Patent-Based Technological Overview of Propolis–Cyclodextrin Inclusion Complexes with Pharmaceutical Potential
Abstract
1. Introduction
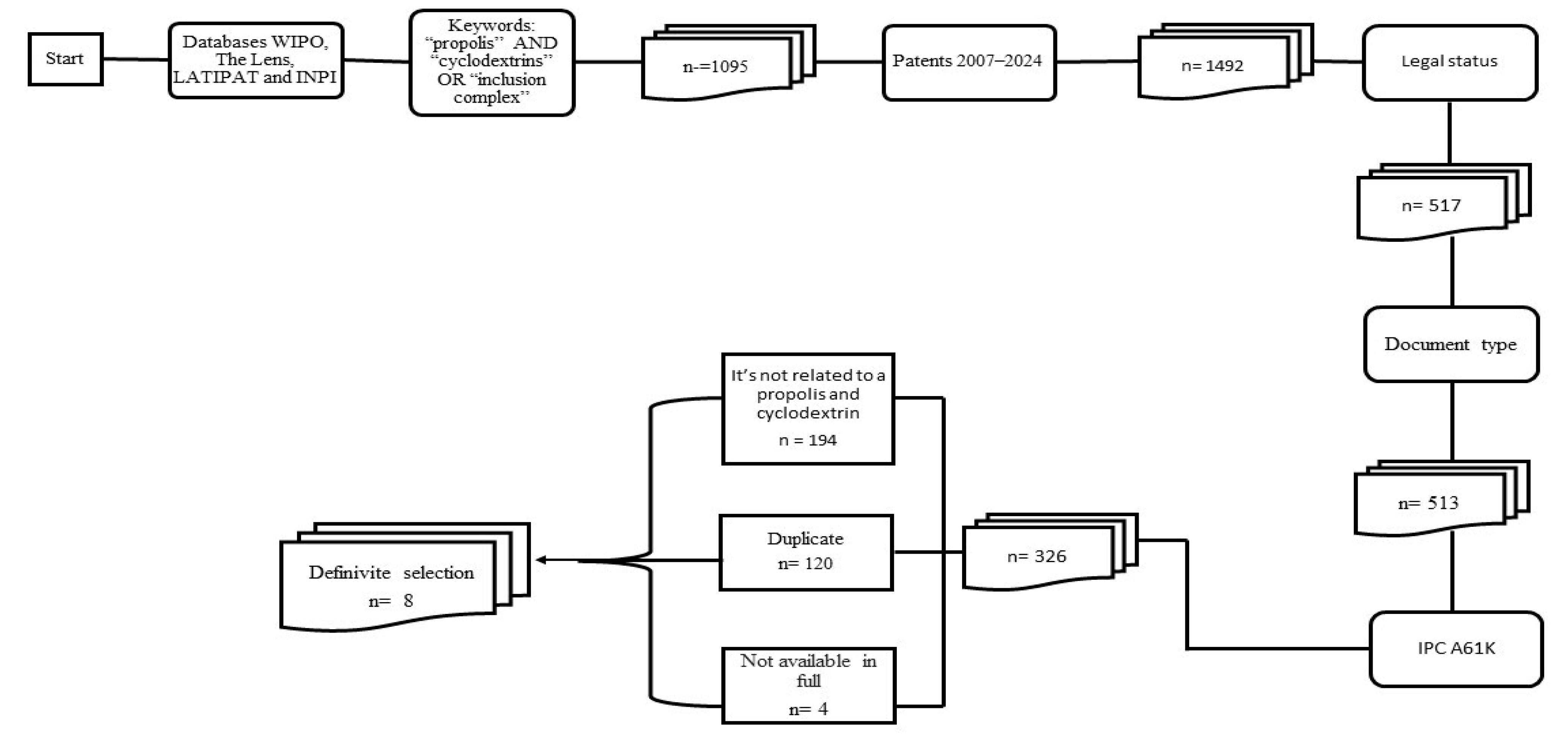
2. Method
3. Results
4. Discussion
4.1. Binary and Ternary Inclusion Complexes
4.2. Propolis-in-Cyclodextrin-in-Liposomes
4.3. Emulsions Stabilized by Cyclodextrins
4.4. Cyclodextrin-Assisted Extraction and Encapsulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayerhoff, Z.D.V.L. Uma Análise Sobre Os Estudos de Prospecção Tecnológica. Cadernos de Prospecção 2008, 1, 7–9. [Google Scholar]
- Do Nascimento, T.G.; Rosário, F.J.P.; De Lima, A.A.; De Moraes, I.Q.S.; De Lira, L.M.S.S.; de Moraes Porto, I.C.C.; de Matos, E.O.; Cedrim, P.C.A.S.; Basílio Júnior, I.D.; de Moura, M.A.B.F.; et al. Patentes e Apropriação de Valor Da Inovação: O Caso Própolis. Cadernos de Prospecção 2018, 11, 87–102. [Google Scholar] [CrossRef]
- Menezes, H. Própolis: Uma Revisão Dos Recentes Estudos de Suas Propriedades Farmacológicas. Arq. Inst. Biol. 2005, 72, 405–411. [Google Scholar] [CrossRef]
- Souza, M.S.; Amaral, K.M.M.; Sousa, E.O.; Pereira, J.M.; Santos, Í.T.; da Silva Cândido, G.; Araújo, S.M.; Silva, R.P.; Cruz, L.P.; de Oliveira Vieira Matos, A.B.; et al. Uso Da Própolis No Tratamento de Lesões Cutâneas: Revisão Integrativa. Revista de casos e Consultoria 2022, 13, 1–16. [Google Scholar]
- Welke, J.E.; Reginatto, S.; Débora, F.; Vicenzi, R.; Soares, J.M. Caracterização Físico-Química de Méis de Apis Mellifera L. Da Região Noroeste Estado Do Rio Grande Do Sul. Ciência Rural 2008, 38, 1737–1741. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An Update on Its Chemistry and Pharmacological Applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How Diverse Is the Chemistry and Plant Origin of Brazilian Propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and Application in the Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2022, 127, 169–180. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Vlaia, L.L.; Vlaia, V.; Olariu, I.V.; Mut, A.M.; Gafitanu, C.A.; Dehelena, C.; Navolan, D.; Lupuleasa, D.; Georgeta, C. Preparation and Characterization of Inclusions Complexes Between Propolis Ethanolic Extracts and 2-Hydroxypropyl-β-Cyclodextrin. Rev. Chim. 2016, 67, 378–381. [Google Scholar]
- de Mélo Silva, I.S.; Gaspar, L.M.; Rocha, A.M.O.; Rda Costa, L.P.; Tada, D.B.; Franceschi, E.; Padilha, F.F. Encapsulation of Red Propolis in Polymer Nanoparticles for the Destruction of Pathogenic Biofilms. AAPS PharmSciTech 2020, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zheng, S.; Zeng, L.; Deng, Z.; Zhang, B.; Li, H. The Phenolic Compounds, Metabolites, and Antioxidant Activity of Propolis Extracted by Ultrasound-Assisted Method. J. Food Sci. 2019, 84, 3850–3865. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Suvarna, P.; Chaudhari, P.; Lewis, S.A. Cyclodextrin-Based Supramolecular Ternary Complexes: Emerging Role of Ternary Agents on Drug Solubility, Stability, and Bioavailability. Crit. Rev. Ther. Drug Carr. Syst. 2022, 39, 1–50. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Ionescu, C.; Samide, A.; Tigae, C.; Spînu, C.I.; Oprea, B. Advancements in Cyclodextrin Complexes with Bioactive Secondary Metabolites and Their Pharmaceutical Applications. Pharmaceutics 2025, 17, 506. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from Molecules to Applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Moreira, R.S.S.; Novais, J.S.; da Silva, R.F.; Nunes, R.P.; de Abreu, L.C.L.; Dias, E.P.; Castro, H.C.; Do Carmo, F.A.; Rodrigues, C.R.; de Sousa, V.P.; et al. Preparation and Evaluation of Red Propolis and Nystatin Cyclodextrin Inclusion Complexes against Oral Microbiome Opportunistic Microorganisms. Food Sci. Technol. 2022, 42, e118022. [Google Scholar] [CrossRef]
- Rimbach, G.; Fischer, A.; Schloesser, A.; Jerz, G.; Ikuta, N.; Ishida, Y.; Matsuzawa, R.; Matsugo, S.; Huebbe, P.; Terao, K. Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet. Int. J. Mol. Sci. 2017, 18, 1141. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Rodrigues, M.R.; Bueno, P.C.P.; De Mello Costa-Machado, A.R.; De Oliveira Lima Leite Vaz, M.M.; Nascimento, A.P.; Barud, H.S.; Berretta-Silva, A.A. Preparation and Thermal Characterization of Inclusion Complex of Brazilian Green Propolis and Hydroxypropyl-β-Cyclodextrin: Increased Water Solubility of the Chemical Constituents and Antioxidant Activity. J. Therm. Anal. Calorim. 2012, 108, 87–94. [Google Scholar] [CrossRef]
- Lazarova, D.L.; Paul, K.; Catchopole, O. Composições Terapêuticas e Usos Das Mesmas. BR112015025025, 18 July 2017. [Google Scholar]
- Suddes, A.J.; Catchpole, O.J. Propolis and Extracts Thereof for the Treatment of Skin Cancers and Impovement of Skin Health. International Patent Application No. WO2016010440A1, 17 July 2015. [Google Scholar]
- Corvi Mora, P.; Carli, F.; Canal, T. Quaternary Compounds Comprising Propolis as the Active Substance. EP1572155A1, 12 February 2002. [Google Scholar]
- Gardikis, K.; Koutsianas, N.; Patera, A.; Dragani, P.; Tsoukalas, A.; Letsious, S. Method for Preparing a Stable Controlled-Release Propolis Colloidal Dispersion System for Various Uses. U.S. Patent 15816858.3, 23 November 2015. [Google Scholar]
- Ki, C.N.; Sung, K.H.; Cho, Y.C. Non-Alcohol Water Soluble Propolis Nano Powder and Method for Preparing the Same. KR20100078349A, 30 June 2008. [Google Scholar]
- Ioannis, M.; Panagiota, D.; Anna, P.; Nikolaos, K. Extraction and Formation of Inclusion Complex of Propolis Active Components with Hydroxypropyl-Beta-Cyclodextrin. WO Patent No. 073051, 7 June 2012. [Google Scholar]
- Rim, L.R.; Won, P.S.; Son, O.A. Method for Manufacturing Water-Soluble Propolis. KR102192760B1, 28 May 2020. [Google Scholar]
- Haoran, F.; Jiadong, Z.; Zhenyan, C.; Xiuhong, W.; Hu Changan, G. Preparation Method of Emulsion Containing Nano-Propolis Extract and Prepared Emulsion. CN110664725A, 1 October 2020. [Google Scholar]
- Ansari, M.J. Formulation and Physicochemical Characterization of Sodium Carboxy Meyhyl Cellulose and β Cyclodextrinmediated Ternary Iclusion Complexes of Silymarin. Int. J. Pharm. Sci. Res. 2016, 7, 984. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Formulation of Propolis with B-Cyclodextrin. Acta Pharm. Technol. 1987, 33, 218–221. [Google Scholar]
- Perak Junaković, E.; Šandor, K.; Terzić, S.; Vujnović, A.; Andrišić, M.; Benić, M.; Fajdić, D.; Sinković, S.; Pehnec, M.; Žarković, I. Influence of Encapsulation of Propolis Extract with 2-Hydroxypropyl-β-Cyclodextrin (HP-β-CD) on Polyphenolic Contents during In Vitro Simulation of Digestion. Appl. Sci. 2023, 13, 9357. [Google Scholar] [CrossRef]
- Coneac, G.; Gafiţanu, E.; Hădărugă, N.G.; Hădărugă, D.I.; Riviş, A.; Bandur, G.; Pînzaru, I.A.; Rusu, G.; Urşica, L.; Păunescu, V.; et al. Propolis Extract/β-Cyclodextrin Nanoparticles: Synthesis, Physico-Chemical, and Multivariate Analyses. J. Agroaliment. Process. Technol. 2008, 14, 58–70. [Google Scholar]
- Kalogeropoulos, N.; Konteles, S.; Mourtzinos, I.; Troullidou, E.; Chiou, A.; Karathanos, V.T. Encapsulation of Complex Extracts in β-Cyclodextrin: An Application to Propolis Ethanolic Extract. J. Microencapsul. 2009, 26, 603–613. [Google Scholar] [CrossRef]
- dos Santos Ferreira, C.I.; Gonzales, A.P.; Mazzobre, M.F.; Ulrih, N.P.; del P. Buera, M. Solubility, Sorption Isotherms and Thermodynamic Parameters of β-Cyclodextrin Complexes with Poplar Propolis Components: Practical Implicances. LWT 2022, 167, 113811. [Google Scholar] [CrossRef]
- CycloChem Bio Co., Ltd. Powderisation of Propolis Extracts by the Use of Cyclodextrins; CycloChem Bio Co., Ltd.: Kobe, Japan, 2019. [Google Scholar]
- Zhu, Z.Y.; Luo, Y.; Liu, Y.; Wang, X.T.; Liu, F.; Guo, M.Z.; Wang, Z.; Liu, A.J.; Zhang, Y.M. Inclusion of Chrysin in β-Cyclodextrin and Its Biological Activities. J. Drug Deliv. Sci. Technol. 2016, 31, 176–186. [Google Scholar] [CrossRef]
- Abbas, Z.S.; Sulaiman, G.M.; Jabir, M.S.; Mohammed, S.A.A.; Khan, R.A.; Mohammed, H.A.; Al-Subaiyel, A. Galangin/β-Cyclodextrin Inclusion Complex as a Drug-Delivery System for Improved Solubility and Biocompatibility in Breast Cancer Treatment. Molecules 2022, 27, 4521. [Google Scholar] [CrossRef]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Anti-Gastrointestinal Cancer Activity of Cyclodextrin-Encapsulated Propolis. J. Funct. Foods 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Ishida, Y.; Gao, R.; Shah, N.; Bhargava, P.; Furune, T.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer Activity in Honeybee Propolis: Functional Insights to the Role of Caffeic Acid Phenethyl Ester and Its Complex With γ-Cyclodextrin. Integr. Cancer Ther. 2018, 17, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular Characterization and Enhancement of Anticancer Activity of Caffeic Acid Phenethyl Ester by γ Cyclodextrin. J. Cancer 2016, 7, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- CycloChem Bio Co. Ltd. Effects of γ-Cyclodextrin on Anti-Cancer Activity of Caffeic Acid Phenethyl Ester; CycloChem Bio Co., Ltd.: Kobe, Japan, 2014. [Google Scholar]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of Cyclodextrins and Drugs after Oral and Parenteral Administration of Drug/Cyclodextrin Complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring Versatile Applications of Cyclodextrins: An Overview. Drug Deliv. 2016, 23, 729–747. [Google Scholar] [CrossRef]
- Trotta, F.; Loftsson, T.; Gaud, R.S.; Trivedi, R.; Shende, P. Integration of Cyclodextrins and Associated Toxicities: A Roadmap for High Quality Biomedical Applications. Carbohydr. Polym. 2022. [Google Scholar] [CrossRef]
- Patil, P.; Kumbhar, S.; Ghorpade, V. Auxiliary Substances for Enhancement of Complexation Efficiency and Dissolution Rate of Drug-Cyclodextrin Complexes. Int. J. Drug Deliv. Technol. 2024, 14, 598–608. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef]
- Di Capua, A.; Bejarano, A.; Adami, R.; Reverchon, E. Preparation and Characterization of Chilean Propolis Coprecipitates Using Supercritical Assisted Atomization. Chem. Eng. Res. Des. 2018, 136, 776–785. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes Incorporating Cyclodextrin-Drug Inclusion Complexes: Current State of Knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Feng, S.S.; Zheng, C.H. A Comparison between Conventional Liposome and Drug-Cyclodextrin Complex in Liposome System. Int. J. Pharm. 2016, 513, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fang, Z.; Bai, C.; Mo, Y.; Liu, D.; Yang, B.; Jia, X.; Feng, L. Novel Nano-Encapsulated Limonene: Utilization of Drug-in-Cyclodextrin-in-Liposome Formulation to Improve the Stability and Enhance the Antioxidant Activity. Int. J. Pharm. 2024, 653, 123914. [Google Scholar] [CrossRef]
- Mccormack, B.; Gregoriadis, G. Entrapment of Cyclodextrin-Drug Complexes into Liposomes: Potential Advantages in Drug Delivery. J. Drug Target. 1994, 2, 449–454. [Google Scholar] [CrossRef]
- Baldim, I.; Oliveira, A.M.; Souto, E.B.; Oliveira, W.P. Cyclodextrins-in-Liposomes: A Promising Delivery System for Lippia Sidoides and Syzygium Aromaticum Essential Oils. Life 2022, 12, 95. [Google Scholar] [CrossRef]
- Alomrani, A.H.; Shazly, G.A.; Amara, A.A.A.F.; Badran, M.M. Itraconazole-Hydroxypropyl-β-Cyclodextrin Loaded Deformable Liposomes: In Vitro Skin Penetration Studies and Antifungal Efficacy Using Candida Albicans as Model. Colloids Surf. B Biointerfaces 2014, 121, 74–81. [Google Scholar] [CrossRef]
- Lim, H.; Jin, S.; Jeong, Y.; Kim, S.B.; Jang, D.J.; Kim, S.T. Preparation of Hydroxypropyl-β-Cyclodextrin-Incorporated Liposomes and Evaluation of Their Rapid Release Property. J. Ind. Eng. Chem. 2021, 100, 59–62. [Google Scholar] [CrossRef]
- Chang, L.; Shepherd, D.; Sun, J.; Ouellette, D.; Grant, K.L.; Tang, X.; Pikal, M.J. Mechanism of Protein Stabilization by Sugars during Freeze-Drying and Storage: Native Structure Preservation, Specific Interaction, and/or Immobilization in a Glassy Matrix? J. Pharm. Sci. 2005, 94, 1427–1444. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Thangaraj, B.; Abdel-Samie, M.A.S.; Cui, H. Improving the Stability of Thyme Essential Oil Solid Liposome by Using β-Cyclodextrin as a Cryoprotectant. Carbohydr. Polym. 2018, 188, 243–251. [Google Scholar] [CrossRef]
- Matloob, A.H.; Mourtas, S.; Klepetsanis, P.; Antimisiaris, S.G. Increasing the Stability of Curcumin in Serum with Liposomes or Hybrid Drug-in-Cyclodextrin-in-Liposome Systems: A Comparative Study. Int. J. Pharm. 2014, 476, 108–115. [Google Scholar] [CrossRef]
- Hammoud, Z.; Kayouka, M.; Trifan, A.; Sieniawska, E.; Ben Jemâa, J.M.; Elaissari, A.; Greige-Gerges, H. Encapsulation of α-Pinene in Delivery Systems Based on Liposomes and Cyclodextrins. Molecules 2021, 26, 6840. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Drug-in-Cyclodextrin-in-Liposomes as a Carrier System for Volatile Essential Oil Components: Application to Anethole. Food Chem. 2017, 218, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical Characteristics, Applications and Research Trends of Edible Pickering Emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Yuan, C.; Cheng, C.; Cui, B. Pickering Emulsions Stabilized by Cyclodextrin Nanoparticles: A Review. Starch/Staerke 2021, 73, 2100077. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.; Perez Gonzalez, A.J.; Huang, Q. Kafirin Nanoparticles-Stabilized Pickering Emulsions: Microstructure and Rheological Behavior. Food Hydrocoll. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Jug, M.; Yoon, B.K.; Jackman, J.A. Cyclodextrin-Based Pickering Emulsions: Functional Properties and Drug Delivery Applications. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 31–50. [Google Scholar] [CrossRef]
- Mathapa, B.G.; Paunov, V.N. Self-Assembly of Cyclodextrin-Oil Inclusion Complexes at the Oil-Water Interface: A Route to Surfactant-Free Emulsions. J. Mater. Chem. A Mater. 2013, 1, 10836–10846. [Google Scholar] [CrossRef]
- Davarpanah, L.; Vahabzadeh, F. Formation of Oil-in-Water (O/W) Pickering Emulsions via Complexation between β-Cyclodextrin and Selected Organic Solvents. Starch/Staerke 2012, 64, 898–913. [Google Scholar] [CrossRef]
- Inoue, M.; Hashizaki, K.; Taguchi, H.; Saito, Y. Emulsion Preparation Using B-Cyclodextrin and Its Derivatives Acting as an Emulsifier. Chem. Pharm. Bull. 2008, 2008, 1335–1337. [Google Scholar] [CrossRef]
- Xi, Y.; Luo, Z.; Lu, X.; Peng, X. Modulation of Cyclodextrin Particle Amphiphilic Properties to Stabilize Pickering Emulsion. J. Agric. = Food Chem. 2018, 66, 228–237. [Google Scholar] [CrossRef]
- Tian, Y.; Yuan, C.; Cui, B.; Lu, L.; Zhao, M.; Liu, P.; Wu, Z.; Li, J. Pickering Emulsions Stabilized by β-Cyclodextrin and Cinnamaldehyde Essential Oil/β-Cyclodextrin Composite: A Comparison Study. Food Chem. 2022, 377, 131995. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ma, X.; Sun, J.; Bai, W. Fabrication and Characterization of Anthocyanin-Loaded Double Pickering Emulsions Stabilized by β-Cyclodextrin. Int. J. Pharm. 2024, 655, 124003. [Google Scholar] [CrossRef] [PubMed]
- Fadiloglu, E.E.; Emir Coban, O. Effects of Whey Protein Coating Incorporated with Propolis-β-Cyclodextrin Emulsion on Quality of Refrigerated Sea Bass Fillets (Dicentrarchus labrax). J. Aquat. Food Prod. Technol. 2022, 31, 702–713. [Google Scholar] [CrossRef]
- Fuente-Ballesteros, A.; Priovolos, I.; Ares, A.M.; Samanidou, V.; Bernal, J. Green Sample Preparation Methods for the Analysis of Bioactive Compounds in Bee Products: A Review. Adv. Sample Prep. 2023, 6, 100060. [Google Scholar] [CrossRef]
- Bankova, V.; Trusheva, B.; Popova, M. Propolis Extraction Methods: A Review. J. Apic. Res. 2021, 60, 734–743. [Google Scholar] [CrossRef]
- Pietta, P.G.; Gardana, C.; Pietta, A.M. Analytical Methods for Quality Control of Propolis. Fitoterapia 2002, 73 (Suppl. 1), S7–S20. [Google Scholar] [CrossRef]
- Cunha, I.B.S.; Sawaya, A.C.H.F.; Caetano, F.M.; Shimizu, M.T.; Marcucci, M.C.; Drezza, F.T.; Povia, G.S.; Carvalho, P.D.O. Factors That Influence the Yield and Composition of Brazilian Propolis Extracts. J. Braz. Chem. Soc. 2004, 15, 964–970. [Google Scholar] [CrossRef]
- Biscaia, D.; Ferreira, S.R.S. Propolis Extracts Obtained by Low Pressure Methods and Supercritical Fluid Extraction. J. Supercrit. Fluids 2009, 51, 17–23. [Google Scholar] [CrossRef]
- Jug, M.; Karas, O.; Kosalec, I. The Influence of Extraction Parameters on Antimicrobial Activity of Propolis Extracts. Nat. Prod. Commun. 2017, 12, 47–50. [Google Scholar] [CrossRef]
- Paviani, L.C.; Fiorito, G.; Sacoda, P.; Cabral, F.A. Different Solvents For Extraction Of Brazilian Green Propolis: Composition And Extraction Yield Of Phenolic Compounds. In Proceedings of the III Iberoamerican Conference on Supercritical Fluid, Cartagena de Indias, Colombia, 1–5 April 2013. [Google Scholar]
- Monroy, Y.M.; Rodrigues, R.A.F.; Rodrigues, M.V.N.; Sant’Ana, A.S.; Silva, B.S.; Cabral, F.A. Brazilian Green Propolis Extracts Obtained by Conventional Processes and by Processes at High Pressure with Supercritical Carbon Dioxide, Ethanol and Water. J. Supercrit. Fluids 2017, 130, 189–197. [Google Scholar] [CrossRef]
- Bayram, N.E.; Gerçek, Y.C.; Bayram, S.; Toğar, B. Effects of Processing Methods and Extraction Solvents on the Chemical Content and Bioactive Properties of Propolis. J. Food Meas. Charact. 2020, 14, 905–916. [Google Scholar] [CrossRef]
- Zin, N.B.M.; Azemin, A.; Rodi, M.M.M.; Mohd, K.S. Chemical Composition and Antioxidant Activity of Stingless Bee Propolis from Different Extraction Methods. Int. J. Eng. Technol. 2018, 7, 90–95. [Google Scholar] [CrossRef]
- Hamzah, N.; Leo, C.P. Microwave-Assisted Extraction of Trigona Propolis: The Effects of Processing Parameters. Int. J. Food Eng. 2015, 11, 861–870. [Google Scholar] [CrossRef]
- Margeretha, I.; Fatma Suniarti, D.; Herda, E.; Mas’ud, Z.A. Optimization and Comparative Study of Different Extraction Methods of Biologically Active Components of Indonesian Propolis Trigona spp. J. Nat. Prod. 2012, 5, 233–242. [Google Scholar]
- Devequi-Nunes, D.; Machado, B.A.S.; De Abreu Barreto, G.; Silva, J.R.; Da Silva, D.F.; Da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical Characterization and Biological Activity of Six Different Extracts of Propolis through Conventional Methods and Supercritical Extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Saito, É.; Sacoda, P.; Paviani, L.C.; Paula, J.T.; Cabral, F.A. Conventional and Supercritical Extraction of Phenolic Compounds from Brazilian Red and Green Propolis. Sep. Sci. Technol. 2021, 56, 3119–3126. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; Barreto, G.D.A.; Costa, S.S.; Da Silva, D.F.; Brandão, H.N.; Da Rocha, J.L.C.; Dellagostin, O.A.; Henriques, J.A.P.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Oroian, M.; Dranca, F.; Ursachi, F. Comparative Evaluation of Maceration, Microwave and Ultrasonic-Assisted Extraction of Phenolic Compounds from Propolis. J. Food Sci. Technol. 2020, 57, 70–78. [Google Scholar] [CrossRef]
- Juodeikaitė, D.; Žilius, M.; Briedis, V. Preparation of Aqueous Propolis Extracts Applying Microwave-Assisted Extraction. Processes 2022, 10, 1330. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Gajger, I.T.; Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New Deep Eutectic Solvent Assisted Extraction of Highly Pure Lignin from Maritime Pine Sawdust (Pinus Pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Q.S.; Wang, L.W.; Chang, S.L.; Wang, P.D.; Zhao, B. Optimization of Cyclodextrin-Assisted Green Extraction of Cannabidiol from Industrial Hemp Leaves: Release Behavior, Permeability, Bioactivity, and Stability. Ind. Crops Prod. 2022, 188, 115709. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Niu, Y.; Ke, Q.; Kou, X. Cyclodextrins as Carriers for Volatile Aroma Compounds: A Review. Carbohydr. Polym. 2021, 269, 118292. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Y.; Tang, K. Selective Extraction of Glabridin from Glycyrrhiza Glabra Crude Extracts by Sulfobutylether-β-Cyclodextrin in a Ternary Extraction System. Process Biochem. 2023, 129, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.-f.; Sang, J. Green Approach for Sample Preparation and Determination of Anthocyanins from Lycium Ruthenicum Murr. Using a β-Cyclodextrin-Based Extraction Method Coupled with UPLC-DAD Analysis. Food Anal. Methods 2018, 11, 2141–2148. [Google Scholar] [CrossRef]
- da Silva, F.C.; Favaro-Trindade, C.S.; de Alencar, S.M.; Thomazini, M.; Balieiro, J.C.C. Physicochemical Properties, Antioxidant Activity and Stability of Spray-Dried Propolis. J. ApiProduct ApiMedical Sci. 2011, 3, 94–100. [Google Scholar] [CrossRef]
- Pant, K.; Thakur, M.; Chopra, H.K.; Nanda, V. Encapsulated Bee Propolis Powder: Drying Process Optimization and Physicochemical Characterization. LWT 2022, 155, 112956. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2010; pp. 3–29. [Google Scholar] [CrossRef]
- Baysan, U.; Zungur Bastıoğlu, A.; Coşkun, N.Ö.; Konuk Takma, D.; Ülkeryıldız Balçık, E.; Sahin-Nadeem, H.; Koç, M. The Effect of Coating Material Combination and Encapsulation Method on Propolis Powder Properties. Powder Technol. 2021, 384, 332–341. [Google Scholar] [CrossRef]
- Nori, M.P.; Favaro-Trindade, C.S.; Matias de Alencar, S.; Thomazini, M.; de Camargo Balieiro, J.C.; Contreras Castillo, C.J. Microencapsulation of Propolis Extract by Complex Coacervation. LWT 2011, 44, 429–435. [Google Scholar] [CrossRef]
- da Cruz Almeida, E.T.; da Silva, M.C.D.; dos Santos Oliveira, J.M.; Kamiya, R.U.; dos Santos Arruda, R.E.; Vieira, D.A.; da Costa Silva, V.; Escodro, P.B.; Basílio-Júnior, I.D.; do Nascimento, T.G. Chemical and Microbiological Characterization of Tinctures and Microcapsules Loaded with Brazilian Red Propolis Extract. J. Pharm. Anal. 2017, 7, 280–287. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; Andrade, G.R.S.; da Cunha Nascimento, C.; Barbosa, P.F.; Jesus, M.S.; Narain, N. Development and Characterization of Microencapsules Containing Spray Dried Powder Obtained from Brazilian Brown, Green and Red Propolis. Food Res. Int. 2018, 109, 278–287. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, T.G.; Redondo, G.D.P.; de Araújo Abreu, C.T.; Silva, V.C.; Lira, G.M.; Meireles Grillo, L.A.; da Conceição, M.M.; Freitas, J.D.; Souza, J.S.; Araújo Júnior, J.X.; et al. Modified Release Microcapsules Loaded with Red Propolis Extract Obtained by Spray-Dryer Technique: Phytochemical, Thermal and Physicochemical Characterizations. J. Therm. Anal. Calorim. 2019, 138, 3559–3569. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Krumreich, F.D.; Zandoná, G.P.; Gularte, M.A.; Borges, C.D.; Zambiazi, R.C. Production of Propolis Extract Microparticles with Concentrated Pea Protein for Application in Food. Food Bioproc. Technol. 2019, 12, 729–740. [Google Scholar] [CrossRef]
- Rodrigues, R.; Bilibio, D.; Plata-oviedo, M.S.V.; Pereira, E.A.; Mitterer-daltoé, M.L.; Perin, E.C.; Carpes, S.T. Microencapsulated and Lyophilized Propolis Co-product Extract as Antioxidant Synthetic Replacer on Traditional Brazilian Starch Biscuit. Molecules 2021, 26, 6400. [Google Scholar] [CrossRef]
- do Nascimento, T.G.; de Almeida, C.P.; da Conceição, M.M.; dos Santos Silva, A.; de Almeida, L.M.; de Freitas, J.M.D.; Grillo, L.A.M.; Dornelas, C.B.; Ribeiro, A.S.; da Silva, J.F.; et al. Caseinates Loaded with Brazilian Red Propolis Extract: Preparation, Protein-Flavonoids Interaction, Antioxidant and Antibacterial Activities. J. Therm. Anal. Calorim. 2022, 147, 1329–1343. [Google Scholar] [CrossRef]
- Gomes Sá, S.H.; Chalella Mazzocato, M.; Saliba, A.S.M.C.; Alencar, S.M.; Sílvia Favaro-Trindade, C. Evaluation of the Release, Stability and Antioxidant Activity of Brazilian Red Propolis Extract Encapsulated by Spray-Drying, Spray-Chilling and Using the Combination of Both Techniques. Food Res. Int. 2023, 164, 112423. [Google Scholar] [CrossRef]
- Maroof, K.; Lee, R.F.S.; Siow, L.F.; Gan, S.H. Microencapsulation of Propolis by Spray Drying: A Review. Dry. Technol. 2022, 40, 1083–1102. [Google Scholar] [CrossRef]
| Application Number (Reference) | Country | Year | International Patent Classification System | Applicants | Type of Cyclodextrin | Indications or Applications | |
|---|---|---|---|---|---|---|---|
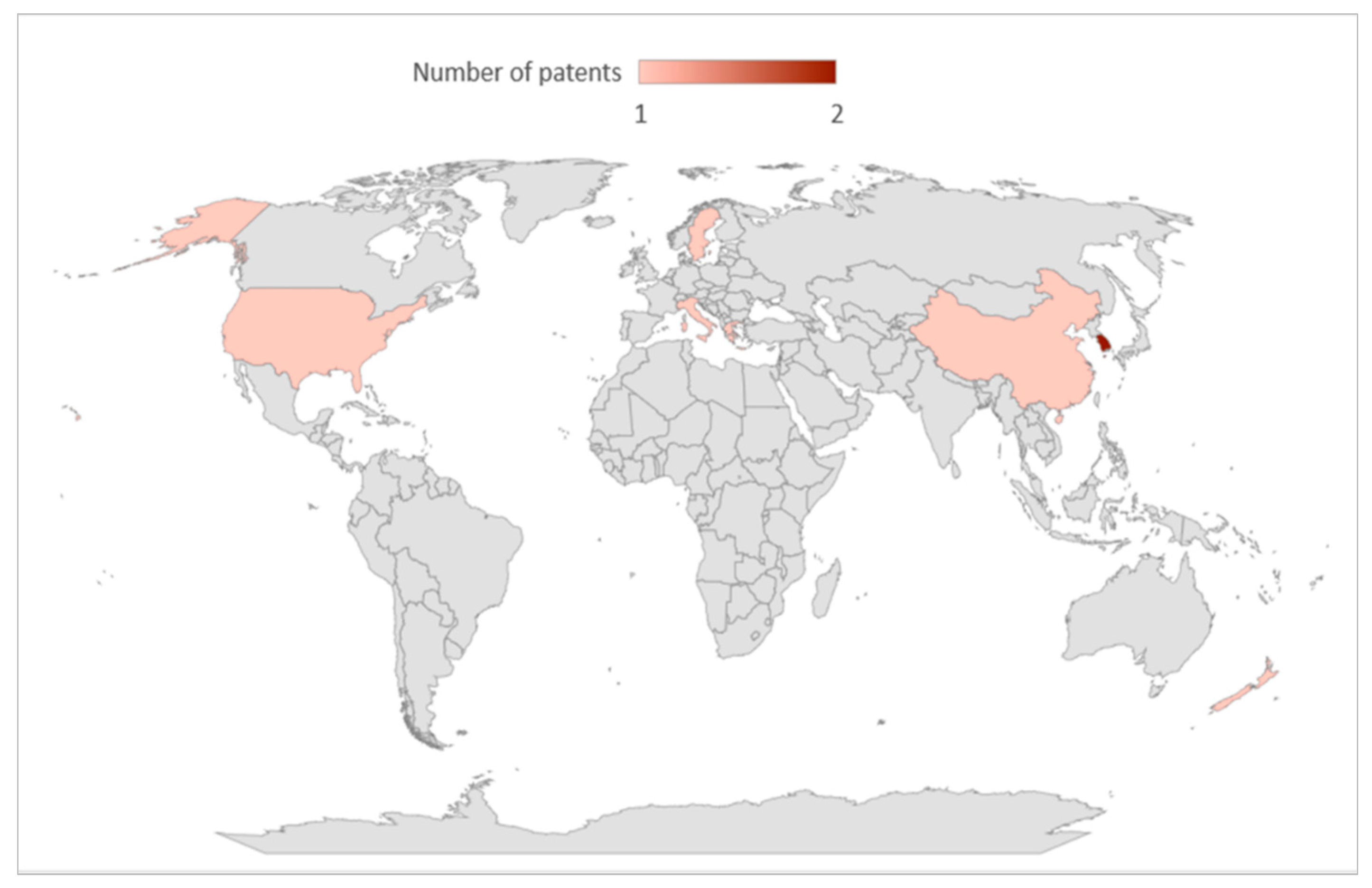
| 1 | US 10420804 B2 [23] | US | 2017–2019 | A61K35/00, A61K47/00, A61P1/00, A61P35/00 | MANUKA HEALTH NEW ZEALAND LTD | γ-CD | Treatment or prevention of gastrointestinal cancer |
| 2 | EP3169321 [24] | European Patent Office (EPO) | 2015–2017 | A61K31/216, A61K31/353, A61K9/00, A61K31/724, A61K31/192, A61K35/644 | MANUKA HEALTH NEW ZEALAND LTD | α-CD β-CD γ-CD | Treatment or prevention of skin cancer |
| 3 | WO 2004/050063 A1 [25] | WIPO | 2004–2007 | A61K8/44, A61K8/98, A61K8/63, A61K8/73, A61K9/00, A61K14/09 | ACTIMEX S.R.L | β-CD | Propolis delivered in a hydrophilic carrier |
| 4 | EP3380081 [26] | EPO | 2015–2018 | A61K9/127, A61K8/04, A61Q19/00 | APIVITA S.A | HPβ-CD β-CD | Antioxidant, antiangiogenic, photoprotective, antimicrobial, and immunostimulant |
| 5 | KR1020100078349 [27] | South Korea | 2010–2012 | A61K 35/644, A61K47/40, A23L1/706, A23P1/02 | GABO FARMS CO., LTD. | β-CD | Production of alcohol-free water-soluble nanopowder, and production thereof |
| 6 | GR 1007520 B [28] | Greece | 2010–2012 | A61K8/988, A23L21/20, A23L33/10, A61K8/738, | APIVITA KALLYNTIKA DIAITITIKA FARMAKA ANONYMI EMPORIKI KAI VIOTECHNIKI ETAIREIA | HPβ-CD | Extraction and formation of inclusion complexes of propolis active components |
| 7 | KR1020210104944 [29] | Republic of Korea | 2020–2021 | A61K 35/644, A23L 33/10, A23P 30/10, A61K 47/69, A61K 11/08, A61K 8/73 | BNCARE AGRICULTURE CO., LTD. | α-CD β-CD | Method for manufacturing water-soluble propolis |
| 8 | CN 110664725 A [30] | China | 2019–2021 | A61K8/98, A61K8/73, A61K8/9789, A61K8/9794 | BEIJING ZHONGMI TECH DEVELOPMENT CO LTD | β-CD | Emulsion containing nano-propolis extract |
| Formulation | Solubility |
|---|---|
| Propolis | 465 μg/mL |
| 1-Quaternary composition: propolis, ammonium glycyrrhizate, β-CD, and L-glycine (1:1:7.5:0.5 w/w) | 1.695 μg/mL |
| 2-Quaternary composition: propolis, ammonium glycyrrhizate, β-CD, and glutamic acid (1:1:7.5:0.5 w/w) | 2.430 μg/mL |
| 3-Tertiary composition: propolis, β-CD, and L-glycine (1:7.5:0.5 w/w) | 1.584 μg/mL |
| 4-Tertiary composition: propolis, β-CD, and glutamic acid (1:7.5:0.5 w/w) | 1.999 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, S.; Barreto, I.C.; Gama, N.; Santos, K.; Oliveira, C.M.d.; Costa, I.S.; Vila Nova, M.; Santos, R.; Borges, A.; de Alencar Filho, J.M.T.; et al. Patent-Based Technological Overview of Propolis–Cyclodextrin Inclusion Complexes with Pharmaceutical Potential. Pharmaceutics 2025, 17, 898. https://doi.org/10.3390/pharmaceutics17070898
Costa S, Barreto IC, Gama N, Santos K, Oliveira CMd, Costa IS, Vila Nova M, Santos R, Borges A, de Alencar Filho JMT, et al. Patent-Based Technological Overview of Propolis–Cyclodextrin Inclusion Complexes with Pharmaceutical Potential. Pharmaceutics. 2025; 17(7):898. https://doi.org/10.3390/pharmaceutics17070898
Chicago/Turabian StyleCosta, Salvana, Ighor Costa Barreto, Nataly Gama, Kathylen Santos, Cleomárcio Miguel de Oliveira, Isabela Silva Costa, Monique Vila Nova, Ruane Santos, Arthur Borges, José Marcos Teixeira de Alencar Filho, and et al. 2025. "Patent-Based Technological Overview of Propolis–Cyclodextrin Inclusion Complexes with Pharmaceutical Potential" Pharmaceutics 17, no. 7: 898. https://doi.org/10.3390/pharmaceutics17070898
APA StyleCosta, S., Barreto, I. C., Gama, N., Santos, K., Oliveira, C. M. d., Costa, I. S., Vila Nova, M., Santos, R., Borges, A., de Alencar Filho, J. M. T., & do Nascimento, T. G. (2025). Patent-Based Technological Overview of Propolis–Cyclodextrin Inclusion Complexes with Pharmaceutical Potential. Pharmaceutics, 17(7), 898. https://doi.org/10.3390/pharmaceutics17070898







