The Influence of Moisturizer Co-Application Protocols on In Vitro Penetration of Betamethasone in Porcine Skin
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.1.1. Excipients of BET Formulations
- (a)
- BET dipropionate cream: Non-ionic self-emulsifying wax, liquid petrolatum, decyl oleate, propylene glycol, phenoxyethanol, ethyl paraben, methylparaben, butylparaben, propylparaben, simethicone, and water.
- (b)
- BET dipropionate ointment: Petrolatum and polyethylene.
2.1.2. Composition of Moisturizers
- (a)
- Emollient Moisturizer (Cetaphil® Moisturizing Cream): Cetaphil Moisturizing Cream is an oil-in-water (O/W) emulsion composed of aqua, glycerin, petrolatum, dicaprylyl ether, dimethicone, glyceryl stearate, cetyl alcohol, Helianthus annuus seed oil, PEG-30 stearate, panthenol, niacinamide, Prunus amygdalus dulcis oil, tocopherol, tocopheryl acetate, pantolactone, dimethiconol, acrylates/C10–30 alkyl acrylate crosspolymer, carbomer, propylene glycol, BHT, disodium EDTA, benzyl alcohol, phenoxyethanol, sodium hydroxide, citric acid. FIL.1765.V00.
- (b)
- Occlusive Moisturizer (Nivea® Cream): This Nivea moisturizer is a water-in-oil (W/O) emulsion composed of aqua, paraffinum liquidum, glycerin, isododecane, isopropyl palmitate, PEG-40 sorbitan perisostearate, cera microcristallina, polyglyceryl-3 diisostearate, Prunus amygdalus dulcis oil, sodium hyaluronate, tocopherol, magnesium sulfate, sodium citrate, citric acid, tocopheryl acetate, potassium sorbate, ethylhexylglycerin, linalool, limonene, geraniol, benzyl alcohol, citronellol, alpha-isomethyl ionone, benzyl benzoate, BHT, parfum.
2.1.3. Porcine Skin
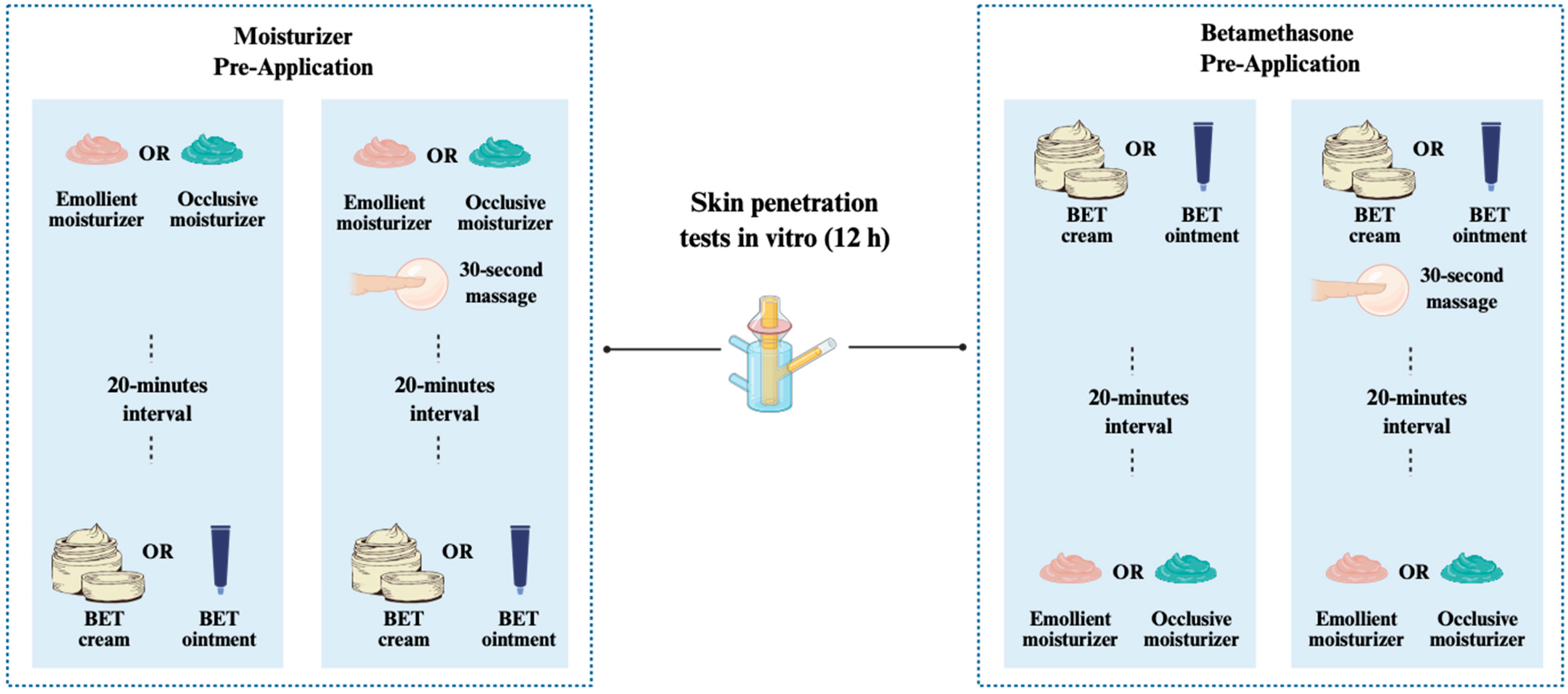
2.2. In Vitro Skin Penetration Test
Experimental Design
2.3. Analytical Method
2.4. Statistical Analysis
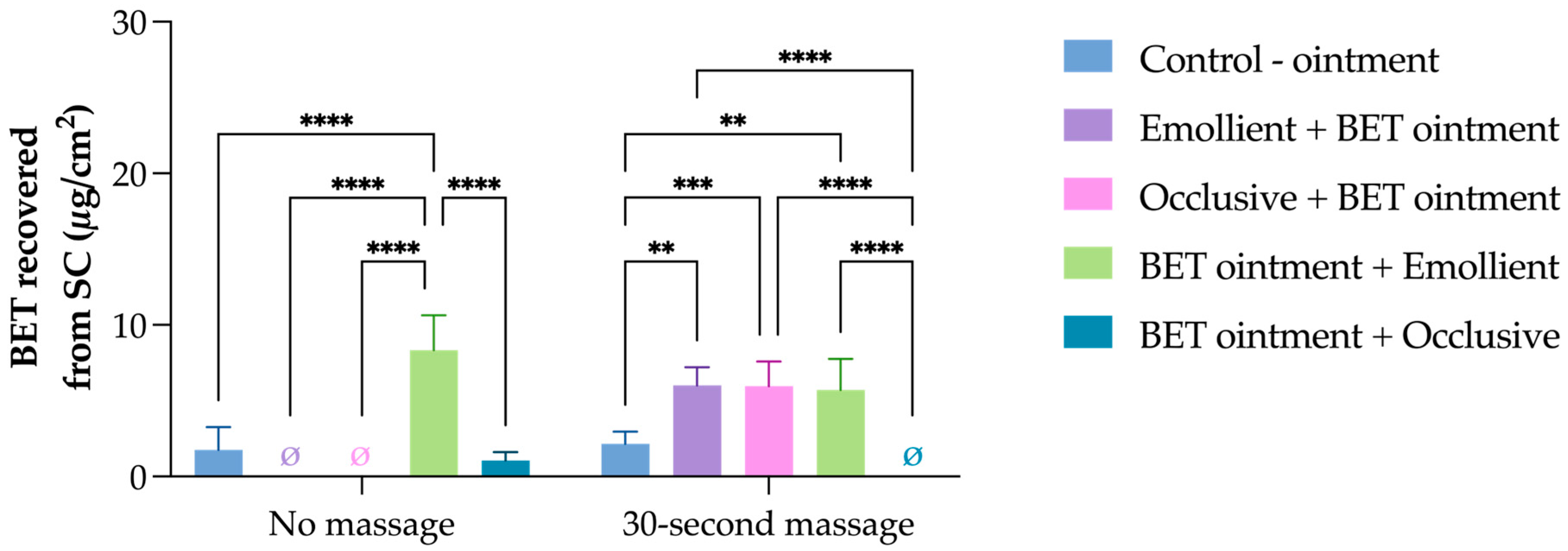
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elias, P.M.; Wakefield, J.S. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791.e1. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician. 2020, 101, 590–598. [Google Scholar] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Katayama, I.; Aihara, M.; Ohya, Y.; Saeki, H.; Shimojo, N.; Shoji, S.; Taniguchi, M.; Yamada, H. Japanese guidelines for atopic dermatitis 2017. Allergol. Int. 2017, 66, 230–247. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. IJMS 2020, 21, 2867. [Google Scholar] [CrossRef]
- Chu, D.K.; Schneider, L.; Asiniwasis, R.N.; Boguniewicz, M.; De Benedetto, A.; Ellison, K.; Frazier, W.T.; Greenhawt, M.; Huynh, J.; Kim, E.; et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann. Allergy Asthma Immunol. 2024, 132, 274–312. [Google Scholar] [CrossRef]
- Tang, X.; Lin, L.; Yu, F.; Ma, Y.; Liu, Z.; Xu, X. Allergic-related skin diseases: Global disease burden from 1990 to 2021 and future trends. World Allergy Organ. J. 2025, 18, 101072. [Google Scholar] [CrossRef]
- Gabros, S.; Nessel, T.A.; Zito, P.M. Topical Corticosteroids [Internet]; StatPearls: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532940/ (accessed on 30 May 2025).
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.-I.; Conlan, S.; NISC Comparative Sequencing Program; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef]
- Maskey, A.R.; Mo, X.; Li, X.-M. Preclinical Models of Atopic Dermatitis Suitable for Mechanistic and Therapeutic Investigations. J. Inflamm. Res. 2024, 17, 6955–6970. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and Its Relationship with Passive Drug Permeation. Pharm. Res. 2010, 28, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Nakamura, E.; Iwase, H.; Arima-Osonoi, H.; Sakuragi, M. Effect of water content on stratum corneum penetration mechanism of W/O type microemulsions. RSC Adv. 2023, 13, 17742–17749. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef]
- Siegfried, E.C.; Jaworski, J.C.; Kaiser, J.D.; Hebert, A.A. Systematic review of published trials: Long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Sidbury, R.; Davis, D.M.; Cohen, D.E.; Cordoro, K.M.; Berger, T.G.; Bergman, J.N.; Chamlin, S.L.; Cooper, K.R.; Feldman, S.R.; Hanifin, J.M.; et al. Guidelines of care for the management of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 71, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Camilion, J.V.; Khanna, S.; Anasseri, S.; Laney, C.; Mayrovitz, H.N. Physiological, Pathological, and Circadian Factors Impacting Skin Hydration. Cureus 2022, 14, e27666. Available online: https://www.cureus.com/articles/98088-physiological-pathological-and-circadian-factors-impacting-skin-hydration (accessed on 30 May 2025). [CrossRef]
- Pons-Guiraud, A. Dry skin in dermatology: A complex physiopathology. Acad. Dermatol. Venereol. 2007, 21, 1–4. [Google Scholar] [CrossRef]
- Gentile, L.L.; Cecatto, A.P. Creme hidratante com extrato de calêndula: Os benefícios da calêndula para a pele. Braz. J. Hea. Rev. 2023, 6, 32022–32042. [Google Scholar] [CrossRef]
- Charman, C.; Williams, H. The use of corticosteroids and corticosteroid phobia in atopic dermatitis. Clin. Dermatol. 2003, 21, 193–200. [Google Scholar] [CrossRef] [PubMed]
- McMullen, E.; Gawkrodger, D.J. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis: Physical friction and contact dermatitis. Br. J. Dermatol. 2006, 154, 154–156. [Google Scholar] [CrossRef]
- Kaushik, V.; Ganashalingam, Y.; Schesny, R.; Raab, C.; Sengupta, S.; Keck, C.M. Influence of Massage and Skin Hydration on Dermal Penetration Efficacy of Nile Red from Petroleum Jelly—An Unexpected Outcome. Pharmaceutics 2021, 13, 2190. [Google Scholar] [CrossRef]
- Angelo, T.; El-Sayed, N.; Jurisic, M.; Koenneke, A.; Gelfuso, G.M.; Cunha-Filho, M.; Taveira, S.F.; Lemor, R.; Schneider, M.; Gratieri, T. Effect of physical stimuli on hair follicle deposition of clobetasol-loaded Lipid Nanocarriers. Sci. Rep. 2020, 10, 176. [Google Scholar] [CrossRef]
- Phuong, C.; Maibach, H.I. Effect of massage on percutaneous penetration and skin decontamination: Man and animal. Cutan. Ocul. Toxicol. 2015, 35, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Agência Navional de Vigilância Sanitária. ANVISA. Resolução da Diretoria Colegiada (RDC) n 35, de 7 de Agosto de 2015. Available online: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/concea/arquivos/pdf/legislacao/resolucao-rdc-no-35-de-7-de-agosto-de-2015-anvisa.pdf/view (accessed on 27 May 2025).
- National Research Guide. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; Available online: http://www.nap.edu/catalog/12910 (accessed on 27 May 2025).
- European Parliament and Council. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010L0063 (accessed on 27 May 2025).
- Dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; dos Santos Ré, A.C.; Vieira Anjos, J.L.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef]
- Brain, K.; Walters, K.A.; Watkinson, A.C. Methods for Studying Percutaneous Absorption. In Dermatological and Transdermal Formulations; CRC Press: Boca Raton, FL, USA, 2002; pp. 197–270. [Google Scholar]
- Catherine Mack Correa, M.; Nebus, J. Management of Patients with Atopic Dermatitis: The Role of Emollient Therapy. Dermatol. Res. Pract. 2012, 2012, 836931. [Google Scholar] [CrossRef]
- Kurebayashi, A.K.; Phan, K.; Abdoh, A.; Andreo-Filho, N.; Lopes, P.S.; Mohammed, Y.; Leite-Silva, V.R. Strategic Approaches in Formulation Development for Atopic Dermatitis. Cosmetics 2024, 11, 113. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, K.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in Patients with Inflammatory Skin Diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Diana Draelos, Z. Therapeutic Moisturizers. Dermatol. Clin. 2000, 18, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Patient burden and quality of life in atopic dermatitis in US adults. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Mengeaud, V.; Phulpin, C.; Bacquey, A.; Boralevi, F.; Schmitt, A.; Taieb, A. An Innovative Oat-Based Sterile Emollient Cream in the Maintenance Therapy of Childhood Atopic Dermatitis. Pediatr. Dermatol. 2015, 32, 208–215. [Google Scholar] [CrossRef]
- Rubel, D.; Thirumoorthy, T.; Soebaryo, R.W.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Consensus guidelines for the management of atopic dermatitis: An Asia–Pacific perspective. J. Dermatol. 2013, 40, 160–171. [Google Scholar] [CrossRef]
- Morais, G.G.; Santos, O.D.H.; Masson, D.S.; Oliveira, W.P.; Filho, P.A.R. Development of O/W Emulsions with Annato Oil (Bixa orellana) Containing Liquid Crystal. J. Dispers. Sci. Technol. 2005, 26, 591–596. [Google Scholar] [CrossRef]
- Flynn, T.C.; Petros, J.; Clark, R.E.; Viehman, G.E. Dry skin and moisturizers. Clin. Dermatol. 2001, 19, 387–392. [Google Scholar] [CrossRef]
- Lodén, M. Role of Topical Emollients and Moisturizers in the Treatment of Dry Skin Barrier Disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Li, B.S.; Cary, J.H.; Maibach, H.I. Should we instruct patients to rub topical agents into skin? The evidence. J. Dermatol. Treat. 2019, 30, 328–332. [Google Scholar] [CrossRef]
- Salas, T.; Bordes, C.; Arquier, D.; Caillier, L.; Mandica, F.; Bolzinger, M.-A. Effect of massage on retinol skin penetration. Int. J. Pharm. 2023, 642, 123106. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Draelos, M.M.; Steele, F.; Georgiou, M.; Praestegaard, M. Enhanced Skin Deposition of Betamethasone Dipropionate from a Novel Formulation and Drug Delivery Technology. Dermatol. Ther. 2023, 13, 1763–1771. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Singhvi, G. Dermato-pharmacokinetic: Assessment tools for topically applied dosage forms. Expert. Opin. Drug Deliv. 2021, 18, 423–426. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rost, D.L.; Barbalho, G.N.; Andrade, J.F.M.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. The Influence of Moisturizer Co-Application Protocols on In Vitro Penetration of Betamethasone in Porcine Skin. Pharmaceutics 2025, 17, 874. https://doi.org/10.3390/pharmaceutics17070874
Rost DL, Barbalho GN, Andrade JFM, Cunha-Filho M, Gelfuso GM, Gratieri T. The Influence of Moisturizer Co-Application Protocols on In Vitro Penetration of Betamethasone in Porcine Skin. Pharmaceutics. 2025; 17(7):874. https://doi.org/10.3390/pharmaceutics17070874
Chicago/Turabian StyleRost, Daiane L., Geisa N. Barbalho, Jayanaraian F. M. Andrade, Marcilio Cunha-Filho, Guilherme M. Gelfuso, and Tais Gratieri. 2025. "The Influence of Moisturizer Co-Application Protocols on In Vitro Penetration of Betamethasone in Porcine Skin" Pharmaceutics 17, no. 7: 874. https://doi.org/10.3390/pharmaceutics17070874
APA StyleRost, D. L., Barbalho, G. N., Andrade, J. F. M., Cunha-Filho, M., Gelfuso, G. M., & Gratieri, T. (2025). The Influence of Moisturizer Co-Application Protocols on In Vitro Penetration of Betamethasone in Porcine Skin. Pharmaceutics, 17(7), 874. https://doi.org/10.3390/pharmaceutics17070874





