In Vitro Assessment of a Doubly Adjuvanted Self-Emulsified Nanoemulsion as a Delivery Vehicle for Antigenic Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Protein/DMPG Complexes
2.3. Preparation and Characterization of Protein/DMPG-Loaded ST-SNEDDSs
2.4. Theoretical Prediction of Protein Release from the Nanoemulsion Droplets
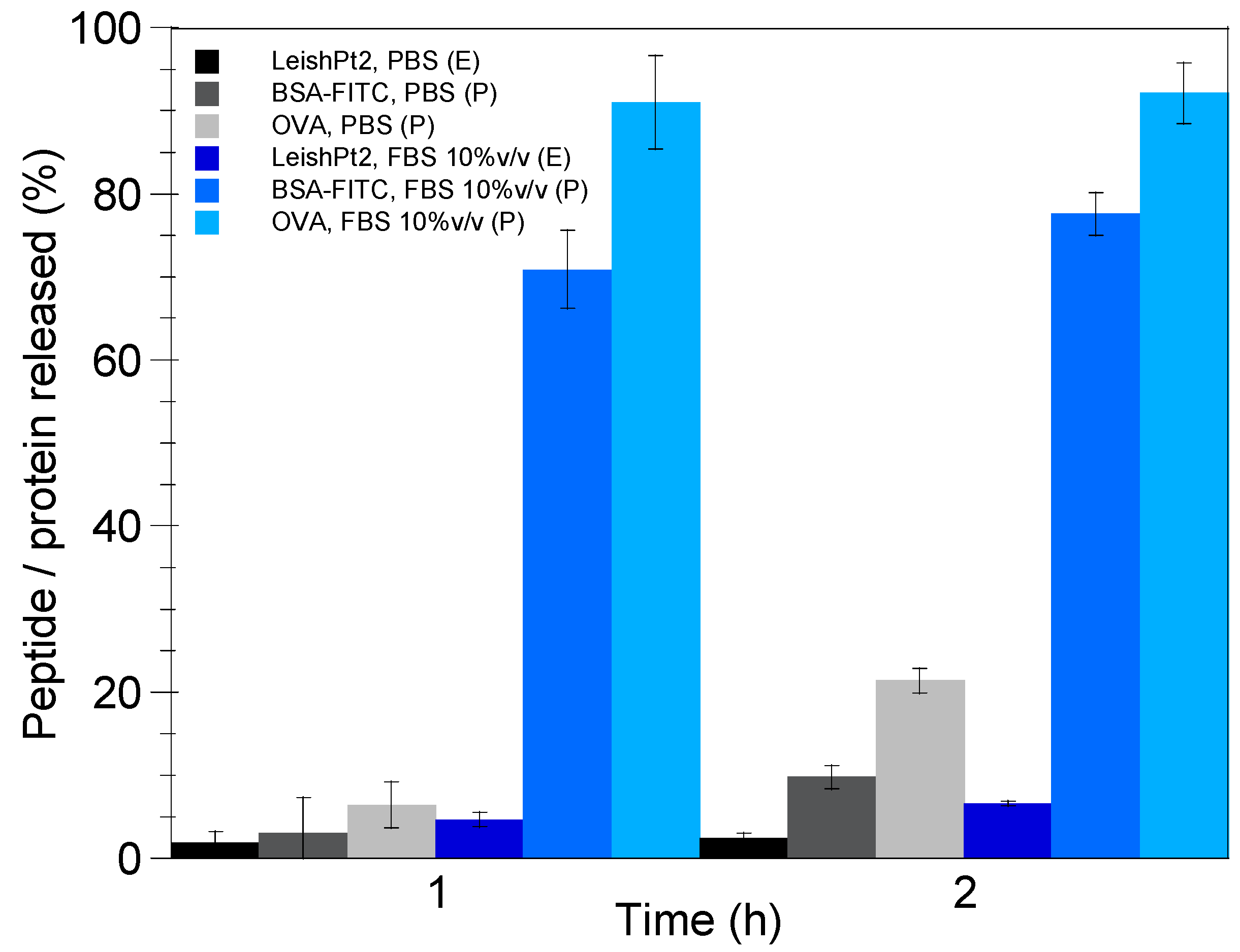
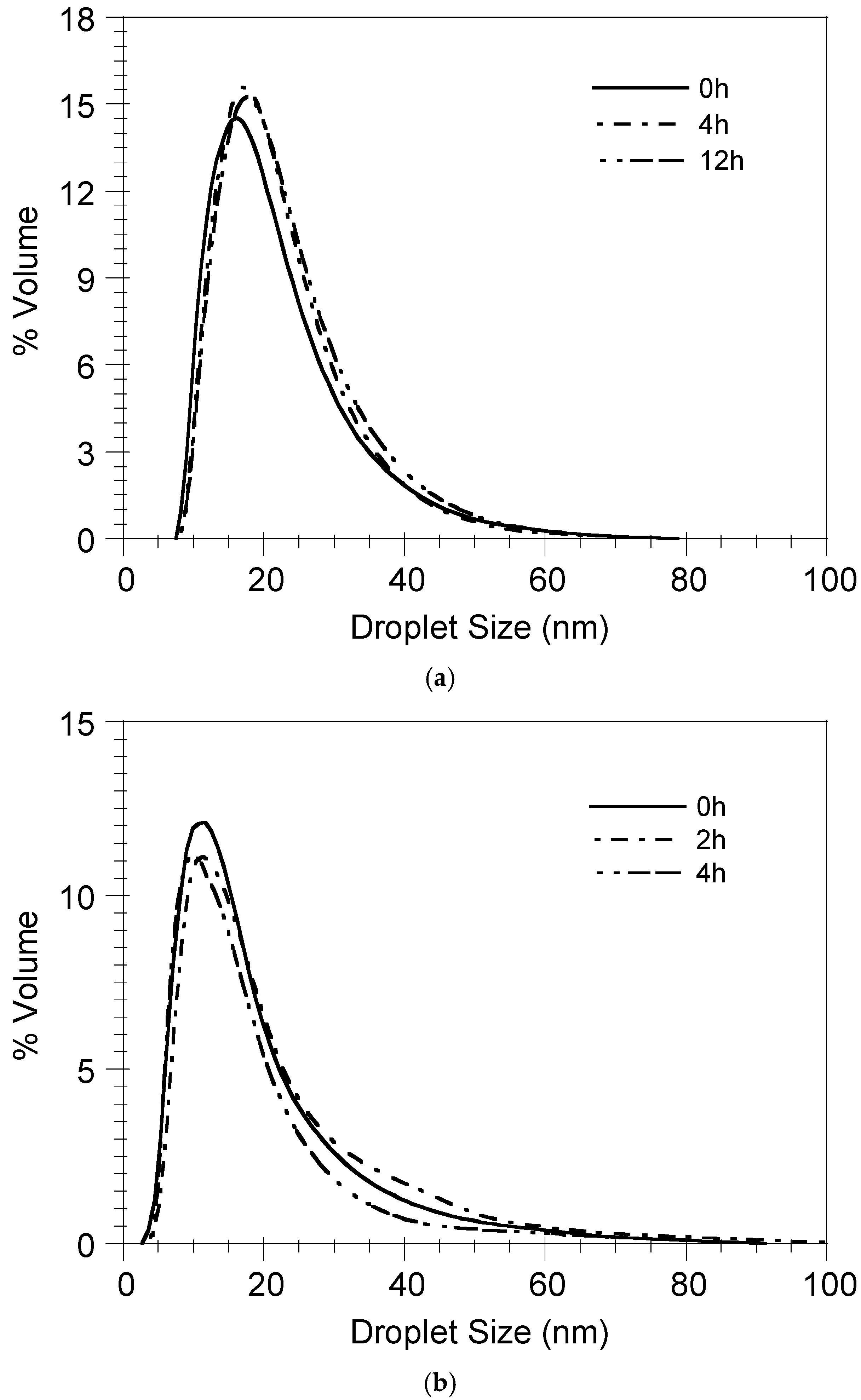
2.5. In Vitro Release Study
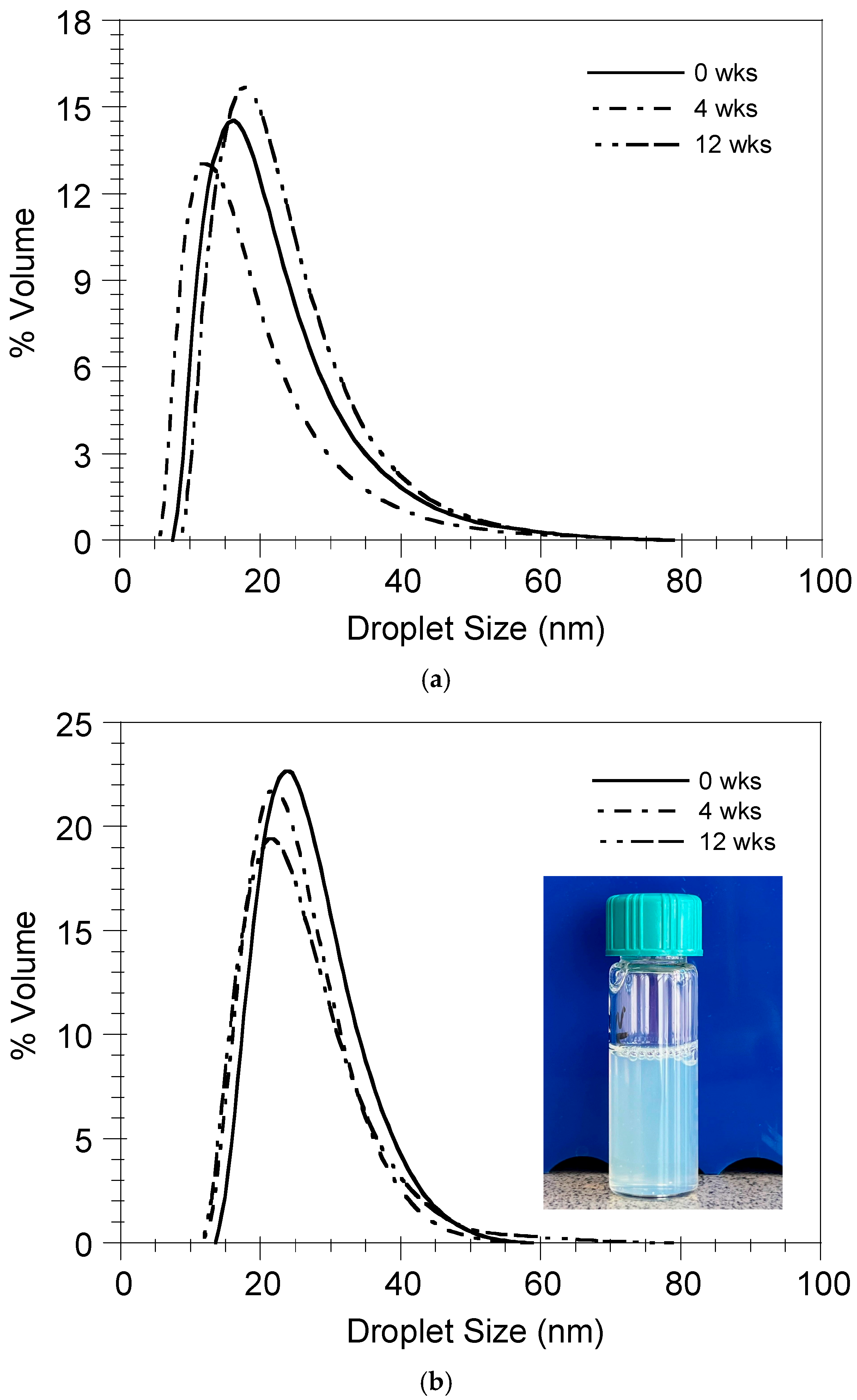
2.6. Nanoemulsion Stability
2.7. Uptake Studies
3. Results and Discussion
3.1. Synthesis and Characterization of Protein/DMPG Complexes
3.2. Preparation and Characterization of Protein/DMPG-Loaded ST-SNEDDS
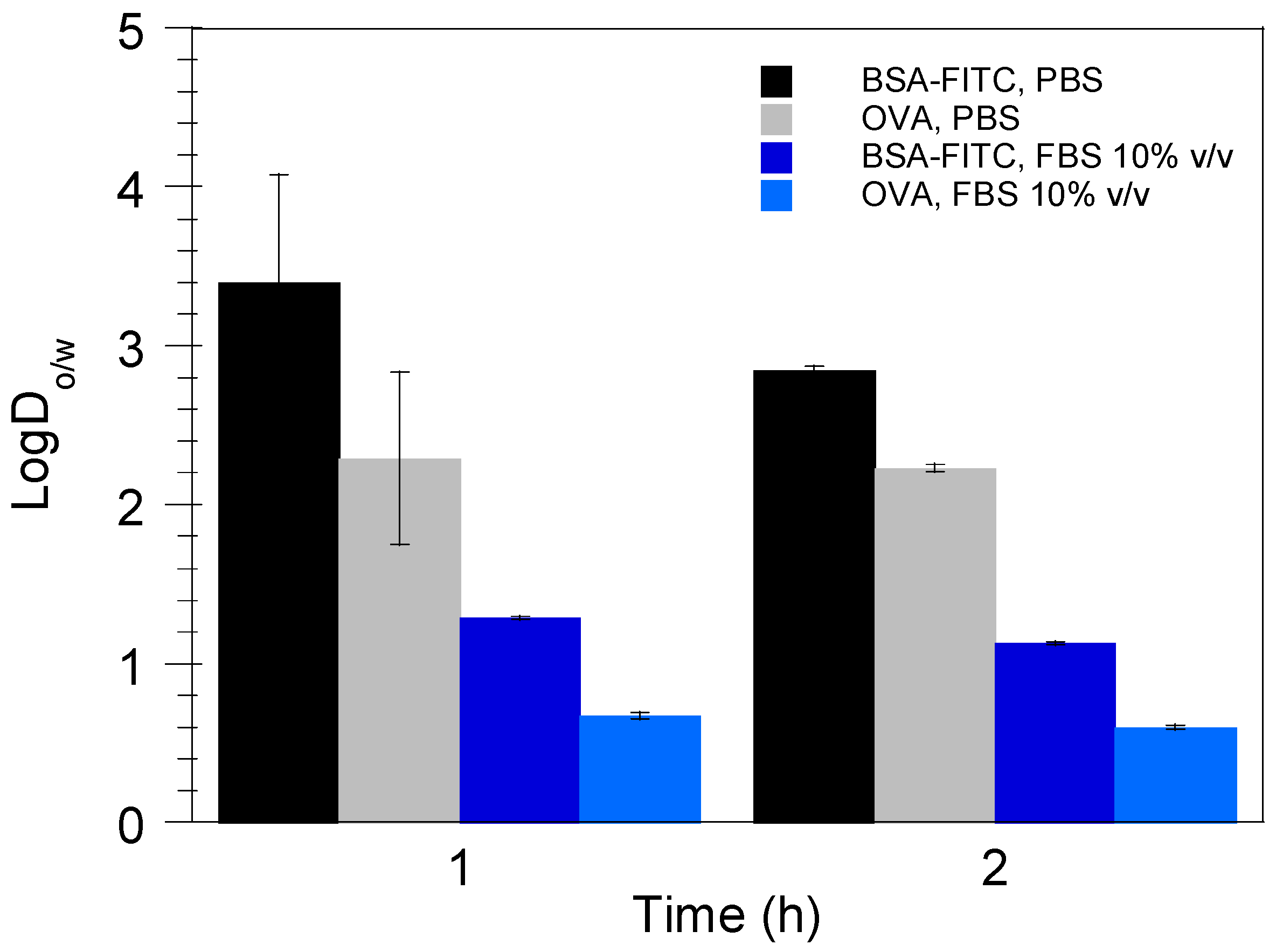
3.3. Theoretical Prediction of Protein Release from the Nanoemulsion Droplets
3.4. Nanoemulsion Stability
3.5. Uptake and Localization of ST-SNEDDS-BSA-FITC by Macrophages In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LeishPts | Multi-epitope Leishmania peptides |
| ST-SNEDDS | Doubly adjuvanted self-nanoemulsifying drug delivery systems |
| BSA-FITC | Bovine serum albumin–fluorescein isothiocyanate conjugate |
| OVA | Ovalbumin |
| DMPG | Dimyristoyl phosphatidylglycerol |
| PBS | Phosphate-buffered saline |
| FBS | Fetal bovine serum |
| DNA | Deoxyribonucleic acid |
| DC | Dendritic cell |
| SLNs | Solid lipid nanoparticles |
| SNEDDS | Self-nanoemulsifying drug delivery systems |
| SEDDS | Self-emulsifying drug delivery systems |
| IFNγ | Interferon gamma |
| LogDo/w | Distribution coefficient of the protein between the oil and aqueous phases |
| APC | Antigen presenting cell |
| Cy-5 | Cyanine-5 carboxylic acid |
| FTIR | Fourier-transform infrared |
| KBr | Potassium bromide |
| HPLC | High-performance liquid chromatography |
| UV | Ultraviolet |
| DSD | Droplet size distribution |
| PCS | Photon correlation spectroscopy |
| MWCO | Molecular weight cut-off |
| LeishPt2 | Multi-epitope Leishmania peptide |
References
- Oliveira, S.S.C.; Ferreira, C.S.; Branquinha, M.H.; Santos, A.L.S.; Chaud, M.V.; Jain, S.; Cardoso, J.C.; Kovaċeviċ, A.B.; Soutod, E.B.; Severino, P. Overcoming multi-resistant leishmanial treatment by nanoencapsulation of potent antimicrobials. J. Chem. Technol. Biotechnol. 2020, 96, 2123–2140. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Badiee, A.; Khamesipour, A. Development of nano-carriers for Leishmania vaccine delivery. Expert Opin. Drug Deliv. 2020, 17, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Costa, L.; Ryan, N.; Halsey, G.; Satoskar, A.; Oghumu, S. Nanoparticulate drug delivery systems for the treatment of neglected tropical protozoan diseases. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e144118. [Google Scholar] [CrossRef]
- Saleem, K.; Khursheed, Z.; Hano, C.; Anjum, L.; Anjum, S. Applications of nanomaterials in leishmaniasis: A focus on recent advances and challenges. Nanomaterials 2019, 9, 1749. [Google Scholar] [CrossRef]
- Yoon, K.-W.; Chu, K.B.; Eom, G.-D.; Mao, J.; Moon, E.-K.; Kim, S.S.; Quan, F.-S. CpG-adjuvanted virus-like particle vaccine induces protective immunity against Leishmania donovani infection. J. Infect. Dis. 2025, 231, 998–1007. [Google Scholar] [CrossRef]
- Ayala, A.; Llanes, A.; Lleonart, R.; Restrepo, C.M. Advances in Leishmania Vaccines: Current Development and Future Prospects. Pathogens 2024, 13, 812. [Google Scholar] [CrossRef]
- Velez, R.; Gallego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 15, 540–557. [Google Scholar] [CrossRef]
- Coelho, E.A.F.; Christodoulides, M. Vaccines for Canine Leishmaniasis in Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges, Part III; Christodoulides, M., Ed.; Springer: Cham, Switzerland, 2023; p. 281. [Google Scholar] [CrossRef]
- CaniLeish—Procedural Steps Taken and Scientific Information After the Authorization. Available online: https://www.ema.europa.eu/en/documents/procedural-steps-after/canileish-epar-procedural-steps-taken-and-scientific-information-after-authorisation_en.pdf (accessed on 23 June 2025).
- Leish-Tec®—Why was the Leishmaniasis Vaccine Suspended? Available online: https://www.portalinsights.com.br/perguntas-frequentes/porque-a-vacina-leishmaniose-foi-suspensa (accessed on 23 June 2025).
- Letifend Canine Leishmaniasis Vaccine (Recombinant Protein). Available online: https://www.ema.europa.eu/en/documents/overview/letifend-epar-summary-public_en.pdf (accessed on 23 June 2025).
- Lefitend Annex I Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/letifend-epar-product-information_en.pdf (accessed on 23 June 2025).
- Agallou, M.; Margaroni, M.; Tsanaktsidou, E.; Kammona, O.; Kiparissides, C.; Karagouni, E. A liposomal vaccine promotes immune responses via dendritic cell activation in draining lymph nodes. J. Control. Release 2023, 356, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.R.; Caillet, C.; Kusters, I.C.; Haensler, J. Emulsion-based adjuvants for influenza vaccines. Expert. Rev. Vaccines 2009, 8, 483–492. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.A.; Pollard, A.J. AS03- and MF59-adjuvanted influenza vaccines in children. Front. Immunol. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Garcon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert. Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef]
- Yam, K.K.; Gupta, J.; Winter, K.; Allen, E.; Brewer, A.; Beaulieu, E.; Mallett, C.P.; Burt, D.S.; Ward, B.J. AS03-adjuvanted, very-low-dose influenza vaccines induce distinctive immune responses compared to unadjuvanted high-dose vaccines in BALB/c mice. Front. Immunol. 2015, 6, 207. [Google Scholar] [CrossRef]
- Kammona, O.; Tsanaktsidou, E. Nanotechnology-aided diagnosis, treatment and prevention of leishmaniasis. Int. J. Pharm. 2021, 605, 120761. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Bourganis, V.; Kammona, O.; Kiparissides, C. Lipid-based nanocarriers for the oral administration of biopharmaceutics. Nanomedicine 2016, 11, 3009–3032. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T. Mucus Permeating Nanocarriers for Controlled Delivery. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2015. Available online: https://ikee.lib.auth.gr/record/280193/files/GRI-2015-15552.pdf (accessed on 23 June 2025).
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef]
- Lupo, N.; Tkadlečková, V.N.; Jelkmann, M.; Laffleur, F.; Hetényi, G.; Kubová, K.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems: In vivo evaluation of their potential for oral vaccination. Acta Biomater. 2019, 94, 425–434. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Jalil, A. Do drug release studies from SEDDS make any sense? J. Control. Release 2018, 271, 55–59. [Google Scholar] [CrossRef]
- Karamanidou, T.; Karidi, K.; Bourganis, V.; Kontonikola, K.; Kammona, O.; Kiparissides, C. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur. J. Pharm. Biopharm. 2015, 97, 223–229. [Google Scholar] [CrossRef]
- Menzel, C.; Holzeisen, T.; Laffleur, F.; Zaichik, S.; Abdulkarim, M.; Gumbleton, M.; Bernkop-Schnürch, A. In vivo evaluation of an oral self-emulsifying drug delivery system (SEDDS) for exenatide. J. Control. Release 2018, 277, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv. Drug. Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Haddadzadegan, S.; To, D.; Jörgensen, A.M.; Wibel, R.; Laffleur, F.; Bernkop-Schnürch, A. Comparative analysis of PEG-free and PEG-based self-emulsifying drug delivery systems for enhanced oral bioavailability of therapeutic (Poly) peptides. Small 2024, 20, 2307618. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Karagouni, E. Intramuscular immunization with a liposomal multi-epitope chimeric protein induces strong cellular immune responses against visceral leishmaniasis. Vaccines 2023, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Margaroni, M.; Tsanaktsidou, E.; Agallou, M.; Kiparissides, C.; Kammona, O.; Karagouni, E. Development of a novel squa-lene/a-tocopherol-based self-emulsified nanoemulsion incorporating Leishmania peptides for induction of antigen-specific cellular immune responses. Int. J. Pharm. 2024, 649, 123621. [Google Scholar] [CrossRef]
- McReynolds, L.; O’Malley, B.W.; Nisbet, A.D.; Fothergill, J.E.; Givol, D.; Fields, S.; Robertson, M.; Brownlee, G.G. Sequence of chicken ovalbumin mRNA. Nature 1978, 2713, 723–728. [Google Scholar] [CrossRef]
- Sodium phosphate. Cold Spring Harb. Protoc. 2006. [CrossRef]
- Surewicz, W.K.; Epand, R.M.; Orlowski, R.C.; Mantsch, H.H. Structural properties of acidic phospholipids in complexes with calcitonin: A Fourier transform infrared spectroscopic investigation. Biochim. Biophys. Acta 1987, 899, 307–310. [Google Scholar] [CrossRef]
- Paolorossi, M.; Montich, G.G. Conformational changes of β2-human glycoprotein I and lipid order in lipid-protein complexes. Biochim. Biophys. Acta 2011, 1808, 2167–2177. [Google Scholar] [CrossRef]
- Malkawi, A.; Jalil, A.; Nazir, I.; Matuszczak, B.; Kennedy, R.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems: Hydrophobic drug polymer complexes provide a sustained release In vitro. Mol. Pharm. 2020, 17, 3709–3719. [Google Scholar] [CrossRef]
- Shoemaker, B.A.; Panchenko, A.R. Deciphering protein–protein interactions. Part I. Experimental techniques and databases. PLoS Comput. Biol. 2007, 3, e42. [Google Scholar] [CrossRef]
- Loughney, J.W.; Lancaster, C.; Ha, S.; Rustandi, R.R. Residual bovine serum albumin (BSA) quantitation in vaccines using automated capillary western technology. Anal. Biochem. 2014, 461, 49–56. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Niemeyer, B. A novel correlation for protein diffusion coefficients based on molecular weight and radius of gyration. Biotechnol. Prog. 2003, 19, 544–548. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids. 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A Simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Helder, M.N.; Naderinezhad, S.; Sheikhha, M.H.; Forouzanfar, T.; Zandieh-Doulabi, B. A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 169–177. [Google Scholar] [CrossRef]
- Mu, H.; Wang, Y.; Chu, Y.; Jiang, Y.; Hua, H.; Chu, L.; Wang, K.; Wang, A.; Liu, W.; Li, Y.; et al. Multivesicular liposomes for sustained release of bevacizumab in treating laser-induced choroidal neovascularization. Drug Deliv. 2018, 25, 1372–1383. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Skalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef]
- Bastola, R.; Seo, J.E.; Keum, T.; Noh, G.; Choi, J.W.; Shin, J.I.; Kim, J.H.; Lee, S. Preparation of squalene oil-based emulsion adjuvants employing a self-emulsifying drug delivery system and assessment of Mycoplasma hyopneumoniae-specific antibody titers in BALB/c mice. Pharmaceutics 2019, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed]




| Nanoemulsion | Droplet Diameter (nm) | PDI | Zeta Potential (mV) | ST-SNEDDS Conc. in the Nanoemulsion (mg/mL) | Protein Loading in the oily Nanodroplets (wt%) | Protein Encapsulation Efficiency (%) | Cy5 Conc. in the Nanoemulsion (mg/mL) |
|---|---|---|---|---|---|---|---|
| ST-SNEDDS-BSA-FITC/DMPG | 27.3 ± 1.2 | 0.07 ± 0.02 | −3.5 ± 0.1 | 330 | 0.16 ± 0.02 | 95.3 ± 2.7 | - |
| ST-SNEDDS-BSA-FITC/DMPG-Cy5 | 24.9 ± 0.2 | 0.21 ± 0.05 | −3.8 ± 0.2 | 330 | 0.56 ± 0.00 | 98.4 ± 0.2 | 0.167 |
| ST-SNEDDS-OVA/DMPG | 27.3 ± 0.8 | 0.06 ± 0.03 | −1.5 ± 1.1 | 330 | 0.25 ± 0.06 | 95.1 ± 1.8 | - |
| ST-SNEDDS | 28.6 ± 0.1 | 0.03 ± 0.02 | 1.8 ± 1.0 | 330 | - | - | - |
| ST-SNEDDS-CY5 | 24.3 ± 0.8 | 0.23 ± 0.01 | 0.3 ± 1.0 | 330 | - | - | 0.167 |
| Formulation | Vw/Vo | Protein Loading (%w/w) | k | n | R2 |
|---|---|---|---|---|---|
| ST3-BSA-FITC/DMPG | 10 | 0.18 | 0.024 | 0.230 | 0.9630 |
| ST3-BSA-FITC/DMPG-Cy5 | 10 | 0.56 | 0.009 | 0.304 | 0.9652 |
| ST3-OVA/DMPG | 10 | 0.29 | 0.030 | 0.369 | 0.9589 |
| ST3-BSA-FITC/DMPG | 100 | 0.16 | 0.082 | 0.403 | 0.9985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsanaktsidou, E.; Margaroni, M.; Karagouni, E.; Kiparissides, C.; Kammona, O. In Vitro Assessment of a Doubly Adjuvanted Self-Emulsified Nanoemulsion as a Delivery Vehicle for Antigenic Proteins. Pharmaceutics 2025, 17, 870. https://doi.org/10.3390/pharmaceutics17070870
Tsanaktsidou E, Margaroni M, Karagouni E, Kiparissides C, Kammona O. In Vitro Assessment of a Doubly Adjuvanted Self-Emulsified Nanoemulsion as a Delivery Vehicle for Antigenic Proteins. Pharmaceutics. 2025; 17(7):870. https://doi.org/10.3390/pharmaceutics17070870
Chicago/Turabian StyleTsanaktsidou, Evgenia, Maritsa Margaroni, Evdokia Karagouni, Costas Kiparissides, and Olga Kammona. 2025. "In Vitro Assessment of a Doubly Adjuvanted Self-Emulsified Nanoemulsion as a Delivery Vehicle for Antigenic Proteins" Pharmaceutics 17, no. 7: 870. https://doi.org/10.3390/pharmaceutics17070870
APA StyleTsanaktsidou, E., Margaroni, M., Karagouni, E., Kiparissides, C., & Kammona, O. (2025). In Vitro Assessment of a Doubly Adjuvanted Self-Emulsified Nanoemulsion as a Delivery Vehicle for Antigenic Proteins. Pharmaceutics, 17(7), 870. https://doi.org/10.3390/pharmaceutics17070870








