Abstract
Spaceflight-associated dry eye syndrome (SADES) has been reported among astronauts during both International Space Station (ISS) and Space Transportation System (STS) missions. As future missions extend beyond low Earth orbit, the physiological challenges of spaceflight include microgravity, radiation, and environmental stressors, which may further exacerbate the development of ocular surface disease. A deeper understanding of the underlying pathophysiology, along with the exploration of innovative countermeasures, is critical. In this review, we examine nanomedicine as a promising countermeasure for managing ophthalmic conditions in space, with the goal of enhancing visual health and mission readiness for long-duration exploration-class missions.
1. Introduction
The ocular surface is a fragile interface; the multi-layered tear film affects visual acuity and comfort. Composed of lipid, aqueous, and mucin layers, the tear film is continuously regulated through blinking, drainage, and evaporation. A single stimulus may result in altered function through feedback mechanisms and reflex loops [1] For instance, cytokines such as IL-10, IL-17A, and IFNγ are highly correlated with each other and associated with some DED signs [2] Under Earth’s gravity, these systems are well-characterized and vital for ocular surface health.
In microgravity, however, these homeostatic systems face significant disruption. The absence of gravity alters the basic physics of astronaut physiology. As a result, astronauts experience cephalad fluid shifts, cardiac deconditioning, loss of musculoskeletal mass, and changes in circadian rhythm [3,4]. Ocular physiology is not exempt; spaceflight has been associated with optic disk edema, globe flattening, and refractive changes, collectively described under spaceflight-associated neuro–ocular syndrome (SANS) [5]. Additionally, microgravity disrupts normal fluid mechanics on the ocular surface, potentially impairing tear film formation, distribution, and clearance [6,7]. These changes occur in parallel with space-induced immune dysregulation and oxidative stress, which may further compromise ocular health [8,9].
However, there is a paucity of literature on anterior segment conditions during spaceflight. This review aims to synthesize current knowledge on tear film dynamics in microgravity, with a focus on the underlying physical forces that govern tear behavior in space, especially the shift in dominance from gravity to surface tension and capillarity. We also explore how these altered dynamics contribute to dry eye syndrome, impaired tear clearance, and increased susceptibility to ocular surface disease during spaceflight. Finally, we highlight emerging strategies to mitigate these risks, including nanomedicine-based drug delivery systems designed to optimize ocular surface hydration, reduce inflammation, and restore tear film stability in the unique environment of microgravity. These insights may inform future approaches to preserving visual performance and ocular health during long-duration missions.
2. Fluid Dynamics in Spaceflight
In Earth’s gravity, fluid dynamics are governed by a balance of inertial, viscous, and gravitational forces. We can model hemodynamics using fluid dynamic equations [10] In microgravity, gravitational forces become negligible, and capillary and viscous forces dominate [11] This shift is reflected in several governing equations:
2.1. Navier–Stokes in Microgravity
In the low Reynolds number environment of tear film dynamics, inertial forces become negligible, and flow behavior is governed by the Stokes flow limit of the Navier–Stokes equations [12]:
where
ρ(∂v/∂t + (v · ∇)v) = −∇p + μ∇2v + fsurfacetension
- v: velocity field of the tear film
- ρ: density of the tears
- μ: dynamic viscosity
- ∇p: pressure gradient (reduced in microgravity)
- fsurfacetension: capillary force at fluid interfaces
In a microgravity setting, where bulk motion is minimal, the inertial terms diminish, simplifying the equation to:
0 = −∇p + μ∇2v + fcapillary
This reflects a Stokes flow regime where surface tension plays a dominant role [13]
2.2. Capillary Number
The Capillary Number (Ca) quantifies the ratio of viscous to surface tension forces. The capillary number for tear film deposition is typically 1.9 × 10−3 [14,15]:
where
Ca = μU/γ
- μ: dynamic viscosity
- U: characteristic velocity (e.g., blink-induced tear motion)
- γ: surface tension
In microgravity several changes are postulated to occur: surface tension remains constant, but characteristic velocity may decrease due to the absence of gravity-induced drainage. This results in the capillary number decreasing because of dominant surface tension forces. This can impair tear spreading and drainage, leading to abnormal ocular surface wetting [16]
2.3. Bond Number
The Bond Number (Bo) evaluates the relative importance of gravitational to surface tension forces:
where
Bo = Δρ g L2/γ
- Δρ: density difference between tears and air
- g: gravitational acceleration (approaches zero in space)
- L: characteristic length scale (e.g., tear meniscus height)
- γ: surface tension
In microgravity:
g → 0 ⇒ Bo → 0
This confirms that gravity’s influence on tear dynamics becomes negligible, and surface tension becomes the primary force governing tear film behavior. Surface tension completely dominates, leading to tear pooling and lack of drainage [17] These principles predict a fundamental reorganization of tear film dynamics, including stagnation, pooling, and impaired drainage [18] The changes in fluid dynamics in spaceflight lead to uneven tears forming dome-shaped pools on the eye surface, incomplete redistribution during blinking, altered lipid spreading, impaired corneal wound healing, and impaired tear clearance, leading to and potentiating SADES [6,19]
2.4. Immune Dysregulation and Risk of Infection in Microgravity
Perturbations of the immune system are thought to arise in the context of spaceflight. Elevations in the plasma concentrations of TNF, IL-1α, and IL-1β have been observed among astronauts [20] These key inflammatory cytokines are typically released during early immune responses and essential in the clearance of many pathogens [20] Furthermore, in the period immediately after spaceflight, experimental records have demonstrated diminished natural killer cell function, altered peripheral leukocyte distribution, diminished monocyte function, diminished granulocyte function, and dysregulated T cell intracellular signaling [21] Clinical symptoms associated with immune dysregulation were reported affecting 46% of crew members including the reactivation of herpetic viruses [22,23] For viral keratitis, the immune system plays a crucial role, including both natural killer T cells and cytotoxic T cells [24] The modulation of cytokines, namely IL-6, is known to reduce the damage seen in corneas with fungal keratitis [24] Furthermore, inflammation may mediate and cause variability in drug response and toxicity by altering the regulation of drug-metabolizing enzymes and transporters [25] Along with changes in the tear film, immune system dysregulation may increase the risk of complications from infectious keratitis during exploration class missions (Figure 1).
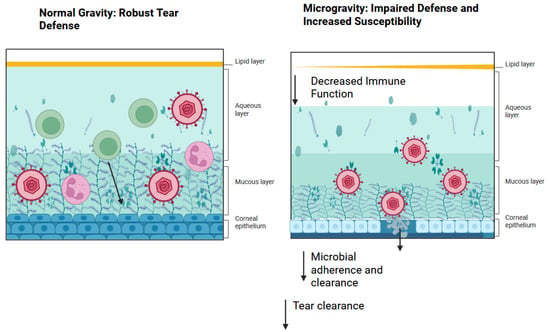
Figure 1.
Pathogenesis of viral keratosis in spaceflight. As a result of decreased tear film secretions, immunity on the ocular surface becomes impaired, leading to decreased microbial adherence and clearance. Created in BioRender. Lee, R. (2025) https://BioRender.com/r61p8im.
2.5. Impact on Mission
Given the changes in the tear film during microgravity, tears will accumulate on the ocular surface, forming large, dome-shaped pools. The thicker tear film may distort the refractive surface of the cornea, leading to blurry and fluctuating vision [26] Visual integrity is critical to the operational success of space missions, where tasks often demand high visual acuity, rapid focus adjustment, and consistent ocular comfort in dynamic environments (Figure 2) [27] Its impact goes beyond ocular discomfort, as DED has been consistently linked to reduced contrast sensitivity, increased light scatter, and higher-order aberrations, all of which compromise visual performance. These disturbances can manifest as blurring, glares, or halos, potentially interfering with navigation, manual docking, and digital equipment interpretation onboard spacecraft [28]

Figure 2.
NASA astronaut Chris Cassidy, Expedition 36 flight engineer, performs a visual exam using an eye chart (out of frame) in the Destiny laboratory of the International Space Station. Vision is crucial to the success of the astronaut mission. Image courtesy of NASA.
3. Keratitis in Spaceflight
- a.
- Viral Keratitis
The reactivation of latent herpesviruses, including the varicella zoster virus (VZV) and Epstein–Barr virus, has been consistently observed in astronauts during and after spaceflight. This phenomenon is hypothesized to be driven by spaceflight-induced immune dysregulation, characterized by increased levels of stress hormones (cortisol, catecholamines), a Th1 to Th2 immune shift, and reduced cytotoxic T cell and NK cell function, which are essential for controlling latent viral infections [20,29]. Though astronauts are generally asymptomatic, the presence of VZV DNA and infectious VZV particles in saliva during and after spaceflight has been well-documented. In a study by Cohrs et al. [30], infectious VZV was cultured from the saliva of two astronauts within 2 days of landing following a 13-day mission, despite the absence of clinical zoster [30]. HSV-1 DNA has also been intermittently detected, and anti-HSV-1 antibody titers have been shown to rise during spaceflight, further supporting subclinical reactivation [23,30] These findings suggest that astronauts can shed infectious viruses without clinical signs, a phenomenon with potential implications for ocular health [30]
Viral keratitis, a corneal inflammation caused by the reactivation of HSV or VZV in the trigeminal ganglion, remains a theoretical but significant concern in the spaceflight environment. While no documented cases of ocular herpes reactivation (e.g., herpes simplex keratitis or herpes zoster ophthalmicus) have occurred during flight to date, the detection of VZV and HSV-1 in astronaut saliva and the known neurotropic reactivation pathways of these viruses raise concerns for reactivation in the ophthalmic branch of the trigeminal nerve [31] Stress, radiation exposure, and sleep deprivation, common in microgravity missions, are all established triggers of ocular viral reactivation on Earth [32] Should ocular reactivation occur in space, the consequences could be severe, including corneal scarring or opacity, decreased visual acuity, endothelial damage or uveitis, and difficulties in diagnosis and management without a slit-lamp biomicroscope [33,34] Furthermore, treatment options aboard spacecrafts may be limited. While antivirals like acyclovir or valacyclovir could theoretically be stored in mission medical kits, their effectiveness depends on early recognition, which may be compromised by limited ophthalmologic equipment and crew training.
The risk of viral keratitis may be amplified on long-duration missions, where cumulative immune suppression, prolonged confinement, and radiation exposure intersect. Notably, reactivation rates for VZV and CMV increase significantly during International Space Station (ISS) missions compared to shorter shuttle flights, and VZV has been found in up to 65% of astronauts on long missions [35] These findings emphasize the need for pre-flight vaccinations, real-time salivary monitoring, and the development of point-of-care viral diagnostics for ocular pathogens.
- b.
- Bacterial Keratitis
Bacterial keratitis, an infection of the cornea that can lead to rapid vision loss if untreated, presents a particularly concerning risk during long-duration spaceflight. On Earth, bacterial keratitis is commonly caused by pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae, especially in contact lens wearers or individuals with ocular surface compromise [36,37] In space, the risk is heightened due to a confluence of host-related, microbial, and environmental factors unique to the space environment [38]
Spaceflight induces a well-documented suppression of both innate and adaptive immunity. Microgravity, psychological stress, radiation exposure, and disrupted circadian rhythms contribute to alterations in immune regulation, particularly in the attenuation of pathways such as NF-κB and reduced antigen presentation [20] This dysregulation compromises the host’s ability to fight infections and has been associated with the reactivation of latent viruses and potentially opportunistic bacterial pathogens. Increased cortisol and stress-related immunosuppression may predispose astronauts to ocular infections, including bacterial keratitis [39]
Spacecrafts such as the ISS represent closed, semi-sterile ecosystems where microbial contamination arises from astronauts’ skin, GI flora, delivered supplies, and onboard equipment. Despite rigorous sterilization protocols, studies have identified viable microbial communities including Pseudomonas spp., Klebsiella pneumoniae, Staphylococcus spp., and even multidrug-resistant organisms [40] Many of these bacteria have been isolated from spacecraft surfaces and air and are capable of forming biofilm-structured microbial communities resistant to disinfection and antibiotics [41] Biofilm formation has been documented on the ISS, with potential for colonization on ocular surfaces, especially under compromised tear film conditions.
Several experiments have demonstrated that microgravity enhances bacterial virulence and adaptability. For instance, Pseudomonas aeruginosa, a leading cause of contact lens-related keratitis, shows increased biofilm formation, stress resistance, and growth rate under simulated microgravity. This may lead to altered morphologies, which maintain effects in microgravity [42] Similarly, Salmonella enterica becomes more virulent in mice under modeled microgravity, while E. coli and S. aureus exhibit altered antibiotic susceptibility profiles in flight conditions [43] These changes are partly driven by the upregulation of global stress regulators such as the RNA binding protein, Hfq, and quorum-sensing systems, which enhance pathogenicity in confined environments [44]
There are historical precedents for serious infections in space. During the Apollo 13 mission, one astronaut developed a Pseudomonas infection, raising early concerns about bacterial adaptation in space [45] While keratitis was not explicitly documented, the causative organism is a known ocular pathogen. On the ISS and Mir, various bacterial genera, including Staphylococcus epidermidis and Propionibacterium acnes, were found on multiple surfaces [46] These commensals can become pathogenic under immunocompromised conditions, potentially affecting ocular structures like the cornea. A decline in beneficial bacteria, such as Faecalibacterium prausnitzii and Lactobacillus spp., correlates with increased inflammatory susceptibility and reduced epithelial defense, further heightening the risk of keratitis [47]
In summary, bacterial keratitis represents a credible threat during spaceflight due to immune suppression, microbial adaptation, and compromised ocular defense. Future missions must prioritize infection prevention protocols, real-time microbial diagnostics, and robust pharmacological strategies to ensure ocular health and crew safety.
- c.
- Fungal Keratitis
Fungal keratitis is a vision-threatening ocular infection typically caused by filamentous fungi, such as Aspergillus, and Fusarium,, or by yeast-like fungi, such as Candida. In terrestrial settings, risk factors include ocular trauma, contact lens use, and immunosuppression [48] Although fungal keratitis remains rare in spaceflight, emerging data on microbial persistence and astronaut-associated fungal colonization raises concerns regarding the potential for opportunistic ocular infections during long-duration missions [49] Several studies have demonstrated the presence of fungal biota aboard the International Space Station (ISS), including Aspergillus, Penicillium, Cryptococcus, and Rhodotorula species [50] These organisms are commonly found in terrestrial environments but are capable of opportunistic infection, particularly in immunocompromised individuals. HEPA filtration and controlled humidity aboard the ISS mitigate airborne spread; however, fungal spores are lightweight and remain suspended in microgravity, posing potential inhalation and mucosal exposure risks [51] Longitudinal studies of astronaut microbiota have confirmed the transient colonization of Candida albicans and other fungi in nasal, pharyngeal, and salivary samples, although in-flight fungal isolation rates were significantly lower compared to pre- and postflight samples [52] Notably, C. albicans was detected in saliva using onboard loop-mediated isothermal amplification assays, emphasizing the feasibility of in situ fungal monitoring.
Environmental isolates such as Rhodotorula mucilaginosa have been recovered from crew members in-flight, suggesting potential colonization or secondary contamination from environmental surfaces [53]. Despite the reduced diversity and viability of cultivable fungi in space likely due to unfavorable growth conditions such as limited nutrients, low humidity, and controlled air quality fungi capable of biofilm formation or possessing melanin-based protective adaptations may persist [54] In fact, melanized fungi such as Cryptococcus neoformans have demonstrated increased viability under spaceflight conditions compared to their non-melanized counterparts [54] Melanin appears to confer resistance to oxidative and radiation-induced stress, supporting its role in fungal survival in space environments [55]
The implications for ocular health are particularly relevant for long-duration missions. Candida albicans, for instance, is a known cause of endogenous fungal endophthalmitis and keratitis, particularly in hosts with disrupted epithelial barriers or altered immunity [56] Given that astronauts experience immune modulation, including impaired antigen presentation, reduced NK cell function, and increased stress hormones, there exists a theoretical vulnerability to fungal infections, including keratitis, especially in the context of corneal micro-abrasions or contact lens wear [57] Table 1 compares the reactivation of infectious keratitis on Earth to those in spaceflight.

Table 1.
Comparison of microbial keratitis on Earth versus Spaceflight.
Furthermore, spaceflight-associated dry eye syndrome may exacerbate the risk of fungal keratitis by compromising the protective tear film and ocular surface defenses. The reduction in blink-induced meibum distribution and decreased tear clearance in microgravity may create a microenvironment more susceptible to colonization [58] Though no documented cases of fungal keratitis have been reported in spaceflight to date, the presence of viable opportunistic fungi, immune suppression, and ocular surface alterations warrant continued surveillance.
4. Nanomedicine
In recent years, nanotechnology has emerged as a promising platform for targeted ocular drug delivery in spaceflight, where conventional formulations are limited by rapid tear clearance and altered ocular surface dynamics. Various nanocarriers including liposomes, chitosan-based nanoparticles, dendrimers, and polymeric micelles have been engineered to enhance drug retention, penetration, and sustained release on the ocular surface [59,60,61] While this is a field of ongoing research, cutting-edge methods for the synthesis of organic nanoparticles include: supramolecular self-assembled aggregates, polymeric nanoparticles, DNA-polymer conjugates, and dendrimers [62] Liposomes, for example, have a tendency to self-assemble, allowing for control and modification of their core composition [63]. These chitosan nanoparticles, for instance, are mucoadhesive and can increase pre-corneal residence time, whereas dendrimers offer precise control over size and surface functionalization, enabling multivalent drug binding [63,64] Methods such as ionic gelation (for chitosan NPs), solvent evaporation (for liposomes), and nanoprecipitation (for polymeric micelles) allow for the fine-tuning of drug release profiles and high relative encapsulation efficiencies [65,66] Early approaches in ocular nanomedicine with liposomes and dendrimers, focused on overcoming tear drainage and improving corneal permeability; however, more recent innovations include contact lens embedded nanoparticles, carbon-based nanostructures, and inorganic nanostructures that allow sustained and minimally invasive delivery, critical advantages for long-duration missions [67,68,69] While comparative studies in space analogs remain limited, these systems hold significant potential for improving keratitis prophylaxis and treatment in microgravity.
- a.
- Dry Eye Syndrome
Spaceflight-associated dry eye syndrome (SADES) represents a unique clinical challenge due to the altered physiology and environmental stressors encountered in microgravity. Astronauts experience changes in tear film stability, meibomian gland dysfunction, increased tear evaporation, and reduced blinking frequency, compounded by environmental stressors such as low humidity, decreased air pressures, extravehicular activities, high CO2 levels, and radiation exposure [70] These factors collectively exacerbate ocular surface inflammation and tear film instability, predisposing astronauts to DED during and after missions [71]
Conventional topical therapies, such as artificial tears or corticosteroid drops, are impractical in microgravity due to fluid handling limitations and suboptimal pharmacokinetics. The low ocular surface residence time and potential for bottle misapplication pose logistical and clinical hurdles [72] Additionally, many medication eye drops have limited shelf lives. During exploration class missions, such as the minimum 21-month trip to Mars, current formulations will not suffice. Furthermore, space-induced immune dysregulation and oxidative stress can reduce the efficacy of standard anti-inflammatory agents [73]
Nanomedicine presents a promising countermeasure tailored to the unique constraints of spaceflight. Advanced nanoformulations, such as nanoemulsions, liposomes, dendrimers, chitosan, and nanostructured lipid carriers (NLCs), may enhance drug bioavailability, improve ocular surface adhesion, and offer controlled-release capabilities [74,75]
Notably, FDA-approved nanoemulsions like Restasis and Cequa deliver cyclosporine A in stable nanosized particles, significantly improving ocular tissue penetration and tear film retention, features potentially advantageous in low-gravity environments where dosing frequency must be minimized [65] Recent innovations such as hyaluronic acid-coated liposomes, ROS-scavenging cerium oxide nanoparticles, and thermoresponsive hydrogels loaded with corticosteroids or FK506 have demonstrated efficacy in mitigating inflammation and oxidative damage in terrestrial DED models [76]
These platforms are particularly relevant to SADES, where prolonged oxidative stress and inflammation may result from both microgravity-induced immune dysregulation and radiation exposure. Moreover, contact lens-based delivery systems incorporating drug-loaded nanoparticles offer an elegant solution to zero-gravity drug retention challenges [77] These lenses can continuously release therapeutic agents over days, reduce the need for manual instillation, and protect the ocular surface from microdebris and dehydration [78]
Despite the promise, the clinical translation of nano-based therapies to space remains limited. Key challenges include ensuring temperature stability over long-duration missions, biocompatibility with altered astronaut physiology, and rigorous in-flight delivery validation. As NASA and international space agencies plan for extended lunar and Martian exploration, the deployment of nanoformulated ocular therapeutics may become essential in safeguarding astronaut ocular health.
- b.
- Keratitis
Keratitis poses a significant risk to astronaut ocular health, particularly under spaceflight conditions that compromise tear film integrity, alter immune responses, and elevate oxidative stress. In microgravity, impaired lymphatic drainage, increased exposure to radiation, and changes in stress hormone levels collectively attenuate the immune system and potentiate microbial colonization on the ocular surface [31] Fungal keratitis, in particular, is difficult to treat with conventional topical therapies due to poor corneal penetration and rapid drug clearance. Nanomedicine offers a transformative solution for space-based ocular infections. ROS-responsive nanocarriers such as the nanocarrier, GC-EB-VOR, formulated with glycol chitosan and 4-carboxyphenylboronic acid pinacol ester, enable targeted, sustained release of antifungal agents like voriconazole at the site of infection [79] ROS-reactive formulations, such as GC-EB, are highly effective in spaceflight where there is a higher prevalence of reactive oxygen species [80] These nanodrugs enhance corneal retention, scavenge ROS, and minimize inflammatory damage, effectively overcoming spaceflight-induced pharmacokinetic challenges [80] Future application of such stimuli-responsive nanosystems may enable compact, long-duration ophthalmic treatment strategies aboard spacecrafts, significantly improving astronaut ocular safety and preserving vision in extended missions.
- c.
- Spaceflight Associated Neuro–Ocular Syndrome
Spaceflight-associated neuro–ocular syndrome (SANS) is a constellation of neuro-ophthalmic changes increasingly recognized among astronauts on long-duration missions. These changes include optic disk edema, globe flattening, choroidal folds, and hyperopic shift, attributed in part to cephalad fluid redistribution in microgravity and subsequent alterations in cerebrospinal fluid (CSF) dynamics [81] Elevated intracranial pressure (ICP) relative to intraocular pressure (IOP), along with impaired venous outflow and glymphatic drainage, is hypothesized to play a central role in the pathophysiology of SANS [82] However, the syndrome remains incompletely understood, and effective pharmacological interventions have not been established [83]
Acetazolamide, a carbonic anhydrase inhibitor traditionally used for glaucoma and idiopathic intracranial hypertension, has been proposed as a potential countermeasure for SANS due to its dual action in reducing both IOP and CSF production. Acetazolamide has been applied previously to astronauts with elevated ICP, with mixed results. However, systemic administration of acetazolamide in astronauts poses logistical and physiological challenges, including fluid electrolyte imbalance, renal load, and undesirable sulfonamide-related side effects are particularly concerning in austere space environments where medical interventions are limited [84]
In this context, nanotechnology-enabled localized delivery of acetazolamide may offer a transformative approach to mitigate SANS. The encapsulation of acetazolamide in elastin-like recombinamer (ELR)-based nanocarriers via the supercritical antisolvent (SAS) technique offers a paradigm shift in ocular pharmacotherapy suited for spaceflight conditions [85] These ELR-based nanoparticles, measuring around 42 nm and exhibiting long-term colloidal stability (zeta potential −33 mV), can traverse ocular surface barriers and allow for sustained drug release, thereby avoiding systemic toxicity while maintaining pharmacologic efficacy [85] The biocompatibility and thermoresponsive behavior of ELRs further enable their performance in dynamic environments such as microgravity, where ocular surface hydration, tear film stability, and fluid mechanics are altered [86]
In-flight administration of acetazolamide-loaded ELRs may achieve targeted reduction in IOP without the risks of systemic administration. Moreover, combining this approach with intravitreal or subconjunctival implants using materials such as PLGA or ELR hydrogels could provide long-acting drug reservoirs, which is critical for mission durations extending beyond six months. These delivery systems may also help fine-tune the translaminar pressure gradient between IOP and ICP, an emerging therapeutic target in the management of SANS [87] As spaceflight increasingly ventures toward extended lunar and Martian habitation, integrating nanotechnological drug delivery platforms such as ELR-based acetazolamide carriers holds great promise. They offer precise pharmacokinetics, enhanced ocular bioavailability, and reduced toxicity, aligning with the demands of personalized aerospace medicine. Future studies should evaluate the pharmacodynamics of these systems under simulated microgravity and validate their efficacy in both preventing and reversing SANS-related neuro–ocular changes.
5. Conclusions
Given the high likelihood of tear film disruption and ocular surface instability during spaceflight, we have outlined the key biophysical mechanisms underlying these changes in microgravity. This review outlines the complications that may arise from altered tear dynamics, such as impaired vision, increased susceptibility to infection, and inflammation, and explores emerging nanomedicine-based countermeasures. We also propose that future studies evaluate the integration of these advanced therapeutics into NASA’s existing medical kits to address spaceflight associated conditions such as SANS (spaceflight-associated neuro–ocular syndrome) and SADES (spaceflight-associated dry eye syndrome). With these innovations, we may be better equipped to preserve ocular health and visual performance during long-duration exploration-class missions. Future clinical validation and the integration of nanopharmaceuticals into astronaut medical kits may become indispensable for next-generation planetary missions.
Author Contributions
R.L. was responsible for writing—the original draft and reviewing and editing, data curation, conceptualization, and investigation. R.K. was responsible for writing—reviewing and editing. J.S. was responsible for writing—reviewing and editing. J.O. was responsible for conceptualization and writing—reviewing and editing. E.W. was responsible for writing—reviewing and editing. A.T. was responsible for supervision and writing—reviewing and editing. All authors agree to be accountable for the content and conclusions of the article. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SADES | Spaceflight-Associated Dry Eye Syndrome |
| SANS | Spaceflight-Associated Neuro–ocular Syndrome |
| IFNγ | Interferon Gamma |
| IL-10 | Interleukin-10 |
| IL-17A | Interleukin-17A |
| NK cells | Natural Killer Cells |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| ROS | Reactive Oxygen Species |
| NLCs | Nanostructured Lipid Carriers |
| ELR | Elastin-Like Recombinamer |
| SAS | Supercritical Antisolvent (technique) |
| PLGA | Poly(lactic-co-glycolic acid) |
References
- Rolando, M.; Zierhut, M. The Ocular Surface and Tear Film and Their Dysfunction in Dry Eye Disease. Surv. Ophthalmol. 2001, 45, S203–S210. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.S.; Wei, Y.; Ying, G.-S.; Maguire, M.G.; Asbell, P.A. Association of Tear Cytokine Concentrations with Symptoms and Signs of Dry Eye Disease: Baseline Data from the Dry Eye Assessment and Management (DREAM) Study. Curr. Eye Res. 2023, 48, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Foldager, N.; Andersen, T.A.; Jessen, F.B.; Ellegaard, P.; Stadeager, C.; Videbaek, R.; Norsk, P. Central Venous Pressure in Humans during Microgravity. J. Appl. Physiol. 1996, 81, 408–412. [Google Scholar] [CrossRef]
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Tarver, W.; Rabiei, P.; Riascos, R.F.; Galdamez, L.A.; Brunstetter, T. Spaceflight Associated Neuro-Ocular Syndrome (SANS) and the Neuro-Ophthalmologic Effects of Microgravity: A Review and an Update. npj Microgravity 2020, 6, 7. [Google Scholar] [CrossRef]
- Lee, R.; Ong, J.; Waisberg, E.; Lee, A.G. Corneal Wound Healing in Spaceflight: Implications of Microgravity-Induced Molecular Signaling Modulations for Corneal Health. Eye 2024, 38, 2851–2853. [Google Scholar] [CrossRef]
- Panzo, N.; Memon, H.; Ong, J.; Suh, A.; Sampige, R.; Lee, R.; Waisberg, E.; Kadipasaoglu, C.M.; Berdahl, J.; Chévez-Barrios, P.; et al. Molecular and Biomechanical Changes of the Cornea and Lens in Spaceflight. Life Sci. Space Res. 2025, 45, 151–157. [Google Scholar] [CrossRef]
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How Does Spaceflight Affect the Acquired Immune System? npj Microgravity 2020, 6, 14. [Google Scholar] [CrossRef]
- Lee, R.; Ong, J.; Waisberg, E.; Mader, T.; Berdahl, J.; Suh, A.; Panzo, N.; Memon, H.; Sampige, R.; Katsev, B.; et al. Potential Risks of Ocular Molecular and Cellular Changes in Spaceflight. Semin. Ophthalmol. 2025, 1–11. [Google Scholar] [CrossRef]
- He, Y.; Northrup, H.; Le, H.; Cheung, A.K.; Berceli, S.A.; Shiu, Y.T. Medical Image-Based Computational Fluid Dynamics and Fluid-Structure Interaction Analysis in Vascular Diseases. Front. Bioeng. Biotechnol. 2022, 10, 855791. [Google Scholar] [CrossRef]
- Langbein, D. Fluid Statics and Dynamics in Microgravity. J. Phys. Condens. Matter 1990, 2, SA491–SA498. [Google Scholar] [CrossRef]
- Talbott, K.; Xu, A.; Anderson, D.M.; Seshaiyer, P. Modelling the Evaporation of a Tear Film over a Contact Lens. Math. Med. Biol. A J. IMA 2015, 32, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M.; Winter, N.; Bliss, G. Tear Film Stability and Tear Surface Tension. Curr. Eye Res. 1989, 8, 507–515. [Google Scholar] [CrossRef]
- Creech, J.L.; Do, L.T.; Fatt, I.; Radke, C.J. In Vivo Tear-Film Thickness Determination and Implications for Tear-Film Stability. Curr. Eye Res. 1998, 17, 1058–1066. [Google Scholar] [CrossRef]
- Wong, H.; Fatt, I.; Radke, C.J. Deposition and Thinning of the Human Tear Film. J. Colloid Interface Sci. 1996, 184, 44–51. [Google Scholar] [CrossRef]
- Xu, X.; Li, G.; Zuo, Y.Y. Biophysical Properties of Tear Film Lipid Layer I. Surface Tension and Surface Rheology. Biophys. J. 2022, 121, 439–450. [Google Scholar] [CrossRef]
- Sahlin, S.; Chen, E. Gravity, Blink Rate, and Lacrimal Drainage Capacity. Am. J. Ophthalmol. 1997, 124, 758–764. [Google Scholar] [CrossRef]
- Maurice, D.M. The Dynamics and Drainage of Tears. Int. Ophthalmol. Clin. 1973, 13, 103. [Google Scholar] [CrossRef]
- Lee, R.; Ong, J.; Waisberg, E.; Lee, A.G. Spaceflight Associated Dry Eye Syndrome (SADES): Radiation, Stressors, and Ocular Surface Health. Life Sci. Space Res. 2024, 43, 75–81. [Google Scholar] [CrossRef]
- Marchal, S.; Choukér, A.; Bereiter-Hahn, J.; Kraus, A.; Grimm, D.; Krüger, M. Challenges for the Human Immune System after Leaving Earth. npj Microgravity 2024, 10, 106. [Google Scholar] [CrossRef]
- Crucian, B.; Babiak-Vazquez, A.; Johnston, S.; Pierson, D.L.; Ott, C.M.; Sams, C. Incidence of Clinical Symptoms during Long-Duration Orbital Spaceflight. Int. J. Gen. Med. 2016, 9, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tierney, B.T.; Overbey, E.G.; Dantas, E.; Fuentealba, M.; Park, J.; Narayanan, S.A.; Wu, F.; Najjar, D.; Chin, C.R.; et al. Single-Cell Multi-Ome and Immune Profiles of the Inspiration4 Crew Reveal Conserved, Cell-Type, and Sex-Specific Responses to Spaceflight. Nat. Commun. 2024, 15, 4954. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent Virus Reactivation in Astronauts on the International Space Station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Menko, A.S. Immune Responses to Injury and Their Links to Eye Disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- Dunvald, A.-C.D.; Järvinen, E.; Mortensen, C.; Stage, T.B. Clinical and Molecular Perspectives on Inflammation-Mediated Regulation of Drug Metabolism and Transport. Clin. Pharmacol. Ther. 2022, 112, 277–290. [Google Scholar] [CrossRef]
- Goto, E.; Yagi, Y.; Matsumoto, Y.; Tsubota, K. Impaired Functional Visual Acuity of Dry Eye Patients. Am. J. Ophthalmol. 2002, 133, 181–186. [Google Scholar] [CrossRef]
- Shah, J.; Ong, J.; Lee, R.; Suh, A.; Waisberg, E.; Gibson, C.R.; Berdahl, J.; Mader, T.H. Risk of Permanent Corneal Injury in Microgravity: Spaceflight-Associated Hazards, Challenges to Vision Restoration, and Role of Biotechnology in Long-Term Planetary Missions. Life 2025, 15, 602. [Google Scholar] [CrossRef]
- Barratt, M.R.; Baker, E.S.; Pool, S.L. (Eds.) Principles of Clinical Medicine for Space Flight; Springer New York: New York, NY, USA, 2019; ISBN 978-1-4939-9887-6. [Google Scholar]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Mehta, S.K.; Schmid, D.S.; Gilden, D.H.; Pierson, D.L. Asymptomatic Reactivation and Shed of Infectious Varicella Zoster Virus in Astronauts. J. Med. Virol. 2008, 80, 1116–1122. [Google Scholar] [CrossRef]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Jones, C. Intimate Relationship Between Stress and Human Alpha-Herpes Virus 1 (HSV-1) Reactivation from Latency. Curr. Clin. Microbiol. Rep. 2023, 10, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.R. The Clinical Features of Viral Keratitis and a Concept of Their Pathogenesis. Proc. R. Soc. Med. 1958, 51, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D. Herpes Simplex Virus Keratitis. Home Healthc. Now. 2019, 37, 281. [Google Scholar] [CrossRef] [PubMed]
- Pavletić, B.; Runzheimer, K.; Siems, K.; Koch, S.; Cortesão, M.; Ramos-Nascimento, A.; Moeller, R. Spaceflight Virology: What Do We Know about Viral Threats in the Spaceflight Environment? Astrobiology 2022, 22, 210–224. [Google Scholar] [CrossRef]
- Hatami, H.; Ghaffari Jolfayi, A.; Ebrahimi, A.; Golmohammadi, S.; Zangiabadian, M.; Nasiri, M.J. Contact Lens Associated Bacterial Keratitis: Common Organisms, Antibiotic Therapy, and Global Resistance Trends: A Systematic Review. Front. Ophthalmol. 2021, 1. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Harmis, N.; Cowell, B.A.; Williams, T.; Holden, B.A. Bacterial Interactions with Contact Lenses; Effects of Lens Material, Lens Wear and Microbial Physiology. Biomaterials 2001, 22, 3235–3247. [Google Scholar] [CrossRef]
- Taylor, P.W. Impact of Space Flight on Bacterial Virulence and Antibiotic Susceptibility. IDR 2015, 8, 249–262. [Google Scholar] [CrossRef]
- Cruzat, A.; Witkin, D.; Baniasadi, N.; Zheng, L.; Ciolino, J.B.; Jurkunas, U.V.; Chodosh, J.; Pavan-Langston, D.; Dana, R.; Hamrah, P. Inflammation and the Nervous System: The Connection in the Cornea in Patients with Infectious Keratitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5136–5143. [Google Scholar] [CrossRef]
- Lee, R.; Ong, J.; Waisberg, E.; Fanning, S.L.; Lee, A.G. Spaceflight Associated Dry Eye Syndrome (SADES): Outflow Biophysics and Infection Risk. J. Space Saf. Eng. 2025, 43, 75–81. [Google Scholar] [CrossRef]
- Marra, D.; Ferraro, R.; Caserta, S. Biofilm Contamination in Confined Space Stations: Reduction, Coexistence or an Opportunity? Front. Mater. 2024, 11. [Google Scholar] [CrossRef]
- Flores, P.; Luo, J.; Mueller, D.W.; Muecklich, F.; Zea, L. Space Biofilms—An Overview of the Morphology of Pseudomonas Aeruginosa Biofilms Grown on Silicone and Cellulose Membranes on Board the International Space Station. Biofilm 2024, 7, 100182. [Google Scholar] [CrossRef] [PubMed]
- Barrila, J.; Sarker, S.F.; Hansmeier, N.; Yang, S.; Buss, K.; Briones, N.; Park, J.; Davis, R.R.; Forsyth, R.J.; Ott, C.M.; et al. Evaluating the Effect of Spaceflight on the Host–Pathogen Interaction between Human Intestinal Epithelial Cells and Salmonella Typhimurium. NPJ Microgravity 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bacterial Quorum Sensing in Complex and Dynamically Changing Environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Fang, X.; Zhang, D.; Chang, D.; Wang, J.; Li, T.; Guo, Y.; Dai, W.; Liu, C. Genome Sequence of Pseudomonas Aeruginosa Strain LCT-PA41, with Changed Metabolism after Space Flight. Genome Announc. 2014, 2, e01124-13. [Google Scholar] [CrossRef]
- Morrison, M.D.; Thissen, J.B.; Karouia, F.; Mehta, S.; Urbaniak, C.; Venkateswaran, K.; Smith, D.J.; Jaing, C. Investigation of Spaceflight Induced Changes to Astronaut Microbiomes. Front. Microbiol. 2021, 12, 659179. [Google Scholar] [CrossRef]
- Bharindwal, S.; Goswami, N.; Jha, P.; Pandey, S.; Jobby, R. Prospective Use of Probiotics to Maintain Astronaut Health during Spaceflight. Life 2023, 13, 727. [Google Scholar] [CrossRef]
- Jurkunas, U.; Behlau, I.; Colby, K. Fungal Keratitis: Changing Pathogens and Risk Factors. Cornea 2009, 28, 638. [Google Scholar] [CrossRef]
- Sugita, T.; Yamazaki, T.; Makimura, K.; Cho, O.; Yamada, S.; Ohshima, H.; Mukai, C. Comprehensive Analysis of the Skin Fungal Microbiota of Astronauts during a Half-Year Stay at the International Space Station. Med. Mycol. 2016, 54, 232–239. [Google Scholar] [CrossRef]
- Checinska Sielaff, A.; Urbaniak, C.; Mohan, G.B.M.; Stepanov, V.G.; Tran, Q.; Wood, J.M.; Minich, J.; McDonald, D.; Mayer, T.; Knight, R.; et al. Characterization of the Total and Viable Bacterial and Fungal Communities Associated with the International Space Station Surfaces. Microbiome 2019, 7, 50. [Google Scholar] [CrossRef]
- Godard, B. Allergy and Hypersensitivity Diseases in Space: Physiological Changes, Clinical Aspects, and Mechanisms with Countermeasures. J. Allergy Hypersensitivity Dis. 2024, 2, 100007. [Google Scholar] [CrossRef]
- Crabbé, A.; Nielsen-Preiss, S.M.; Woolley, C.M.; Barrila, J.; Buchanan, K.; McCracken, J.; Inglis, D.O.; Searles, S.C.; Nelman-Gonzalez, M.A.; Ott, C.M.; et al. Spaceflight Enhances Cell Aggregation and Random Budding in Candida Albicans. PLoS ONE 2013, 8, e80677. [Google Scholar] [CrossRef] [PubMed]
- Daudu, R.; Parker, C.W.; Singh, N.K.; Wood, J.M.; Debieu, M.; O’Hara, N.B.; Mason, C.E.; Venkateswaran, K. Draft Genome Sequences of Rhodotorula Mucilaginosa Strains Isolated from the International Space Station. Microbiol. Resour. Announc. 2020, 9, e00570-20. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Dragotakes, Q.; Friello, P.J.; Casadevall, A. Melanin Protects Cryptococcus Neoformans from Spaceflight Effects. Env. Microbiol. Rep. 2022, 14, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Panzella, L.; Napolitano, A.; Payne, G.F. Redox Activities of Melanins Investigated by Electrochemical Reverse Engineering: Implications for Their Roles in Oxidative Stress. J. Investig. Dermatol. 2020, 140, 537–543. [Google Scholar] [CrossRef]
- Sun, R.L.; Jones, D.B.; Wilhelmus, K.R. Clinical Characteristics and Outcome of Candida Keratitis. Am. J. Ophthalmol. 2007, 143, 1043–1045. [Google Scholar] [CrossRef]
- Hassan, H.M.J.; Papanikolaou, T.; Mariatos, G.; Hammad, A.; Hassan, H. Candida Albicans Keratitis in an Immunocompromised Patient. Clin. Ophthalmol. 2010, 4, 1211–1215. [Google Scholar] [CrossRef]
- Su, Y.; Liang, Q.; Su, G.; Wang, N.; Baudouin, C.; Labbé, A. Spontaneous Eye Blink Patterns in Dry Eye: Clinical Correlations. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5149–5156. [Google Scholar] [CrossRef]
- Ameeduzzafar; Imam, S.S.; Bukhari, S.N.A.; Ali, A. Preparation and Evaluation of Novel Chitosan: Gelrite Ocular System Containing Besifloxacin for Topical Treatment of Bacterial Conjunctivitis: Scintigraphy, Ocular Irritation and Retention Assessment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 959–967. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Vicario-de-la-Torre, M.; Andrés-Guerrero, V.; Sánchez-Nieves, J.; Guzmán-Navarro, M.; de la Mata, F.J.; Gómez, R.; de Las Heras, B.; Argüeso, P.; Ponchel, G.; et al. Novel Water-Soluble Mucoadhesive Carbosilane Dendrimers for Ocular Administration. Mol. Pharm. 2016, 13, 2966–2976. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic Acid in Soluplus(®) Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef]
- Euliss, L.E.; DuPont, J.A.; Gratton, S.; DeSimone, J. Imparting Size, Shape, and Composition Control of Materials for Nanomedicine. Chem. Soc. Rev. 2006, 35, 1095. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef] [PubMed]
- Chaplot, S.P.; Rupenthal, I.D. Dendrimers for Gene Delivery—A Potential Approach for Ocular Therapy? J. Pharm. Pharmacol. 2014, 66, 542–556. [Google Scholar] [CrossRef]
- Aksungur, P.; Demirbilek, M.; Denkbaş, E.B.; Vandervoort, J.; Ludwig, A.; Unlü, N. Development and Characterization of Cyclosporine A Loaded Nanoparticles for Ocular Drug Delivery: Cellular Toxicity, Uptake, and Kinetic Studies. J. Control Release 2011, 151, 286–294. [Google Scholar] [CrossRef]
- Carneiro, G.; Silva, E.L.; Pacheco, L.A.; Souza-Fagundes, E.M.; Corrêa, N.C.R.; Goes, A.M.; Oliveira, M.C.; Ferreira, L.A.M. Formation of Ion Pairing as an Alternative to Improve Encapsulation an d Anticancer Activity of All-Trans Retinoic Acid Loaded in Solid Lipid Nanoparticles. Int. J. Nanomed. 2012, 7, 6011–6020. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Ocular Drug Delivery: Nanomedicine Applications. Nanomedicine 2007, 2, 11–21. [Google Scholar] [CrossRef]
- De Hoon, I.; Barras, A.; Swebocki, T.; Vanmeerhaeghe, B.; Bogaert, B.; Muntean, C.; Abderrahmani, A.; Boukherroub, R.; De Smedt, S.; Sauvage, F.; et al. Influence of the Size and Charge of Carbon Quantum Dots on Their Corneal Penetration and Permeation Enhancing Properties. ACS Appl. Mater. Interfaces 2023, 15, 3760–3771. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Vivero-Lopez, M.; Concheiro, A. Contact Lenses That Transform Gold into Nanoparticles for Prophylaxis of Light-Related Events and Photothermal Therapy. Int. J. Pharm. 2023, 641, 123048. [Google Scholar] [CrossRef]
- Ax, T.; Ganse, B.; Fries, F.N.; Szentmáry, N.; de Paiva, C.S.; March de Ribot, F.; Jensen, S.O.; Seitz, B.; Millar, T.J. Dry Eye Disease in Astronauts: A Narrative Review. Front. Physiol. 2023, 14, 1281327. [Google Scholar] [CrossRef]
- Ong, J.; Mader, T.; Gibson, C.R.; Suh, A.; Panzo, N.; Memon, H.; Lee, R.; Soares, B.; Waisberg, E.; Sampige, R.; et al. The Ocular Surface during Spaceflight: Post-Mission Symptom Report, Extraterrestrial Risks, and in-Flight Therapeutics. Life Sci. Space Res. 2025, 46, 169–186. [Google Scholar] [CrossRef]
- Memon, H.; Ong, J.; Waisberg, E.; Panzo, N.; Sarker, P.; Zaman, N.; Tavakkoli, A.; Lee, A.G. Biophysics of Ophthalmic Medications During Spaceflight: Principles of Ocular Fluid Dynamics and Pharmacokinetics in Microgravity. Life Sci. Space Res. 2024, 42, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Makedonas, G.; Sams, C.F.; Pierson, D.L.; Simpson, R.; Stowe, R.P.; Smith, S.M.; Zwart, S.R.; Krieger, S.S.; Rooney, B.; et al. Countermeasures-Based Improvements in Stress, Immune System Dysregulation and Latent Herpesvirus Reactivation Onboard the International Space Station - Relevance for Deep Space Missions and Terrestrial Medicine. Neurosci. Biobehav. Rev. 2020, 115, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a Potential Ophthalmic Delivery System for Dorzolamide Hydrochloride. AAPS PharmSciTech 2009, 10, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Ismail, S.; Fattal, E. Functionalizing Liposomes with Anti-CD44 Aptamer for Selective Targeti Ng of Cancer Cells. Bioconjugate Chem. 2015, 26, 1307–1313. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Zhu, Y.; Liu, Y.; Liang, H.; Zhu, F. A Novel FK506 Loaded Nanomicelles Consisting of Amino-Terminated Poly(Ethylene Glycol)-Block-Poly(D,L)-Lactic Acid and Hydroxypropyl Methylcellulose for Ocular Drug Delivery. Int. J. Pharm. 2019, 562, 1–10. [Google Scholar] [CrossRef]
- Abdi, B.; Mofidfar, M.; Hassanpour, F.; Kirbas Cilingir, E.; Kalajahi, S.K.; Milani, P.H.; Ghanbarzadeh, M.; Fadel, D.; Barnett, M.; Ta, C.N.; et al. Therapeutic Contact Lenses for the Treatment of Corneal and Ocular Surface Diseases: Advances in Extended and Targeted Drug Delivery. Int. J. Pharm. 2023, 638, 122740. [Google Scholar] [CrossRef]
- Arora, R.; Jain, S.; Monga, S.; Narayanan, R.; Raina, U.K.; Mehta, D.K. Efficacy of Continuous Wear PureVision Contact Lenses for Therapeutic Use. Contact Lens Anterior Eye 2004, 27, 39–43. [Google Scholar] [CrossRef]
- Andrade, L.M.; Rocha, K.A.D.; De Sá, F.A.P.; Marreto, R.N.; Lima, E.M.; Gratieri, T.; Taveira, S.F. Voriconazole-Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery. Cornea 2016, 35, 866–871. [Google Scholar] [CrossRef]
- Niu, P.; Wu, Y.; Zeng, F.; Zhang, S.; Liu, S.; Gao, H. Development of Nanodrug-Based Eye Drops with Good Penetration Properties and ROS Responsiveness for Controllable Release to Treat Fungal Keratitis. NPG Asia Mater. 2023, 15, 1–15. [Google Scholar] [CrossRef]
- Mader, T.H.; Gibson, C.R.; Pass, A.F.; Kramer, L.A.; Lee, A.G.; Fogarty, J.; Tarver, W.J.; Dervay, J.P.; Hamilton, D.R.; Sargsyan, A.; et al. Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-Duration Space Flight. Ophthalmology 2011, 118, 2058–2069. [Google Scholar] [CrossRef]
- Lee, A.G.; Tarver, W.J.; Mader, T.H.; Gibson, C.R.; Hart, S.F.; Otto, C.A. Neuro-Ophthalmology of Space Flight. J. Neuro-Ophthalmol. 2016, 36, 85. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Waisberg, E.; Ong, J.; Paladugu, P.; Amiri, D.; Saintyl, J.; Yelamanchi, J.; Nahouraii, R.; Jagadeesan, R.; Tavakkoli, A. Artificial Intelligence-Based Methodologies for Early Diagnostic Precision and Personalized Therapeutic Strategies in Neuro-Ophthalmic and Neurodegenerative Pathologies. Brain Sci. 2024, 14, 1266. [Google Scholar] [CrossRef] [PubMed]
- Whitson, P.A.; Charles, J.B.; Williams, W.J.; Cintron, N.M. Changes in Sympathoadrenal Response to Standing in Humans after Spaceflight. J. Appl. Physiol. 1995, 79, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R.; Quinteros, D.; Gutiérrez, J.; Martínez, S.; Rodríguez Rojo, S.; Ignacio Tártara, L.; Palma, S.; Javier Arias, F. Acetazolamide Encapsulation in Elastin like Recombinamers Using a Supercritical Antisolvent (SAS) Process for Glaucoma Treatment. Int. J. Pharm. 2024, 657, 124098. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface Impact on Nanoparticle-Based Magnetic Resonance Imaging Contrast Agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- da Silva, P.H.R.; de Castro, M.A.; Ribeiro, M.C.S.; Gonçalves, L.T.A.; de Melo, L.A.; Freitas-Marques, M.B.; Pedrosa, T.A.; Pianetti, G.A.; Fialho, S.L.; Yoshida, M.I.; et al. Acetazolamide-Loaded Intravitreal Implants for the Treatment of Glaucoma: Formulation, Physicochemical Characterization and Assessment of in Vitro and in Vivo Safety. Int. J. Pharm. 2025, 674, 125507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).