Optimizing Burn Wound Healing: The Critical Role of pH and Rheological Behavior in Plant-Derived Topical Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction
Harvesting and Preparation of Plant Material (Sambucus Nigra Bark)
2.2. Phytochemical Profile of Extracts
2.3. Materials an Methods Used in the Formulations
2.4. Determination of Partition Coefficient LogP and Skin Permeability Potential
2.5. Physicochemical and Rheological Characterizationation Methods
2.6. Methodology for Physicochemical and Rheological Characterization
2.6.1. Determination of Macroscopic Properties and pH
2.6.2. Rheological Measurements
2.6.3. pH Analysis: Formulations and Burn Wounds
2.7. Animal Study and Clinical Parameters
2.7.1. In Vivo Study and Experimental Protocol
2.7.2. Anesthesia, Preoperative Preparation, and Pain Management
2.7.3. Burn Induction and Wound Standardization
2.7.4. Treatment Application and Dressing Protocol
2.7.5. Statistical Analysis
3. Results
3.1. Quantitative Phytochemical Profile of Extracts
3.2. Physicochemical and Rheological Properties
3.2.1. Determination of Macroscopic Properties and pH
3.2.2. Standardized Macroscopic Evaluation of Topical Formulations
3.3. Physicochemical Characterization
3.4. Rheological Characterization
3.5. Comparative pH Evaluation
3.5.1. Association Between Wound pH Category and Burn Surface Area at Specific Timepoints
3.5.2. Comparative Analysis of Skin Surface pH and Temperature Dynamics in Relation to Plant Extracts and Formulation Types
3.6. Clinical and Histological Evaluation of Burn Healing
3.6.1. Impact of pH Variations in Hydrogel Formulations on Vascularization, Epithelialization, and Inflammation
3.6.2. Correlation Between Formulation pH, Rheological Characteristics, and Healing Progression
4. Discussion
4.1. Role of LogP and Additional Physicochemical Parameters in Assessing Skin Permeability
4.2. Correlation of Rheological Assessment in Literature Topical Formulation
4.3. Clinical Significance of pH and Temperature Monitoring in Effective Wound Healing Management
4.4. Comparative Analysis with Existing Plant-Based Hydrogels and Oleogels
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Blank-OG-OO | Blank oleogel olive oil formulation |
| BS | Boswellia serrata |
| Compritol 888 ATO | Glyceryl dibehenate excipient |
| DEGEE | Diethylene glycol monoethyl ether |
| GV | Galium verum |
| HG | Hydrogel |
| LC-MS | Liquid chromatography-mass spectrometry |
| LogP | Logarithm of partition coefficient |
| OC | Ocimum basilicum |
| OG | Oleogel |
| pH | Potential of hydrogen |
| PVP-I | Liposome polyvinyl-pyrrolidone-iodine |
| RAPID-3D | Rat printed induction device-3D |
| SD | Standard deviation |
| SNB | Sambucus nigra brunch bark |
| SNF | Sambucus nigra flower |
| SFO | Sunflower oil |
| TA | Topical antimicrobial |
References
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.G.; Haalboom, M.; Bowler, P.G.; Gamerith, C.; Sigl, E.; Heinzle, A.; Burnet, M.W.M. Elevated wound fluid pH correlates with increased risk of wound infection. Wound Med. 2019, 26, 100166. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Lei, D.; Liu, D.; Zhang, J.; Zhang, L.; Man, M.Q. Benefits of topical natural ingredients in epidermal permeability barrier. Front. Physiol. 2024, 14, 1275506. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Scalamandré, A.; Bogie, K.M. Smart Technologies in Wound Prevention and Care. In Innovations and Emerging Technologies in Wound Care; Gefen, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–244. [Google Scholar]
- Safta, D.A.; Vlase, A.-M.; Pop, A.; Cherfan, J.; Carpa, R.; Iurian, S.; Bogdan, C.; Vlase, L.; Moldovan, M.-L. Optimized Sambucus nigra L., Epilobium hirsutum L., and Lythrum salicaria L. Extracts: Biological Effects Supporting Their Potential in Wound Care. Antioxidants 2025, 14, 521. [Google Scholar] [CrossRef]
- Marțiș, G.S.; Mureșan, V.; Marc, R.M.; Mureșan, C.C.; Pop, C.R.; Buzgău, G.; Mureșan, A.E.; Ungur, R.A.; Muste, S. The Physicochemical and Antioxidant Properties of Sambucus nigra L. and Sambucus nigra Haschberg during Growth Phases: From Buds to Ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef]
- Ammon, H.P. Boswellic Acids and Their Role in Chronic Inflammatory Diseases. Adv. Exp. Med. Biol. 2016, 928, 291–327. [Google Scholar] [CrossRef]
- Mannino, G.; Occhipinti, A.; Maffei, M.E. Quantitative Determination of 3-O-Acetyl-11-Keto-βBoswellic Acid (AKBA) and Other Boswellic Acids in Boswellia sacra Flueck (syn. B. carteri Birdw) and Boswellia serrata Roxb. Molecules 2016, 21, 1329. [Google Scholar] [CrossRef] [PubMed]
- Petkova, M.K.; Grozeva, N.H.; Tzanova, M.T.; Todorova, M.H. A Review of Phytochemical and Pharmacological Studies on Galium verum L., Rubiaceae. Molecules 2025, 30, 1856. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Camero, C.; Germanò, M.P.; Rapisarda, A.; D’Angelo, V.; Amira, S.; Benchikh, F.; Braca, A.; De Leo, M. Anti-angiogenic activity of iridoids from Galium tunetanum. Rev. Bras. Farm. 2018, 28, 374–377. [Google Scholar] [CrossRef]
- Antonescu, A.-I.; Miere, F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Antonescu, A.; Vicas, S.I.; Bodog, F.; Sindhu, R.K.; et al. Perspectives on the Combined Effects of Ocimum basilicum and Trifolium pratense Extracts in Terms of Phytochemical Profile and Pharmacological Effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef]
- Ajaz, N.; Tiwari, V.; Saxena, V. Advanced Topical Formulations for Wound Healing: An Overview. Asian J. Pharm. Sci. 2021, 16, 119–136. [Google Scholar] [CrossRef]
- Teixeira, L.G.; Rezende, S.; Fernandes, Â.; Fernandes, I.P.; Barros, L.; Barreira, J.C.M.; Leimann, F.V.; Ferreira, I.C.F.R.; Barreiro, M.-F. Water-in-Oil-in-Water Double Emulsions as Protective Carriers for Sambucus nigra L. Coloring Systems. Molecules 2022, 27, 552. [Google Scholar] [CrossRef]
- Roșca, O.-J.; Nistor, A.; Coneac, G.-H.; Olariu, I.-V.; Cotan, A.-M.; Racoviceanu, R.; Heredea, E.R.; Ciudoiu, A.; Didea, G.; Lupou, C.-M.; et al. Wound Healing Properties of Plant-Based Hydrogel and Oleogel Formulations in a Rat Scald Burn Model. Pharmaceutics 2025, 17, 597. [Google Scholar] [CrossRef]
- Viteri, F.; Sánchez, N.E.; Alexandrino, K. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Leaf and Bark Samples of Sambucus nigra Using High-Performance Liquid Chromatography (HPLC). Methods Protoc. 2023, 6, 17. [Google Scholar] [CrossRef]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon balm extracts prevent breast cancer progression in vitro and in ovo on chorioallantoic membrane Assay. Evid.-Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef]
- Oniga, I.; Puscas, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pantone, L.L.C. Pantone Matching System® Color Chart; Pantone Inc.: Carlstadt, NJ, USA, 2023; Available online: https://www.pantone.com/color-systems/pantone-color-systems-explained (accessed on 2 May 2024).
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; Wiley-Interscience: Hoboken, NY, USA, 2000; p. 117. [Google Scholar]
- Sinko, P.J. Rheology. In Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 7th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017; Chapter 17; pp. 433–466. [Google Scholar]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Clarity and degree of opalescence of liquids. In European Pharmacopoeia (Ph. Eur.), 11th ed.; EDQM Council of Europe: Strasbourg, France, 2022; General Chapter 2.2.1; p. 29. [Google Scholar]
- The United States Pharmacopeial Convention. The United States Pharmacopoeia and National Formulary USP 43–NF 38; The United States Pharmacopeial Convention: Rockville, MD, USA, 2019; p. 6415. [Google Scholar]
- Lawless, H.T.; Heymann, H. Descriptive analysis. In Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010; Chapter 10; pp. 227–257. [Google Scholar]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Odor evaluation techniques. In Sensory Evaluation Practices, 5th ed.; Academic Press: London, UK, 2020; Chapter 8; pp. 177–208. [Google Scholar]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Descriptive analysis techniques. In Sensory Evaluation Techniques, 5th ed.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 5; pp. 141–174. [Google Scholar]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Measurement of Consistency by Penetrometry. In European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022; p. 360. [Google Scholar]
- Parente, M.E.; Ochoa Andrade, A.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive Hydrogels for Cosmetic Applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Potentiometric Determination of pH. In European Pharmacopeia, 11th ed.; EDQM Council of Europe: Strasbourg, France, 2022; p. 29. [Google Scholar]
- Roșca, O.-J.; Nistor, A.; Brandabur, C.; Heredea, R.E.; Hoinoiu, B.; Șoica, C. Rat 3D Printed Induction Device (RAPID-3D): A 3D-Printed Device for Uniform and Reproducible Scald Burn Induction in Rats with Histological and Microvascular Validation. Biology 2025, 14, 378. [Google Scholar] [CrossRef]
- NC3Rs. Revision of the ARRIVE Guidelines. Available online: https://nc3rs.org.uk/our-portfolio/revision-arrive-guidelines (accessed on 1 January 2025).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate the solubility and pemeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Karrer, S.; Heinlin, J.; Landthaler, M.; Babilas, P. The impact of the pH value on skin integrity and cutaneous wound healing. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanism and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Gethin, G. The Significance of Surface pH in Chronic Wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel Chemical Permeation enhancers for Transdermal Drug Delivery. Asian J. Pharm. Sci. 2018, 13, 51–64. [Google Scholar] [CrossRef]
- Pecoraro, B.; Tutone, M.; Hoffman, E.; Hutter, V.; Almerico, A.M.; Traynor, M. Predicting skin permeability by means of computational approaches: Reliability and caveats in pharmaceutical studies. J. Chem. Inf. Model. 2019, 59, 968–981. [Google Scholar] [CrossRef]
- Patel, A.; Iliopoulos, F.; Caspers, P.J.; Puppes, G.J.; Lane, M.E. In vitro–in vivo correlation in dermal delivery: The role of excipients. Pharmaceutics 2021, 13, 542. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Jane, M.E. Franz cell diffusion testing and quantitative confocal Raman spectroscopy: In vitro-in vivo correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface Sci. 2009, 147–148, 214–227. [Google Scholar] [CrossRef]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.B.; Cunha, R.L. Tailoring properties of mixed-component oleogels: Wax and monoglyceride interactions towards flaxseed oil structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Hinestroza, J.P.; Martín-Alfonso, J.E. Impact of vegetable oil type on the rheological and tribological behavior of montmorillonite-based oleogels. Gels 2022, 8, 504. [Google Scholar] [CrossRef]
- Sánchez, R.; Franco, J.M.; Delgado, M.A.; Valencia, C.; Gallegos, C. Rheology of oleogels based on sorbitan and glycerol monostearates and vegetable oils for lubricating applications. Grasas Aceites 2011, 62, 328–336. [Google Scholar] [CrossRef]
- Chaibundit, C.; Ricardo, N.M.; Muryn, C.A.; Madec, M.B.; Yeates, S.G.; Booth, C. Effect of ethanol on the gelation of aqueous solutions of Pluronic F127. J. Colloid Interface Sci. 2010, 351, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified Poloxamer 407. Heliiyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Tang, N.; Zheng, Y.; Jiang, X.; Zhou, C.; Jin, H.; Jin, K.; Wu, W.; Haick, H. Wearable sensors and systems for wound healing-related pH and temperature detection. Micromachines 2021, 12, 430. [Google Scholar] [CrossRef]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Watters, C.; Yuan, T.T.; Rumbaugh, K.P. Beneficial and deleterious bacterial-host interactions in chronic wound pathophysiology. Chronic Wound Care Manag. Res. 2015, 2, 53–62. [Google Scholar] [CrossRef]
- Martínez-Jiménez, M.A.; Aguilar-García, J.; Valdés-Rodríguez, R.; Metlich-Medlich, M.A.; Dietsch, L.J.P.; Gaitán-Gaona, F.I.; Kolosovas-Machuca, E.S.; González, F.J.; Sánchez-Aguilar, J.M. Local Use of insulin in wounds of diabetic patients: Higher temperature, fibrosis, and angiogenesis. Plast. Reconstr. Surg. 2013, 132, 1015e–1019e. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, G.; Sanada, H.; Iizaka, S.; Kadono, T.; Higashino, T.; Koyanagi, H.; Haga, N. Predicting Delayed Pressure Ulcer Healing Using Thermography: A Prospective Cohort Study. J. Wound Care 2010, 19, 465–472. [Google Scholar] [CrossRef]
- Wilmore, D.W.; Aulick, L.H.; Mason, A.D., Jr.; Pruitt, B.A., Jr. Influence of the Burn Wound on Local and Systemic Responses to Injury. Ann. Surg. 1977, 186, 444. [Google Scholar] [CrossRef] [PubMed]
- Wijlens, A.M.; Holloway, S.; Bus, S.A.; van Netten, J.J. An Explorative Study on the Validity of Various Definitions of a 2.2 °C Temperature Threshold as Warning Signal for Impending Diabetic Foot Ulceration. Int. Wound J. 2017, 14, 1346–1351. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Holtz-Neiderer, K.; Wendel, C.; Mohler, M.J.; Kimbriel, H.R.; Lavery, L.A. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am. J. Med. 2007, 120, 1042–1046. [Google Scholar] [CrossRef]
- Heberlé, G.; Dos Santos, M.A.; Magri, S. Cosmetic formulations containing blueberry extracts (Vaccinium myrtillus L.). TOJSAT 2012, 2, 1–6. [Google Scholar]
- Da-Lozzo, E.J.; Moledo, R.C.; Faraco, C.D.; Ortolani-Machado, C.F.; Bresolin, T.M.; Silveira, J.L. Curcumin/Xanthan-Galactomannan hydrogels: Rheological analysis and biocompatibility. Carbohydr. Polym. 2013, 93, 279–284. [Google Scholar] [CrossRef]
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.K.; Achim, M.; Mirel, S. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242. [Google Scholar] [CrossRef]
- Andleeb, M.; Shoaib Khan, H.M.; Daniyal, M. Development, Characterization and Stability Evaluation of Topical Gel Loaded with Ethosomes Containing Achillea millefolium L. Extract. Front. Pharmacol. 2021, 12, 603227. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Bargan, A.; Homocianu, M.; Aflori, M.; Rîmbu, C.M.; Enache, A.A.; Vlad-Bubulac, T. Electrospun Polyvinyl Alcohol Loaded with Phytotherapeutic Agents for Wound Healing Applications. Nanomaterials 2021, 11, 3336. [Google Scholar] [CrossRef]
- Alexa, E.; Bota, V.; Sumalan, R.M.; Obistioiu, D.; Negrea, M.; Cocan, I.; Borcan, F.; Cozma, A.; Radulov, I. Natural Emulsions Based on Essential Oils as Antifungal and Antimycotoxicogenic Agents on Wheat for Bakery Industry. Foods 2022, 11, 2926. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Verma, S. Formulation of a Novel Emulgel for Wound Healing: A Synergistic Blend of Three Unique Botanical Extracts. PEXACY Int. J. Pharm. Sci. 2023, 2, 153–166. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasă, A.M.; Suteu, D. Antioxidants from Galium verum as Ingredients for the Design of New Dermatocosmetic Products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, I.A.; Antonescu, A.; Miere, F.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; et al. Evaluation of Wound Healing Potential of Novel Hydrogel Based on Ocimum basilicum and Trifolium pratense Extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and Evaluation of Ocimum basilicum-Based Emulgel for Wound Healing Using Animal Model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H.; Duarte, N.; Serra, A.T.; Ferreira, A.; Bronze, M.R.; Custódio, L.; Gaspar, M.M.; Simões, S.; Rijo, P.; Ascensão, L.; et al. Further Evidence of Possible Therapeutic Uses of Sambucus nigra L. Extracts by the Assessment of the In Vitro and In Vivo Anti-Inflammatory Properties of Its PLGA and PCL-Based Nanoformulations. Pharmaceutics 2020, 12, 1181. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of pH, exudate composition and temperature in wound healing: A systematic review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef]







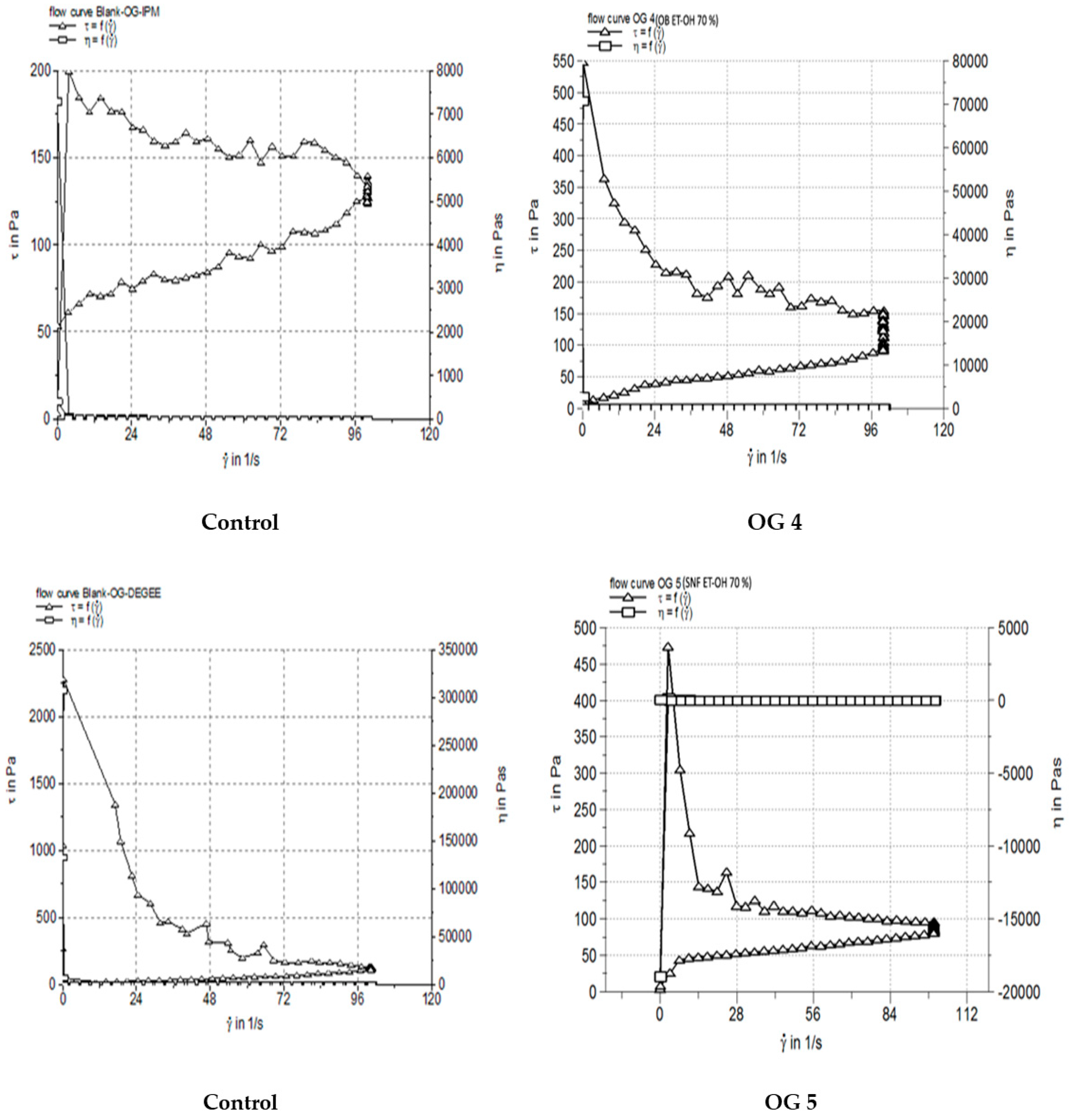





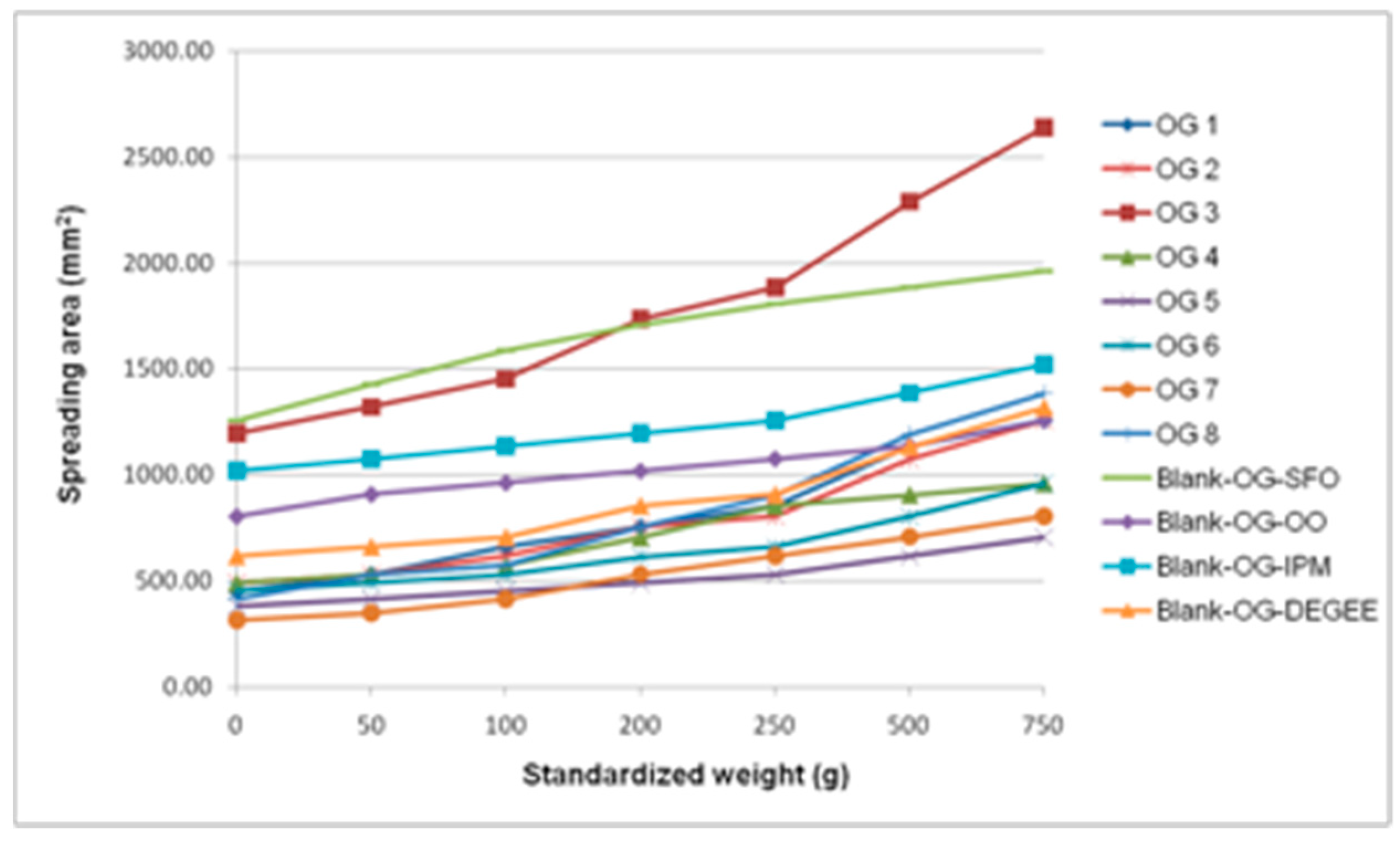

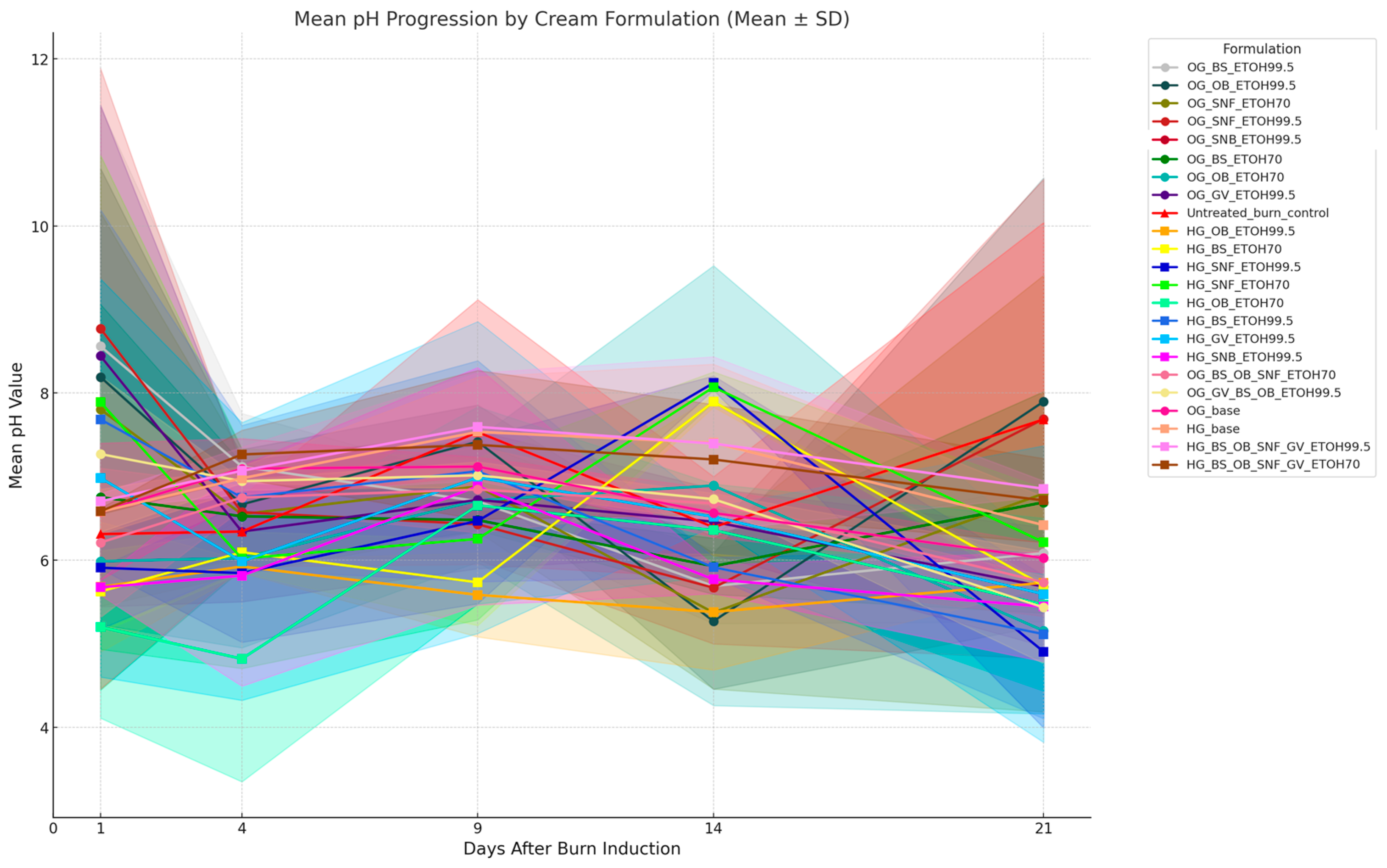





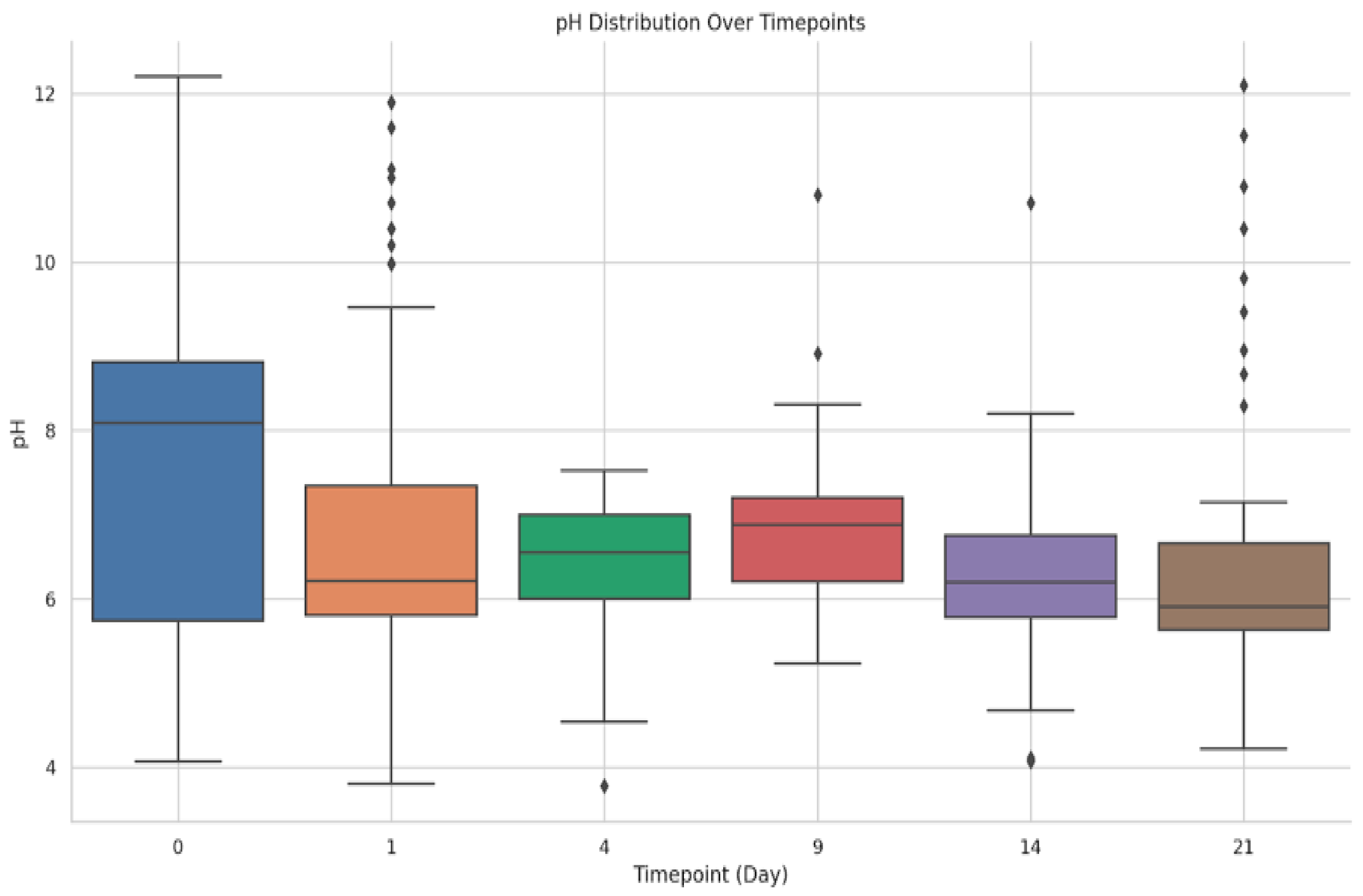




| Formulation | Extraction Method | Extract and Concentration (w/w) | Gel Components | In Vivo Unique Name | Description |
|---|---|---|---|---|---|
| OG | ETOH99.5 | OB 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_OB_ETOH99.5 | Oleogel with Ocimum basilicum extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | BS 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_BS_ETOH99.5 | Oleogel with Boswellia serrata extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | SNF 5% | Olive oil 80% Glyceryl dibehenate 15% | OG_SNF_ETOH99.5 | Oleogel with Sambucus nigra flower extract in absolute ethanol, based on glyceryl dibehenate and olive oil |
| OG | ETOH99.5 | SNB 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_SNB_ETOH99.5 | Oleogel with Sambucus nigra bark brunch extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | GV 5% | Olive oil 80% Glyceryl dibehenate 15% | OG_GV_ETOH99.5 | Oleogel with Galium verum extract in absolute ethanol, based on glyceryl dibehenate and olive oil |
| OG | ETOH70 | OB 5% | Isopropyl myristate 80% Glyceryl dibehenate 15% | OG_OB_ETOH70 | Oleogel with Ocimum basilicum extract in 70% ethanol, based on glyceryl dibehenate and isopropyl myristate |
| OG | ETOH70 | BS 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_BS_ETOH70 | Oleogel with Boswellia serrata extract in 70% ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH70 | SNF 5% | Diethylene glycol monoethyl ether 80% Glyceryl dibehenate 15% | OG_SNF_ETOH70 | Oleogel with Sambucus nigra flower extract in 70% ethanol, based on glyceryl dibehenate and diethylene glycol monoethyl ether |
| OG | ETOH99.5 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Sunflower oil 20% Olive oil 20% Isopropyl myristate 20% Diethylene glycol monoethyl ether 20% Glyceryl dibehenate 15% | OG_BS_OB_SNF_GV_ETOH99.5 | Oleogel with 4 plant extracts in absolute ethanol |
| OG | ETOH70 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Sunflower oil 20% Olive oil 20% Isopropyl myristate 20% Diethylene glycol monoethyl ether 20% Glyceryl dibehenate 15% | OG_BS_OB_SNF_GV_ETOH70 | Oleogel with 4 plant extracts in 70% ethanol |
| HG | ETOH99.5 | OB 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_OB_ETOH99.5 | Hydrogel with Ocimum basilicum extract in absolute ethanol |
| HG | ETOH99.5 | BS 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_ETOH99.5 | Hydrogel with Boswellia serrata extract in absolute ethanol |
| HG | ETOH99.5 | SNF 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_SNF_ETOH99.5 | Hydrogel with Sambucus nigra flower extract in absolute ethanol |
| HG | ETOH99.5 | GV 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_GV_ETOH99.5 | Hydrogel with Galium verum extract in absolute ethanol |
| HG | ETOH70 | OB 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_OB_ETOH70 | Hydrogel with Ocimum basilicum extract in 70% ethanol |
| HG | ETOH70 | BS 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_ETOH70 | Hydrogel with Boswellia serrata extract in 70% ethanol |
| HG | ETOH70 | SNF 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_SNF_ETOH70 | Hydrogel with Sambucus nigra flower extract in 70% ethanol |
| HG | ETOH99.5 | SNB 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_SNB_ETOH99.5 | Hydrogel with Sambucus nigra bark brunch extract in absolute ethanol |
| HG | ETOH70 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_OB_SNF_GV_ETOH70 | Hydrogel with 4 plant extracts in 70% ethanol |
| HG | ETOH99.5 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_OB_SNF_GV_ETOH99.5 | Hydrogel with 4 plant extracts in absolute ethanol |
| Pre-burn | N/A | Baseline | N/A | Pre-burn_baseline | Control for the healthy skin |
| Untreated_ Burn | N/A | Untreated_Burn | N/A | Untreated_burn_control | Control for the untreated burn |
| OG | N/A | OG_base | Sunflower oil 21.5% Olive oil 21.5% Isopropyl myristate 21.5% Diethylene glycol monoethyl ether 21.5% Glyceryl dibehenate 14% | OG_base | Control oleogel (without bioactive component) |
| HG | N/A | HG_base | Purified water 65% Poloxamer 407 25% Glycerol 10% | HG_base | Control hydrogel (without bioactive component), based on poloxamer 407 and glycerol, purified water |
| No. | Compound Name | Molecular Weight (g/mol) | Permeability Potential | Log Po/w |
|---|---|---|---|---|
| 1 | Caftaric acid | 311 | High | −0.29 |
| 2 | Gentisic acid | 153 | High | 0.74 |
| 3 | Chlorogenic acid | 353 | High | −0.43 |
| 4 | Caffeic acid | 179 | High | 0.93 |
| 5 | P-coumaric acid | 163 | High | 1.26 |
| 6 | Ferulic acid | 193 | High | 1.36 |
| 7 | Sinapic acid | 223 | High | 1.31 |
| 8 | Hyperoside | 463 | High | −0.38 |
| 9 | Isoquercitrin | 463 | High | −0.48 |
| 10 | Rutin | 609 | Low | −1.51 |
| 11 | Rosmarinic acid | 359 | High | 5.15 |
| 12 | Quercitrin | 447 | High | −0.05 |
| 13 | Quercetol | 301 | High | 1.23 |
| 14 | Luteolin | 285 | High | 1.73 |
| 15 | Kaempferol | 285 | High | 1.58 |
| 16 | Apigenin | 269 | High | 2.11 |
| 17 | Gaelic acid | 169 | High | 0.21 |
| 18 | Epicatechin | 289 | High | 0.83 |
| 19 | Beta-Resorcylic | 153 | High | 0.77 |
| 20 | Resveratrol | 227 | High | 2.48 |
| Qualitative Description (Original) | Suggested Pantone Reference |
|---|---|
| Pearly white | Pantone 11-0601 |
| Straw yellow | Pantone 13-0922 |
| White | Pantone 11-0601 |
| Dull-yellow | Pantone 13-0850 |
| Orange | Pantone 16-1364 |
| Green-moss | Pantone 17-0530 |
| Brown | Pantone 18-0930 |
| Pale brown | Pantone 15-1213 |
| Brownish-dark | Pantone 19-1015 |
| Brownish-green | Pantone 18-0435 |
| Colorless | Pantone Transparent |
| Term Used | Definition | Example Formulations |
|---|---|---|
| Transparent | Completely clear, no turbidity | Blank-HG |
| Translucent | Slightly cloudy but still clear | HG-PL 3–8 |
| Slightly opaque | Mild turbidity, partially opaque | Blank-OG-SFO, HG-PL 1, HG-PL 2 |
| Opaque | Completely opaque, no transparency | OG 1–8, Blank-OG-OO, Blank-OG-IPM, Blank-OG-DEGEE |
| Original Odor Description | Improved Standardized Description |
|---|---|
| Sunflower oil characteristic | Mild vegetal, characteristic of sunflower oil |
| Olive oil characteristic | Mild vegetal, characteristic of olive oil |
| Isopropyl myristate characteristic | Neutral, characteristic of IPM |
| DEGEE characteristic | Slightly chemical, neutral odor |
| Specific, slightly aromatic | Mild herbal aroma |
| Specific aromatic | Herbal, fresh aroma |
| Specific aromatic (brownish) | Earthy, herbal aroma |
| Specific aromatic (brown-dark) | Woody, earthy aroma |
| Odorless | Odorless (neutral) |
| Formulation | Viscosiy (Pa·s) | Thixotropy (Pa/s) | Penetration Depth (mm) |
|---|---|---|---|
| Blank-OG-SFO | 1.071733 ± 0.014 | 8019 | 370.0 ± 7.24 |
| Blank-OG-OO | 0.799833 ± 0.009 | 5845 | 321.0 ± 8.07 |
| Blank-OG-IPM | 1.3042 ± 0.041 | 7367 | 339.0 ± 3.65 |
| Blank-OG-DEGEE | 1.217 ± 0.081 | 54,860 | 330.0 ± 6.41 |
| OG 1 | 2.583 ± 0.084 | 10,850 | 107.0 ± 5.64 |
| OG 2 | 2.586 ± 0.066 | 6977 | 106.7 ± 5.03 |
| OG 3 | 0.640 ± 0.041 | 2900 | 256.3 ± 4.73 |
| OG 4 | 1.185 ± 0.182 | 17,370 | 113.0 ± 2.28 |
| OG 5 | 0.867 ± 0.043 | 7717 | 103.7 ± 1.53 |
| OG 6 | 2.062 ± 0.091 | 17,300 | 118.0 ± 2.14 |
| OG 7 | 2.457 ± 0.081 | 15,910 | 113.7 ± 1.53 |
| OG 8 | 1.645 ± 0.068 | 13,710 | 130.0 ± 2.66 |
| Blank HG-PL | 10.179 ± 0.027 | 9932 | 126.3 ± 1.58 |
| HG-PL 1 | 13.855 ± 0.415 | 94,340 | 84.7 ± 3.05 |
| HG-PL 2 | 10.839 ± 0.275 | 88,420 | 85.3 ± 2.52 |
| HG-PL 3 | 11.389 ± 0.097 | 17,410 | 97.7 ± 1.15 |
| HG-PL 4 | 5.723 ± 0.028 | 30,040 | 99.3 ± 1.53 |
| HG-PL 5 | 7.274 ± 0.187 | 77,680 | 119.7 ± 0.58 |
| HG-PL 6 | 9.341 ± 0.055 | 14,740 | 113.3 ± 2.08 |
| HG-PL 7 | 11.467 ± 0.059 | 15,360 | 112.0 ± 1.05 |
| HG-PL 8 | 9.073 ± 0.019 | 10,470 | 117.3 ± 2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roșca, O.-J.; Coneac, G.-H.; Racoviceanu, R.; Nistor, A.; Olariu, I.-V.; Cotan, A.-M.; Negrea-Ghiulai, R.; Dehelean, C.A.; Vlaia, L.L.; Șoica, C.M. Optimizing Burn Wound Healing: The Critical Role of pH and Rheological Behavior in Plant-Derived Topical Formulations. Pharmaceutics 2025, 17, 853. https://doi.org/10.3390/pharmaceutics17070853
Roșca O-J, Coneac G-H, Racoviceanu R, Nistor A, Olariu I-V, Cotan A-M, Negrea-Ghiulai R, Dehelean CA, Vlaia LL, Șoica CM. Optimizing Burn Wound Healing: The Critical Role of pH and Rheological Behavior in Plant-Derived Topical Formulations. Pharmaceutics. 2025; 17(7):853. https://doi.org/10.3390/pharmaceutics17070853
Chicago/Turabian StyleRoșca, Oana-Janina, Georgeta-Hermina Coneac, Roxana Racoviceanu, Alexandru Nistor, Ioana-Viorica Olariu, Ana-Maria Cotan, Roxana Negrea-Ghiulai, Cristina Adriana Dehelean, Lavinia Lia Vlaia, and Codruța Marinela Șoica. 2025. "Optimizing Burn Wound Healing: The Critical Role of pH and Rheological Behavior in Plant-Derived Topical Formulations" Pharmaceutics 17, no. 7: 853. https://doi.org/10.3390/pharmaceutics17070853
APA StyleRoșca, O.-J., Coneac, G.-H., Racoviceanu, R., Nistor, A., Olariu, I.-V., Cotan, A.-M., Negrea-Ghiulai, R., Dehelean, C. A., Vlaia, L. L., & Șoica, C. M. (2025). Optimizing Burn Wound Healing: The Critical Role of pH and Rheological Behavior in Plant-Derived Topical Formulations. Pharmaceutics, 17(7), 853. https://doi.org/10.3390/pharmaceutics17070853






.jpg)





