Phage Therapy in Managing Multidrug-Resistant (MDR) Infections in Cancer Therapy: Innovations, Complications, and Future Directions
Abstract
1. Introduction
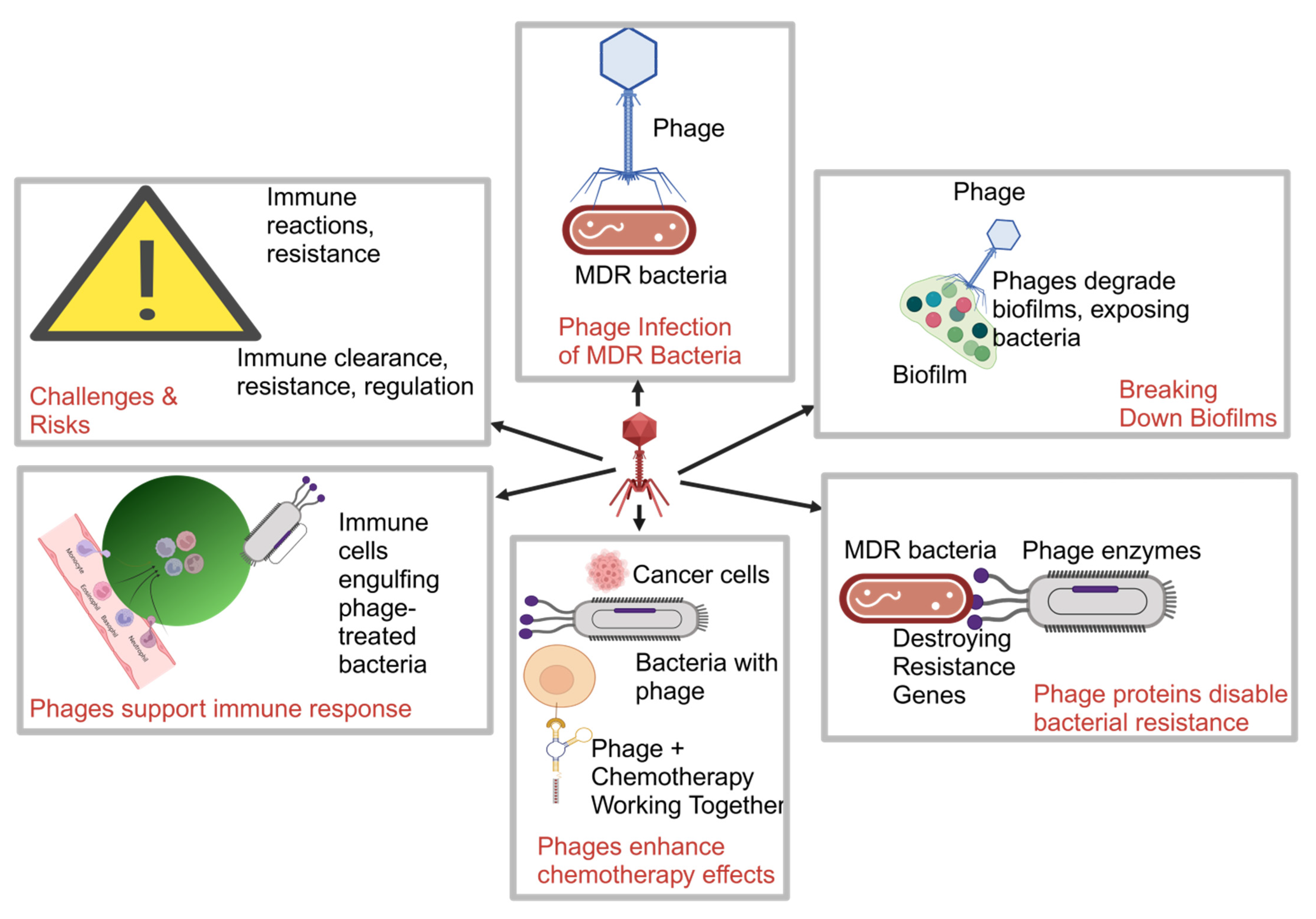
2. Mechanisms of Phage Therapy in Overcoming MDR
2.1. Phage Therapy for MDR Pathogens
2.2. Key Mechanisms in Managing MDR Infections in Cancer Patients
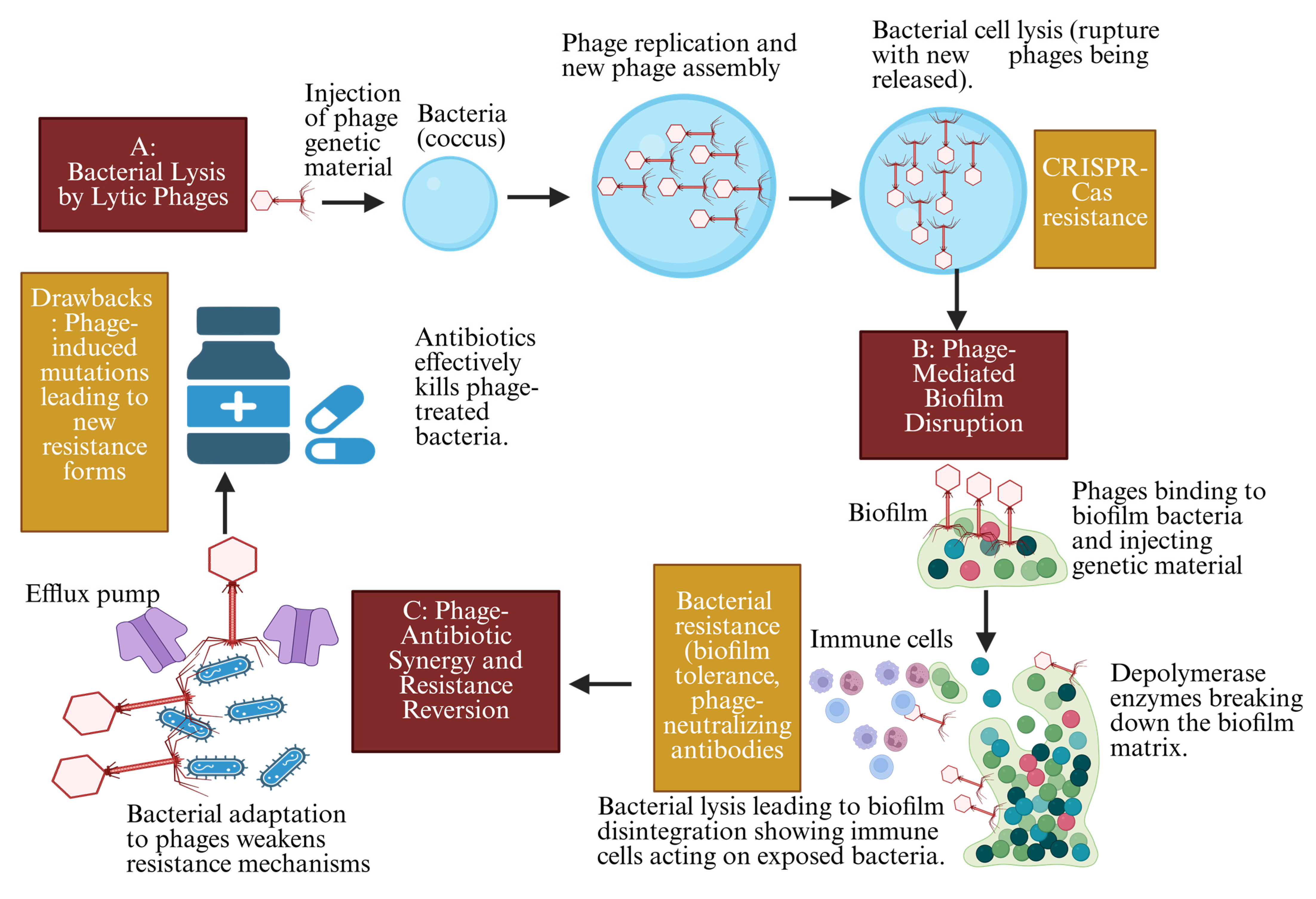
2.2.1. Targeted Lysis of MDR Bacteria in Immunocompromised Cancer Patients
2.2.2. Phage Disruption of Biofilms in MDR-Associated Cancer Infections
2.2.3. Synergy with Antibiotics to Overcome MDR Infections
2.2.4. Drawbacks of Phage Therapy Mechanisms in MDR Infections in Cancer Patients
2.3. Case Studies: Successful Applications of Phage Therapy in MDR Cancer-Related Infections
2.3.1. Phage Therapy in MDR Bacteremia in Cancer Patients
2.3.2. Phage Therapy for MDR Pseudomonas aeruginosa Pneumonia
2.3.3. Treatment of MDR Staphylococcus aureus Surgical Site Infections
2.3.4. Breakthrough Applications in MDR Infections Related to Cancer Therapy
2.3.5. Summary of Case Studies
3. Innovations in Phage Therapy
3.1. Novel Delivery Systems
3.2. CRISPR-Cas9 as a Genetic Tool for Targeting MDR Resistance Genes
3.3. Synthetic Biology Advances
3.4. Combination Therapies
4. Complications and Limitations
4.1. Immune Reactions and Phage Stability
4.2. Ethical Considerations in Personalized Phage Therapy
4.3. Logistical Complications in Hospital Settings
4.4. Case Examples: Problems in Clinical Trials
4.5. Overcoming Drawbacks: The Path Forward
5. Critical Analysis of Current Literature
5.1. Research Gaps and the Need for Large-Scale Clinical Trials
5.2. Conflicting Data on Phage Efficacy and Resistance Risks
5.3. Future Directions in Oncology Applications
6. Future Work and Potential Directions
6.1. Innovative Approaches
6.2. Future Research Directions
6.3. Recommendations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug-Resistant |
| RBPs | receptor-binding proteins |
| LPS | Lipopolysaccharides |
| EPS | extracellular polymeric substance |
| CRISPR-Cas | Clustered Regularly Interspaced Short Palindromic Repeats—CRISPR-associated proteins |
| SSIs | Surgical site infections |
| MRSA | methicillin-resistant Staphylococcus aureus |
| PAS | Phage–antibiotic synergy |
| GM | genetically modified |
| RCTs | randomized controlled trials |
References
- Lopera, C.; Monzó, P.; Aiello, T.F.; Chumbita, M.; Peyrony, O.; Gallardo-Pizarro, A.; Pitart, C.; Cuervo, G.; Morata, L.; Bodro, M.; et al. Prevalence and impact of multidrug-resistant bacteria in solid cancer patients with bloodstream infection: A 25-year trend analysis. Microbiol. Spectr. 2024, 12, e0296123. [Google Scholar] [CrossRef] [PubMed]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Chigozie, U.V.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Perdikouri, E.I.A.; Arvaniti, K.; Lathyris, D.; Kiouti, F.A.; Siskou, E.; Haidich, A.B.; Papandreou, C. Infections Due to Multidrug-Resistant Bacteria in Oncological Patients: Insights from a Five-Year Epidemiological and Clinical Analysis. Microorganisms 2019, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Olawade, D.B.; Fapohunda, O.; Egbon, E.; Ebiesuwa, O.A.; Usman, S.O.; Faronbi, A.O.; Fidelis, S.C. Phage therapy: A targeted approach to overcoming antibiotic resistance. Microb. Pathog. 2024, 197, 107088. [Google Scholar] [CrossRef]
- Chegini, Z.; Khoshbayan, A.; Moghadam, M.T.; Farahani, I.; Jazireian, P.; Shariati, A. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: A review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 45. [Google Scholar] [CrossRef]
- Liu, C.G.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar] [CrossRef]
- Faruk, O.; Jewel, Z.A.; Bairagi, S.; Rasheduzzaman, M.; Bagchi, H.; Tuha, A.S.M.; Hossain, I.; Bala, A.; Ali, S. Phage treatment of multidrug-resistant bacterial infections in humans, animals, and plants: The current status and future prospects. Infect. Med. 2025, 4, 100168. [Google Scholar] [CrossRef]
- Jo, S.J.; Kwon, J.; Kim, S.G.; Lee, S.-J. The Biotechnological Application of Bacteriophages: What to Do and Where to Go in the Middle of the Post-Antibiotic Era. Microorganisms 2023, 11, 2311. [Google Scholar] [CrossRef]
- Dunne, M.; Prokhorov, N.S.; Loessner, M.J.; Leiman, P.G. Reprogramming bacteriophage host range: Design principles and strategies for engineering receptor binding proteins. Curr. Opin. Biotechnol. 2021, 68, 272–281. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kengne, M.F.; Tsobeng, O.D.; Dadjo, B.S.T.; Kuete, V.; Mbaveng, A.T.; Chen, T. Multidrug Resistant Enteric Bacteria from Cancer Patients Admitted in Douala Laquintinie Hospital, Littoral Region of Cameroon. Can. J. Infect. Dis. Med. Microbiol. 2024, 2024, 2084884. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Ramirez, P. Efficacy and Experience of Bacteriophages in Biofilm-Related Infections. Antibiotics 2024, 13, 125. [Google Scholar] [CrossRef]
- Kapoor, A.; Mudaliar, S.B.; Bhat, V.G.; Chakraborty, I.; Prasad, A.S.B.; Mazumder, N. Phage therapy: A novel approach against multidrug-resistant pathogens. 3 Biotech 2024, 14, 256. [Google Scholar] [CrossRef]
- Islam, S.; Fan, J.; Pan, F. The power of phages: Revolutionizing cancer treatment. Front. Oncol. 2023, 13, 1290296. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Concha-Eloko, R.; Bernabéu-Gimeno, M.; Fernández-Cuenca, F.; Cañada-García, J.E.; García-Cobos, S.; Sanjuán, R.; Domingo-Calap, P.; Van Tyne, D. Targeted phage hunting to specific Klebsiella pneumoniae clinical isolates is an efficient antibiotic resistance and infection control strategy. Microbiol. Spectr. 2024, 12, e0025424. [Google Scholar] [CrossRef]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-spectrum antibacterial agents. MedChemComm 2018, 9, 12–21. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Derived Depolymerases against Bacterial Biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef]
- Shrestha, L.; Fan, H.-M.; Tao, H.-R.; Huang, J.-D. Recent Strategies to Combat Biofilms Using Antimicrobial Agents and Therapeutic Approaches. Pathogens 2022, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Kieft, K.; Breister, A.; Anantharaman, K.; Vemuganti, R. Gut virome dysbiosis following focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2022, 42, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Khanna, S. Gut microbiome and Clostridioides difficile infection: A closer look at the microscopic interface. Ther. Adv. Gastroenterol. 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M.; et al. A Comprehensive Review on Phage Therapy and Phage-Based Drug Development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef]
- Sabnis, A.; Hagart, K.L.; Klöckner, A.; Becce, M.; Evans, L.E.; Furniss, R.C.D.; Mavridou, D.A.; Murphy, R.; Stevens, M.M.; Davies, J.C.; et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. eLife 2021, 10, e65836. [Google Scholar] [CrossRef]
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y. Case analysis of hepatotoxicity caused by vancomycin. J. Med. Case Rep. 2024, 18, 267. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Fowoyo, P.T. Phage Therapy: Clinical Applications, Efficacy, and Implementation Hurdles. Open Microbiol. J. 2024, 18, e18742858281566. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska, B.; Mohammed, M. The War between Bacteria and Bacteriophages. In Growing and Handling of Bacterial Cultures; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Fernandes, S.; São-José, C. Enzymes and Mechanisms Employed by Tailed Bacteriophages to Breach the Bacterial Cell Barriers. Viruses 2018, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Mafe, A.N.; Büsselberg, D. Microbiome Integrity Enhances the Efficacy and Safety of Anticancer Drug. Biomedicines 2025, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Ahn, J. Evolutionary Dynamics between Phages and Bacteria as a Possible Approach for Designing Effective Phage Therapies against Antibiotic-Resistant Bacteria. Antibiotics 2022, 11, 915. [Google Scholar] [CrossRef]
- Sahoo, K.; Meshram, S. The Evolution of Phage Therapy: A Comprehensive Review of Current Applications and Future Innovations. Cureus 2024, 16, e70414. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.-C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Kakkar, A.; Kandwal, G.; Nayak, T.; Jaiswal, L.K.; Srivastava, A.; Gupta, A. Engineered bacteriophages: A panacea against pathogenic and drug resistant bacteria. Heliyon 2024, 10, e34333. [Google Scholar] [CrossRef]
- Wang, X.; Tang, J.; Dang, W.; Xie, Z.; Zhang, F.; Hao, X.; Sun, S.; Liu, X.; Luo, Y.; Li, M.; et al. Isolation and Characterization of Three Pseudomonas aeruginosa Viruses with Therapeutic Potential. Microbiol. Spectr. 2023, 11, e0463622. [Google Scholar] [CrossRef]
- Salazar, K.C.; Ma, L.; Green, S.I.; Zulk, J.J.; Trautner, B.W.; Ramig, R.F.; Clark, J.R.; Terwilliger, A.L.; Maresso, A.W.; Goldman, G.H. Antiviral Resistance and Phage Counter Adaptation to Antibiotic-Resistant Extraintestinal Pathogenic Escherichia coli. mBio 2021, 12, e00211-21. [Google Scholar] [CrossRef]
- Wang, X.; Leptihn, S. Defense and anti-defense mechanisms of bacteria and bacteriophages. J. Zhejiang Univ. B 2024, 25, 181–196. [Google Scholar] [CrossRef]
- Watson, B.N.; Steens, J.A.; Staals, R.H.; Westra, E.R.; van Houte, S. Coevolution between bacterial CRISPR-Cas systems and their bacteriophages. Cell Host Microbe 2021, 29, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. Bacteriophage-mediated approaches for biofilm control. Front. Cell. Infect. Microbiol. 2024, 14, 1428637. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- Dhar, Y.; Han, Y. Current developments in biofilm treatments: Wound and implant infections. Eng. Regen. 2020, 1, 64–75. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Sawa, T.; Moriyama, K.; Kinoshita, M. Current status of bacteriophage therapy for severe bacterial infections. J. Intensiv. Care 2024, 12, 44. [Google Scholar] [CrossRef]
- Liu, C.; Hong, Q.; Chang, R.Y.K.; Kwok, P.C.L.; Chan, H.-K. Phage–Antibiotic Therapy as a Promising Strategy to Combat Multidrug-Resistant Infections and to Enhance Antimicrobial Efficiency. Antibiotics 2022, 11, 570. [Google Scholar] [CrossRef]
- Kumar, V.; Yasmeen, N.; Pandey, A.; Chaudhary, A.A.; Alawam, A.S.; Rudayni, H.A.; Islam, A.; Lakhawat, S.S.; Sharma, P.K.; Shahid, M. Antibiotic adjuvants: Synergistic tool to combat multi-drug resistant pathogens. Front. Cell. Infect. Microbiol. 2023, 13, 1293633. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Noei, M.; Chegini, Z. Bacteriophages: The promising therapeutic approach for enhancing ciprofloxacin efficacy against bacterial infection. J. Clin. Lab. Anal. 2023, 37, e24932. [Google Scholar] [CrossRef]
- Eghbalpoor, F.; Gorji, M.; Alavigeh, M.Z.; Moghadam, M.T. Genetically engineered phages and engineered phage-derived enzymes to destroy biofilms of antibiotics resistance bacteria. Heliyon 2024, 10, e35666. [Google Scholar] [CrossRef] [PubMed]
- Santamaría-Corral, G.; Senhaji-Kacha, A.; Broncano-Lavado, A.; Esteban, J.; García-Quintanilla, M. Bacteriophage–Antibiotic Combination Therapy against Pseudomonas aeruginosa. Antibiotics 2023, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Wu, S.M.; Soni, I.; Wang-Crocker, C.; Matern, T.; Beck, J.P.; Loc-Carrillo, C. Phage and Antibiotic Combinations Reduce Staphylococcus aureus in Static and Dynamic Biofilms Grown on an Implant Material. Viruses 2023, 15, 460. [Google Scholar] [CrossRef]
- Zhu, M.; Tse, M.W.; Weller, J.; Chen, J.; Blainey, P.C. The future of antibiotics begins with discovering new combinations. Ann. New York Acad. Sci. 2021, 1496, 82–96. [Google Scholar] [CrossRef]
- Fabiyi, K.; Sintondji, K.; Agbankpe, J.; Assogba, P.; Koudokpon, H.; Lègba, B.; Gbotche, E.; Baba-Moussa, L.; Dougnon, V. Harnessing Bacteriophages to Combat Antibiotic-Resistant Infections in Africa: A Comprehensive Review. Antibiotics 2024, 13, 795. [Google Scholar] [CrossRef]
- Bleriot, I.; Pacios, O.; Blasco, L.; Fernández-García, L.; López, M.; Ortiz-Cartagena, C.; Barrio-Pujante, A.; García-Contreras, R.; Pirnay, J.-P.; Wood, T.K.; et al. Improving phage therapy by evasion of phage resistance mechanisms. JAC-Antimicrob. Resist. 2023, 6, dlae017. [Google Scholar] [CrossRef]
- Tang, M.; Yao, Z.; Liu, Y.; Ma, Z.; Zhao, D.; Mao, Z.; Wang, Y.; Chen, L.; Zhou, T.; Tamma, P.D. Host immunity involvement in the outcome of phage therapy against hypervirulent Klebsiella pneumoniae infections. Antimicrob. Agents Chemother. 2024, 68, e0142923. [Google Scholar] [CrossRef]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Abedon, S.T. Pharmacologically Aware Phage Therapy: Pharmacodynamic and Pharmacokinetic Obstacles to Phage Antibacterial Action in Animal and Human Bodies. Microbiol. Mol. Biol. Rev. 2019, 83, e00012-19. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Bacteriophages and the One Health Approach to Combat Multidrug Resistance: Is This the Way? Antibiotics 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Ragothaman, M.; Yoo, S.Y. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines 2023, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Emencheta, S.C.; Onugwu, A.L.; Kalu, C.F.; Ezinkwo, P.N.; Eze, O.C.; Vila, M.M.D.C.; Balcão, V.M.; Attama, A.A.; Onuigbo, E.B. Bacteriophages as nanocarriers for targeted drug delivery and enhanced therapeutic effects. Mater. Adv. 2024, 5, 986–1016. [Google Scholar] [CrossRef]
- Seo, S.K.; Liu, C.; Dadwal, S.S. Infectious Disease Complications in Patients with Cancer. Crit. Care Clin. 2021, 37, 69–84. [Google Scholar] [CrossRef]
- Ma, Z.; Lai, C.; Zhang, J.; Han, Y.; Xin, M.; Wang, J.; Wu, Z.; Luo, Y. High mortality associated with inappropriate initial antibiotic therapy in hematological malignancies with Klebsiella pneumoniae bloodstream infections. Sci. Rep. 2024, 14, 13041. [Google Scholar] [CrossRef]
- Kim, M.K.; Chen, Q.; Echterhof, A.; Pennetzdorfer, N.; McBride, R.C.; Banaei, N.; Burgener, E.B.; Milla, C.E.; Bollyky, P.L. A blueprint for broadly effective bacteriophage-antibiotic cocktails against bacterial infections. Nat. Commun. 2024, 15, 9987. [Google Scholar] [CrossRef]
- Cornejo-Juárez, P.; González-Oros, I.; Mota-Castañeda, P.; Vilar-Compte, D.; Volkow-Fernández, P. Ventilator-associated pneumonia in patients with cancer: Impact of multidrug resistant bacteria. World J. Crit. Care Med. 2020, 9, 43–53. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Palma, M.; Qi, B. Advancing Phage Therapy: A Comprehensive Review of the Safety, Efficacy, and Future Prospects for the Targeted Treatment of Bacterial Infections. Infect. Dis. Rep. 2024, 16, 1127–1181. [Google Scholar] [CrossRef]
- Kim, M.K.; Suh, G.A.; Cullen, G.D.; Rodriguez, S.P.; Dharmaraj, T.; Chang, T.H.W.; Li, Z.; Chen, Q.; Green, S.I.; Lavigne, R.; et al. Bacteriophage therapy for multidrug-resistant infections: Current technologies and therapeutic approaches. J. Clin. Investig. 2025, 135, e187996. [Google Scholar] [CrossRef] [PubMed]
- Pantvaidya, G.; Joshi, S.; Nayak, P.; Kannan, S.; DeSouza, A.; Poddar, P.; Prakash, G.; Vijaykumaran, P.; Nair, D.; Vaish, R.; et al. Surgical Site Infections in patients undergoing major oncological surgery during the COVID-19 paNdemic (SCION): A propensity-matched analysis. J. Surg. Oncol. 2022, 125, 327–335. [Google Scholar] [CrossRef]
- Kumar, S.; Mahato, R.P.; Ch, S.; Kumbham, S. Current strategies against multidrug-resistant Staphylococcus aureus and advances toward future therapy. Microbe 2025, 6, 100281. [Google Scholar] [CrossRef]
- Ling, H.; Lou, X.; Luo, Q.; He, Z.; Sun, M.; Sun, J. Recent advances in bacteriophage-based therapeutics: Insight into the post-antibiotic era. Acta Pharm. Sin. B 2022, 12, 4348–4364. [Google Scholar] [CrossRef] [PubMed]
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage Therapy for a Multidrug-Resistant Acinetobacter baumannii Craniectomy Site Infection. Open Forum Infect. Dis. 2018, 5, ofy064. [Google Scholar] [CrossRef]
- Eiselt, V.A.; Bereswill, S.; Heimesaat, M.M. Phage therapy in lung infections caused by multidrug-resistant Pseudomonas aeruginosa—A literature review. Eur. J. Microbiol. Immunol. 2024, 14, 1–12. [Google Scholar] [CrossRef]
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.-P.; Costechareyre, D.; Molle, V. Bacteriophage Therapy for Staphylococcus aureus Infections: A Review of Animal Models, Treatments, and Clinical Trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314. [Google Scholar] [CrossRef]
- Pattnaik, A.; Pati, S.; Samal, S.K. Bacteriophage as a potential biotherapeutics to combat present-day crisis of multi-drug resistant pathogens. Heliyon 2024, 10, e37489. [Google Scholar] [CrossRef]
- Zulk, J.J.; Patras, K.A.; Maresso, A.W.; Hinton, D. The rise, fall, and resurgence of phage therapy for urinary tract infection. EcoSal Plus 2024, 12, eesp-0029. [Google Scholar] [CrossRef]
- Gou, Z.; Yao, P.; Xiong, L.; Wang, X.; Yuan, Q.; Sun, F.; Cheng, Y.; Xia, P. Potential of a phage cocktail in the treatment of multidrug-resistant Klebsiella pneumoniae pulmonary infection in mice. BMC Microbiol. 2025, 25, 151. [Google Scholar] [CrossRef]
- Paprocka, P.; Durnaś, B.; Mańkowska, A.; Król, G.; Wollny, T.; Bucki, R. Pseudomonas aeruginosa Infections in Cancer Patients. Pathogens 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Troeman, D.P.R.; Hazard, D.; Timbermont, L.; Malhotra-Kumar, S.; van Werkhoven, C.H.; Wolkewitz, M.; Ruzin, A.; Goossens, H.; Bonten, M.J.M.; Harbarth, S.; et al. Postoperative Staphylococcus aureus Infections in Patients with and Without Preoperative Colonization. JAMA Netw. Open 2023, 6, e2339793. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the critically ill: Complex infections get complicated. Front. Microbiol. 2023, 14, 1196774. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B.; Piątek, R. Phage Therapy as a Novel Strategy in the Treatment of Urinary Tract Infections Caused by E. Coli. Antibiotics 2020, 9, 304. [Google Scholar] [CrossRef]
- Hetta, H.F.; Rashed, Z.I.; Ramadan, Y.N.; Al-Kadmy, I.M.S.; Kassem, S.M.; Ata, H.S.; Nageeb, W.M. Phage Therapy, a Salvage Treatment for Multidrug-Resistant Bacteria Causing Infective Endocarditis. Biomedicines 2023, 11, 2860. [Google Scholar] [CrossRef]
- Elshamy, A.A.; Kamal, S.K.; Mahmoud, M.T.; Elhasany, A.M.; Shady, A.A.; Mohamed, S.A.; Abd-Elmaaboud, H.A.; El-Awady, N.E.; Mohamed, R.A.; El-Mirghany, S.A.; et al. Recent insights on phage therapy against multidrug-resistant Acinetobacter baumannii. AMB Express 2025, 15, 44. [Google Scholar] [CrossRef]
- Ntim, O.K.; Awere-Duodu, A.; Osman, A.-H.; Donkor, E.S. Antimicrobial resistance of bacterial pathogens isolated from cancer patients: A systematic review and meta-analysis. BMC Infect. Dis. 2025, 25, 296. [Google Scholar] [CrossRef]
- Venkataraman, S.; Shahgolzari, M.; Yavari, A.; Hefferon, K. Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering 2025, 12, 469. [Google Scholar] [CrossRef]
- Yang, Y.; Du, H.; Zou, G.; Song, Z.; Zhou, Y.; Li, H.; Tan, C.; Chen, H.; Fischetti, V.A.; Li, J. Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review. J. Control Release 2023, 353, 634–649. [Google Scholar] [CrossRef]
- AlQurashi, D.M.; AlQurashi, T.F.; Alam, R.I.; Shaikh, S.; Tarkistani, M.A.M. Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. J. Nanotheranostics 2025, 6, 9. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Liang, W. The Application of the CRISPR-Cas System in Antibiotic Resistance. Infect. Drug Resist. 2022, 15, 4155–4168. [Google Scholar] [CrossRef] [PubMed]
- Moitra, A.; Chakraborty, A.; Dam, B. CRISPR-Cas9 system: A potent tool to fight antibiotic resistance in bacteria. Microbe 2024, 5, 100184. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Fang, Y.; Zhang, H.; Xu, Y.; Chen, L.; Liang, W. Elimination of blaKPC−2-mediated carbapenem resistance in Escherichia coli by CRISPR-Cas9 system. BMC Microbiol. 2023, 23, 310. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Kayode, H.H.; Okesanya, O.J.; Ukoaka, B.M.; Eshun, G.; Mourid, M.R.; Adigun, O.A.; Ogaya, J.B.; Mohamed, Z.O.; Lucero-Prisno, D. CRISPR-Cas Systems in the Fight Against Antimicrobial Resistance: Current Status, Potentials, and Future Directions. Infect. Drug Resist. 2024, 17, 5229–5245. [Google Scholar] [CrossRef]
- Lenneman, B.R.; Fernbach, J.; Loessner, M.J.; Lu, T.K.; Kilcher, S. Enhancing phage therapy through synthetic biology and genome engineering. Curr. Opin. Biotechnol. 2021, 68, 151–159. [Google Scholar] [CrossRef]
- Peng, H.; Chen, I.A.; Qimron, U. Engineering Phages to Fight Multidrug-Resistant Bacteria. Chem. Rev. 2025, 125, 933–971. [Google Scholar] [CrossRef]
- Ooi, V.Y.; Yeh, T.-Y. Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 9938. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Meile, S.; Du, J.; Dunne, M.; Kilcher, S.; Loessner, M.J. Engineering therapeutic phages for enhanced antibacterial efficacy. Curr. Opin. Virol. 2022, 52, 182–191. [Google Scholar] [CrossRef]
- Alqahtani, A. Bacteriophage treatment as an alternative therapy for multidrug-resistant bacteria. Saudi Med. J. 2023, 44, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Ferous, S.; Petsimeri, A.; Gioula, G.; Tsakris, A. Phage-Based Therapy in Combination with Antibiotics: A Promising Alternative against Multidrug-Resistant Gram-Negative Pathogens. Pathogens 2024, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.-H.; Kotey, F.C.N.; Odoom, A.; Darkwah, S.; Yeboah, R.K.; Dayie, N.T.K.D.; Donkor, E.S. The Potential of Bacteriophage-Antibiotic Combination Therapy in Treating Infections with Multidrug-Resistant Bacteria. Antibiotics 2023, 12, 1329. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Mdarhri, H.A.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Assimi, H.G.; Hamam, M.; Boussettine, R.; Filali-Ansari, N.; Lahlou, F.A.; Diawara, I.; et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics 2022, 11, 1826. [Google Scholar] [CrossRef]
- Chen, S.; Yao, C.; Tian, N.; Zhang, C.; Chen, Y.; Wang, X.; Jiang, Y.; Zhang, T.; Zeng, T.; Song, Y. The interplay between persistent pathogen infections with tumor microenvironment and immunotherapy in cancer. Cancer Med. 2024, 13, e70154. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Cinquerrui, S.; Mancuso, F.; Vladisavljević, G.T.; Bakker, S.E.; Malik, D.J. Nanoencapsulation of Bacteriophages in Liposomes Prepared Using Microfluidic Hydrodynamic Flow Focusing. Front. Microbiol. 2018, 9, 2172. [Google Scholar] [CrossRef]
- Khambhati, K.; Bhattacharjee, G.; Gohil, N.; Dhanoa, G.K.; Sagona, A.P.; Mani, I.; Le Bui, N.; Chu, D.; Karapurkar, J.K.; Jang, S.H.; et al. Phage engineering and phage-assisted CRISPR-Cas delivery to combat multidrug-resistant pathogens. Bioeng. Transl. Med. 2023, 8, e10381. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, L.; Zhang, B.-L.; Yu, J.; Wei, F.; Sun, Y.; Chen, W.; Wang, S. Advance on Engineering of Bacteriophages by Synthetic Biology. Infect. Drug Resist. 2023, 16, 1941–1953. [Google Scholar] [CrossRef]
- Karthika, C.; Malligarjunan, N.; Prasath, N.H.; Pandian, S.K.; Gowrishankar, S. Phage (cocktail)-antibiotic synergism: A new frontier in addressing Klebsiella pneumoniae resistance. Front. Microbiol. 2025, 16, 1588472. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and their potential to modulate the microbiome and immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piątek, B. Phage Therapy—Challenges, Opportunities and Future Prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Sharma, A.; Jasrotia, S.; Kumar, A. Effects of Chemotherapy on the Immune System: Implications for Cancer Treatment and Patient Outcomes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2551–2566. [Google Scholar] [CrossRef]
- Le, H.T.; Venturini, C.; Lubian, A.F.; Bowring, B.; Iredell, J.; George, J.; Ahlenstiel, G.; Read, S.A. Differences in Phage Recognition and Immunogenicity Contribute to Divergent Human Immune Responses to Escherichia coli and Klebsiella pneumoniae Phages. Eur. J. Immunol. 2025, 55, e202451543. [Google Scholar] [CrossRef]
- Ali, Y.; Inusa, I.; Sanghvi, G.; Mandaliya, V.B.; Bishoyi, A.K. The current status of phage therapy and its advancement towards establishing standard antimicrobials for combating multi drug-resistant bacterial pathogens. Microb. Pathog. 2023, 181, 106199. [Google Scholar] [CrossRef]
- Loh, B.; Gondil, V.S.; Manohar, P.; Khan, F.M.; Yang, H.; Leptihn, S.; Elkins, C.A. Encapsulation and Delivery of Therapeutic Phages. Appl. Environ. Microbiol. 2021, 87, e01979-20. [Google Scholar] [CrossRef]
- Shuwen, H.; Kefeng, D. Intestinal phages interact with bacteria and are involved in human diseases. Gut Microbes 2022, 14, 2113717. [Google Scholar] [CrossRef]
- Schorr, L.; Mathies, M.; Elinav, E.; Puschhof, J. Intracellular bacteria in cancer—Prospects and debates. npj Biofilms Microbiomes 2023, 9, 76. [Google Scholar] [CrossRef]
- Knezevic, P.; Hoyle, N.S.; Matsuzaki, S.; Gorski, A. Editorial: Advances in Phage Therapy: Present Challenges and Future Perspectives. Front. Microbiol. 2021, 12, 701898. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.; Clark, J.R.; Bull, S.; Jones, J.D. Ethical argument for establishing good manufacturing practice for phage therapy in the UK. J. Med. Ethics 2025, 51, jme-2023-109423. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, A.; Healy, T.; Ebrahimi, G.; Rezvankhah, S.; Shahraki, A.H.; Mirsaeidi, M. Phage therapy: Breathing new tactics into lower respiratory tract infection treatments. Eur. Respir. Rev. 2024, 33, 240029. [Google Scholar] [CrossRef]
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-quality health systems in the Sustainable Development Goals era: Time for a revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef]
- Ari, M.M.; Dadgar, L.; Elahi, Z.; Ghanavati, R.; Taheri, B.; Laranjo, M. Genetically Engineered Microorganisms and Their Impact on Human Health. Int. J. Clin. Pract. 2024, 2024, 1–38. [Google Scholar] [CrossRef]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. BioDrugs 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Torres-Barceló, C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg. Microbes Infect. 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Mutti, M.; Corsini, L. Robust Approaches for the Production of Active Ingredient and Drug Product for Human Phage Therapy. Front. Microbiol. 2019, 10, 2289. [Google Scholar] [CrossRef]
- Iszatt, J.J.; Larcombe, A.N.; Chan, H.-K.; Stick, S.M.; Garratt, L.W.; Kicic, A. Phage Therapy for Multi-Drug Resistant Respiratory Tract Infections. Viruses 2021, 13, 1809. [Google Scholar] [CrossRef]
- Rosner, D.; Clark, J. Formulations for Bacteriophage Therapy and the Potential Uses of Immobilization. Pharmaceuticals 2021, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.N.; Tai, A.S.; Chang, B.J.; Stick, S.M.; Kicic, A. Overcoming Challenges to Make Bacteriophage Therapy Standard Clinical Treatment Practice for Cystic Fibrosis. Front. Microbiol. 2021, 11, 593988. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Al-Tawaha, A.R.M.S.; Selvaraj, M.; Malik, T. Addressing the global challenge of bacterial drug resistance: Insights, strategies, and future directions. Front. Microbiol. 2025, 16, 1517772. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.; de la Torriente, A.P.; Chalka, A.; Vallejo-Trujillo, A.; McAteer, S.P.; Paterson, G.K.; Low, A.S.; Gally, D.L. Predictive phage therapy for Escherichia coli urinary tract infections: Cocktail selection for therapy based on machine learning models. Proc. Natl. Acad. Sci. USA 2024, 121, e2313574121. [Google Scholar] [CrossRef]
- Omerovic, E.; Petrie, M.; Redfors, B.; Fremes, S.; Murphy, G.; Marquis-Gravel, G.; Lansky, A.; Velazquez, E.; Perera, D.; Reid, C.; et al. Pragmatic randomized controlled trials: Strengthening the concept through a robust international collaborative network: PRIME-9—Pragmatic Research and Innovation through Multinational Experimentation. Trials 2024, 25, 80. [Google Scholar] [CrossRef]
- Ibrahim, R.; Aranjani, J.M.; Valappil, V.K.; Nair, G. Unveiling the potential bacteriophage therapy: A systematic review. Future Sci. OA 2025, 11, 2468114. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical Experience of Personalized Phage Therapy Against Carbapenem-Resistant Acinetobacter baumannii Lung Infection in a Patient with Chronic Obstructive Pulmonary Disease. Front. Cell. Infect. Microbiol. 2021, 11, 631585. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Fabijan, A.P.; Khalid, A.; Maddocks, S.; Ho, J.; Gilbey, T.; Sandaradura, I.; Lin, R.C.; Ben Zakour, N.; Venturini, C.; Bowring, B.; et al. Phage therapy for severe bacterial infections: A narrative review. Med. J. Aust. 2020, 212, 279–285. [Google Scholar] [CrossRef]
- Gorodnichev, R.B.; Krivulia, A.O.; Kornienko, M.A.; Abdraimova, N.K.; Malakhova, M.V.; Zaychikova, M.V.; Bespiatykh, D.A.; Manuvera, V.A.; Shitikov, E.A. Phage-antibiotic combinations against Klebsiella pneumoniae: Impact of methodological approaches on effect evaluation. Front. Microbiol. 2025, 16, 1530819. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Khoshbayan, A.; Chegini, Z.; Farahani, I.; Shariati, A. Bacteriophages, a New Therapeutic Solution for Inhibiting Multidrug-Resistant Bacteria Causing Wound Infection: Lesson from Animal Models and Clinical Trials. Drug Des. Dev. Ther. 2020, 14, 1867–1883. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Babakhani, S.; Moradi, L.; Karami, S.; Shahbandeh, M.; Mirshekar, M.; Mohebi, S.; Moghadam, M.T. Bacteriophage as a Novel Therapeutic Weapon for Killing Colistin-Resistant Multi-Drug-Resistant and Extensively Drug-Resistant Gram-Negative Bacteria. Curr. Microbiol. 2021, 78, 4023–4036. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- Kurilovich, E.; Geva-Zatorsky, N. Effects of bacteriophages on gut microbiome functionality. Gut Microbes 2025, 17, 2481178. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Jiang, Z.; Tong, H.; Ma, X.; Liu, Y. Tumor microbiome: Roles in tumor initiation, progression, and therapy. Mol. Biomed. 2025, 6, 9. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B.; Nagórka, M. Phages as potential life-saving therapeutic option in the treatment of multidrug-resistant urinary tract infections. Acta Biochim. Pol. 2025, 72, 14264. [Google Scholar] [CrossRef]
- Branda, F.; Scarpa, F. Implications of Artificial Intelligence in Addressing Antimicrobial Resistance: Innovations, Global Challenges, and Healthcare’s Future. Antibiotics 2024, 13, 502. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohammed, M.; Oleiwi, J.K.; Osman, A.F.; Adam, T.; Betar, B.O.; Gopinath, S.C.; Ihmedee, F.H. Enhancing antimicrobial resistance strategies: Leveraging artificial intelligence for improved outcomes. South Afr. J. Chem. Eng. 2025, 51, 272–286. [Google Scholar] [CrossRef]
- Mani, I. Phage and phage cocktails formulations. Prog. Mol. Biol. Transl. Sci. 2023, 200, 159–169. [Google Scholar] [CrossRef]
- Wandro, S.; Ghatbale, P.; Attai, H.; Hendrickson, C.; Samillano, C.; Suh, J.; Dunham, S.J.B.; Pride, D.T.; Whiteson, K.; Gaglia, M.M. Phage Cocktails Constrain the Growth of Enterococcus. mSystems 2022, 7, e0001922. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Shang, J.; Wang, K.; Zhou, Q.; Wei, Y. The Role of Quorum Sensing in Phage Lifecycle Decision: A Switch Between Lytic and Lysogenic Pathways. Viruses 2025, 17, 317. [Google Scholar] [CrossRef] [PubMed]
- Alisigwe, C.V.; Ikpa, C.S.; Otuonye, U.J. Examining alternative approaches to antibiotic utilisation: A critical evaluation of phage therapy and antimicrobial peptides combination as potential alternatives. Microbe 2025, 6, 100254. [Google Scholar] [CrossRef]
- Cianci, R.; Caldarelli, M.; Brani, P.; Bosi, A.; Ponti, A.; Giaroni, C.; Baj, A. Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance. Biomedicines 2025, 13, 1202. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules 2025, 15, 688. [Google Scholar] [CrossRef]
- Liping, Z.; Sheng, Y.; Yinhang, W.; Yifei, S.; Jiaqun, H.; Xiaojian, Y.; Shuwen, H.; Jing, Z. Comprehensive retrospect and future perspective on bacteriophage and cancer. Virol. J. 2024, 21, 278. [Google Scholar] [CrossRef]
- Shein, A.M.S.; Hongsing, P.; Khatib, A.; Phattharapornjaroen, P.; Miyanaga, K.; Cui, L.; Shibuya, K.; Amarasiri, M.; Monk, P.N.; Kicic, A.; et al. Phage therapy could be key to conquering persistent bacterial lung infections in children. npj Antimicrob. Resist. 2024, 2, 31. [Google Scholar] [CrossRef]
- Liu, K.; Wang, C.; Zhou, X.; Guo, X.; Yang, Y.; Liu, W.; Zhao, R.; Song, H. Bacteriophage therapy for drug-resistant Staphylococcus aureus infections. Front. Cell. Infect. Microbiol. 2024, 14, 1336821. [Google Scholar] [CrossRef]
- Vázquez, R.; Díez-Martínez, R.; Domingo-Calap, P.; García, P.; Gutiérrez, D.; Muniesa, M.; Ruiz-Ruigómez, M.; Sanjuán, R.; Tomás, M.; Tormo-Mas, M.Á.; et al. Essential Topics for the Regulatory Consideration of Phages as Clinically Valuable Therapeutic Agents: A Perspective from Spain. Microorganisms 2022, 10, 717. [Google Scholar] [CrossRef]
- Gelman, D.; Yerushalmy, O.; Alkalay-Oren, S.; Rakov, C.; Ben-Porat, S.; Khalifa, L.; Adler, K.; Abdalrhman, M.; Coppenhagen-Glazer, S.; Aslam, S.; et al. Clinical Phage Microbiology: A suggested framework and recommendations for the in-vitro matching steps of phage therapy. Lancet Microbe 2021, 2, e555–e563. [Google Scholar] [CrossRef]

| Feature | Phage Therapy | Conventional Antibiotics |
|---|---|---|
| Target Specificity | High; targets specific bacterial strains, e.g., K. pneumoniae-specific phages used in bacteremia cases [16] | Broad or narrow spectrum; may kill both pathogenic and beneficial bacteria [17] |
| Resistance Development | Low; phages co-evolve with bacterial mutations; resistance is often transient and manageable [18] | High bacterial resistance (e.g., MRSA, ESBL-E. coli) is escalating globally [19] |
| Biofilm Penetration | Highly effective; phage-derived depolymerases degrade biofilms, e.g., shown in P. aeruginosa lung infections [20] | Limited; antibiotics often fail to penetrate biofilm matrices, leading to relapse [21] |
| Impact on Microbiome | Minimal; preserves commensals; shown to reduce dysbiosis in murine studies [22] | Significant; alters gut flora; may cause C. difficile overgrowth and secondary infections [23] |
| Effectiveness in Immunocompromised Patients | Promising; successful in leukemia and chemotherapy patients; efficacy may improve with adjunctive immune support [24] | Often reduced; due to high resistance and microbiome disruption, e.g., failure of colistin in neutropenic patients [25] |
| Immune Response and Side Effects | Mild; generally well tolerated; occasional immune neutralization may limit dosing [26] | Variable; allergic reactions, nephrotoxicity, and hepatotoxicity are common with drugs like aminoglycosides [27] |
| Regulatory Approval and Clinical Use | Limited; in Phase I/II trials; FDA-approved for compassionate use; lacks standardized protocols [28] | Established; broad clinical use, regulatory approval, and dosing guidelines worldwide [29] |
| Patient Summary | Infection Type | Pathogen (Resistance Profile) | Phage Type | Mode of Delivery | Therapy Duration | Adjunct Therapy | Clinical Outcome and Follow-Up |
|---|---|---|---|---|---|---|---|
| A leukemia patient undergoing immunosuppressive therapy | Bloodstream infection | Klebsiella pneumoniae (MDR) | Personalized phage cocktail | Intravenous infusion | 7 days | Carbapenem antibiotic | Complete bacterial clearance with significant clinical recovery; sustained response at 30-day follow-up [81] |
| A chemotherapy patient with neutropenia | Pneumonia | Pseudomonas aeruginosa (MDR) | Natural lytic phage | Inhalation (nebulizer) | 10 days | None | Marked reduction in bacterial load; full respiratory recovery noted within 2 weeks [82] |
| Post-operative cancer patient | Surgical site infection | Staphylococcus aureus (MRSA) | Phage cocktail | Topical + Intravenous | 14 days | Aminoglycoside | Faster wound healing observed; bacterial susceptibility to antibiotics restored post-therapy [83] |
| Advanced lung cancer patient post-chemotherapy | Lung infection | Pseudomonas aeruginosa (MDR) | Natural + Engineered phage combo | Inhalation + IV | 10–14 days | Colistin and immune support | Complete bacterial eradication and restored lung function; follow-up confirmed sustained recovery [77] |
| Solid tumor patient with sepsis | Bloodstream infection | Acinetobacter baumannii (MDR/XDR) | Compassionate-use phage therapy | Intravenous | 7–10 days | Tigecycline | Pan-resistant infection eradicated; notable systemic improvement and no adverse effects [84] |
| Colorectal cancer patient | Urinary tract infection | Escherichia coli (ESBL+) | Engineered phage (clinical trial) | Intravesical instillation | 5 days | None | Infection resolved with minimal adverse effects; no recurrence observed during 30-day monitoring [85] |
| Innovation | Description | Impact on Treating MDR Infections |
|---|---|---|
| Liposome and Nanoparticle Delivery | Encapsulation of bacteriophages in liposomes or polymeric nanoparticles for targeted delivery | Enhances phage stability and bioavailability, enabling effective delivery to MDR infection sites [109] |
| CRISPR-Cas9-Enhanced Phages | Engineered phages integrated with CRISPR-Cas9 systems to cleave bacterial DNA at specific loci | Provides precise genome targeting, significantly reduces resistance development in MDR pathogens [110] |
| Synthetic Biology Phages | Phages modified using synthetic biology to improve lysis capability and reduce immunogenicity | Boosts therapeutic efficiency and host compatibility against MDR bacteria [111] |
| Phage–Antibiotic Synergism | Strategic combination of phages with conventional antibiotics for enhanced antibacterial effects | Restores or enhances antibiotic efficacy, overcoming resistance in MDR strains [112] |
| Integration with Immunotherapy | Utilization of phages to stimulate or modulate host immune responses during infection control | Offers potential synergy with immunotherapies for better clearance of MDR infections and biofilms [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafe, A.N.; Büsselberg, D. Phage Therapy in Managing Multidrug-Resistant (MDR) Infections in Cancer Therapy: Innovations, Complications, and Future Directions. Pharmaceutics 2025, 17, 820. https://doi.org/10.3390/pharmaceutics17070820
Mafe AN, Büsselberg D. Phage Therapy in Managing Multidrug-Resistant (MDR) Infections in Cancer Therapy: Innovations, Complications, and Future Directions. Pharmaceutics. 2025; 17(7):820. https://doi.org/10.3390/pharmaceutics17070820
Chicago/Turabian StyleMafe, Alice N., and Dietrich Büsselberg. 2025. "Phage Therapy in Managing Multidrug-Resistant (MDR) Infections in Cancer Therapy: Innovations, Complications, and Future Directions" Pharmaceutics 17, no. 7: 820. https://doi.org/10.3390/pharmaceutics17070820
APA StyleMafe, A. N., & Büsselberg, D. (2025). Phage Therapy in Managing Multidrug-Resistant (MDR) Infections in Cancer Therapy: Innovations, Complications, and Future Directions. Pharmaceutics, 17(7), 820. https://doi.org/10.3390/pharmaceutics17070820










