257/TQJ230 [70] | Ongoing | Liver (LPA mRNA) | Acute coronary syndrome; cardiovascular diseases; coronary artery disease (coronary heart disease); elevated serum lipoprotein(a); genetic polymorphisms; hepatic impairment; hyperlipoproteinemia (hyperlipidemia, lipoproteinemia); inflammation; ischemic stroke; liver cirrhosis; myocardial infarction; peripheral arterial disease (PAD); renal impairment; stenosis | SC | Undisclosed | Ligand-Conjugated Antisense (LICA) (GalNac) |
| Suvodirsen/WVE-210201 [71] | Stopped | Muscles (DMD mRNA) | Duchenne muscular dystrophy (DMD) | IV | Undisclosed | NA |
| Aganirsen/GS-101 [72,73] | Ongoing | Eye (IRS1 mRNA) | Ischemic central retinal vein occlusion; neovascular glaucoma | Eye, Topical | Naked RNA | NA |
| Alicaforsen/ISIS-2302 [74] | Ongoing | Colon (undisclosed) | Inflammatory bowel disease; pouchitis; ulcerative colitis | Rectal | Naked RNA | NA |
| Olezarsen */AKCEA-APOCIII-LRx/IONIS APOCIII-LRx/ISIS 678354 [75,76,77] | Ongoing | Liver (APOC3 mRNA) | Atherosclerosis; atherosclerotic cardiovascular disease (ASCVD); cardiovascular diseases; coronary artery disease (coronary heart disease); elevated triglycerides; familial chylomicronemia syndrome (FCS); familial lipoprotein lipase deficiency (hyperlipoproteinemia type I); hypertriglyceridemia; ischemic stroke; pancreatitis; peripheral arterial disease (PAD) | SC | Undisclosed | Ligand-conjugated antisense (LICA) (GalNac) |
| Drug Names | Clinical Trial Status | Target Site (Target) | Disease Indication | Deliv. Route | Deliv. System | Conjugation/Termination |
|---|---|---|---|---|---|---|
| Revusiran/ALN-TTRsc/SAR438714/siTTRsc [78] | Stopped | Liver (TTR mRNA) | Amyloidosis; cardiac amyloidosis; familial amyloid polyneuropathy (hereditary transthyretin-mediated amyloid polyneuropathy); hereditary ATTR amyloidosis | SC | Undisclosed | GalNAc |
| Teprasiran/I5NP/QPI-1002 [79] | Ongoing | Kidney (p53 mRNA) | Acute kidney injury (AKI); renal failure | IV | Undisclosed | NA |
| Tivanisiran/SYL1001 [80] | Ongoing | Eye (TRPV1 mRNA) | Dry eye disease; ocular Pain; Sjögren’s syndrome | Eye, topical | Naked RNA | NA |
| Nedosiran */DCR-PHXC [81] | Ongoing | Liver (LDHA mRNA) | Genetic-related diseases; kidney disorders; primary hyperoxaluria; primary hyperoxaluria type 1; primary hyperoxaluria type 2; primary hyperoxaluria type 3; renal disease | SC | Undisclosed | GalNAc |
| Olpasiran/AMG 890/ARO-LPA [82,83] | Ongoing | Liver (LPA mRNA) | Atherosclerotic cardiovascular disease (ASCVD); cardiovascular diseases; elevated serum lipoprotein(a); hepatic impairment; myocardial infarction; renal impairment | SC | Undisclosed | Targeted RNAi Molecule (TRiMTM) platform (GalNac) |
| ARO-APOC3 [32] | Ongoing | Liver (APOC3 mRNA) | Dyslipidemia; familial chylomicronemia syndrome (FCS); hypertriglyceridemia | SC | Undisclosed | Targeted RNAi Molecule (TRiMTM) platform |
| Fazirsiran/ADS-001/ARO-AAT/TAK-999 [32,82] | Ongoing | Liver (SERPINA1 mRNA) | Alpha-1 antitrypsin deficiency; liver fibrosis; PiZZ alpha-1 antitrypsin deficiency (ZZ type alpha-1 antitrypsin deficiency) | SC | Undisclosed | Targeted RNAi Molecule (TRiMTM) platform (GalNac) |
| Cemdisiran/ALN-CC5 [84,85,86] | Ongoing | Liver (C5 mRNA) | Anemia; atypical hemolytic uremic syndrome (aHUS); immunoglobulin A nephropathy (Berger disease); myasthenia gravis; paroxysmal nocturnal hemoglobinuria (PNH); renal impairment; thrombocytopenia; tuberculous meningitis | SC | Undisclosed | GalNAc |
| QPI-1007 [32,87] | Ongoing | Eye (CASP2 mRNA) | Glaucoma; non-arteritic anterior ischemic optic neuropathy (NAION) | IVt | Naked RNA | NA |
| Fitusiran */ALN-AT3SC/SAR439774 [87,88] | Ongoing | Liver (antithrombin III mRNA) | Hemophilia; hemophilia A; hemophilia B | SC | Undisclosed | GalNAc |
| INN Name | Approv. | Target Site (Target) | Disease | Posology | Deliv. Route | Backbone Mod. | 2′ Mod. | 5′/3′ Mod. |
|---|---|---|---|---|---|---|---|---|
| Casimersen (Amondys 45) | FDA 2021 | Muscle fibers (DMD Exon 45) | Duchenne muscular dystrophy | 30 mg/kg q.w. | IV | PMO | NA | Short PEG tail |
| Eteplirsen (Exondys 51) | FDA 2016 | Muscle fibers (DMD Exon 51) | Duchenne muscular dystrophy | 30 mg/kg q.w. | IV | PMO | NA | Short PEG tail |
| Golodirsen (Vyondys 53) | FDA 2019 | Muscle fibers (DMD Exon 53) | Duchenne muscular dystrophy | 30 mg/kg q.w. | IV | PMO | NA | Short PEG tail |
| Milasen [104] | FDA 2017 | SNC (CLN7) | Batten’s disease (patient-customized) | 42 mg quarterly after induction phase | ITh | PS | 2′-O-MOE | NA |
| Nusinersen (Spinraza) | FDA 2016 EMA 2017 | SNC (SMN2 pre-mRNA) | Spinal muscular atrophy | 12 mg every four months after an induction phase | ITh | PS | 2′-O-MOE | NA |
| Viltolarsen (Viltepso) [105] | FDA 2020 | Muscle fibers (DMD Exon 53) | Duchenne muscular dystrophy | 80 mg/kg q.w. | IV | PMO | NA | NA |
| Fomivirsen (Vitravene) | FDA 1998 EMA 1999 | Eye (CMV mRNA) | Cytomegalovirus retinitis | 0.165/0.330 mg/eye q.2w after induction phase | IVt | PS | NA | NA |
| Inotersen (Tegsedi) | FDA 2018 EMA 2018 | Liver (TTR mRNA) | Hereditary transthyretin-mediated amyloidosis | 285 mg q.w. | SC | PS | 2′-O-MOE | NA |
| Mipomersen (Kynamro) | FDA 2013 | Liver/intestine (apo-B-100 mRNA) | Familial hypercholesterolemia | 200 mg q.w. | SC | PS | 2′-O-MOE | NA |
| Volanesorsen (Waylivra) | EMA 2019 | Liver/intestine (apoC-III mRNA) | Familial chylomicronemia | 285 mg q.2w after induction phase | SC | PS | 2′-O-MOE | NA |
| INN Name | Approv. | Target Site (Target) | Disease | Posology | Deliv. Route | Backbone Mod. | 2′ MOD. | 5′/3′ Mod. |
|---|---|---|---|---|---|---|---|---|
| Givosiran (Givlaari) [35] | FDA 2019 EMA 2020 | Liver/hepatocytes (ALS1 mRNA) | Acute hepatic porphyria | 2.5 mg/kg q.m. | SC | PS | 2′-F, 2′-Ome | NA |
| Inclisiran (Leqvio) [32,36] | EMA 2020 | Liver/hepatocytes (PCSK9 mRNA) | Primary hypercholesterolemia | 284 mg every six months after induction phase | SC | PS | 2′-F, 2′-Ome | NA |
| Lumasiran (Oxlumo) [37] | FDA 2020 EMA 2020 | Liver/hepatocytes (HAO1 mRNA) | Primary hyperoxaluria | 3 to 6 mg/kg quarterly after induction phase | SC | PS | 2′-F, 2′-Ome | NA |
| Vutrisiran (Amvuttra) [38,39,40] | FDA 2022 EMA 2022 | Liver/hepatocytes (TTR mRNA) | hATTR amyloidosis | 25 mg quarterly | SC | PS | 2′-F, 2′-Ome | NA |
| Patisiran (Onpattro) | FDA 2018 EMA 2018 | Liver (TTR mRNA) | hATTR amyloidosis | 0.3 mg/kg every three weeks | IV | NA | 2′-Ome | NA |
| INN Name | Clinical Trial Status | Target Site (Target) | Disease | Deliv. Route | Backbone Mod. | 2′ Mod. | 5′/3′ Mod. |
|---|---|---|---|---|---|---|---|
| Tofersen */BIIB067/IONIS-SOD1Rx/ISIS 333611/BIIB067/IONIS-SOD1Rx/ISIS 333611 [52] | Ongoing | CNS (SOD1) | Amyotrophic lateral sclerosis (ALS); superoxide dismutase 1-amyotropic lateral sclerosis (SOD1-ALS) | ITh | PS | 2′-O-MOE | NA |
| Tominersen/IONIS-HTTRx/ISIS 443139/RG6042/RO7234292 [52,53] | Ongoing | CNS (HTT) | Huntington’s disease | ITh | PS | 2′-O-MOE | NA |
| Zilganersen/ION-1166998/ION373 [54] | Ongoing | CNS (GFAP) | Alexander disease | ITh | PS | 2′-O-MOE | NA |
| Sepofarsen/QR-110 [55] | Ongoing | Eye (CEP290) | Blindness; congenital eye disorders; eye disorders; Leber congenital amaurosis (LCA); Leber congenital amaurosis 10 (LCA10); neurological disorders; retinal degeneration; retinal disease; retinal dystrophy; sensation disorders; vision disorders | IO | PS | 2′-O-Me | NA |
| OT-101/AP 12009/AP-12009/ATB-301/TASO-001/TGF-β2 targeting anti-sense oligonucleotide/Trabedersen [56] | Ongoing | Tumor (TGF-B2) | Advanced malignant pleural mesothelioma; advanced solid tumors; cancer indications; colorectal cancer; diffuse intrinsic pontine glioma; glioma; melanoma; metastatic solid tumors; pancreatic cancer; pleural malignant mesothelioma; progressive myopia; SARS-CoV-2 (COVID-19) infection; solid tumors | IT | PS | NA | NA |
| Drisapersen/GSK2402968/Kyndrisa/PRO051 [57,58] | Stopped | Muscles (DMD exon 51) | Duchenne muscular dystrophy (DMD); muscular dystrophies | SC | PS | 2′-O-MOE | NA |
| Ultevursen/QR-421a [59,60] | Ongoing | Eye (USH2A exon 13) | Blindness; congenital eye disorders; deafness; eye disorders; hereditary conditions; retinal disease; retinitis pigmentosa; Usher syndrome type 2; Usher syndrome type 2a; vision disorders | IO | PS | 2′-O-MOE | NA |
| Bepirovirsen/GSK3228836/IONIS-HBVRx/ISIS 505358/ISIS-GSK3Rx/ISIS-HBVRx [61] | Ongoing | Liver (HBsAg) | Hepatic impairment; hepatitis B; liver cirrhosis; liver disorders | SC | PS | 2′-O-MOE | NA |
| Mongersen/GED-0301 [62] | Ongoing | GI epithelial cells (SMAD7) | Crohn’s disease; ulcerative colitis | Oral | PS | NA | NA |
| IONIS-PKK-LRx/Donidalorsen/ISIS 721744 [63,64] | Ongoing | Liver (KLKB1) | Cardiovascular diseases; hereditary angioedema; SARS-CoV-2 (COVID-19) infection | SC | PS | 2′-O-MOE | NA |
| Eplontersen */AKCEA-TTR-LRx/ION-682884/IONIS-TTR-LRx/ISIS-TTR(Rx) [65,66] | Ongoing | Liver (TTR mRNA) | Amyloidosis; familial amyloid polyneuropathy (hereditary transthyretin-mediated amyloid polyneuropathy); transthyretin amyloid cardiomyopathy | SC | PS | 2′-O-MOE | NA |
| ION363/Jacifusen [67] | Ongoing | CNS (FUS) | Amyotrophic lateral sclerosis (ALS) | ITh | PS | 2′-O-MOE | NA |
| Custirsen/OGX-011/TV-1011 [68,69] | Stopped | Tumor (sCLU) | Adenocarcinoma of the prostate; advanced non-small cell lung cancer (NSCLC); cancer indications; cardiac conduction and repolarization; castrate prostate cancer; metastatic castrate prostate cancer; metastatic non-small cell lung cancer; prostate cancer; solid tumors | IV | PS | 2′-O-MOE | NA |
| Pelacarsen/AKCEA-APO(a)-LRx/ASO 144367/IONIS-APO(a)-LRx/ISIS 681257/TQJ230 [70] | Ongoing | Liver (LPA) | Acute coronary syndrome; cardiovascular diseases; coronary artery disease (coronary heart disease); elevated serum lipoprotein(a); genetic polymorphisms; hepatic impairment; hyperlipoproteinemia (hyperlipidemia, lipoproteinemia); inflammation; ischemic stroke; liver cirrhosis; myocardial infarction; peripheral arterial disease (PAD); renal impairment; stenosis | SC | PS | 2′-O-MOE | NA |
| Suvodirsen/WVE-210201 [71] | Stopped | Muscles (DMD) | Duchenne muscular dystrophy | IV | PS | 2′-O-Me, 2′-F | NA |
| Aganirsen/GS-101 [72,73] | Ongoing | Eye (IRS1) | Ischemic central retinal vein occlusion; neovascular glaucoma | Eye, topical | PS | NA | NA |
| Alicaforsen/ISIS-2302 [74] | Ongoing | Colon (undisclosed) | Inflammatory bowel disease; pouchitis; ulcerative colitis | Rectal | PS | NA | NA |
| Olezarsen */AKCEA-APOCIII-LRx/IONIS APOCIII-LRx/ISIS 678354 [75,76,77] | Ongoing | Liver (APOC3) | Atherosclerosis; atherosclerotic cardiovascular disease (ASCVD); cardiovascular diseases; coronary artery disease (coronary heart disease); elevated triglycerides; familial chylomicronemia syndrome (FCS); familial lipoprotein lipase deficiency (hyperlipoproteinemia type I); hypertriglyceridemia; ischemic stroke; pancreatitis; peripheral arterial disease (PAD) | SC | PS | 2′-O-MOE | NA |
| INN Name | Clinical Trial Status | Target Site (Target) | Disease | Deliv. Route | Backbone Mod. | 2′ Mod. | 5′/3′ Mod. |
|---|---|---|---|---|---|---|---|
| Tivanisiran/SYL1001 [80] | Ongoing | Eye (TRPV1 mRNA) | Dry eye disease; ocular pain; Sjögren’s syndrome | Eye, topical | NA | NA | NA |
| Nedosiran */DCR-PHXC [81] | Ongoing | Hepatocytes (Liver) (LDHA mRNA) | Genetic-related diseases; kidney disorders; primary hyperoxaluria; primary hyperoxaluria type 1; primary hyperoxaluria type 2; primary hyperoxaluria type 3; renal disease | SC | PS | 2′-O-Me, 2′-F | NA |
| Olpasiran/AMG 890/ARO-LPA [82,83] | Ongoing | Hepatocytes (Liver) (LPA mRNA) | Atherosclerotic cardiovascular disease (ASCVD); cardiovascular diseases; elevated serum lipoprotein(a); hepatic impairment; myocardial infarction; renal Impairment | SC | PS | 2′-F, 2′-O-Me | NA |
| ARO-APOC3 [32] | Ongoing | Liver (APOC3 mRNA) | Dyslipidemia; familial chylomicronemia syndrome (FCS); hypertriglyceridemia | SC | PS | PS, 2′-OMe, 2′-F | Inverted base |
| Fazirsiran/ADS-001/ARO-AAT/TAK-999 [32,82] | Ongoing | Liver (SERPINA1 mRNA) | Alpha-1 antitrypsin deficiency; liver fibrosis; PiZZ alpha-1 antitrypsin deficiency (ZZ type alpha-1 antitrypsin deficiency) | SC | PS | PS, 2′-OMe, 2′-F, inverted base | Inverted base |
| Cemdisiran/ALN-CC5 [84,85,86] | Ongoing | Liver (C5 mRNA) | Anemia; atypical hemolytic uremic syndrome (aHUS); immunoglobulin A nephropathy (Berger disease); myasthenia gravis; paroxysmal nocturnal hemoglobinuria (PNH); renal impairment; thrombocytopenia; tuberculous meningitis | SC | PS | 2′-O-Me, 2′-F, PS | NA |
| QPI-1007 [32,87] | Ongoing | Eye (CASP2 mRNA) | Glaucoma; non-arteritic anterior ischemic optic neuropathy (NAION) | IVt | NA | 2′-O-Me, | 5′ inverted deoxybasic sense |
| Fitusiran */ALN-AT3SC/SAR439774 [87,88] | Ongoing | Liver (AT III mRNA)) | Hemophilia; hemophilia A; hemophilia B | SC | PS | 2′-O-Me, 2′-F | NA |
| INN Name | Approv. | Target Site | Indication | Posology | Deliv. Route | Deliv. System | 2′/Backbone Mod. | 5′/3′ Mod. | Base Mod. |
|---|---|---|---|---|---|---|---|---|---|
| Elasomeran (Spikevax) [140] | FDA 2020 EMA 2020 | Undisclosed | COVID-19 vaccine | 0./0.2 mg two doses 28 days apart | IM | LNP | Undisclosed | 5′-cap, Poly A tail | N1-methylpseudouridine |
| Tozinameran (Comirnaty) [141] | FDA 2020 EMA 2020 | Undisclosed | COVID-19 vaccine | 0.03 mg 2 doses at least 21 days apart | IM | LNP | Undisclosed | 5′-cap2, Poly A tail | N1-methylpseudouridine |
| INN Name | Clinical Trial Status | Target Site | Indication | Deliv. Route | Deliv. System | 2′/Backbone Mod. | 5′/3′ Mod. | Base Mod. |
|---|---|---|---|---|---|---|---|---|
| BNT162b1 [142] | Ongoing | Undisclosed | Respiratory disease; SARS-CoV-2 (COVID-19) infection; viral diseases | IM | LNP | Undisclosed | Undisclosed | N1-Methylpseudouridine |
| mRNA-1273.213 [143,144,145] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | LNP | Undisclosed | Undisclosed | N1-Methylpseudouridine |
| RQ3013 [146] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | LNP | Undisclosed | Undisclosed | N1-Methylpseudouridine |
| LVRNA009 [147,148,149] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undisclosed | Undisclosed | Undisclosed | LNP |
| Pfizer BioNtech Omicron BA.1-adapted bivalent COVID-19 vaccine | Approved; ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | Undisclosed | Undisclosed | Undisclosed | Undisclosed | LNP |
| Oslo University Hospital tumor (glioblastoma) stem cells/hTERT/Survivin mRNA transfected autologous DCs [150] | Ongoing | Ex vivo cell therapy | Glioblastoma multiforme | ID | Undisclosed | Undisclosed | Undisclosed | LNP |
| Elasomeran/Imelasomeran/Imelasomeran/mRNA-1273.529/Spikevax Bivalent Original/Omicron BA.1 | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | Undisclosed | Undisclosed | Undisclosed | N1-Methylpseudouridine | LNP |
| DS-5670/Daiichi Sankyo COVID-19 mRNA vaccine [151] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undisclosed | Undisclosed | Uridine-modified | LNP |
| ABO1020 [152] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undisclosed | Undisclosed | Undisclosed | LNP |
| Rocapuldencel-T/AGS-003/AGS-003-LNG/CoImmune CMN-001 [153] | Ongoing | Ex vivo cell therapy | Advanced renal cell carcinoma; clear cell renal cell carcinoma; infiltrating bladder urothelial carcinoma; kidney cancer; metastatic kidney cancer; metastatic renal cell carcinoma; muscle-invasive bladder cancer; non-small cell lung cancer (NSCLC); renal cancer; renal cell carcinoma (RCC); urothelial carcinoma of the bladder; urothelial cell carcinoma | SC | Undis-closed | Undisclosed | Undisclosed | Electroporation; naked RNA |
| mRNA-1273.211 [143] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undis-closed | Undisclosed | N1-Methylpseudouridine | LNP |
| Pfizer Quadrivalent Influenza modRNA Vaccine [154] | Ongoing | Undisclosed | Influenza; SARS-CoV-2 (COVID-19) Infection | IM | Undisclosed | Undisclosed | modRNA | Undisclosed |
| BNT161 [155,156,157] | Ongoing | Undisclosed | Influenza; SARS-CoV-2 (COVID-19) infection | IM | Undis-closed | Undisclosed | modRNA | LNP |
| mRNA-1010 | Ongoing | Undisclosed | Cytomegalovirus disease (CMV disease); influenza; respiratory syncytial virus (RSV) infection; SARS-CoV-2 (COVID-19) infection | IM | Undis-closed | Undisclosed | Undisclosed | LNP |
| mRNA-1647 | Ongoing | Undisclosed | Cytomegalovirus disease (CMV disease); cytomegalovirus infections; influenza; respiratory syncytial virus (RSV) infection | IM | Undis-closed | Undisclosed | Undisclosed | LNP |
| mRNA-1273.617.2 [143,158] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undisclosed | Undisclosed | N1-Methylpseudouridine | LNP |
| Imelasomeran/elasomeran/mRNA-1273.214 [143,159] | Approved; ongoing | Undisclosed | Asthma; cystic fibrosis (CF); diabetes mellitus; HIV infection; influenza; Middle East respiratory syndrome (MERS, MERS-CoV); respiratory syncytial virus (RSV) infection; SARS-CoV-2 (COVID-19) infection | IM | Undis-closed | Undisclosed | N1-Methylpseudouridine | LNP |
| Walvax COVID-19 Vaccine/ARCoV/ARCoV mRNA vaccine/AWcorna [22,160] | Approved; ongoing | Undisclosed | Pneumonia; SARS-CoV-2 (COVID-19) infection; viral pneumonia | IM | NA | NA | NA | LNP |
| mRNA-1345 [161] | Ongoing | Undisclosed | Chronic obstructive pulmonary disease; congestive heart failure (CHF); cytomegalovirus disease (CMV disease); influenza; respiratory syncytial virus (RSV) infection; SARS-CoV-2 (COVID-19) infection | IM | Undis-closed | Undisclosed | Nucleoside-modified | LNP |
| PTX-COVID19-B [162] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undis-closed | Undisclosed | Undisclosed | LNP |
| SW-BIC-213 mRNA vaccine [163,164,165,166] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undisclosed | Undisclosed | Undisclosed | Lipopolyplex |
| CVnCoV/CureVac COVID-19 mRNA vaccine/CV07050101 [167] | Discontinued (clinical) | Undisclosed | Respiratory disease; SARS-CoV-2 (COVID-19) infection | IM | NA | NA | NA | LNP |
| SYS6006 [168,169] | Ongoing | Undisclosed | SARS-CoV-2 (COVID-19) infection | IM | Undisclosed | Undisclosed | Undisclosed | LNP |
| INN Name | Approv. | Target Site (Target) | Disease | Posology | Delivery Route | Backbone Mod. | 2′ Mod. | 5′/3′ Mod. |
|---|---|---|---|---|---|---|---|---|
| Defibrotide (Defitelio) [192] | FDA 2016 EMA 2013 | Liver endothelium/blood (adenosine A1/A2 receptor) | Sinusoidal obstruction syndrome | 25 mg/kg daily, up to 6 week | IV | Undisclosed | Undisclosed | Undisclosed |
| Pegaptanib (Macugen) | FDA 2004 | Eye (heparin-binding domain of VEGF-165) | Age-related macular degeneration | 0.3 mg every six months | IVt | NA | 2′-O-Me, 2′-F, | 3′-inverted nucleotide; PEG conjugate |
| INN Name | Clinical Trial Status | Target Site | Disease | Deliv. Route | Backbone Mod. | 2′ Mod. | 5′/3′ Mod. |
|---|---|---|---|---|---|---|---|
| Pegnivacogin/REG1 (RB006) [193,194] | Ongoing | Blood (factor IXA) | Angina (angina pectoris); coronary artery disease (coronary heart disease) | IV | NA | 2′-F, 2′-O-Me | 3′ inverted deoxythymidine; PEG conjugate |
| Fovista®/E10030, Pegpleranib [195] | Ongoing | Eye (PDGF) | Choroidal neovascularization; dry age-related macular degeneration (atrophic macular degeneration); neovascular age-related macular degeneration (wet age-related macular degeneration) | IO | PEG loops | 2′-F, 2′-O-Me | 3′ inverted deoxythymidine; PEG conjugate |
| Zimura/Avacincaptad pegol [116,196] | Ongoing | Eye (C5 inhibitor) | Age-related macular degeneration (AMD); cancer indications; choroidal neovascularization; dry age-related macular degeneration (atrophic macular degeneration); hereditary ATTR amyloidosis; idiopathic polypoidal choroidal vasculopathy; macular degeneration; neovascular age-related macular degeneration (wet age-related macular degeneration); Stargardt macular dystrophy (Stargardt disease, fundus flavimaculatus) | IVt | NA | 2′-O-Me | 3′ inverted deoxythymidine; PEG conjugate |
| INN Name | Approval(s) | Target Site (Target) | Disease | Posology | Deliv. Route | Deliv. System | Conjugation/Termination |
|---|---|---|---|---|---|---|---|
| Defibrotide (Defitelio) | FDA 2016 EMA 2013 | Liver endothelium/blood (Adenosine A1/A2 receptor) | Sinusoidal obstruction syndrome | 25 mg/kg daily, up to 6 week | IV | Naked in citrate buffer | NA |
| Pegaptanib (Macugen) | FDA 2004 | Eye (Heparin-binding domain of VEGF-165) | Age-related macular degeneration | 0.3 mg every six months | IVt | Naked in NaCl phosphate buffer | NA |
| INN Name | Clinical Trial Status | Target Site (Target) | Disease | Deliv. Route | Deliv. System | Conjugation/Termination |
|---|---|---|---|---|---|---|
| Pegnivacogin/REG1 (RB006) [193,194] | Ongoing | Blood (Factor IXA) | Angina (angina pectoris); coronary artery disease (coronary heart disease) | IV | Undisclosed | NA |
| Fovista®/E10030, Pegpleranib [195] | Ongoing | Eye (PDGF) | Choroidal neovascularization; dry age-related macular degeneration (atrophic macular degeneration); neovascular age-related macular degeneration (wet age-related macular degeneration) | IO | Undisclosed | NA |
| Zimura/Avacincaptad pegol [116,196] | Ongoing | Eye (C5 inhibitor) | Age-related macular degeneration (AMD); cancer indications; choroidal neovascularization; dry age-related macular degeneration (atrophic macular degeneration); hereditary ATTR amyloidosis; idiopathic polypoidal choroidal vasculopathy; macular degeneration; neovascular age-related macular degeneration (wet age-related macular degeneration); Stargardt macular dystrophy (Stargardt disease, fundus flavimaculatus) | IVt | Undisclosed | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufeu, M.; Herkenne, C.; Kalia, Y.N. Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics. Pharmaceutics 2025, 17, 903. https://doi.org/10.3390/pharmaceutics17070903
Tufeu M, Herkenne C, Kalia YN. Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics. Pharmaceutics. 2025; 17(7):903. https://doi.org/10.3390/pharmaceutics17070903
Chicago/Turabian StyleTufeu, Maxime, Christophe Herkenne, and Yogeshvar N. Kalia. 2025. "Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics" Pharmaceutics 17, no. 7: 903. https://doi.org/10.3390/pharmaceutics17070903
APA StyleTufeu, M., Herkenne, C., & Kalia, Y. N. (2025). Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics. Pharmaceutics, 17(7), 903. https://doi.org/10.3390/pharmaceutics17070903










