Hydrogel Conjugation: Engineering of Hydrogels for Drug Delivery
Abstract
1. Introduction
2. Conjugation of Small Molecules to Hydrogels
2.1. Hydrogels in Wound-Healing and Drug Delivery
2.2. Hydrogels for Targeted Delivery
2.3. Stimuli-Responvise Hydrogels
| Hydrogel Constituents | Drugs | Method of Conjugation * | Release | Administration Routes | Stimuli-Responsive | |
|---|---|---|---|---|---|---|
| Zilactin-B Gel® by Zila Pharmaceuticals (Phoenix, AZ, USA) [17] | (Hydroxypropyl)methylcellulose (HPMC) | Benzocaine | drug dispersed in gel solution | 4–6 h pain-free on the oral mucosa | Buccal | |
| Thermosensitive poloxamer 407-based gel for ultrasound-mediated inner ear drug delivery [29] | Albumin-shelled microbubble gel, poloxamer | dexamethasone | sustained release of dexamethasone from the middle ear. On post-treatment days 1 and 7, the treated groups showed significantly higher drug levels than the injection group. | intratympanic | temperature | |
| Kaletra® film-coated tablets by AbbVie Ltd. (Chicago, IL, USA) [33] | Polyvinyl alcohol (PVA) | Lopinavir/ritonavir | Obtained steady-state pharmacokinetic properties and parameters of lopinavir | Oral | ||
| pH-sensitive hydrogel films for oral administration [34] | sodium tripolyphosphate cross-linked ternary blended chitosan, guar gum, polyvinylpyrrolidone | Ciprofloxacin hydrochloride | drug dissolved in aqueous gel solution | 30% of the drug was released in the first 30 min in simulated gastric fluid, and sustained release was observed in simulated intestinal fluid and phosphate-buffered saline | Oral 1 | pH |
| pH-sensitive hemicellulose/graphene oxide-based hydrogel for oral administration [35] | Physically cross-linked hemicellulose and graphene oxide | Vitamin B12 | Controlled intestinal release and decreased ineffective stomach release of the drug. | Oral | pH | |
| Drinkable liquid in situ-forming tough (LIFT) hydrogels [36] | Chemically cross-linked alginate, four-arm-PEG-maleimide | Lumefantrine | drug dispersed in gel solution | Hydrogel formulations resulted in peak plasma drug concentrations at 24 h, meanwhile free drug resulted in peak plasma concentrations at 5–7 h post-administration. Hydrogels can deliver comparable total drug doses as free drug at lower plasma concentrations. | Oral | pH |
| Astero® by Gensco Pharma (Doral, FL, USA) [37] | Polyethylene glycol (PEG) | Lidocaine hydrochloride | drug dissolved in aqueous gel solution | Fast pain relief with an onset of action within 3–5 min. | Transdermal | |
| Electro-responsive conductive hydrogel patch [38] | photo cross-linked gelatin methacrylate, alginate, and silver nanowire | Doxorubicin | drug dissolved or dispersed in aqueous gel solution; cationic doxorubicin interacts with hydrogel anionic groups 2 | Hydrogel patch controlled on-off drug release. Controlled drug release through hydrogel osmotic pressure and structural changes by electrical stimulation. | Transdermal | Electrical stimulation |
| Drug loaded- magnetite nanoparticles dispersed in hydrogel beads [39] | PNIPAM, methylene bisacrylamide, sodium alginate | Dexamethasone | drug dissolved or dispersed in the aqueous pre-gel solution | Light exposure enables on-demand drug release. Cumulative drug release was 24% for the first 10 h and 50% in 40 h, then reaching an equilibrium value of 66% in 120 h. The release rate could be adjusted by light intensity. | Transdermal | Light |
| Encare® by Blairex Laboratories Inc. (Columbus, IN, USA) [40,41] | PEG | Nonoxynol-9 | drug dissolved in aqueous gel solution | 1 suppository should be inserted at least 10 min before intercourse and provide effective contraception for up to 1 h after insertion. | Vaginal | |
| OncoGel™, a controlled-release depot formulation of paclitaxel in ReGel™, Protherics Salt Lake City, Inc. (Salt Lake City, UT, USA) [42,43,44] | PLGA and PEG | paclitaxel | drug dissolved or dispersed in ReGel™ | provides a depot of ReGel™ for the continuous release of paclitaxel directly to the tumor and surrounding tissue for 6 weeks. | Intralesional | temperature |
| Thermosensitive poloxamer-hyaluronic acid-kappa-carrageenan-based hydrogel anti-adhesive agent loaded with 5-fluorouracil [4] | Poloxamer, hyaluronic acid, kappa-carrageenan | 5-fluorouracil | drug dissolved in aqueous gel solution | The drug was initially burst released, the steady-state was achieved after 3–4 h, sustained release up to 3 days | Intraperitoneal | temperature |
| Silane-graphene dispersed cross-linked vinyl carboxymethyl chitosan and PNIPAM hydrogel [45] | Silane-graphene, vinyl carboxymethyl chitosan, PNIPAM | ciprofloxacin hydrochloride | The cumulative drug release increased rapidly in the first 7 h and remained constant after 7 h | Intranasal | temperature | |
| Bioadhesive hydrogels for spinal cord injury [46] | Chemically modified hyaluronic acid with dopamine | Ibuprofen | dopamine chemically conjugated to hyaluronic backbone via ester or amide bonds; ibuprofen dissolved or dispersed in the aqueous pre-gel solution | Intrathecal | ||
| Calcium-responsive composite hydrogel for acute spinal cord injury treatment [47,48] | Alginate, chitosan, and genipin (cross-linking agent) | In situ forming (rapid cross-linking) creates a cross-linking gradient, making the formation of a homogeneously cross-linked hydrogel difficult. The saturation of alginate/calcium cross-linking on the hydrogel’s surface significantly limits diffusion, resulting in slow drug release. 3 | Intrathecal | Ca2+ concentration | ||
| Ozurdex®, an intravitreal implant [49,50] | PLGA | dexamethasone | drug embedded in gel matrix using hot-melt extrusion | Ozurdex® is injected directly into the vitreous humor. The implant slowly releases dexamethasone for up to six months. | Intravitreal | |
| Nanoparticle-loaded ring implant placed between partially polymerized hydrogel contact lenses [51] | ethyl cellulose-nanoparticles incorporated in poly-hydroxyethyl methyl acrylate (HEMA), and poly[HEMA-co-methacrylic acid] loaded in polypropylene lens mold. | Timolol maleate | pre-formed gel soaked in drug solution | In vitro sustained drug release within the therapeutic window for 168 h and in vivo tear fluid release for more than 192 h | Ocular | |
| Thermosensitive chitosan/gelatin hydrogel eye drop [52] | Chitosan, gelatin, glycerol | Latanoprost | drug dissolved or dispersed in the aqueous pre-gel solution | sustained-release profile both in vitro and in vivo for 7 days | Ocular | temperature |
3. Conjugation of Biologics to Hydrogels
3.1. Nucleic Acids-Conjugated Hydrogels
| DNA/RNA Hydrogel Constituents | Drugs | Release | Administration Routes | Stimuli-Responsive | |
|---|---|---|---|---|---|
| DNA–RNA hybrid hydrogel for RNA release [53] | polymerized circular DNAs of AS 1411 aptamer and GFP siRNA | GFP siRNA | |||
| ordered structure of DNA hydrogel formed and self-assembled by polyadenine strands and cyanuric acid through hydrogen bonding [57] | polyadenine strands and cyanuric acid | DNA, DNA nanostructures, and gene-silencing nucleic acids (antisense oligonucleotides) | controlled release in a pH-responsive manner | Injection forming in situ depot | pH |
| Core–shell spherical 3D siRNA framework nucleic acids [63] | HCR initiated by siRNA with 4 hairpin DNA monomers, terminated by gDNA, covered with noncationic liposome membranes | doxorubicin | multiple Boolean logic gates arranged in a sequence, where the output of one gate acts as the input for the next, allow controlled release of doxorubicin and DNA in the presence of logical stimulators (glutathione, ATP, and survivin mRNA) | intravenous injection | tumor microenvironments and folate receptor overexpression |
| Injectable DNA supramolecular hydrogel vaccine system [73] | Y-scaffold DNA and DNA linkers assemble through non-covalent interactions | Antigens | Injection forming in situ depot | ||
| Immunostimulatory receptor molecules (CpG motifs) can be integrated into the DNA sequences within the hydrogel to enhance immune response. | |||||
| pH-responsive DNA hydrogel for mRNA delivery [74] | nanosphere formation from cross-linking X-shaped DNA scaffolds containing a pH-responsive i-motif sequence and DNA linkers | mRNA encoding Gluc | The hydrogel was stable under neutral pH, but at pH 4.5–5, it disintegrated and released the mRNA after being endocytosed into cells through the lysosome. | - | pH |
| “I-gel” enabling in situ siRNA production [75] | DNA hydrogel scaffolds incorporated with plasmid DNA encoding siRNA gene | Plasmid DNA, siRNA | |||
| Injectable DNA–chitosan hybrid hydrogel [76] | DNA gel cross-linked with chitosan | dexamethasone | The gel degraded rapidly without coating. The release of dexamethasone corresponded with the gel degradation. | Injection | |
| Photothermal polydopamine-coated DNA gel [77] | siRNA cross-linked with DNA-grafted polycaprolactone, coated with polydopamine and surface PEGylation | siRNA | Slow release. siRNAs were released after 36 h. Acidic conditions induce photothermal conversion, polydopamine degradation, and siRNA release. | Intravenous injection | Light energy heat |
| self-assembled, dendrimer complexed RNA-hydrogel [78] | miRNAs formed complexation with polyamidoamin dendrimers | Two types of miRNAs: a miR mimic and an antagomiR | tumor suppressor miR was released first, then oncogenic miR was released | Intraperitoneal injection | |
| Self-assembled, layered RNA dendrimers for layer-by-layer release [79] | 4 generations of RNA dendrimers | paclitaxel | RNA assembly degraded, leading to the exposure of RNA-paclitaxel linkage. Paclitaxel was released from the linkage through hydrolysis with low cytokine release. | Injection | |
3.2. Protein- and Peptide-Conjugated Hydrogels
| Protein/Peptide-Hydrogel Conjugates | Self-Assembly and Physical Entrapment | Chemical Conjugation |
| Methods |
|
3.3. Cell-Conjugated Hydrogels
| Cell–Hydrogel Conjugates | Hydrogel Constituents | Cell Type | Method of Conjugations | Specifications | Administration Routes | Stimuli-Responsive |
|---|---|---|---|---|---|---|
| Multi-layer hydrogel-loaded mesenchymal stem cells for cartilage repair [107] | PEG di-methacrylate, methacrylate chondroitin sulfate | Mesenchymal stem cells | Physical entrapment (encapsulation) | |||
| Injectable stem cell-based hydrogel delivering miRNA for cartilage repair [108] | a cholesterol-modified miRNA mimic, bone marrow–homing and stem cell–homing peptides assembly | Synovium-derived mesenchymal stem cells | Sustained delivery of miRNA and recruitment of stem cells for cartilage repair | Intra-articular injection | ||
| Injectable TGF-β1 conjugated chitosan, collagen hydrogel for articular cartilage regeneration [109] | TGF-β1 growth and chondrogenic factors conjugated chitosan, collagen | Mesenchymal stem cells | Mesenchymal stem cells embedded in the gel matrix (physical entrapment); TGF-β1 growth and chondrogenic factors are chemically linked to chitosan through covalent bonds (covalent conjugation) | The delivery of TGF-β1 growth factor and collagen promoted cellular aggregation and deposition of cartilaginous extracellular matrix | Injection | |
| Alginate-collagen encapsulation of human pancreatic islet cells for transplantation [110] | Alginate, collagen 1 | Islet cells | cells suspended in gel solution (Physical entrapment) | Collagen improves islet cell survival [110]. Although, alginate is often used for islet cell encapsulation. Alginate cannot support islet cell survival [111]. | ||
| 3D hydrogel tissue model for liver tissue engineering [105,112] | Gelatin methacryloyl | Hepatocytes, fibroblasts | The tissue model maintained over 90% cell viability. | |||
| T-cell stimulating hydrogel matrix for immunotherapy [113] | Thiolated hyaluronic acid cross-linked with PEG diacrylate | T cells | T cell activation | T cell stimulatory signals | ||
| T-cell-responsive hydrogels for in situ T-cell expansion and enhanced antitumor efficacy [114] | Dynabeads, PEG, alginate | T cells | Dynabeads embedded in the gel solution; specific bio-affinity between Dynabeads (T-cell-specific antibody-coated beads) and T-cells; T-cells entrapped in the gel matrix | Enabled controlled release of T cell activation and facilitated T cell expansion as well es enhanced antitumor efficacy. | Injectable | T cell stimulatory signals |
| Hydrogel-releasing CAR-T cells and anti-PDL1-conjugated platelets [115] | Acrylated hyaluronic acid | CAR T cells, cytokine interleukin-15 (IL-15), platelets conjugated with the checkpoint inhibitor programmed death-ligand 1 2 | physical entrapment | sustained release of CAR-T cells targeting the human chondroitin sulfate proteoglycan 4 and IL-15 | Intraoperative patch | cytokine IL-15 |
| Biopolymeric implant for the delivery of T-cell therapy [116] | Alginate, collagen | T cells | Target delivery to tumor site for the release, expansion, and dispersion of T cells | Implantation | ||
| Injectable hydrogels for controlled co-delivery of CAR-T cells and stimulatory cytokines [117] | HPMC, RGD-PEG-PLA nanoparticles 3 | CAR-T cells, cytokine IL-15 | IL-15 slowly diffused and released, activated T cells. CAR-T cells were continuously released over 8 days from 2 formulations with >85% viability, enabling 4.5-fold enhancement CAR-T cell exposure compared to standard bolus administration. | Injection | cytokine IL-15 | |
| Injectable thermosensitive hydrogels for controlled delivery of NK cells against solid tumor [118] | Hydroxyapatite-modified chitosan | NK cells | NK cell stimulation and release for tumor immunotherapy. | Injection | temperature | |
| Localized in situ gelling PEG-based hydrogel for multiple sclerosis [119] | PEG | Dendritic cells treated with IL-10 | PEG attached to dendritic cells (bio affinity-based conjugation) | Cells were delivered locally, altered the injection site recruited, increased endogenous immune cell profile within 2 days. | Injection |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLGA | poly (lactic-co-glycolic acid) |
| PLA | poly (lactic acid) |
| PGA | poly (glycolic acid) |
| HPMC | (hydroxypropyl)methylcellulose |
| PNIPAM | poly(N-isopropyl acrylamide) |
| PEG | polyethylene glycol |
| HEMA | hydroxyethyl methyl acrylate |
| PCR | polymerase chain reaction |
| HCR | hybridization chain reaction |
| RCA | rolling circle amplification |
| MCA | multi-primed chain amplification |
| MDA | multiple displacement amplification |
| C-HCR | clamped-hybridization chain reaction |
| NK cells | natural killer cells |
| IL-15 | interleukin-15 |
References
- Vaginal Itching Relief, Dryness & Odor Protection. Available online: https://www.vagisil.com (accessed on 14 February 2025).
- Brodwall, J.; Alme, G.; Gedde-Dahl, S.; Smith, J.; Lilliedahl, N.P.; Kunz, P.A.; Sunderraj, P. A comparative study of polyacrylic acid (Viscotears) liquid gel versus polyvinylalcohol in the treatment of dry eyes. Acta Ophthalmol. Scand. 1997, 75, 457–461. [Google Scholar] [CrossRef] [PubMed]
- ACUVUE® Brand Contact Lenses. Available online: https://www.acuvue.com (accessed on 14 February 2025).
- Dinh, L.; Hong, J.; Min Kim, D.; Lee, G.; Jung Park, E.; Hyuk Baik, S.; Hwang, S.J. A novel thermosensitive poloxamer-hyaluronic acid- kappa-carrageenan-based hydrogel anti-adhesive agent loaded with 5-fluorouracil: A preclinical study in Sprague-Dawley rats. Int. J. Pharm. 2022, 621, 121771. [Google Scholar] [CrossRef] [PubMed]
- GranuGEL® Gel—Wound|ConvaTec. Available online: https://www.convatec.com/en-gb/products/advanced-wound-care/wound-type/pc-wound-leg-ulcers/granugel-gel (accessed on 14 February 2025).
- Raeisi, A.; Farjadian, F. Commercial hydrogel product for drug delivery based on route of administration. Front. Chem. 2024, 12, 1336717. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Young, D.J.; Loh, X.J. Chapter 8: From Bench to Bedside—OncoGel™, an In Situ Hydrogel for In Vivo Applications. In Biodegradable Thermogels; Loh, X.J., Young, D.J., Eds.; The Royal Society of Chemistry: London, UK, 2018; pp. 133–144. [Google Scholar]
- Costello, M.A.; Liu, J.; Wang, Y.; Qin, B.; Xu, X.; Li, Q.; Lynd, N.A.; Zhang, F. Reverse engineering the Ozurdex dexamethasone intravitreal implant. Int. J. Pharm. 2023, 634, 122625. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Dinh, L.; Machamasi, R.; Kim, C.J.; Lee, J.-J.; Choi, Y.; Kim, H.; Mahon, L.; Grabenbauer, A.; Yan, B.; Hwang, S.-J. 3D-printed chitosan-pectin-sodium alginate scaffolds for post-surgical peritoneal wound dressing and sustained delivery of oxaliplatin. J. Pharm. Investig. 2024, 54, 857–871. [Google Scholar] [CrossRef]
- Chelu, M.; Magdalena Musuc, A. Biomaterials-Based Hydrogels for Therapeutic Applications; IntechOpen: London, UK, 2024. [Google Scholar]
- Li, J.; Mo, L.; Lu, C.H.; Fu, T.; Yang, H.H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef]
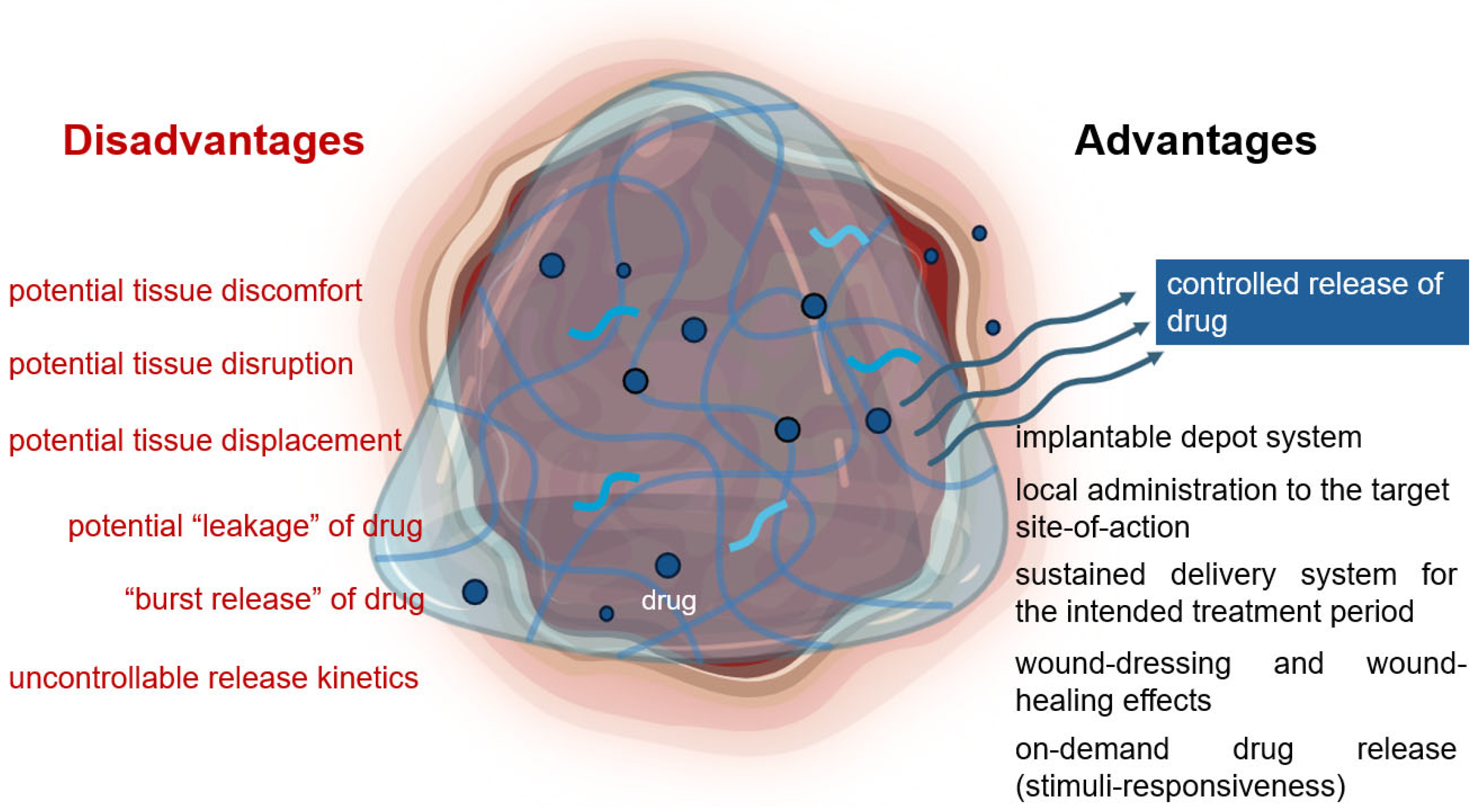
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Rani, R.; Thakur, V.K.; Gupta, M. New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective. ACS Omega 2023, 8, 19145–19167. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef]
- Gilhotra, R.M.; Ikram, M.; Srivastava, S.; Gilhotra, N. A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed. Res. 2014, 28, 81–97. [Google Scholar]
- Oral Balance Moisturizing Gel for Dry Mouth Symptoms. Available online: https://www.biotene.com/dry-mouth-products/moisturizing-gel (accessed on 14 February 2025).
- Chen, W.; Zhang, C.; Peng, S.; Lin, Y.; Ye, Z. Hydrogels in Dental Medicine. Adv. Ther. 2023, 7, 2300128. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Chen, A.; Deng, S.; Lai, J.; Li, J.; Chen, W.; Varma, S.N.; Zhang, J.; Lei, C.; Liu, C.; Huang, L. Hydrogels for Oral Tissue Engineering: Challenges and Opportunities. Molecules 2023, 28, 3946. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Mosi-Roa, Y.; Rubilar, M.; Burgos-Díaz, C. Probiotics as an Adjunct Therapy for the Treatment of Halitosis, Dental Caries and Periodontitis. Probiotics Antimicrob. Proteins 2020, 12, 325–334. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Shih, C.P.; Li, M.W.; Lin, Y.C.; Chuang, H.C.; Wang, C.H. Development of thermosensitive poloxamer 407-based microbubble gel with ultrasound mediation for inner ear drug delivery. Drug Deliv. 2021, 28, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Bressler, E.M.; Adams, S.; Liu, R.; Colson, Y.L.; Wong, W.W.; Grinstaff, M.W. Boolean logic in synthetic biology and biomaterials: Towards living materials in mammalian cell therapeutics. Clin. Transl. Med. 2023, 13, e1244. [Google Scholar] [CrossRef]
- Hollingsworth, N.R.; Wilkanowicz, S.I.; Larson, R.G. Salt- and pH-induced swelling of a poly(acrylic acid) brush via quartz crystal microbalance w/dissipation (QCM-D). Soft Matter 2019, 15, 7838–7851. [Google Scholar] [CrossRef]
- KALETRA (Lopinavir/Ritonavir) Label. Available online: https://clinicalinfo.hiv.gov/en/drugs/lopinavir-ritonavir/patient (accessed on 14 February 2025).
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (chitosan/guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.; Wang, X.; Yin, X.; Fu, M.; Qin, T.; Ji, X.; Yang, G.; Sun, S. A physical crosslinked pH-sensitive hydrogel based on hemicellulose/graphene oxide for controlled oral drug delivery. Int. J. Biol. Macromol. 2025, 289, 138875. [Google Scholar] [CrossRef]
- Liu, G.W.; Pickett, M.J.; Kuosmanen, J.L.P.; Ishida, K.; Madani, W.A.M.; White, G.N.; Jenkins, J.; Park, S.; Feig, V.R.; Jimenez, M.; et al. Drinkable in situ-forming tough hydrogels for gastrointestinal therapeutics. Nat. Mater. 2024, 23, 1292–1299. [Google Scholar] [CrossRef]
- Astero. Gensco Pharma. Available online: https://genscopharma.com/products/astero (accessed on 14 February 2025).
- Ha, J.H.; Lim, J.H.; Lee, J.M.; Chung, B.G. Electro-Responsive Conductive Blended Hydrogel Patch. Polymers 2023, 15, 2608. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Seong, K.Y.; Lee, E.; Yang, S.Y.; Yoon, J. Visible Light-Triggered On-Demand Drug Release from Hybrid Hydrogels and its Application in Transdermal Patches. Adv. Healthc. Mater. 2015, 4, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Chappell, B.T.; Griffin, B.L.; Howard, B. Mechanisms of action of currently available woman-controlled, vaginally administered, non-hormonal contraceptive products. Ther. Adv. Reprod. Health 2022, 16, 26334941221107120. [Google Scholar] [CrossRef] [PubMed]
- Encare by Blairex Laboratories, Inc. FDA Report. Available online: https://fda.report/ (accessed on 21 March 2025).
- Vukelja, S.J.; Anthony, S.P.; Arseneau, J.C.; Berman, B.S.; Cunningham, C.C.; Nemunaitis, J.J.; Samlowski, W.E.; Fowers, K.D. Phase 1 study of escalating-dose OncoGel (ReGel/paclitaxel) depot injection, a controlled-release formulation of paclitaxel, for local management of superficial solid tumor lesions. Anticancer Drugs 2007, 18, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Elstad, N.L.; Fowers, K.D. OncoGel (ReGel/paclitaxel)—Clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009, 61, 785–794. [Google Scholar] [CrossRef]
- Fowers, K. Chapter 9: ReGel™ Hydrogels for In Vivo Applications. In Polymeric and Self Assembled Hydrogels: From Fundamental Understanding to Applications; Loh, X.J., Scherman, O.A., Eds.; The Royal Society of Chemistry: London, UK, 2012; pp. 210–231. [Google Scholar]
- Kang, W.; Liang, J.; Liu, T.; Long, H.; Huang, L.; Shi, Q.; Zhang, J.; Deng, S.; Tan, S. Preparation of silane-dispersed graphene crosslinked vinyl carboxymethyl chitosan temperature-responsive hydrogel with antibacterial properties. Int. J. Biol. Macromol. 2022, 200, 99–109. [Google Scholar] [CrossRef]
- Duarte, D.; Correia, C.; Reis, R.L.; Pashkuleva, I.; Peixoto, D.; Alves, N.M. Bioadhesive Hyaluronic Acid-Based Hydrogels for Spinal Cord Injury. Biomacromolecules 2024, 25, 1592–1601. [Google Scholar] [CrossRef]
- McKay, C.A.; Pomrenke, R.D.; McLane, J.S.; Schaub, N.J.; DeSimone, E.K.; Ligon, L.A.; Gilbert, R.J. An injectable, calcium responsive composite hydrogel for the treatment of acute spinal cord injury. ACS Appl. Mater. Interfaces 2014, 6, 1424–1438. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, X.; Zhang, R.; Huang, Y.; Wu, C. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS PharmSciTech 2010, 11, 610–620. [Google Scholar] [CrossRef]
- Ozurdex. Abbvie. Available online: https://www.ozurdex.com (accessed on 14 February 2025).
- Iovino, C.; Mastropasqua, R.; Lupidi, M.; Bacherini, D.; Pellegrini, M.; Bernabei, F.; Borrelli, E.; Sacconi, R.; Carnevali, A.; D’Aloisio, R.; et al. Intravitreal Dexamethasone Implant as a Sustained Release Drug Delivery Device for the Treatment of Ocular Diseases: A Comprehensive Review of the Literature. Pharmaceutics 2020, 12, 703. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Ko, Y.C.; Chang, Y.F.; Huang, S.H.; Liu, C.J. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Park, Y.; Kim, H.; Nam, H.; Ko, O.; Lee, J.B. Double Controlled Release of Therapeutic RNA Modules through Injectable DNA-RNA Hybrid Hydrogel. ACS Appl. Mater. Interfaces 2020, 12, 55554–55563. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Talebian, S.; Mendes, B.B.; Wallace, G.; Langer, R.; Conde, J.; Shi, J. Hydrogels for RNA delivery. Nat. Mater. 2023, 22, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Pasandideh, R.; Gazi, M.; Teralı, K.; Oladipo, A.A. Fabrication of human genomic DNA encapsulated supermacroporous alginate beads. Mater. Technol. 2020, 36, 331–338. [Google Scholar] [CrossRef]
- Cao, T.; Jia, H.; Dong, Y.; Gui, S.; Liu, D. In Situ Formation of Covalent Second Network in a DNA Supramolecular Hydrogel and Its Application for 3D Cell Imaging. ACS Appl. Mater. Interfaces 2020, 12, 4185–4192. [Google Scholar] [CrossRef]
- Lachance-Brais, C.; Rammal, M.; Asohan, J.; Katolik, A.; Luo, X.; Saliba, D.; Jonderian, A.; Damha, M.J.; Harrington, M.J.; Sleiman, H.F. Small Molecule-Templated DNA Hydrogel with Record Stiffness Integrates and Releases DNA Nanostructures and Gene Silencing Nucleic Acids. Adv. Sci. 2023, 10, e2205713. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, C.; Song, W.; Wang, Q.; Kjems, J.; Gao, S. Chitosan hydrogel as siRNA vector for prolonged gene silencing. Nanobiotechnology 2014, 12, 23. [Google Scholar] [CrossRef]
- Fei, Z.; Gupta, N.; Li, M.; Xiao, P.; Hu, X. Toward highly effective loading of DNA in hydrogels for high-density and long-term information storage. Sci. Adv. 2023, 9, eadg9933. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Q.; Li, Q.; Paluzzi, V.E.; Choi, J.H.; Mao, C. Engineering the Nanoscaled Morphologies of Linear DNA Homopolymers. Macromol. Rapid Commun. 2021, 42, e2100217. [Google Scholar] [CrossRef]
- Zhang, C.; Su, M.; He, Y.; Zhao, X.; Fang, P.A.; Ribbe, A.E.; Jiang, W.; Mao, C. Conformational flexibility facilitates self-assembly of complex DNA nanostructures. Proc. Natl. Acad. Sci. USA 2008, 105, 10665–10669. [Google Scholar] [CrossRef]
- Kahn, J.S.; Hu, Y.; Willner, I. Stimuli-Responsive DNA-Based Hydrogels: From Basic Principles to Applications. Acc. Chem. Res. 2017, 50, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Sun, J.; Ouyang, J.; Na, N. SiRNA-templated 3D framework nucleic acids for chemotactic recognition, and programmable and visualized precise delivery for synergistic cancer therapy. Chem. Sci. 2021, 12, 15353–15361. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bian, A.; Agarwal, A.; Liu, L.; Shen, H.; Wang, L.; Xu, C.; Kotov, N.A. Nanoparticle superstructures made by polymerase chain reaction: Collective interactions of nanoparticles and a new principle for chiral materials. Nano Lett. 2009, 9, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, C.; Liu, D.; Liu, W. Chemical strategies and biomedical applications of DNA hydrogels. Fundam. Res. 2022, 3, 534–536. [Google Scholar] [CrossRef]
- Hamblin, G.D.; Carneiro, K.M.; Fakhoury, J.F.; Bujold, K.E.; Sleiman, H.F. Rolling circle amplification-templated DNA nanotubes show increased stability and cell penetration ability. J. Am. Chem. Soc. 2012, 134, 2888–2891. [Google Scholar] [CrossRef]
- Keller, S.; Wang, J.; Chandra, M.; Berger, R.; Marx, A. DNA polymerase-catalyzed DNA network growth. J. Am. Chem. Soc. 2008, 130, 13188–13189. [Google Scholar] [CrossRef]
- Yuan, B.F.; Xue, Y.; Luo, M.; Hao, Y.H.; Tan, Z. Two DNAzymes targeting the telomerase mRNA with large difference in Mg2+ concentration for maximal catalytic activity. Int. J. Biochem. Cell Biol. 2007, 39, 1119–1129. [Google Scholar] [CrossRef]
- Schubert, S.; Gül, D.C.; Grunert, H.P.; Zeichhardt, H.; Erdmann, V.A.; Kurreck, J. RNA cleaving ‘10-23’ DNAzymes with enhanced stability and activity. Nucleic Acids Res. 2003, 31, 5982–5992. [Google Scholar] [CrossRef]
- Rosenbach, H.; Victor, J.; Etzkorn, M.; Steger, G.; Riesner, D.; Span, I. Molecular Features and Metal Ions That Influence 10-23 DNAzyme Activity. Molecules 2020, 25, 3100. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, L.; Yang, S.; Tang, X.; Chang, K.; Chen, M. Boolean logic gate based on DNA strand displacement for biosensing: Current and emerging strategies. Nanoscale Horiz. 2021, 6, 298–310. [Google Scholar] [CrossRef]
- Fujii, S.; Minami, S.; Yashima, E. Synthetic Nucleic-Acid Droplets: A Bioprogramming Platform for Designer Micro-Liquids. Polym. J. 2025, 57, 577–586. [Google Scholar]
- Shao, Y.; Sun, Z.Y.; Wang, Y.; Zhang, B.D.; Liu, D.; Li, Y.M. Designable Immune Therapeutical Vaccine System Based on DNA Supramolecular Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 9310–9314. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, T.; Song, Y.; Feng, C.; Chen, H.; Zhang, Q.; Chen, G.; Zhu, X. mRNA Delivery by a pH-Responsive DNA Nano-Hydrogel. Small 2021, 17, e2101224. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, M.; Kim, T.; Na, J.; Jung, Y.; Jung, G.Y.; Kim, S.; Park, N. A RNA producing DNA hydrogel as a platform for a high performance RNA interference system. Nat. Commun. 2018, 9, 4331. [Google Scholar] [CrossRef]
- Chen, F.; He, Y.; Li, Z.; Xu, B.; Ye, Q.; Li, X.; Ma, Z.; Song, W.; Zhang, Y. A novel tunable, highly biocompatible and injectable DNA-chitosan hybrid hydrogel fabricated by electrostatic interaction between chitosan and DNA backbone. Int. J. Pharm. 2021, 606, 120938. [Google Scholar] [CrossRef]
- Ding, F.; Gao, X.; Huang, X.; Ge, H.; Xie, M.; Qian, J.; Song, J.; Li, Y.; Zhu, X.; Zhang, C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials 2020, 245, 119976. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Atilano, M.; Song, H.S.; Artzi, N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat. Mater. 2016, 15, 353–363. [Google Scholar] [CrossRef]
- Li, X.; Vieweger, M.; Guo, P. Self-assembly of four generations of RNA dendrimers for drug shielding with controllable layer-by-layer release. Nanoscale 2020, 12, 16514–16525. [Google Scholar] [CrossRef]
- Fisher, S.A.; Baker, A.E.G.; Shoichet, M.S. Designing Peptide and Protein Modified Hydrogels: Selecting the Optimal Conjugation Strategy. J. Am. Chem. Soc. 2017, 139, 7416–7427. [Google Scholar] [CrossRef]
- Holz, E.; Darwish, M.; Tesar, D.B.; Shatz-Binder, W. A Review of Protein- and Peptide-Based Chemical Conjugates: Past, Present, and Future. Pharmaceutics 2023, 15, 600. [Google Scholar] [CrossRef]
- Qi, Y.; Chilkoti, A. Protein-polymer conjugation-moving beyond PEGylation. Curr. Opin. Chem. Biol. 2015, 28, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.J.; Choi, S.H.; Choi, J.S.; Kim, I.; Shin, B.S.; Lee, E.S.; Park, E.S.; Lee, K.C.; Youn, Y.S. Four-arm PEG cross-linked hyaluronic acid hydrogels containing PEGylated apoptotic TRAIL protein for treating pancreatic cancer. Acta Biomater. 2014, 10, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Coolich, M.K.; Lanier, O.L.; Cisneros, E.; Peppas, N.A. PEGylated insulin loaded complexation hydrogels for protected oral delivery. J. Control. Release 2023, 364, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef]
- Cai, B.; Saito, A.; Ikeda, S. Maillard Conjugation of Sodium Alginate to Whey Protein for Enhanced Resistance to Surfactant-Induced Competitive Displacement from Air-Water Interfaces. J. Agric. Food Chem. 2018, 66, 704–710. [Google Scholar] [CrossRef]
- Jain, S.; Zhong, Q. Enhancing the functionality of pea proteins by conjugation with propylene glycol alginate via transacylation reaction assisted with ultrasonication. Food Chem. 2024, 449, 139179. [Google Scholar] [CrossRef]
- Hozumi, K.; Kumai, J.; Yamada, Y.; Nomizu, M. Active Peptide-Conjugated Chitosan Matrices as an Artificial Basement Membrane. Polymers 2015, 7, 281–297. [Google Scholar] [CrossRef]
- Rathinam, S.; Sørensen, K.K.; Hjálmarsdóttir, M.Á.; Thygesen, M.B.; Másson, M. Conjugation of CRAMP18–35 Peptide to Chitosan and Hydroxypropyl Chitosan via Copper-Catalyzed Azide–Alkyne Cycloaddition and Investigation of Antibacterial Activity. Int. J. Mol. Sci. 2024, 25, 9440. [Google Scholar] [CrossRef]
- Hozumi, K.; Nomizu, M. Mixed Peptide-Conjugated Chitosan Matrices as Multi-Receptor Targeted Cell-Adhesive Scaffolds. Int. J. Mol. Sci. 2018, 19, 2713. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, J.; Li, Y.; Wei, P.; Liu, S.; Yang, R. The Formation of Protein-Chitosan Complexes: Their Interaction, Applications, and Challenges. Foods 2024, 13, 3572. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, J.; Huang, R.; Qi, W.; Wang, Y.; Su, R.; He, Z. Calcium-Ion-Triggered Co-assembly of Peptide and Polysaccharide into a Hybrid Hydrogel for Drug Delivery. Nanoscale Res. Lett. 2016, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Aminuddin, M.; Ramya, B.; Liew, M.W.J.; Liu, J.; Venkatraman, S.S. Designing siRNA/chitosan-methacrylate complex nanolipogel for prolonged gene silencing effects. Sci. Rep. 2022, 12, 3527. [Google Scholar] [CrossRef] [PubMed]
- Denizli, M.; Aslan, B.; Mangala, L.S.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Sood, A.K. Chitosan Nanoparticles for miRNA Delivery. Methods Mol. Biol. 2017, 1632, 219–230. [Google Scholar] [PubMed]
- Chen, W.Y.; Chen, Y.T.; Ke, C.J.; Chen, C.Y.; Lin, F.H. The Synthesis and Evaluation of RGD-Conjugated Chitosan Gel as Daily Supplement for Body Weight Control. Materials 2021, 14, 4467. [Google Scholar] [CrossRef]
- Jia, Y.; Taledaohan, A.; Jia, R.; Wang, X.; Jia, Y.; Liu, J.; Wang, Y. Chitosan nanomedicine containing RGD peptide and PAD4 inhibitor based on phenyl boronate coupling inhibition of primary tumor growth and lung metastasis. Biomed. Pharmacother. 2023, 168, 115826. [Google Scholar] [CrossRef]
- Ligorio, C.; Mata, A. Synthetic extracellular matrices with function-encoding peptides. Nat. Rev. Bioeng. 2023, 1, 518–536. [Google Scholar] [CrossRef]
- Du, P.; Diao, L.; Lu, Y.; Liu, C.; Li, J.; Chen, Y.; Chen, J.; Lv, G.; Chen, X. Heparin-based sericin hydrogel-encapsulated basic fibroblast growth factor for in vitro and in vivo skin repair. Heliyon 2023, 9, e13554. [Google Scholar] [CrossRef]
- Tae, G.; Scatena, M.; Stayton, P.S.; Hoffman, A.S. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J. Biomater. Sci. Polym. Ed. 2006, 17, 187–197. [Google Scholar] [CrossRef]
- Choi, W.I.; Kim, M.; Tae, G.; Kim, Y.H. Sustained release of human growth hormone from heparin-based hydrogel. Biomacromolecules 2008, 9, 1698–1704. [Google Scholar] [CrossRef]
- Alina, T.B.; Nash, V.A.; Spiller, K.L. Effects of Biotin-Avidin Interactions on Hydrogel Swelling. Front. Chem. 2020, 8, 593422. [Google Scholar] [CrossRef]
- Jain, E.; Neal, S.; Graf, H.; Tan, X.; Balasubramaniam, R.; Huebsch, N. Copper-Free Azide-Alkyne Cycloaddition for Peptide Modification of Alginate Hydrogels. ACS Appl. Bio Mater. 2021, 4, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E. Bioconjugation of hydrogels for tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, H.; Shen, H.; Wang, B.; Lei, G.; Tuan, R.S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018, 69, 71–82. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, X.; Zhou, X.; Tan, W.; Luo, H.; Lei, S.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar] [CrossRef]
- Steinmetz, N.J.; Aisenbrey, E.A.; Westbrook, K.K.; Qi, H.J.; Bryant, S.J. Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater. 2015, 21, 142–153. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, S.; Qi, Y.; Gong, Z.; Zhang, H.; Liang, K.; Shen, P.; Huang, Y.Y.; Zhang, Z.; Ye, W.; et al. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci. Adv. 2022, 8, eabk0011. [Google Scholar] [CrossRef]
- Kim, J.; Lin, B.; Kim, S.; Choi, B.; Evseenko, D.; Lee, M. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J. Biol. Eng. 2015, 9, 1. [Google Scholar] [CrossRef]
- Llacua, L.A.; Hoek, A.; de Haan, B.J.; de Vos, P. Collagen type VI interaction improves human islet survival in immunoisolating microcapsules for treatment of diabetes. Islets 2018, 10, 60–68. [Google Scholar] [CrossRef]
- Enck, K.; Tamburrini, R.; Deborah, C.; Gazia, C.; Jost, A.; Khalil, F.; Alwan, A.; Orlando, G.; Opara, E.C. Effect of alginate matrix engineered to mimic the pancreatic microenvironment on encapsulated islet function. Biotechnol. Bioeng. 2021, 118, 1177–1185. [Google Scholar] [CrossRef]
- Kasturi, M.; Mathur, V.; Gadre, M.; Srinivasan, V.; Vasanthan, K.S. Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications. Tissue Eng. Regen. Med. 2024, 21, 21–52. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Dong, Y.; Chung, J.W.; Salathe, S.F.; Pruitt, H.C.; Li, X.; Chang, C.; Fraser, A.K.; Bessell, C.A.; Ewald, A.J.; et al. Engineering an Artificial T-Cell Stimulating Matrix for Immunotherapy. Adv. Mater. 2019, 31, e1807359. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Han, J.; Liu, Y.; Bo, Y.; Wang, H. T cell-responsive macroporous hydrogels for in situ T cell expansion and enhanced antitumor efficacy. Biomaterials 2023, 293, 121972. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Archibong, E.; Chen, Q.; Ruan, H.; Ahn, S.; Dukhovlinova, E.; Kang, Y.; Wen, D.; Dotti, G.; et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat. Biomed. Eng. 2021, 5, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Stephan, S.B.; Taber, A.M.; Jileaeva, I.; Pegues, E.P.; Sentman, C.L.; Stephan, M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015, 33, 97–101. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Labanieh, L.; Klysz, D.D.; Roth, G.A.; Xu, P.; Adebowale, O.; Gale, E.C.; Jons, C.K.; Klich, J.H.; Yan, J.; et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 2022, 8, eabn8264. [Google Scholar] [CrossRef]
- Jiao, D.; Hao, M.; Sun, R.; Ren, X.; Wei, Y.; Ding, M.; Yue, X.; Wu, Z.; Li, C.; Gao, L.; et al. Dynamic Hybrid Module-Driven NK Cell Stimulation and Release for Tumor Immunotherapy. Nano Lett. 2024, 24, 5481–5489. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Beskid, N.M.; Blanchfield, J.L.; Rosado, A.M.; García, A.J.; Evavold, B.D.; Babensee, J.E. Localized hydrogel delivery of dendritic cells for attenuation of multiple sclerosis in a murine model. J. Biomed. Mater. Res. A 2021, 109, 1247–1255. [Google Scholar] [CrossRef]
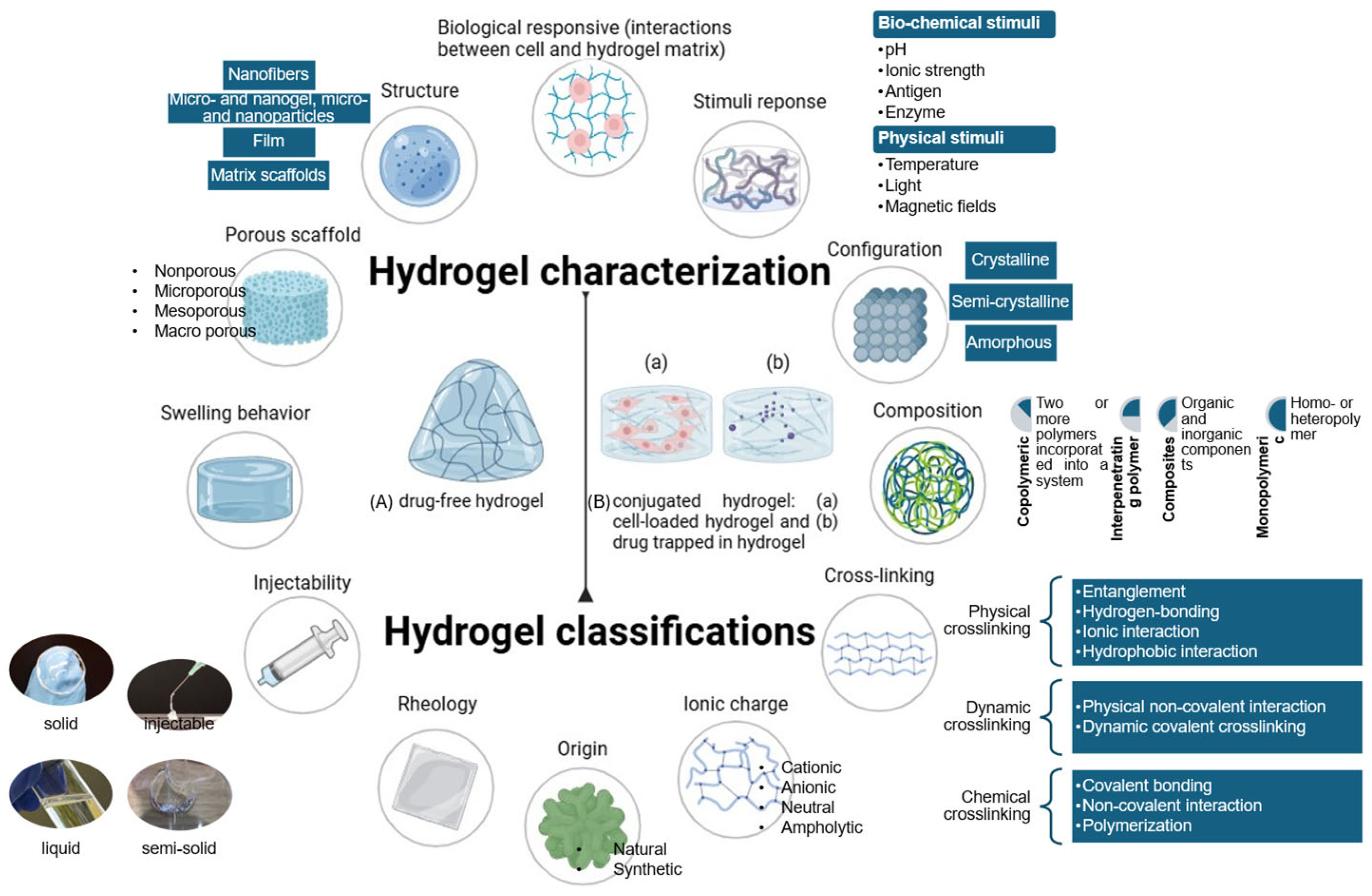



| Hydrogel –drug conjugation | Drug component | |||
| Hydrogel component | Drug type | |||
| Small molecules | chemical compounds, usually <1000 Da | |||
| Biologics | Monomers | single molecule units (e.g., amino acids, nucleotides, etc.) | ||
| Polymers | large biomolecules including peptides, proteins, nucleic acids (e.g., DNA, RNA), and polysaccharides. | |||
| Cells | complex systems made up of polymeric biomolecules. | |||
| Other biotechnological drug delivery systems (carriers) | micelles, liposomes, dendrimers, micro- and nanoparticles… | |||
| DNA–Hydrogel Conjugates | Hydrogels Made Entirely of DNA | Hydrogels Containing DNA as a Functional Graft | Hydrogels with DNA as a Cross-Linker in Polymer Subunits | ||
|---|---|---|---|---|---|
| Methods | Self-assembly | ||||
| Enzymatic reactions | Ligation | ||||
| Hybridization | PCR | ||||
| RCA: amplifying DNA using a circular DNA template | |||||
| Multi-primed chain amplification (MCA) and/or multiple displacement amplification (MDA): significantly amplifying small amounts of DNA, allowing amplification from minimal starting sequences | |||||
| Clamped-hybridization chain reaction (C-HCR): Two DNA hairpins self-assembled into a larger structure through hybridization, with an initiator strand “clamp” to trigger the assembly process, forming a hydrogel. | |||||
| Chemical cross-linkers | ethylene glycol diglycidyl ether (EGDE) and polyethylene glycol diglycidyl ether (PEGDE) | ||||
| tetramethyl ethylenediamine (TEMED) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh, L.; Hwang, S.-J.; Yan, B. Hydrogel Conjugation: Engineering of Hydrogels for Drug Delivery. Pharmaceutics 2025, 17, 897. https://doi.org/10.3390/pharmaceutics17070897
Dinh L, Hwang S-J, Yan B. Hydrogel Conjugation: Engineering of Hydrogels for Drug Delivery. Pharmaceutics. 2025; 17(7):897. https://doi.org/10.3390/pharmaceutics17070897
Chicago/Turabian StyleDinh, Linh, Sung-Joo Hwang, and Bingfang Yan. 2025. "Hydrogel Conjugation: Engineering of Hydrogels for Drug Delivery" Pharmaceutics 17, no. 7: 897. https://doi.org/10.3390/pharmaceutics17070897
APA StyleDinh, L., Hwang, S.-J., & Yan, B. (2025). Hydrogel Conjugation: Engineering of Hydrogels for Drug Delivery. Pharmaceutics, 17(7), 897. https://doi.org/10.3390/pharmaceutics17070897








