Potentials and Challenges in Development of Vesicular Phospholipid Gel as a Novel Dermal Vehicle for Thymol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of T-VPGs
2.3. Size, Zeta Potential, and Membrane Elasticity Determinations
2.4. Encapsulation Efficiency Assessment
2.5. In Vitro Release Studies
Mathematical Modelling of Thymol Release
2.6. Rheometry of T-VPGs
2.6.1. Viscosity
2.6.2. Amplitude Sweep Test
2.7. Ex Vivo Permeation Studies
HPLC Assay for Thymol
2.8. In Vitro Antibacterial Testing
2.9. In Vitro Cytotoxicity Studies
2.9.1. Cell Culturing
2.9.2. Treatment of the Cells and MTT Assay
2.10. In Vitro Scratch Assay
2.11. Storage Stability Studies
2.12. Statistical Analysis
3. Results and Discussion
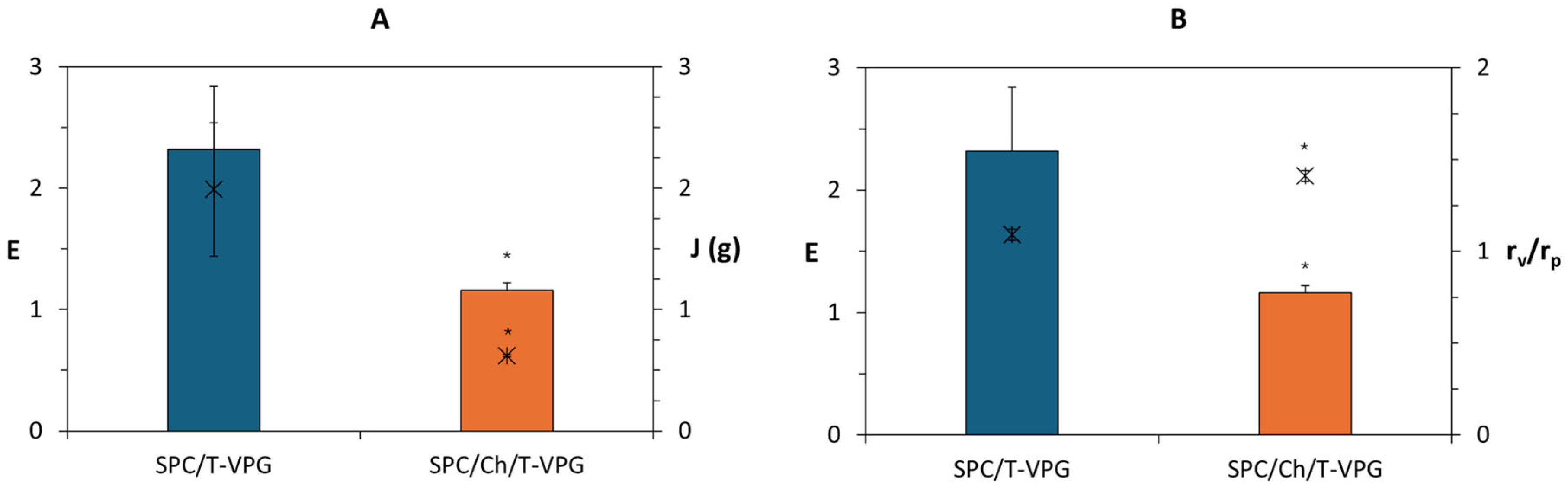
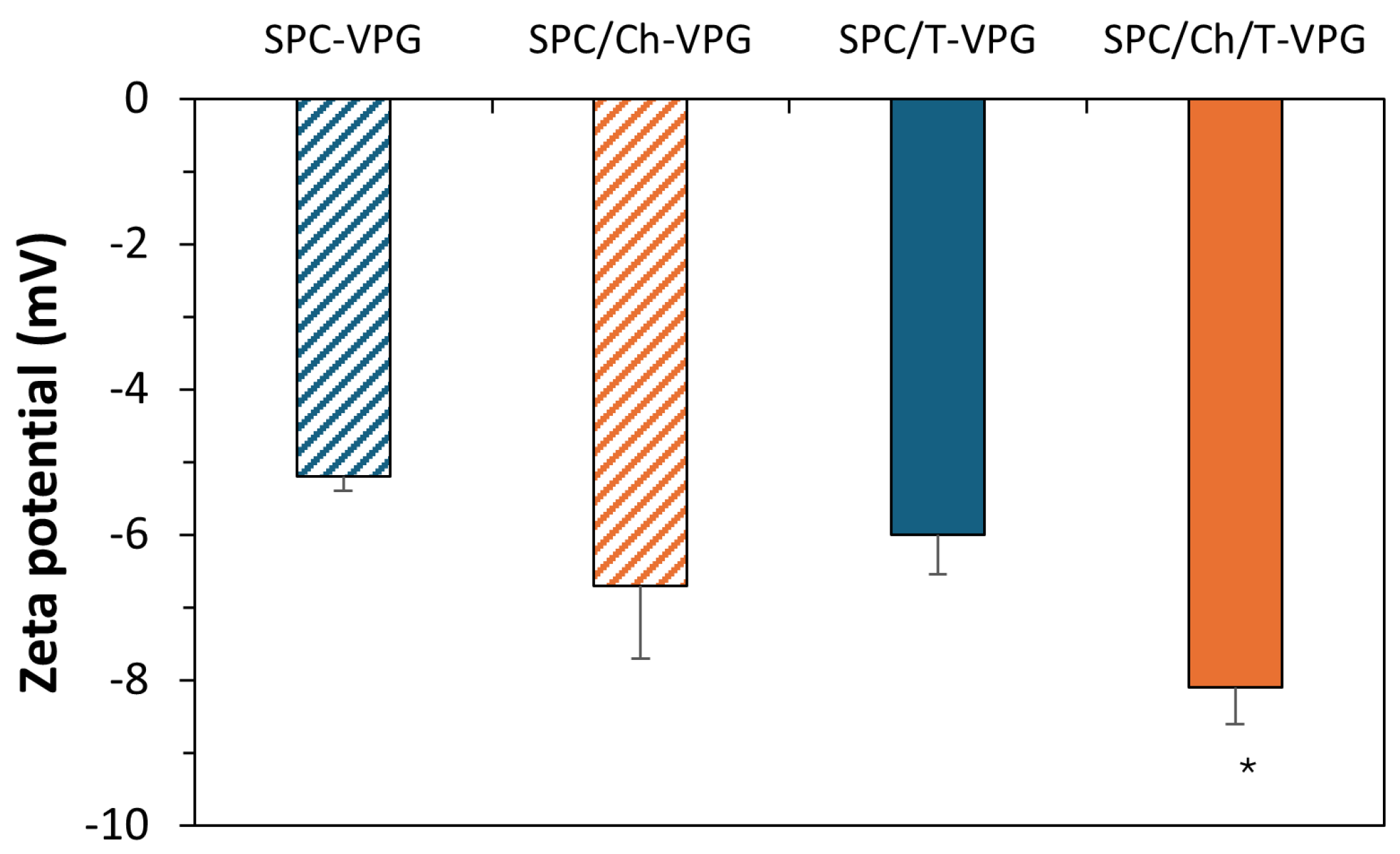
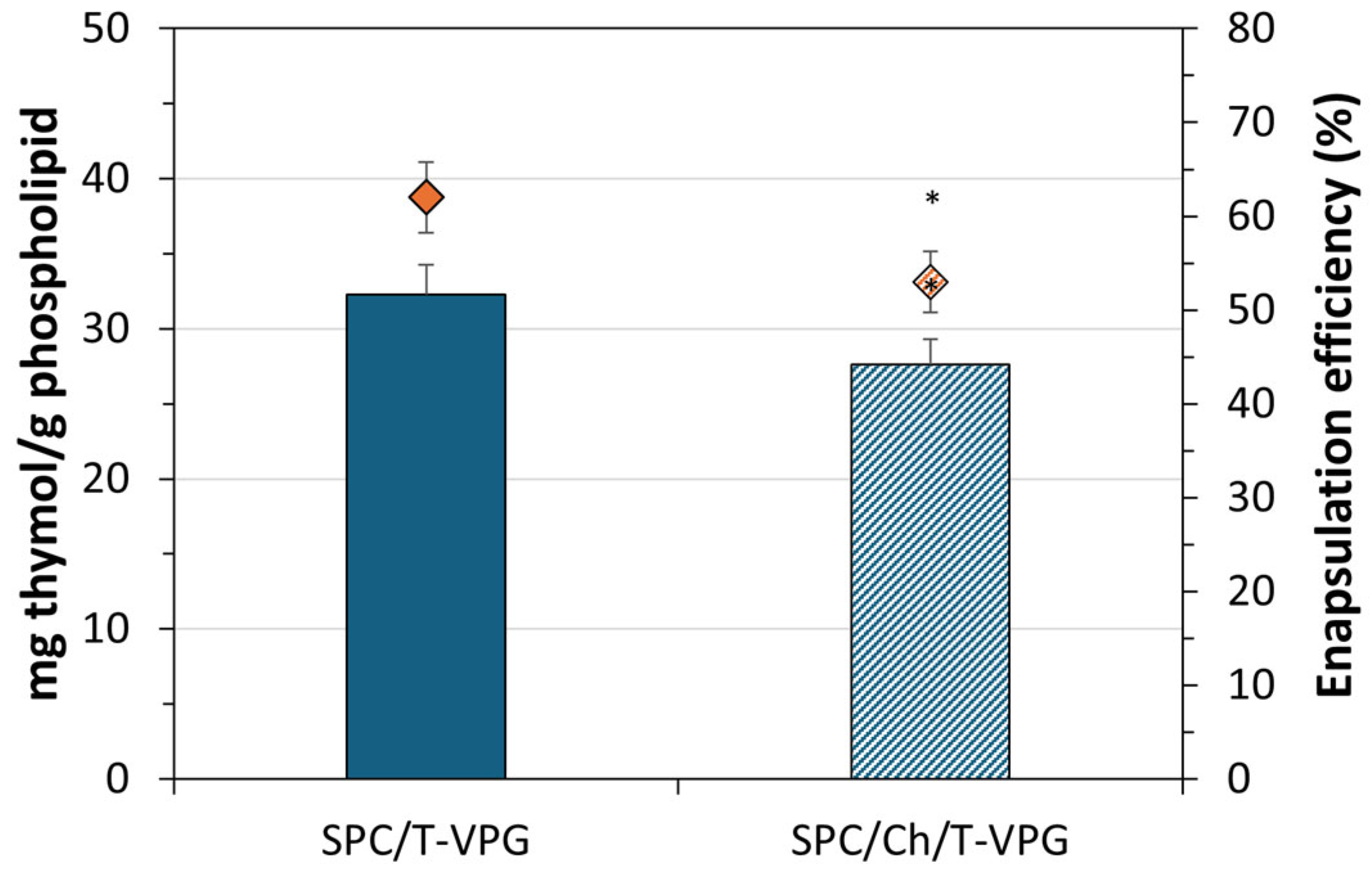
3.1. Characteristics of T-VPG Liposomes
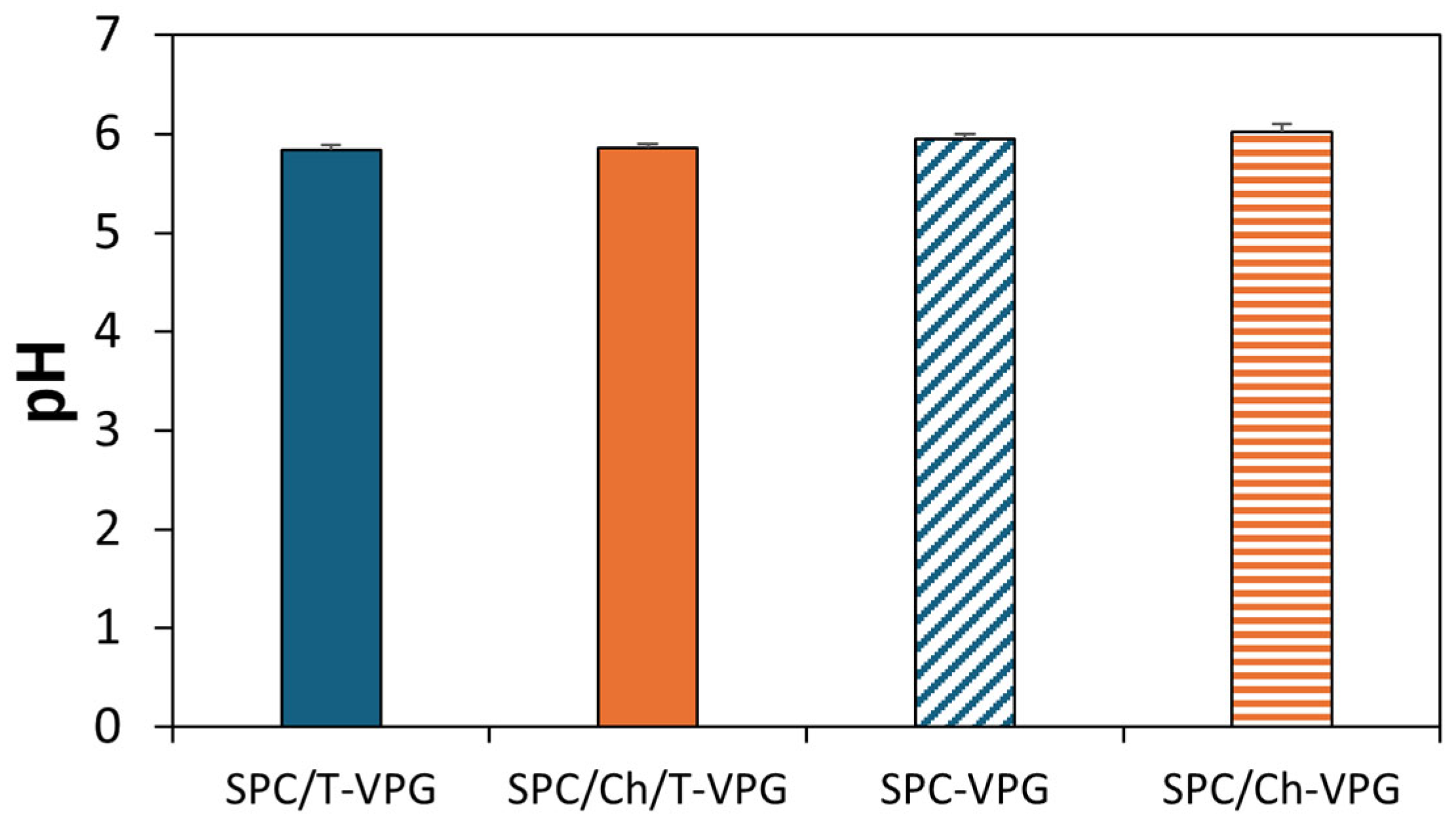
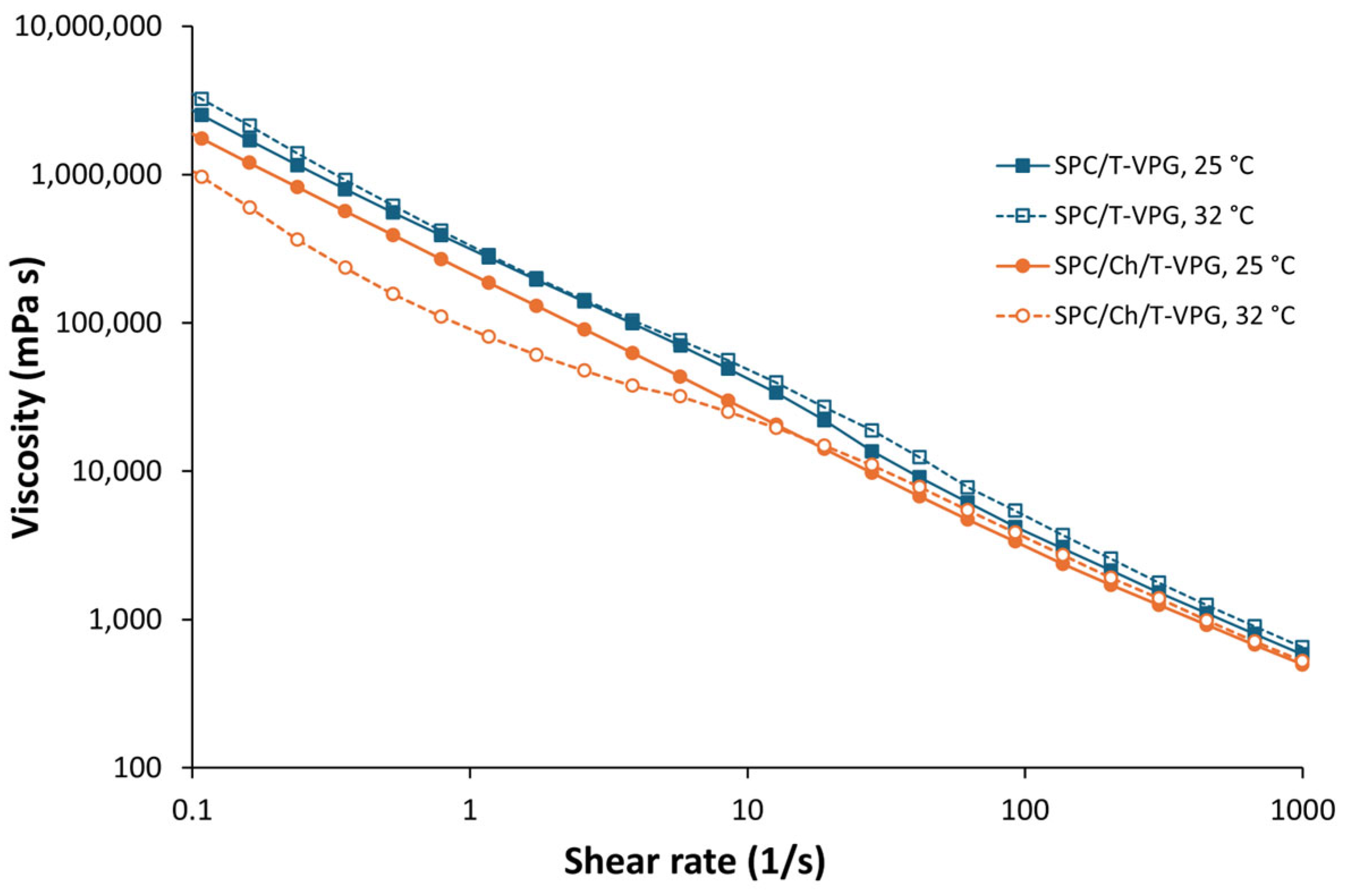
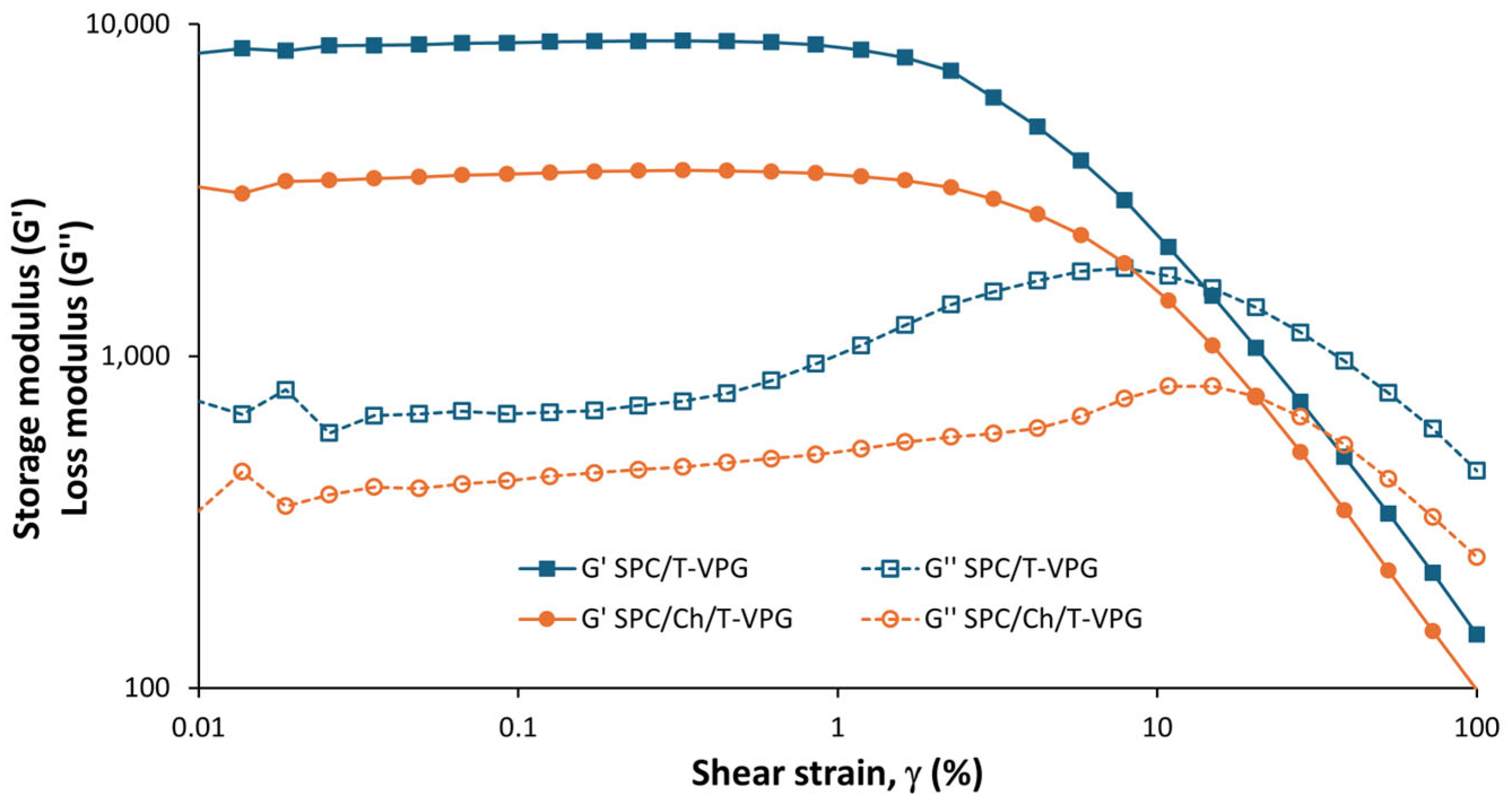
3.2. Characteristics of T-VPGs
3.3. In Vitro Release of Thymol from T-VPGs
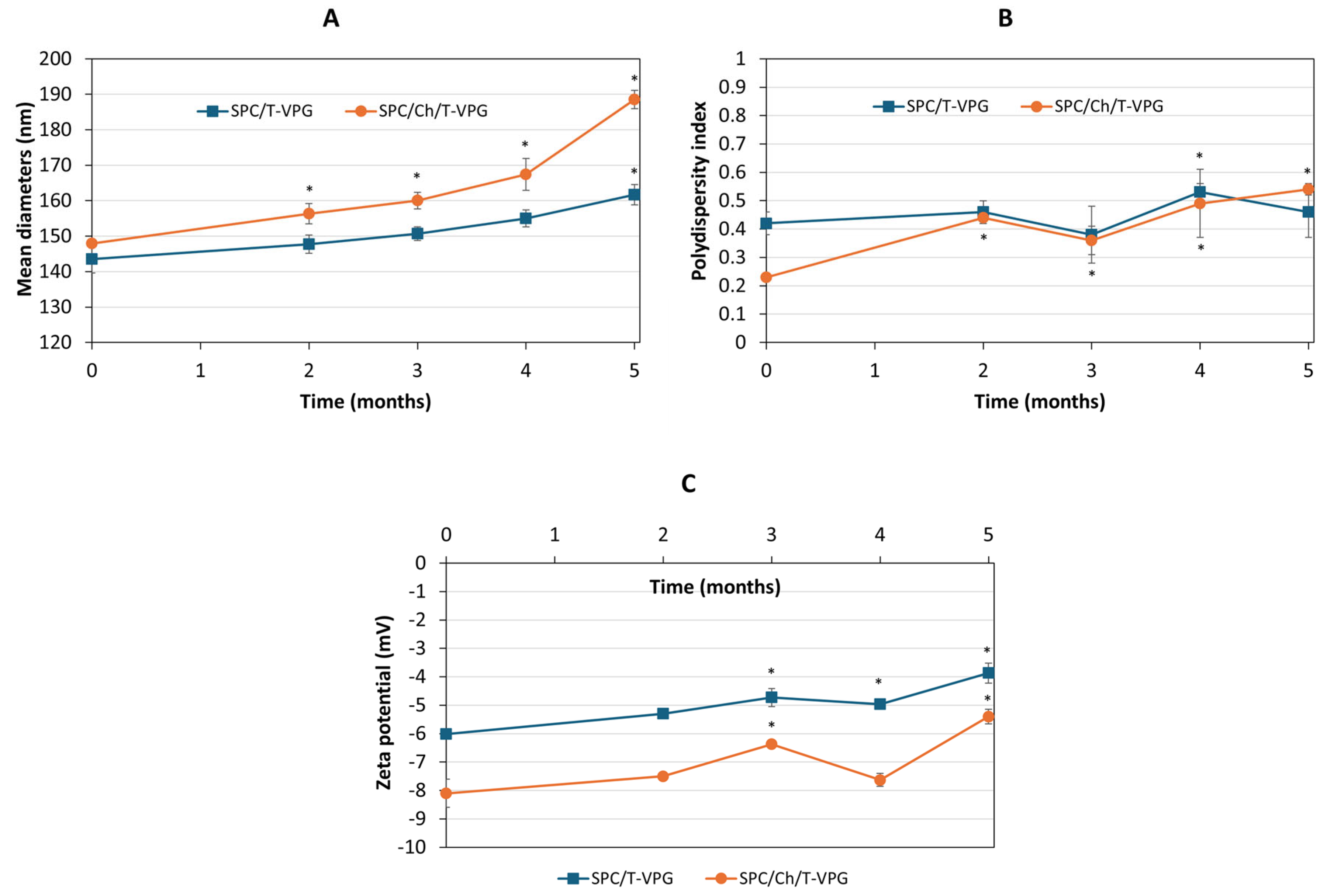
3.4. Storage Stability Evaluation
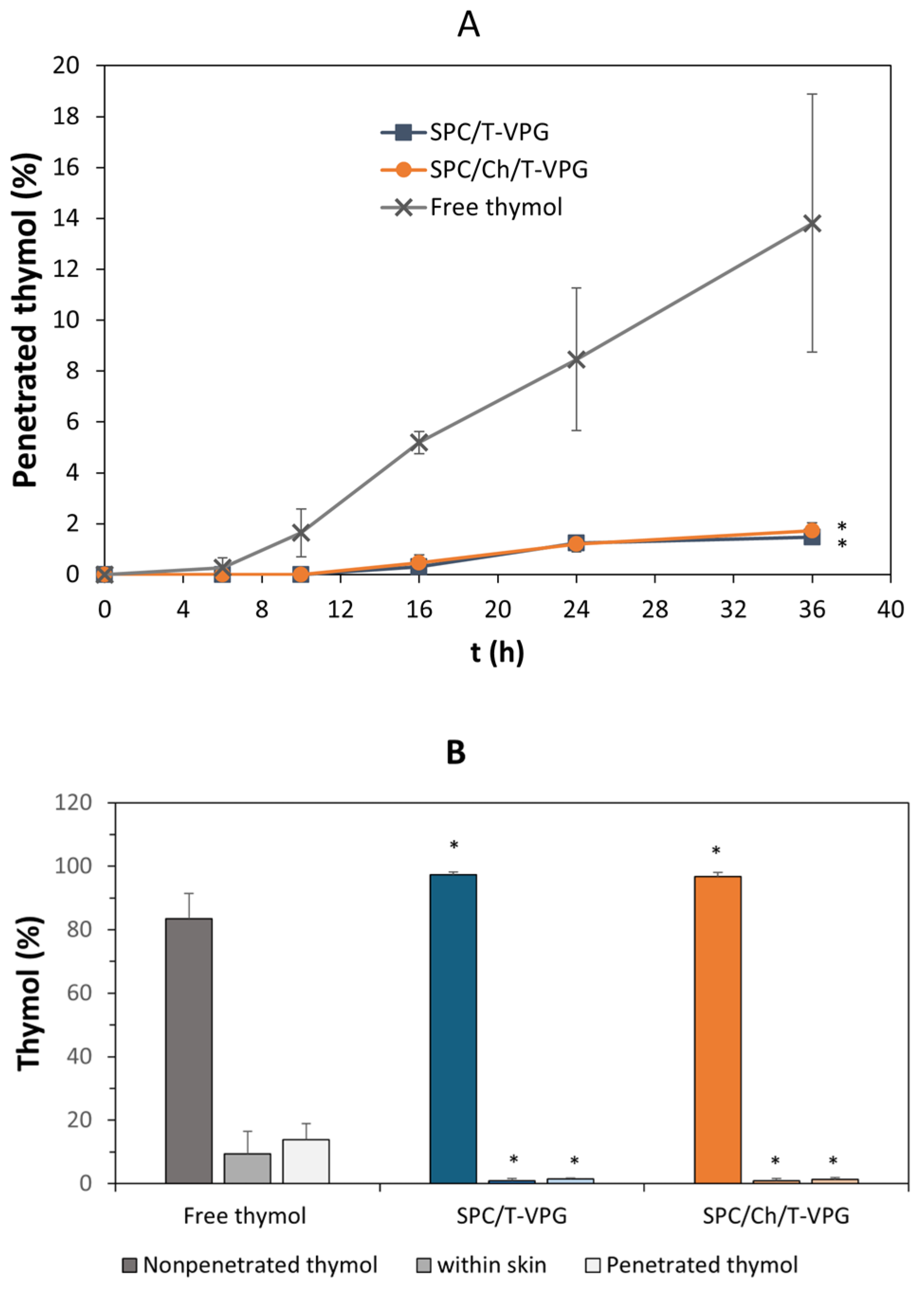
3.5. Ex Vivo Skin Penetration Studies
3.6. In Vitro Antibacterial Assessment of T-VPG Liposomes
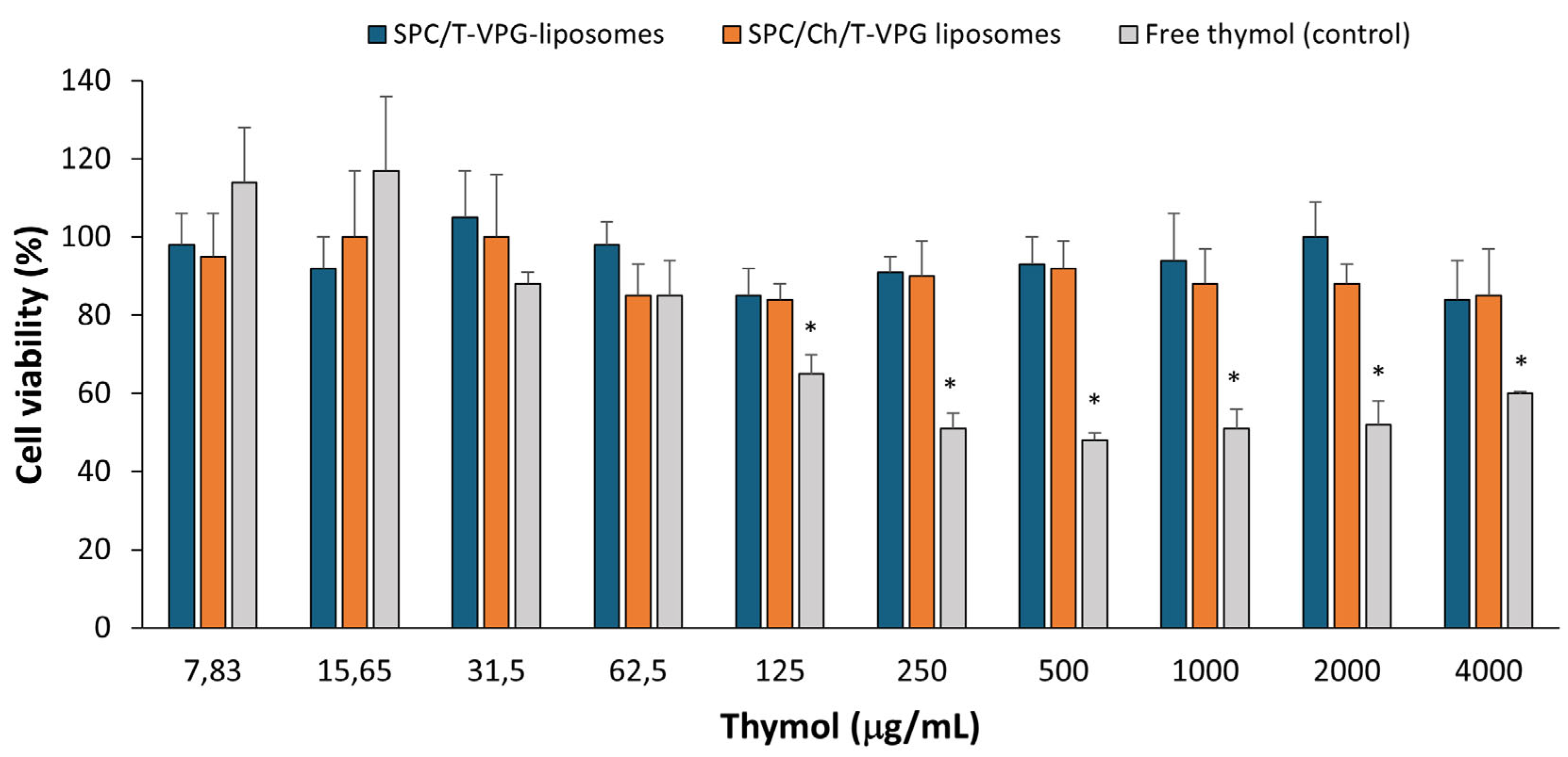
3.7. Biocompatibility Evaluation of T-VPG Liposomes
3.8. In Vitro Healing Ability of T-VPGs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabbai-Armelin, P.R.; Sales, L.S.; Ferrisse, T.M.; De Oliveira, A.B.; De Oliveira, J.R.; Giro, E.M.A.; Brighenti, F.L. A systematic review and meta-analysis of the effect of thymol as an anti-inflammatory and wound healing agent. Phytother. Res. 2022, 36, 3415–3443. [Google Scholar] [CrossRef] [PubMed]
- Najafloo, R.; Behyari, M.; Imani, R.; Nour, S. A mini-review of thymol incorporated materials: Applications in antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2020, 60, 101904. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kim, J.E.; Park, W.H.; Hong, H. Regulation of C6 glioma cell migration by thymol. Oncol. Lett. 2016, 11, 2619–2624. [Google Scholar] [CrossRef]
- Pham, Q.D.; Björklund, S.; Engblom, J.; Topgaard, D.; Sparr, E. Chemical penetration enhancers in stratum corneum—Relation between molecular effects and barrier function. J. Control. Release 2016, 232, 175–187. [Google Scholar] [CrossRef]
- Thymol. In European Pharmacopeia 11.7; Council of Europe: Strasbourg, France, 2024; pp. 4205–4206.
- Garg, A.; Ahmad, J.; Hassan, M.Z. Inclusion complex of thymol and hydroxypropyl-β-cyclodextrin (HP-β-CD) in polymeric hydrogel for topical application: Physicochemical characterization, molecular docking, and stability evaluation. J. Drug Deliv. Sci. Technol. 2021, 64, 102609. [Google Scholar] [CrossRef]
- Folle, C.; Marqués, A.M.; Díaz-Garrido, N.; Espina, M.; Sánchez-López, E.; Badia, J.; Baldoma, L.; Calpena, A.C.; García, M.L. Thymol-loaded PLGA nanoparticles: An efficient approach for acne treatment. J. Nanobiotechnol. 2021, 19, 359. [Google Scholar] [CrossRef]
- Deng, L.L.; Taxipalati, M.; Que, F.; Zhang, H. Physical characterization and antioxidant activity of thymol solubilized Tween 80 micelles. Sci. Rep. 2016, 6, 38160. [Google Scholar] [CrossRef]
- Heckler, C.; Maders Silva, C.M.; Cacciatore, F.A.; Daroit, D.J.; da Silva Malheiros, P. Thymol and carvacrol in nanoliposomes: Characterization and a comparison with free counterparts against planktonic and glass-adhered Salmonella. LWT 2020, 127, 109382. [Google Scholar] [CrossRef]
- Gámez, E.; Elizondo-Castillo, H.; Tascon, J.; García-Salinas, S.; Navascues, N.; Mendoza, G.; Arruebo, M.; Irusta, S. Antibacterial effect of thymol loaded SBA-15 nanorods incorporated in PCL electrospun fibers. Nanomaterials 2020, 10, 616. [Google Scholar] [CrossRef]
- Folle, C.; Sánchez-López, E.; Mallandrich, M.; Díaz-Garrido, N.; Suñer-Carbó, J.; Halbaut, L.; Carvajal-Vidal, P.; Marqués, A.M.; Espina, M.; Badia, J.; et al. Semi-solid functionalized nanostructured lipid carriers loading thymol for skin disorders. Int. J. Pharm. 2024, 651, 123732. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Holæter, A.M.; Škalko-Basnet, N. (Phospho)lipid-based nanosystems for skin administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, Z.; Jøraholmen, M.W.; Božić, D.; Frankol, I.; Golja Gašparović, P.; Škalko-Basnet, N.; Šegvić Klarić, M.; Vanić, Ž. Azithromycin-loaded liposomal hydrogel: A step forward for enhanced treatment of MRSA-related skin infections. Acta Pharm. 2023, 73, 559–579. [Google Scholar] [CrossRef]
- Čačić, A.; Amidžić Klarić, D.; Keser, S.; Radiković, M.; Rukavina, Z.; Jøraholmen, M.W.; Uzelac, L.; Kralj, M.; Škalko-Basnet, N.; Šegvić Klarić, M.; et al. A novel approach for the treatment of aerobic vaginitis: Azithromycin liposomes-in-chitosan hydrogel. Pharmaceutics 2023, 15, 1356. [Google Scholar] [CrossRef]
- Palac, Z.; Hurler, J.; Škalko-Basnet, N.; Filipović-Grčić, J.; Vanić, Ž. Elastic liposomes-in-vehicle formulations destined for skin therapy: The synergy between type of liposomes and vehicle. Drug Dev. Ind. Pharm. 2015, 41, 1247–1253. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Giordani, B.; Pettersen, A.K.; Vitali, B.; Basnet, P.; Škalko-Basnet, N. Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohydr. Polym. 2021, 262, 117939. [Google Scholar] [CrossRef]
- Brandl, M.; Drechsler, M.; Bachmann, D.; Bauer, K.H. Morphology of semisolid aqueous phosphatidylcholine dispersions, a freeze fracture electron microscopy study. Chem. Phys. Lipids 1997, 87, 65–72. [Google Scholar] [CrossRef]
- Brandl, M. Vesicular phospholipid gels: A technology platform. J. Liposome Res. 2007, 17, 15–26. [Google Scholar] [CrossRef]
- Bender, J.; Michaelis, W.; Schubert, R. Morphological and thermal properties of vesicular phospholipid gels studied by DSC, rheometry and electron microscopy. J. Therm. Anal. Calorim. 2002, 68, 603–612. [Google Scholar] [CrossRef]
- Güthlein, F.; Burger, A.M.; Brandl, M.; Fiebig, H.H.; Schubert, R.; Unger, C.; Massing, U. Pharmacokinetics and antitumor activity of vincristine entrapped in vesicular phospholipid gels. Anticancer Drugs 2002, 13, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.; Kimpfler, A.; Massing, U.; Burger, A.M.; Fiebig, H.H.; Brandl, M.; Schubert, R. 5-Fluorouracil in vesicular phospholipid gels for anticancer treatment: Entrapment and release properties. Int. J. Pharm. 2003, 256, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Breitsamer, M.; Winter, G. Vesicular phospholipid gels as drug delivery systems for small molecular weight drugs, peptides and proteins: State of the art review. Int. J. Pharm. 2019, 557, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Wang, Q.; Yang, X.; Qian, Y.; Zhang, G.; Zhu, Q.; Tang, B. Vesicular phospholipid gels as topical ocular delivery system for treatment of anterior uveitis. Colloid. Surf. A Physicochem. Eng. Asp. 2021, 627, 127187. [Google Scholar] [CrossRef]
- Keser, S.; Maravić-Vlahoviček, G.; Lovrić, J.; Vanić, Ž. Vesicular phospholipid gels: A new strategy to improve topical antimicrobial dermatotherapy. Int. J. Pharm. 2024, 667, 124931. [Google Scholar] [CrossRef]
- Buffer solutions. In European Pharmacopeia 11.7; Council of Europe: Strasbourg, France, 2024; pp. 6536–6542.
- Rukavina, Z.; Šegvić Klarić, M.; Filipović-Grčić, J.; Lovrić, J.; Vanić, Ž. Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Pharm. 2018, 553, 109–119. [Google Scholar] [CrossRef]
- Vanić, Ž.; Rukavina, Z.; Manner, S.; Fallarero, A.; Uzelac, L.; Kralj, M.; Amidžić Klarić, D.; Bogdanov, A.; Raffai, T.; Virok, D.P.; et al. Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. Int. J. Nanomed. 2019, 14, 5957–5976. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Lu, T.; Ten Hagen, T.L.M. A novel kinetic model to describe the ultra-fast triggered release of thermosensitive liposomal drug delivery systems. J. Control. Release 2020, 324, 669–678. [Google Scholar] [CrossRef]
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In vitro release study of the polymeric drug nanoparticles: Development and validation of a novel method. Pharmaceutics 2020, 12, 732. [Google Scholar] [CrossRef]
- NCCLS. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute—NCCLS: Wayne, PA, USA, 2007. [Google Scholar]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina Smole, S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Blažević, F.; Milekić, T.; Duvnjak Romić, M.; Juretić, M.; Pepić, I.; Filipović-Grčić, J.; Lovrić, J.; Hafner, A. Nanoparticle-mediated interplay of chitosan and melatonin for improved wound epithelialisation. Carbohydr. Polym. 2016, 146, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Yagublu, V.; Karimova, A.; Hajibabazadeh, J.; Reissfelder, C.; Muradov, M.; Bellucci, S.; Allahverdiyev, A. Overview of physicochemical properties of nanoparticles as drug carriers for targeted cancer therapy. J. Funct. Biomater. 2022, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Chapter 10—Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In Advances in Pharmaceutical Product Development and Research Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: London, UK, 2019; pp. 369–400. [Google Scholar]
- Vanić, Ž.; Hurler, J.; Ferderber, K.; Golja Gašparović, P.; Škalko-Basnet, N.; Filipović-Grčić, J. Novel vaginal drug delivery system: Deformable propylene glycol liposomes-in-hydrogel. J. Liposome Res. 2014, 24, 27–36. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Oogia, C.; Takashima, Y.; Seta, Y. Effects of surface charge and flexibility of liposomes on dermal drug delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 155–162. [Google Scholar] [CrossRef]
- Baldassarre, F.; Schiavi, D.; Ciarroni, S.; Tagliavento, V.; De Stradis, A.; Vergaro, V.; Suranna, G.P.; Balestra, G.M.; Ciccarella, G. Thymol-nanoparticles as effective biocides against the quarantine pathogen Xylella fastidiosa. Nanomaterials 2023, 13, 1285. [Google Scholar] [CrossRef]
- Engel, J.B.; Heckler, C.; Tondo, E.C.; Daroit, D.J.; da Silva Malheiros, P. Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against Salmonella and Staphylococcus aureus adhered to stainless steel. Int. J. Food Microbiol. 2017, 252, 18–23. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Dias, M.; Costa-Barbosa, A.; Collins, T.; Marcos-Arias, C.; Eraso, E.; Pais, C.; Quindós, G.; Sampaio, P. Development and characterization of monoolein-based liposomes of carvacrol, cinnamaldehyde, citral, or thymol with anti-candida activities. Antimicrob. Agents Chemother. 2021, 65, e01628-20. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Brooks, S.G.; Mahmoud, R.H.; Lin, R.R.; Fluhr, J.W.; Yosipovitch, G. The skin acid mantle: An update on skin pH. J. Investig. Dermatol. 2025, 145, 509–521. [Google Scholar] [CrossRef]
- Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of Acidic pH on wound healing in vivo: A novel perspective for wound treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Grit, M.; Crommelin, D.J. Chemical stability of liposomes: Implications for their physical stability. Chem. Phys. Lipids 1993, 64, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Qwist, P.K.; Sander, C.; Okkels, F.; Jessen, V.; Baldursdottir, S.; Rantanen, J. On-line rheological characterization of semi-solid formulations. Eur. J. Pharm. Sci. 2019, 128, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.P.; Raffin, R.P.; Fagan, S.B. Rheological behavior of semisolid formulations containing nanostructured systems. In Nanocosmetics and Nanomedicines; Beck, R., Guterres, S., Pohlmann, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 37–45. [Google Scholar]
- Simões, A.; Miranda, M.; Cardoso, C.; Vitorino, F.V.A. Rheology by design: A regulatory tutorial for analytical method validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales, Institute of Non-Newtonian Fluid Mechanics: Aberystwyth, UK, 2000; pp. 81–105. [Google Scholar]
- Najafinobar, N.; Mellander, L.J.; Kurczy, M.E.; Dunevall, J.; Angerer, T.B.; Fletcher, J.S.; Cans, A.S. Cholesterol alters the dynamics of release in protein independent cell models for exocytosis. Sci. Rep. 2016, 6, 33702. [Google Scholar] [CrossRef]
- Kotla, N.G.; Chandrasekar, B.; Rooney, P.; Sivaraman, G.; Larrañaga, A.; Krishna, K.V.; Pandit, A.; Rochev, Y. Biomimetic lipid-based nanosystems for enhanced dermal delivery of drugs and bioactive agents. ACS Biomater. Sci Eng. 2017, 3, 1262–1272. [Google Scholar] [CrossRef]
- Tian, W.; Schulze, S.; Brandl, M.; Winter, G. Vesicular phospholipid gel-based depot formulations for pharmaceutical proteins: Development and in vitro evaluation. J. Control. Release 2010, 142, 319–325. [Google Scholar] [CrossRef]
- Duvnjak Romić, M.; Šegvić Klarić, M.; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef]
- El-Badry, M.; Fetih, G.; Shakeel, F. Comparative topical delivery of antifungal drug croconazole using liposome and micro-emulsion-based gel formulations. Drug Deliv. 2014, 21, 34–43. [Google Scholar] [CrossRef]
- European Society of Clinical Microbiology and Infectious Diseases (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 9–15. [Google Scholar]
- Bogdanov, A.; Janovák, L.; Vraneš, J.; Meštrović, T.; Ljubin-Sternak, S.; Cseh, Z.; Endrész, V.; Burián, K.; Vanić, Ž.; Virok, D.P. Liposomal encapsulation increases the efficacy of azithromycin against Chlamydia trachomatis. Pharmaceutics 2022, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Moghtaderi, M.; Bazzazan, S.; Sorourian, G.; Sorourian, M.; Akhavanzanjani, Y.; Noorbazargan, H.; Ren, Q. Encapsulation of thymol in gelatin methacryloyl (gelma)-based nanoniosome enables enhanced antibiofilm activity and wound healing. Pharmaceutics 2023, 15, 1699. [Google Scholar] [CrossRef] [PubMed]
- Balko, S.; Kerr, E.; Buchel, E.; Logsetty, S.; Raouf, A. A robust and standardized approach to quantify wound closure using the scratch assay. Methods Protoc. 2023, 6, 87. [Google Scholar] [CrossRef] [PubMed]



| VPGs | SPC (g) | Cholesterol (g) | Thymol (g) | PG (g) | Water (g) |
|---|---|---|---|---|---|
| SPC-VPG | 9.6 | - | - | 1 | 19.4 |
| SPC/Ch-VPG * | 8.6 | 1 | - | 1 | 19.4 |
| SPC/T-VPG | 9.6 | - | 0.5 | 1 | 18.9 |
| SPC/Ch/T-VPG * | 8.6 | 1 | 0.5 | 1 | 18.9 |
| Kinetic Model | SPC/T-VPG | SPC/Ch/T-VPG |
|---|---|---|
| Zero-order | 0.990 | 0.992 |
| First-order | 0.998 | 0.989 |
| Higuchi model | 0.998 | 0.998 |
| Korsmeyer–Peppas | 0.996 (n = 0.61) | 0.995 (n = 0.62) |
| Sample | MIC (µg/mL) | |
|---|---|---|
| S. aureus ATCC 6538 | MRSA MFBF 10679 | |
| Free thymol | 128 | 128 |
| SPC/T-VPG liposomes | >512 | >512 |
| SPC/Ch/T-VPG liposomes | >512 | >512 |
| Gentamicin | 0.25 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keser, S.; Rukavina, Z.; Jozić, M.; Pavlović-Mitrović, L.; Vodolšak, M.; Kranjčec, K.; Stupin Polančec, D.; Maravić-Vlahoviček, G.; Lovrić, J.; Šegvić Klarić, M.; et al. Potentials and Challenges in Development of Vesicular Phospholipid Gel as a Novel Dermal Vehicle for Thymol. Pharmaceutics 2025, 17, 854. https://doi.org/10.3390/pharmaceutics17070854
Keser S, Rukavina Z, Jozić M, Pavlović-Mitrović L, Vodolšak M, Kranjčec K, Stupin Polančec D, Maravić-Vlahoviček G, Lovrić J, Šegvić Klarić M, et al. Potentials and Challenges in Development of Vesicular Phospholipid Gel as a Novel Dermal Vehicle for Thymol. Pharmaceutics. 2025; 17(7):854. https://doi.org/10.3390/pharmaceutics17070854
Chicago/Turabian StyleKeser, Sabina, Zora Rukavina, Marica Jozić, Lea Pavlović-Mitrović, Magda Vodolšak, Kristina Kranjčec, Darija Stupin Polančec, Gordana Maravić-Vlahoviček, Jasmina Lovrić, Maja Šegvić Klarić, and et al. 2025. "Potentials and Challenges in Development of Vesicular Phospholipid Gel as a Novel Dermal Vehicle for Thymol" Pharmaceutics 17, no. 7: 854. https://doi.org/10.3390/pharmaceutics17070854
APA StyleKeser, S., Rukavina, Z., Jozić, M., Pavlović-Mitrović, L., Vodolšak, M., Kranjčec, K., Stupin Polančec, D., Maravić-Vlahoviček, G., Lovrić, J., Šegvić Klarić, M., & Vanić, Ž. (2025). Potentials and Challenges in Development of Vesicular Phospholipid Gel as a Novel Dermal Vehicle for Thymol. Pharmaceutics, 17(7), 854. https://doi.org/10.3390/pharmaceutics17070854








