Development of Bioactive Cotton, Wool, and Silk Fabrics Functionalized with Origanum vulgare L. for Healthcare and Medical Applications: An In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of OE
2.2. OE-Biofunctionalization of Cotton, Wool, and Silk Fabrics and Their Characterization
2.3. In Vivo Wound Healing Study
2.3.1. Animals and Excision Wound Model
- NC (negative control): wounds left untreated;
- PC (positive control): wounds treated daily with 0.5 g of 1% silver sulfadiazine cream;
- WO (wool fabric): wounds treated daily with wool fabric, with dressing replaced daily;
- WO+OE (OE-biofunctionalized wool): wounds treated with OE-biofunctionalized wool fabric, with dressing replaced daily.
2.3.2. Wound Healing Rate and Biochemical Analyses
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. Chemical Profiling and Bioactivity of OE
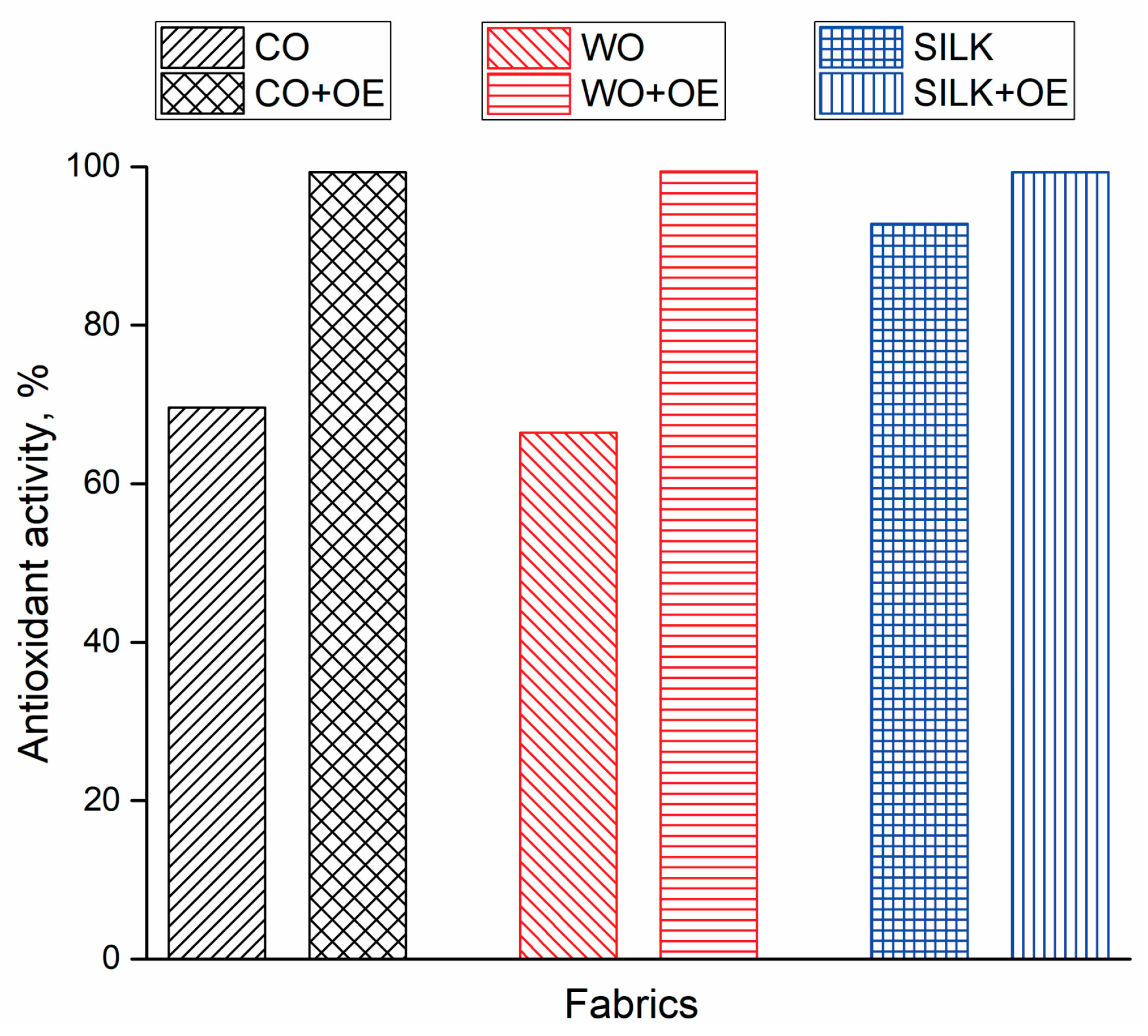
3.2. OE-Biofunctionalized Natural Fabrics
3.3. Testing the Suitability of WO+OE for Application as Wound Dressing
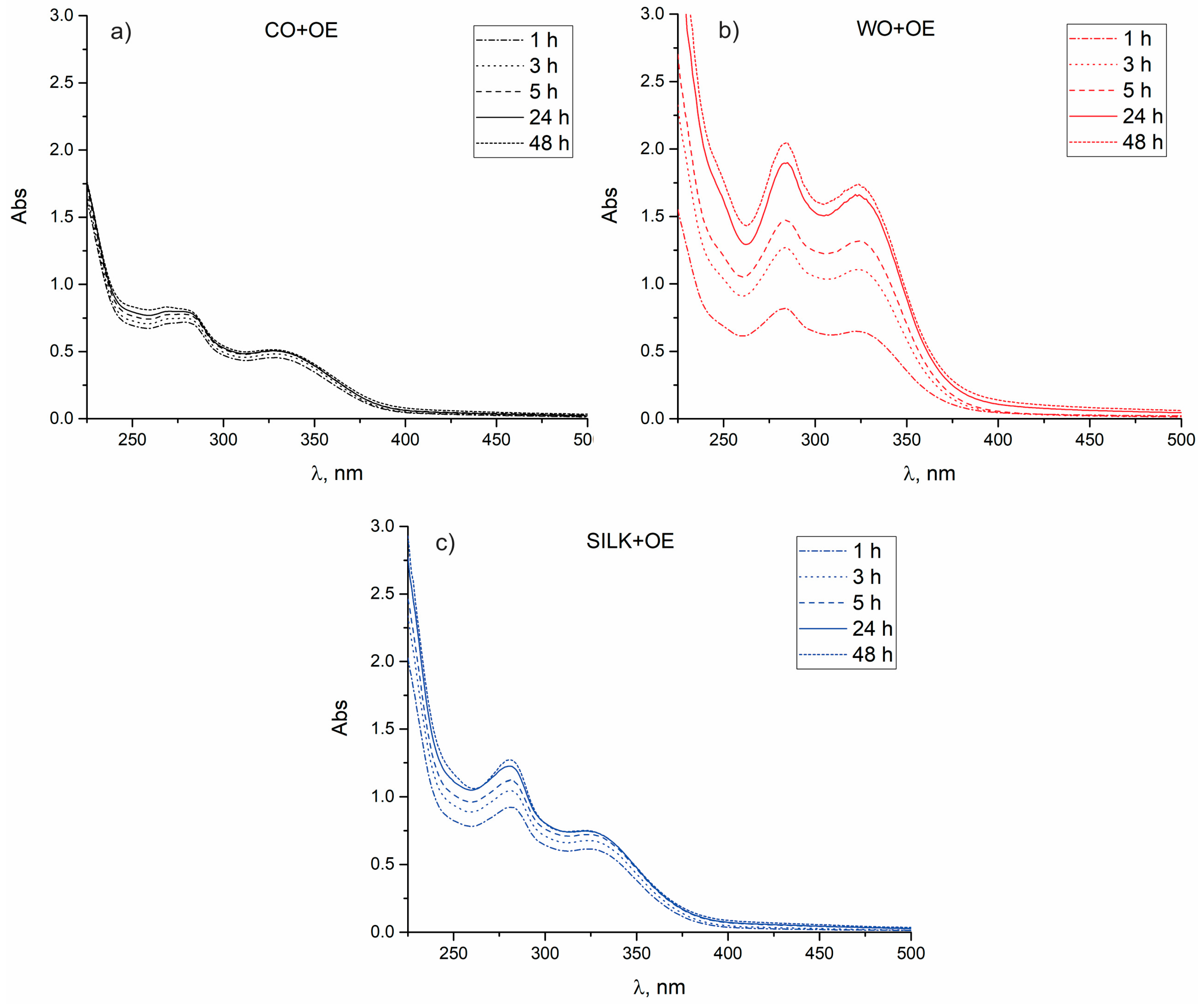
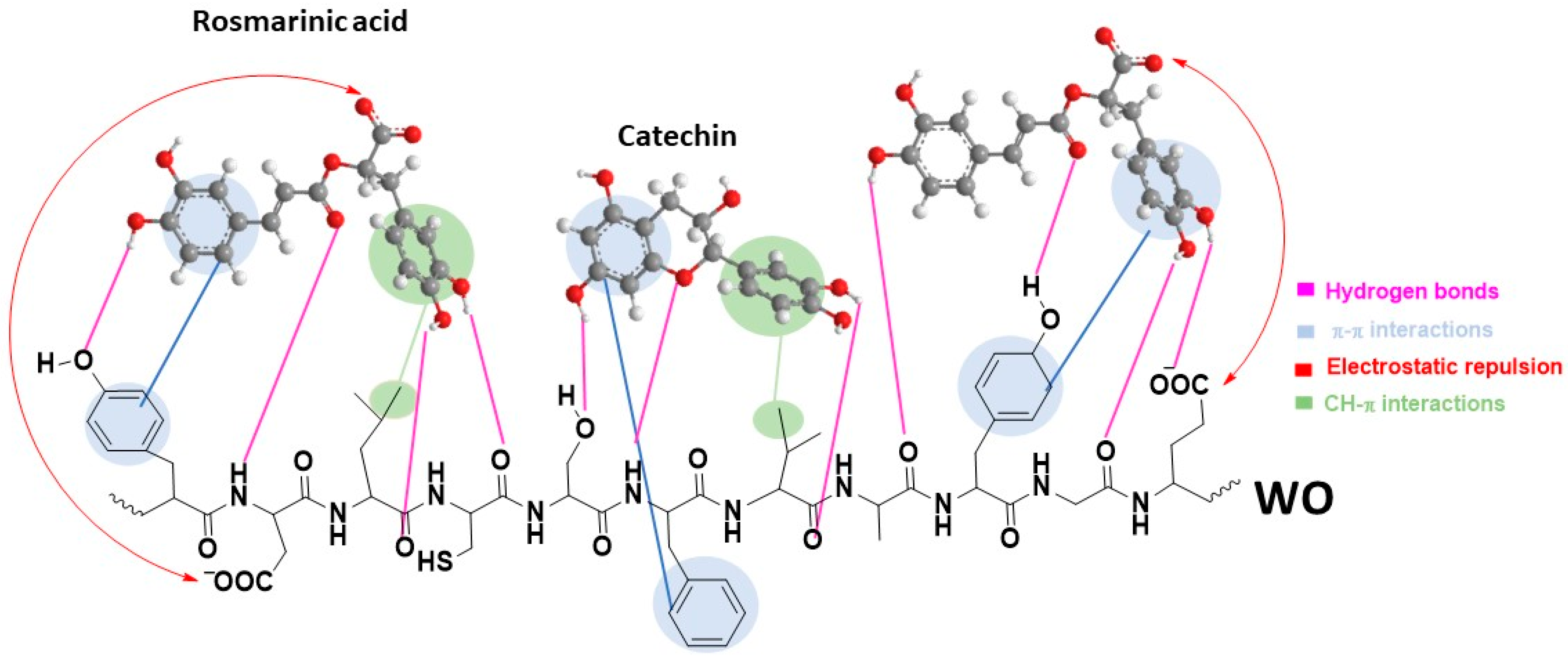
3.3.1. Surface and Chemical Characterization of WO and WO+OE: Insights into OE–Wool Interactions
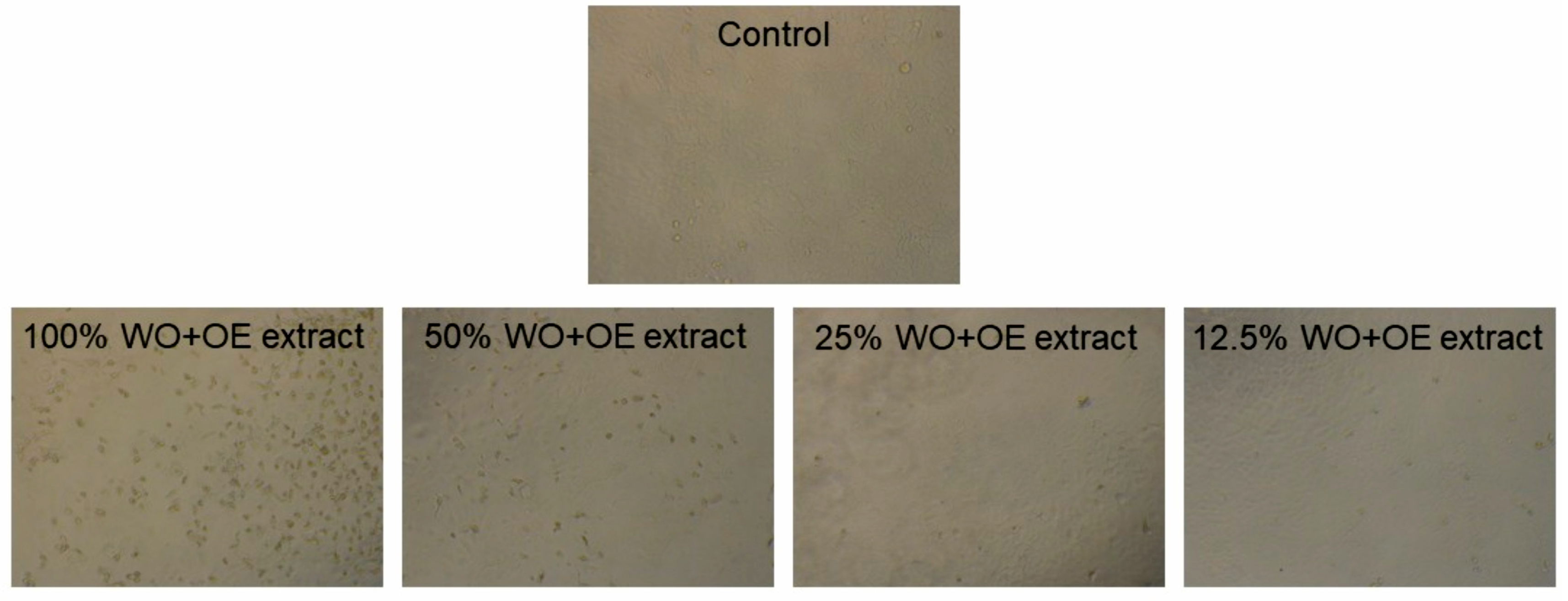
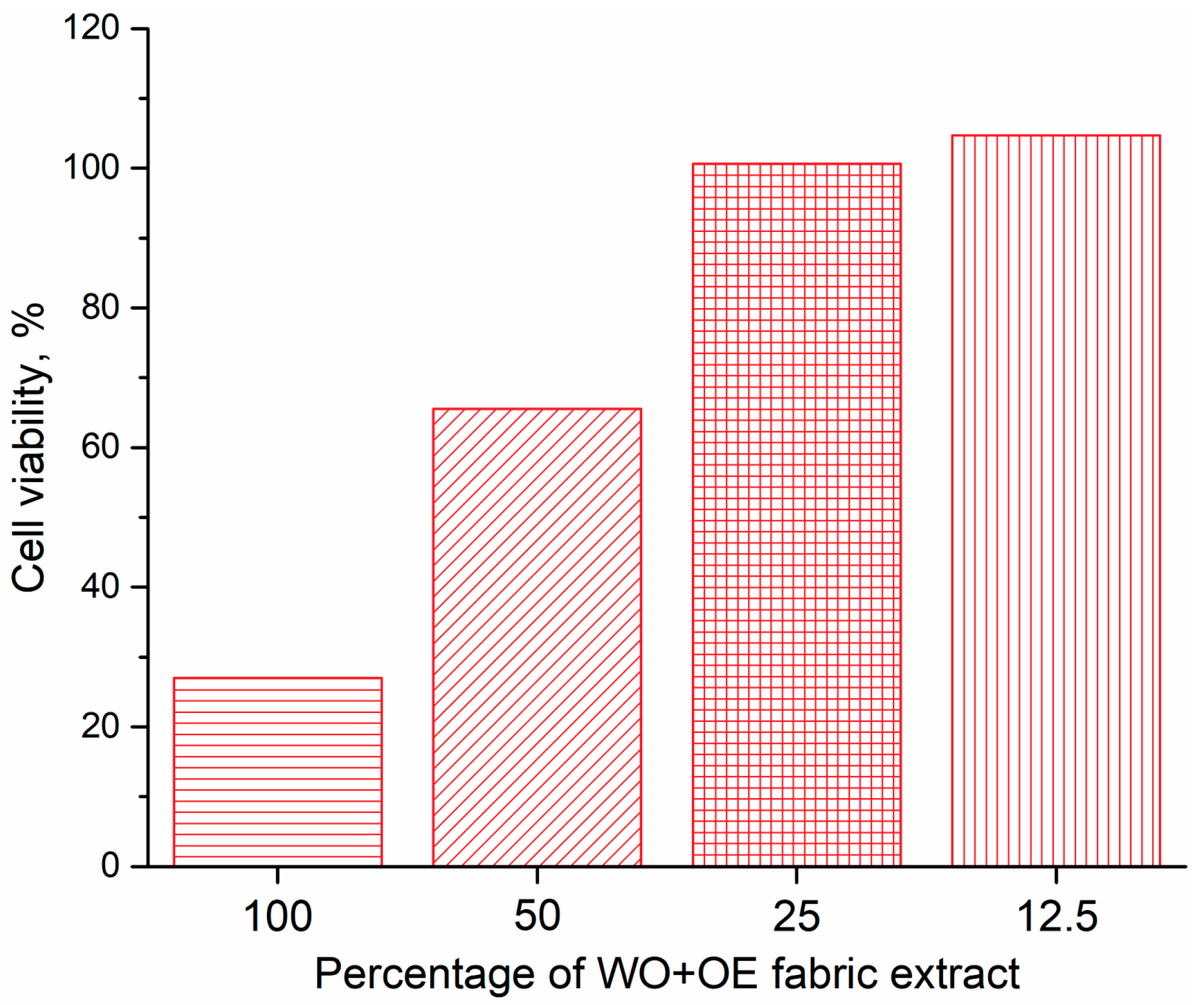
3.3.2. Cytotoxicity of WO+OE
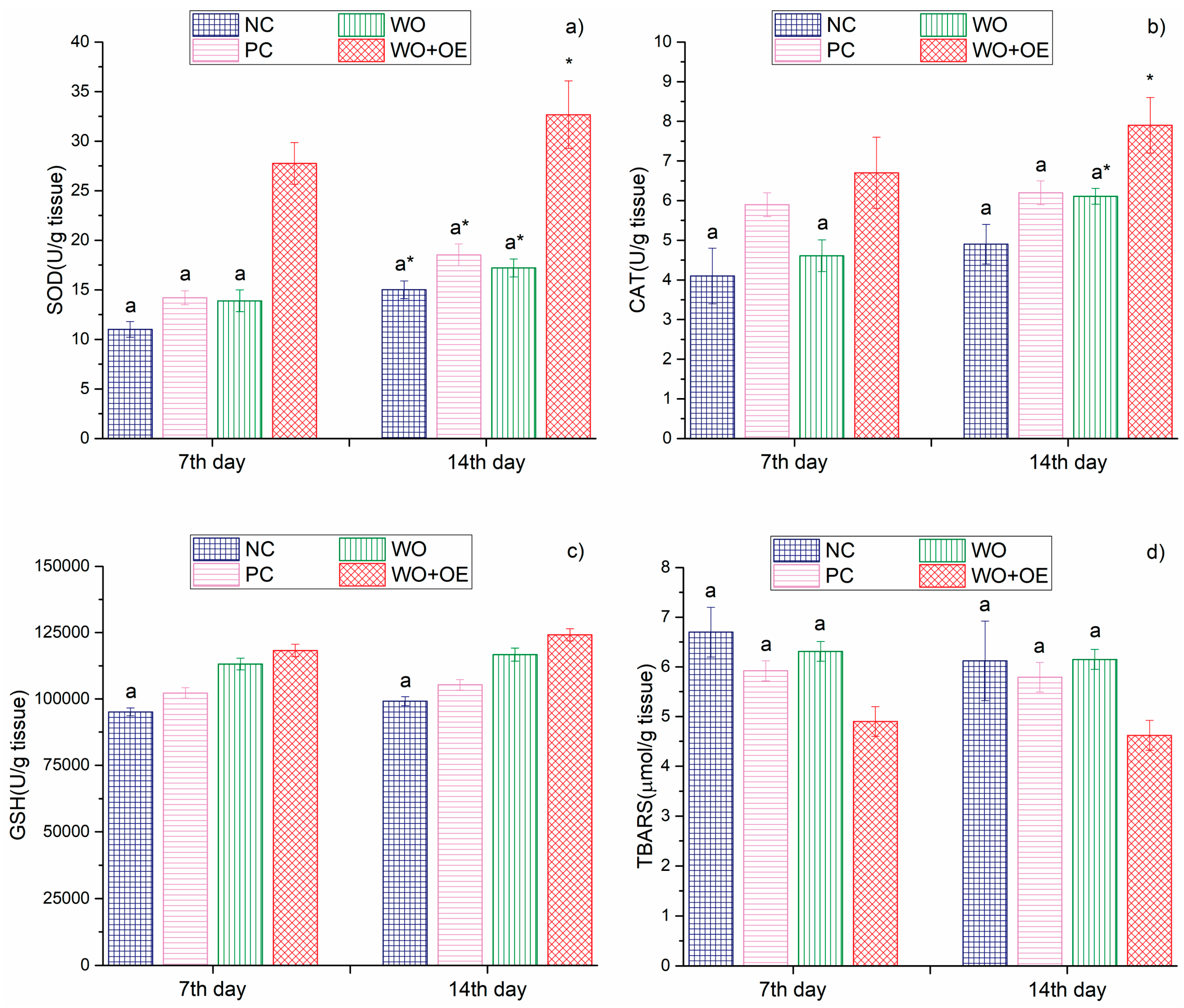
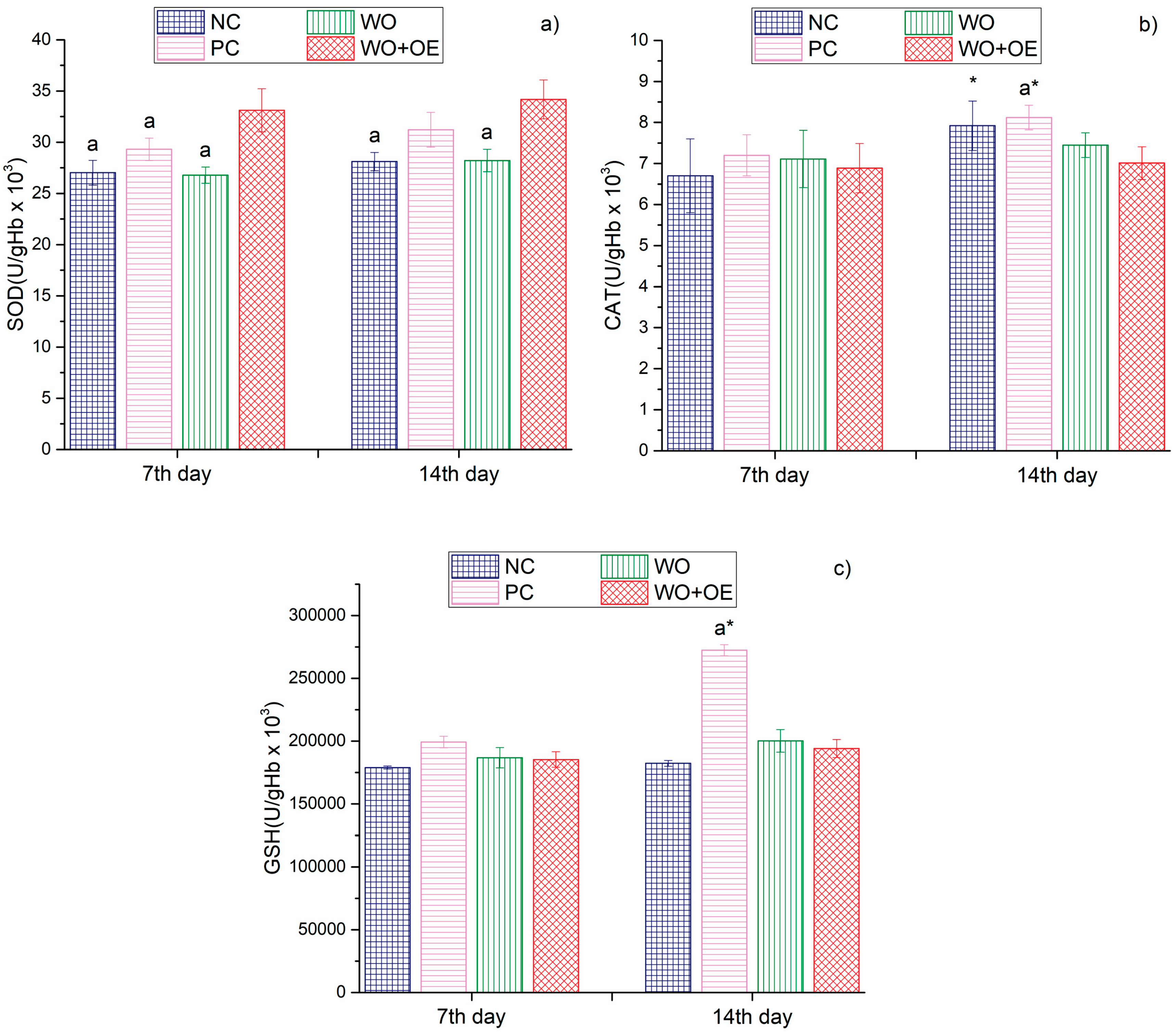
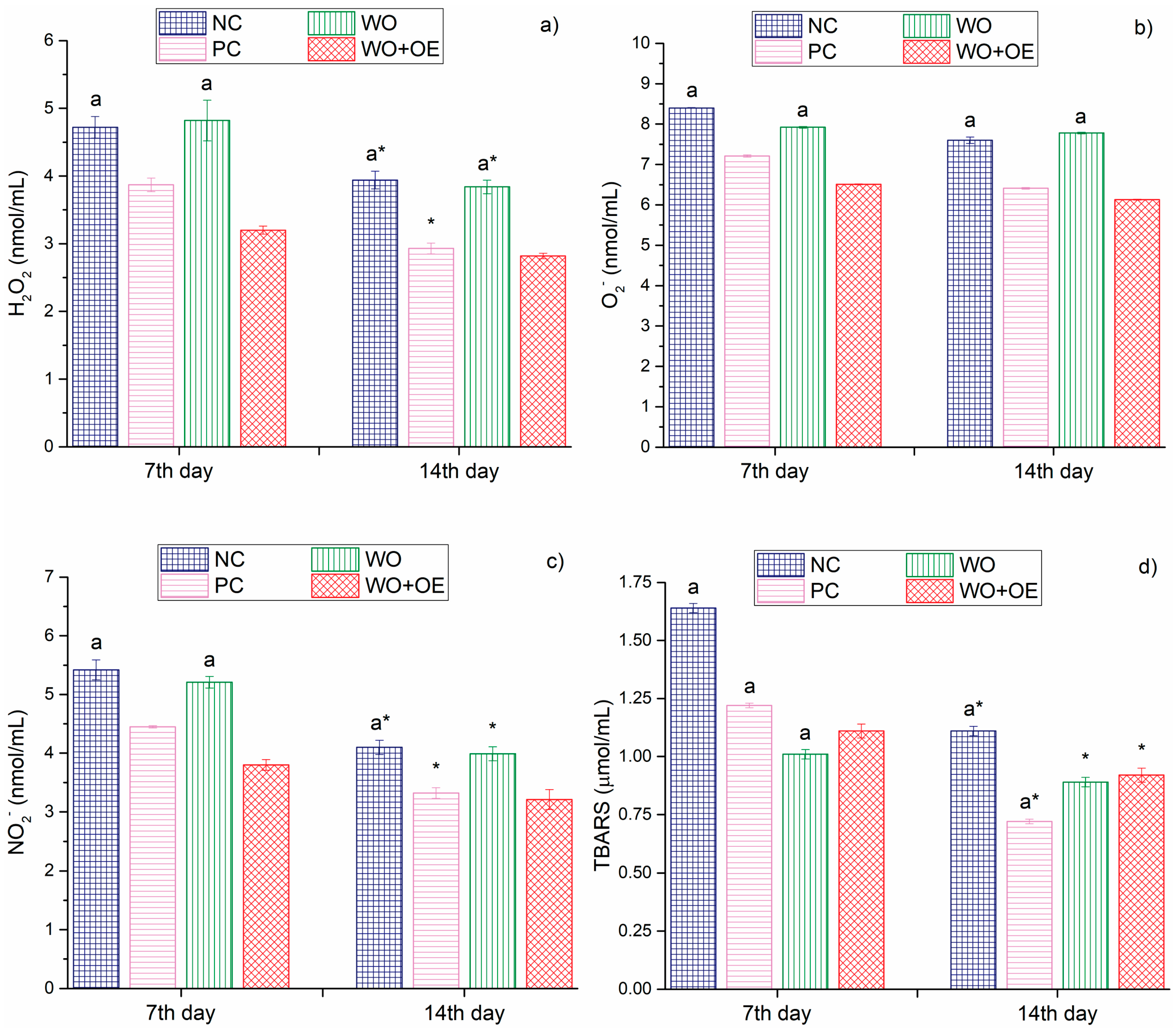
3.3.3. In Vivo Evaluation of WO+OE as Wound Dressing
- (1)
- Many commercially available wound dressings use synthetic antimicrobial agents (e.g., silver nanoparticles, polyhexanide, etc.). WO+OE provides antioxidant, antibacterial, and anti-inflammatory properties using natural plant-based compounds from OE, potentially minimizing the risk of cytotoxicity, allergic reactions, or microbial resistance.
- (2)
- WO has excellent inherent moisture absorption and desorption properties, helping to maintain an optimal moist environment for wound healing and offering excellent patient comfort.
- (3)
- OE provides both antioxidant activity and antibacterial efficacy against S. aureus and E. coli, which can work synergistically to promote wound healing by reducing oxidative stress, inflammation, and infection risks, not always addressed simultaneously by commercial dressings.
- (4)
- Both WO and OE are renewable and biodegradable resources, offering an environmentally friendly alternative to synthetic-based wound dressings. Depending on the production scale, using readily available WO and OE could potentially reduce production costs compared to more complex synthetic or nanomaterial-based dressings.
- (5)
- WO+OE is anticipated to significantly reduce both direct and indirect healthcare costs, lower the frequency of hospital admissions, and ultimately support improved clinical outcomes.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shabih, S.; Hajdari, A.; Mustafa, B.; Quave, C.L. Medicinal plants in the Balkans with antimicrobial properties. In Medicinal Plants as Anti-Infectives; Chassagne, F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 103–138. [Google Scholar]
- Khan, A.; Kumar, D.; Suryavanshi, P.; Padalia, R.C.; Venkatesha, K.T.; Kholiya, S.; Verma, P.P.S.; Singh, S. Spacing and nitrogen application affect Indian oregano (Origanum vulgare L.) biomass, essential oil yield and composition. Ind. Crops Prod. 2024, 218, 119010. [Google Scholar] [CrossRef]
- Liang, Q.; Shen, B.; Xie, Y.; Pan, C.; Xu, H.; Wu, S.; Zhang, O.; Chen, J.; Yin, Z. Regulatory effects and mechanisms of hormones on the growth and rosmarinic acid synthesis in the suspension-cultured cells of Origanum vulgare. Ind. Crops Prod. 2024, 208, 117824. [Google Scholar] [CrossRef]
- Semenzato, G.; Duca, S.D.; Vassallo, A.; Zaccaroni, M.; Mucci, N.; Greco, C.; Padula, A.; Castronovo, L.M.; Chioccioli, S.; Pistelli, L.; et al. Exploring the nexus between the composition of essential oil and the bacterial phytobiome associated with different compartments of the medicinal plants Origanum vulgare ssp. vulgare, O. vulgare ssp. hirtum, and O. heracleoticum. Ind. Crops. Prod. 2023, 191, 115997. [Google Scholar] [CrossRef]
- Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical characterization and biological evaluation of Origanum vulgare L. essential oil formulated as polymeric micelles drug delivery systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.H.; Dahmer, D.; Isawa, L.A.; da Silva, A.B.O.; Souza, L.M.d.S.; Takata, P.H.; Scandorieiro, S.; Deonas, A.N.; Germiniani-Cardozo, J.; Vespero, E.C.; et al. Hydrogel containing biogenic silver nanoparticles and Origanum vulgare essential oil for burn wounds: Antimicrobial efficacy using ex vivo and in vivo methods against multidrug-resistant microorganisms. Pharmaceutics 2025, 17, 503. [Google Scholar] [CrossRef]
- Khan, A.u.R.; Nadeem, M.; Bhutto, M.A.; Yu, F.; Xie, X.; El-Hamshary, H.; El-Faham, A.; Ibrahim, U.A.; Mo, X. Physico-chemical and biological evaluation of PLCL/SF nanofibers loaded with Oregano Essential oil. Pharmaceutics 2019, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crops Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential oils of lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Kamli, M.R.; Malik, M.A.; Srivastava, V.; Sabir, J.S.M.; Mattar, E.H.; Ahmad, A. Biogenic ZnO nanoparticles synthesized from Origanum vulgare abrogates quorum sensing and biofilm formation in opportunistic pathogen Chromobacterium violaceum. Pharmaceutics 2021, 13, 1743. [Google Scholar] [CrossRef]
- Pavun, L.; Spasojević, D.; Ivanovska, A.; Lađarević, J.; Milenković, M.; Uskoković-Marković, S. Characterization of tea water extracts and their utilization for dyeing and functionalization of fabrics of different chemical compositions. Maced. J. Chem. Chem. En. 2024, 42, 263–273. [Google Scholar] [CrossRef]
- Ivanovska, A.; Savić Gajić, I.; Mravik, Ž.; Reljić, M.; Ilić-Tomić, T.; Savić, I.; Luxbacher, T.; Lađarević, J. Transforming discarded walnut green husk into a resource of valuable compounds for colored bioactive textiles with a focus on circular economy concept. Dyes Pigment. 2024, 231, 112406. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol Methods 1989, 119, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Gul Satar, N.Y.; Cangul, I.T.; Topal, A.; Kurt, H.; Ipek, V.; Onel, G.I. The effects of Tarantula cubensis venom on open wound healing in rats. J. Wound Care 2017, 26, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Andjić, M.; Draginić, N.; Kočović, A.; Jeremić, J.; Vučićević, K.; Jeremić, N.; Krstonošić, V.; Božin, B.; Kladar, N.; Čapo, I.; et al. Immortelle essential oil-based ointment improves wound healing in a diabetic rat model. Biomed. Pharmacother. 2022, 150, 112941. [Google Scholar] [CrossRef]
- Patro, G.; Bhattamisra, S.K.; Mohanty, B.K.; Sahoo, H.B. In vitro and in vivo Antioxidant evaluation and estimation of total phenolic, flavonoidal content of Mimosa pudica L. Pharmacognosy Res. 2016, 8, 22–28. [Google Scholar] [CrossRef]
- Andjic, M.; Bradic, J.; Kocovic, A.; Simic, M.; Krstonosic, V.; Capo, I.; Jakovljevic, V.; Lazarevic, N. Immortelle essential-oil-enriched hydrogel for diabetic wound repair: Development, characterization, and in vivo efficacy assessment. Pharmaceutics 2024, 16, 1309. [Google Scholar] [CrossRef]
- Ferreira-Anta, T.; Torres, M.D.; Mourelle, L.; Legido, J.L.; Domínguez, H.; Flórez-Fernández, N. Ecofriendly cascade extraction of antioxidants from Origanum vulgare: Morphological and rheological behavior of microparticles formulations. J. Ind. Eng. Chem. 2024, 137, 174–182. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Walasek-Janusz, M. Chemical composition, biological activity, and potential uses of oregano (Origanum vulgare L.) and oregano essential oil. Pharmaceuticals 2025, 18, 267. [Google Scholar] [CrossRef]
- Panagiotidou, C.; Bouloumpasi, E.; Irakli, M.; Chatzopoulou, P. Characterization of natural bioactive compounds from Greek oregano accessions subjected to advanced extraction techniques. Plants 2024, 13, 3087. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Ruiz-Vargas, A.; Rueda-Robles, A.; Quirantes-Piné, R.; Borrás-Linares, I.; Lozano-Sánchez, J. Bioactive potential of aqueous phenolic extracts of spices for their use in the food industry—A systematic review. Foods 2023, 12, 3031. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Parra, C.; Muñoz, P.; Bustos, L.; Parra, F.; Simirgiotis, M.J.; Escobar, H. UHPLC-DAD characterization of Origanum vulgare L. from Atacama desert Andean region and antioxidant, antibacterial and enzyme inhibition activities. Molecules 2021, 26, 2100. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Aiello, F.; Carullo, G.; Facente, A.; Restuccia, D. Nanotechnologies: An innovative tool to release natural extracts with antimicrobial properties. Pharmaceutics 2021, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, A.; Milenković, J.; Lađarević, J.; Mihajlovski, K.; Dojčinović, B.; Ugrinović, V.; Škaro Bogojević, S.; Kostić, M. Harnessing the power of green and rooibos tea aqueous extracts for obtaining colored bioactive cotton and cotton/flax fabrics intended for disposable and reusable medical textiles. Cellulose 2024, 31, 9523–9542. [Google Scholar] [CrossRef]
- Kola, A.; Vigni, G.; Lamponi, S.; Valensin, D. Protective contribution of rosmarinic acid in rosemary extract against copper-induced oxidative stress. Antioxidants 2024, 13, 1419. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial potential of polyphenols: Mechanisms of action and microbial responses—A narrative review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Potocka, W.; Assy, Z.; Bikker, F.J.; Laine, M.L. Current and potential applications of monoterpenes and their derivatives in oral health care. Molecules 2023, 28, 7178. [Google Scholar] [CrossRef]
- Wiles, D.; Pearson, J.S.; Beddoe, T. Harnessing plant-derived terpenoids for novel approaches in combating bacterial and parasite infections in veterinary and agricultural settings. Curr. Microbiol. 2025, 82, 134. [Google Scholar] [CrossRef] [PubMed]
- Stelmakienė, A.; Ramanauskienė, K.; Briedis, V. Release of rosmarinic acid from semisolid formulations and its penetration through human skin ex vivo. Acta Pharm. 2015, 65, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Harindranath, H.; Susil, A.; Rajeshwari, S.; Sekar, M.; Kumar, B.R.P. Unlocking the potential of Rosmarinic acid: A review on extraction, isolation, quantification, pharmacokinetics and pharmacology. Phytomedicine Plus 2025, 5, 100726. [Google Scholar] [CrossRef]
- Rojas, A.; Misic, D.; de Dicastillo, C.L.; Zizovic, I.; Velásquez, E.; Gutiérrez, D.; Aguila, G.; Vidal, C.P.; Guarda, A.; Galotto, M.J. A review on thymol-based bioactive materials for food packaging. Ind. Crops Prod. 2023, 202, 116977. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Forsch Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef]
- Chang, E.H.; Huang, J.; Lin, Z.; Brown, A.C. Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochim Biophys Acta Gen Subj. 2019, 1863, 191–198. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanauskiene, K.; Kurapkiene, V.; Janulis, V. Dermal penetration studies of potential phenolic compounds ex vivo and their antioxidant activity in vitro. Plants 2022, 11, 1901. [Google Scholar] [CrossRef]
- Polaka, S.; Katare, P.; Pawar, B.; Vasdev, N.; Gupta, T.; Rajpoot, K.; Sengupta, P.; Tekade, R.K. Emerging ROS-modulating technologies for augmentation of the wound healing process. ACS Omega 2022, 7, 30657–30672. [Google Scholar] [CrossRef]
- Ivanovska, A.; Gajić, I.S.; Lađarević, J.; Milošević, M.; Savić, I.; Mihajlovski, K.; Kostić, M. Sustainable dyeing and functionalization of different fibers using orange peel extract’s antioxidants. Antioxidants 2022, 11, 2059. [Google Scholar] [CrossRef]
- Rehan, M.; Abdel-Wahed, A.M.N.; Farouk, A.; El-Zawahry, M.M. Extraction of valuable compounds from Orange peel waste for advanced functionalization of cellulosic surfaces. ACS Sustain. Chem. Eng. 2018, 6, 5911–5928. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.-Y.; Cheng, X.-W.; Tang, R.-C.; Qiao, Y.-F. Adsorption, antibacterial and antioxidant properties of tannic acid on silk fiber. Polymers 2019, 11, 970. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Jankovic-Castvan, I.; Lukic, I. Cornstarch aerogels with thymol, citronellol, carvacrol, and eugenol prepared by supercritical CO2- assisted techniques for potential biomedical applications. Carbohyd. Polym. 2024, 331, 121874. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent advances in the analysis of rosmarinic acid from herbs in the Lamiaceae family. Nat. Prod. Commun. 2019, 14, 1934578X19864216. [Google Scholar] [CrossRef]
- Xiao, Y.; Woods, R.J. Protein—Ligand CH−π interactions: Structural informatics, energy function development, and docking implementation. J. Chem. Theory Comput. 2023, 19, 5503–5515. [Google Scholar] [CrossRef] [PubMed]
- Muzolf-Panek, M.; Gliszczyńska-Świgło, A.; Szymusiak, H.; Tyrakowska, B. The influence of stereochemistry on the antioxidant properties of catechin epimers. Eur. Food Res. Technol. 2012, 235, 1001–1009. [Google Scholar] [CrossRef]
- Machado, V.; Marinho, A.; Vieira de Castro, P.; Silva, T. From fabric to finish: The cytotoxic impact of textile chemicals on humans health. Textiles 2025, 5, 16. [Google Scholar] [CrossRef]
- Fatima, Q.-U.A.; Ahmed, N.; Siddiqui, B.; Rehman, A.U.; Haq, I.U.; Khan, G.M.; Elaissari, A. Enhanced antimicrobial activity of silver sulfadiazine cosmetotherapeutic nanolotion for burn infections. Cosmetics 2022, 9, 93. [Google Scholar] [CrossRef]
- Lu, Y.H.; Hong, Y.; Zhang, T.Y.; Chen, Y.X.; Wei, Z.J.; Gao, C.Y. Rosmarinic acid exerts anti-inflammatory effect and relieves oxidative stress via Nrf2 activation in carbon tetrachloride-induced liver damage. Food Nutr. Res. 2022, 66, 8359. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Chroho, M.; Rouphael, Y.; Petropoulos, S.A.; Bouissane, L. Carvacrol and thymol content affects the antioxidant and antibacterial activity of Origanum compactum and Thymus zygis essential oils. Antibiotics 2024, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Gago, C.; Serralheiro, A.; Miguel, M.d.G. Anti-inflammatory activity of thymol and thymol-rich essential oils: Mechanisms, applications, and recent findings. Molecules 2025, 30, 2450. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, K.X.; Pan, S.; Su, C.; Bi, J.; Lu, X. Thymol disrupts cell homeostasis and inhibits the growth of Staphylococcus aureus. Contrast Media Mol. Imaging 2022, 13, 8743096. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Lorenzo, A.D.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A natural phenolic compound with antimicrobial properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.S.S.; Guimarães, A.G. Effects of carvacrol, thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Pharmacol. 2019, 71, 141–155. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. Proline precursors and collagen synthesis: Biochemical challenges of nutrient supplementation and wound healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef]
- Hamid, A.; Chong, P.L.; Khor, Y.Y.; Kong, P.Y.; Rasli, N.R.; Warif, N.M.A.; Ghazali, A.R.; Jufri, N.F. Biochemical, immunological markers, histology and ultrastructural changes of open wound healing in rats treated with ethyl acetate extract of Zingiber zerumbet rhizomes. Heliyon 2024, 10, e39339. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in Wistar rats. J. Tradit. Compl. Med. 2017, 7, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Ko, Y.G.; Bae, K.H.; Kurisawa, M.; Kwon, O.K.; Kwon, O.H. Green tea catechin-grafted silk fibroin hydrogels with reactive oxygen species scavenging activity for wound healing applications. Biomater. Res. 2022, 26, 62. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Ding, X.; Zhou, L.; Liu, Z.; Jiang, W.; Ai, F.; Cai, K. An antibacterial and antioxidant rosmarinic acid hydrogel normalizes macrophage polarization to expedite diabetic wound healing. J. Colloid Interf. Sci. 2025, 683, 357–371. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Q.; Wei, X.; Ma, Q.; Li, D.; Zhao, J. Rosmarinic acid-grafted dextran/gelatin hydrogel as a wound dressing with improved Pproperties: Strong tissue adhesion, antibacterial, antioxidant and anti-inflammatory. Molecules 2023, 28, 4034. [Google Scholar] [CrossRef]
- Zheng, X.-Q.; Zhang, X.-H.; Gao, H.-Q.; Huang, L.-Y.; Ye, J.-J.; Ye, J.-H.; Lu, J.-L.; Ma, S.-C.; Liang, Y.-R. Green tea catechins and skin health. Antioxidants 2024, 13, 1506. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-scavenging materials for skin wound healing: Advancements and applications. Front. Bioeng. Biotechnol. 2023, 12, 1304835. [Google Scholar] [CrossRef]
- Jabbar, A.A.J.; Abdul-Aziz, A.K.; Abdulla, M.A.; Abdullah, F.O.; Salehen, N.A.; Mothana, R.A.; Houssaini, J.; Hassan, R.R.; Hawwal, M.F.; Fantoukh, O.I.; et al. Sinomenine accelerate wound healing in rats by augmentation of antioxidant, anti-inflammatory, immunuhistochemical pathways. Heliyon 2023, 11, e23581. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, W.; Zhang, Z.; Zhang, S.; Tian, Z.; Liu, Y.; Ho, J.C.; Qu, Y. Regulating the surface of nanoceria and its applications in heterogeneous catalysis. Surf. Sci. Rep. 2018, 73, 1–36. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Mukherjee, P.K.; Kar, A.; Bahadur, S.; Al-Dhabi, N.A.; Duraipandiyan, V. Enhancement of photoprotection potential of catechin loaded nanoemulsion gel against UVA induced oxidative stress. J. Photochem. Photobiol. B 2016, 160, 318–329. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROSdamage regulating ROSsignaling. J. Cell. Biol. 2018, 4, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Maiorino, M.; Ursini, F. Signaling functions of reactive oxygen species. Biochemistry 2010, 9, 835–842. [Google Scholar] [CrossRef]
- Kurahashi, T.; Fujii, J. Roles of antioxidative enzymes in wound healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of hydrogen peroxide formation and elimination in mammalian cells, and its role in various pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/nitrites in food-risk for nitrosative stress and benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]





| Compound | RT, Min | Concentration, µg/mL |
|---|---|---|
| thymol | 12.57 | 0.45 |
| carvacol | 12.20 | 2.86 |
| rosmarinic acid | 5.42 | 137.00 |
| gallic acid | 1.54 | 4.51 |
| salicylic acid | 6.17 | 0.53 |
| isoorientin | 4.02 | 8.97 |
| rutin | 3.90 | 6.81 |
| hydroquinone | 1.80 | 11.48 |
| catechin | 2.63 | 77.34 |
| caffeic acid | 3.25 | 1.58 |
| Fabrics | E. coli, % | S. aureus, % |
|---|---|---|
| CO | / | 25.92 |
| CO+OE | 54.11 | 60.27 |
| WO | / | / |
| WO+OE | 99.99 | 99.99 |
| SILK | / | / |
| SILK+OE | 89.92 | 91.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovska, A.; Petrović, A.; Lazarević-Pašti, T.; Ilic-Tomic, T.; Dimić-Mišić, K.; Lađarević, J.; Bradić, J. Development of Bioactive Cotton, Wool, and Silk Fabrics Functionalized with Origanum vulgare L. for Healthcare and Medical Applications: An In Vivo Study. Pharmaceutics 2025, 17, 856. https://doi.org/10.3390/pharmaceutics17070856
Ivanovska A, Petrović A, Lazarević-Pašti T, Ilic-Tomic T, Dimić-Mišić K, Lađarević J, Bradić J. Development of Bioactive Cotton, Wool, and Silk Fabrics Functionalized with Origanum vulgare L. for Healthcare and Medical Applications: An In Vivo Study. Pharmaceutics. 2025; 17(7):856. https://doi.org/10.3390/pharmaceutics17070856
Chicago/Turabian StyleIvanovska, Aleksandra, Anica Petrović, Tamara Lazarević-Pašti, Tatjana Ilic-Tomic, Katarina Dimić-Mišić, Jelena Lađarević, and Jovana Bradić. 2025. "Development of Bioactive Cotton, Wool, and Silk Fabrics Functionalized with Origanum vulgare L. for Healthcare and Medical Applications: An In Vivo Study" Pharmaceutics 17, no. 7: 856. https://doi.org/10.3390/pharmaceutics17070856
APA StyleIvanovska, A., Petrović, A., Lazarević-Pašti, T., Ilic-Tomic, T., Dimić-Mišić, K., Lađarević, J., & Bradić, J. (2025). Development of Bioactive Cotton, Wool, and Silk Fabrics Functionalized with Origanum vulgare L. for Healthcare and Medical Applications: An In Vivo Study. Pharmaceutics, 17(7), 856. https://doi.org/10.3390/pharmaceutics17070856










