Metformin-Sensitized Chemotherapy of Docetaxel Nanoemulsions Based on a Sequential Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Animals
2.3. Preparation of Nanoemulsions
2.4. In Vitro Therapeutic Efficacy of the Sequential Strategy Against Breast Cancer Cells
2.5. Cellular Uptake and Distribution
2.6. Cell Cycle and Apoptosis Analysis
2.7. In Vivo Anti-Tumor Efficacy of Sequential Strategy in the 4T1 Breast Cancer Mouse Model
2.8. Statistical Analysis
3. Results and Discussion
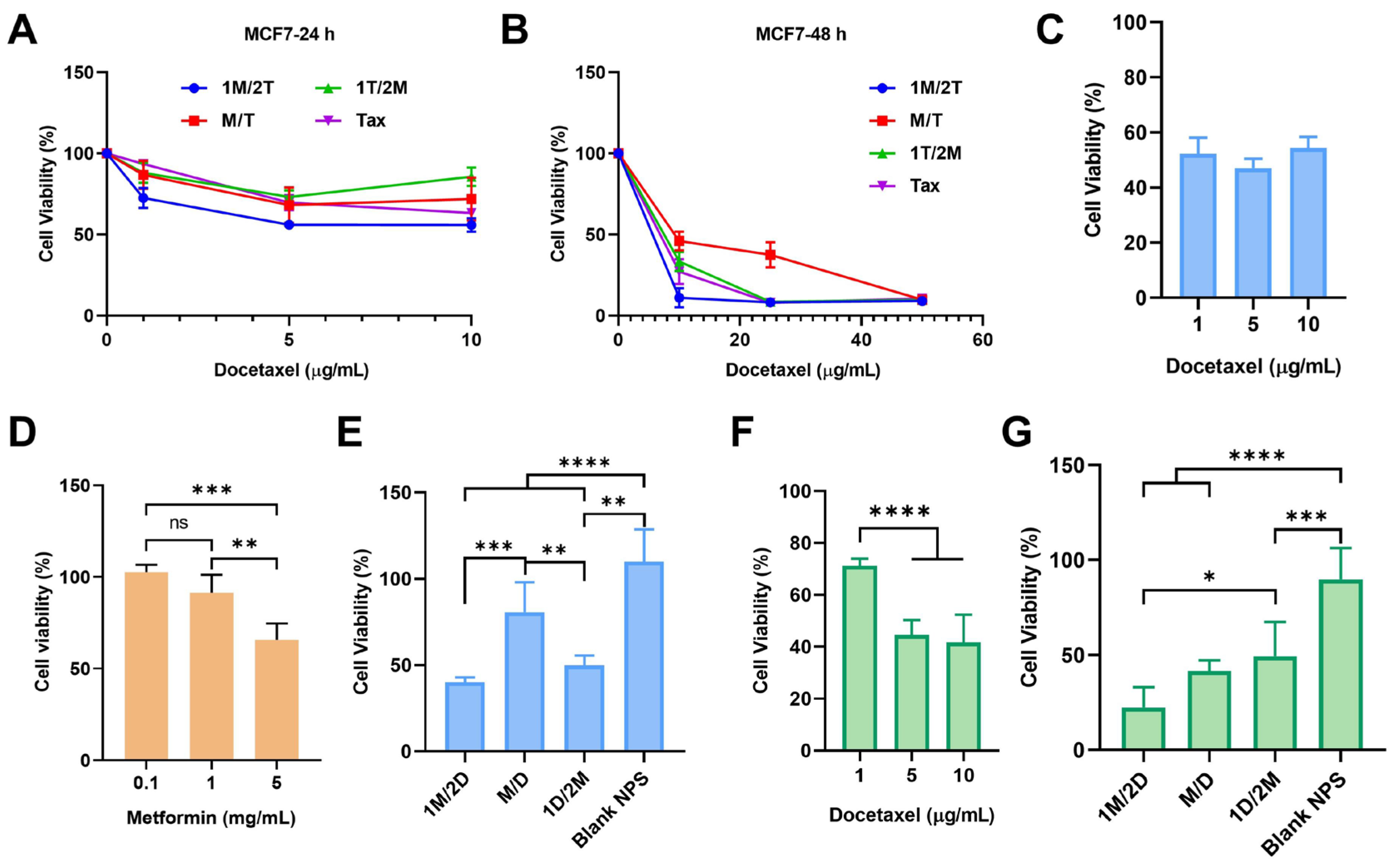
3.1. The Administration Sequence of Metformin Can Affect the Tumor Sensitivity to Chemotherapy
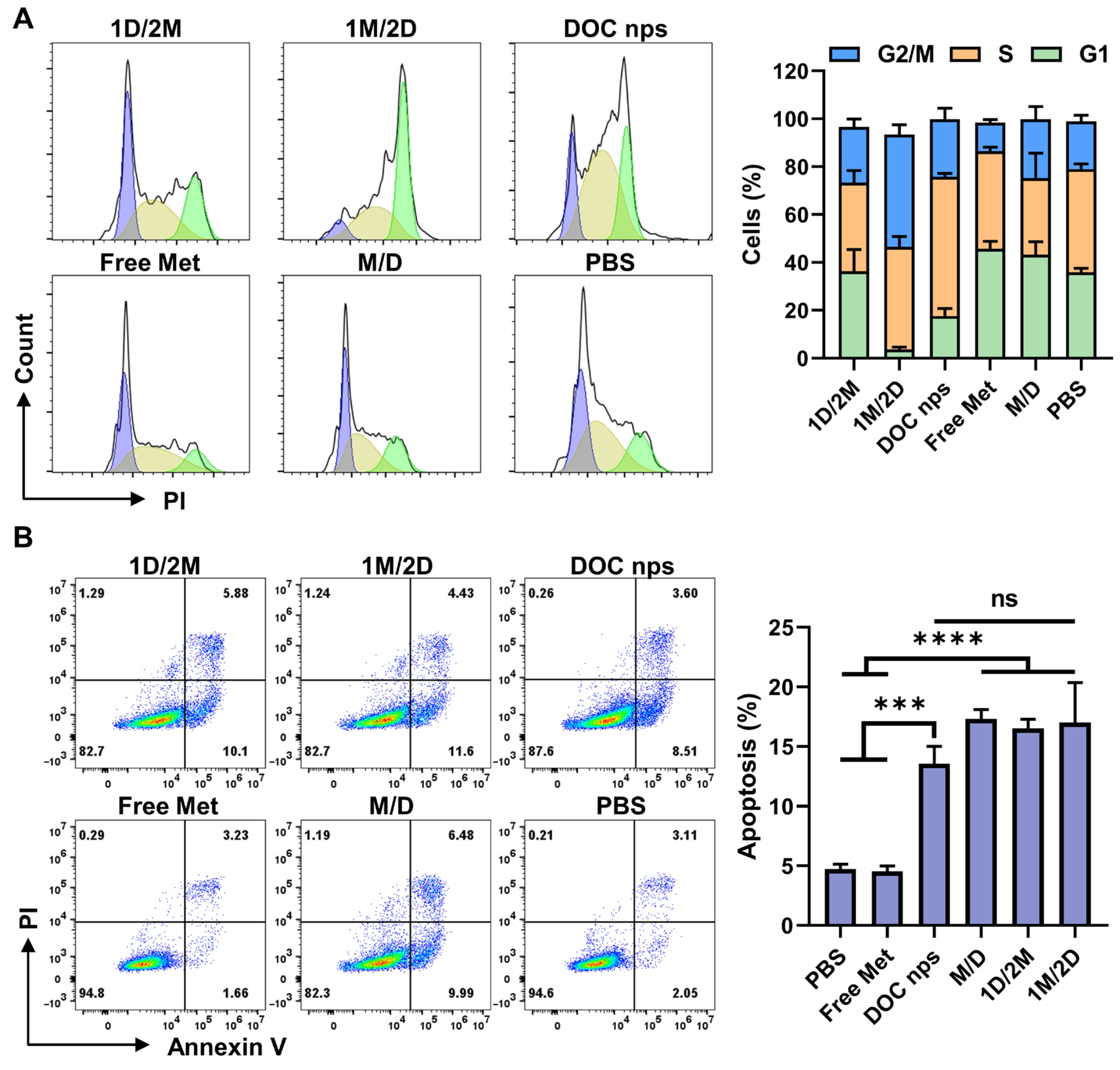
3.2. The 1M/2D Administration Sequence Enhanced the Effect of Docetaxel on Cell Cycle Arrest
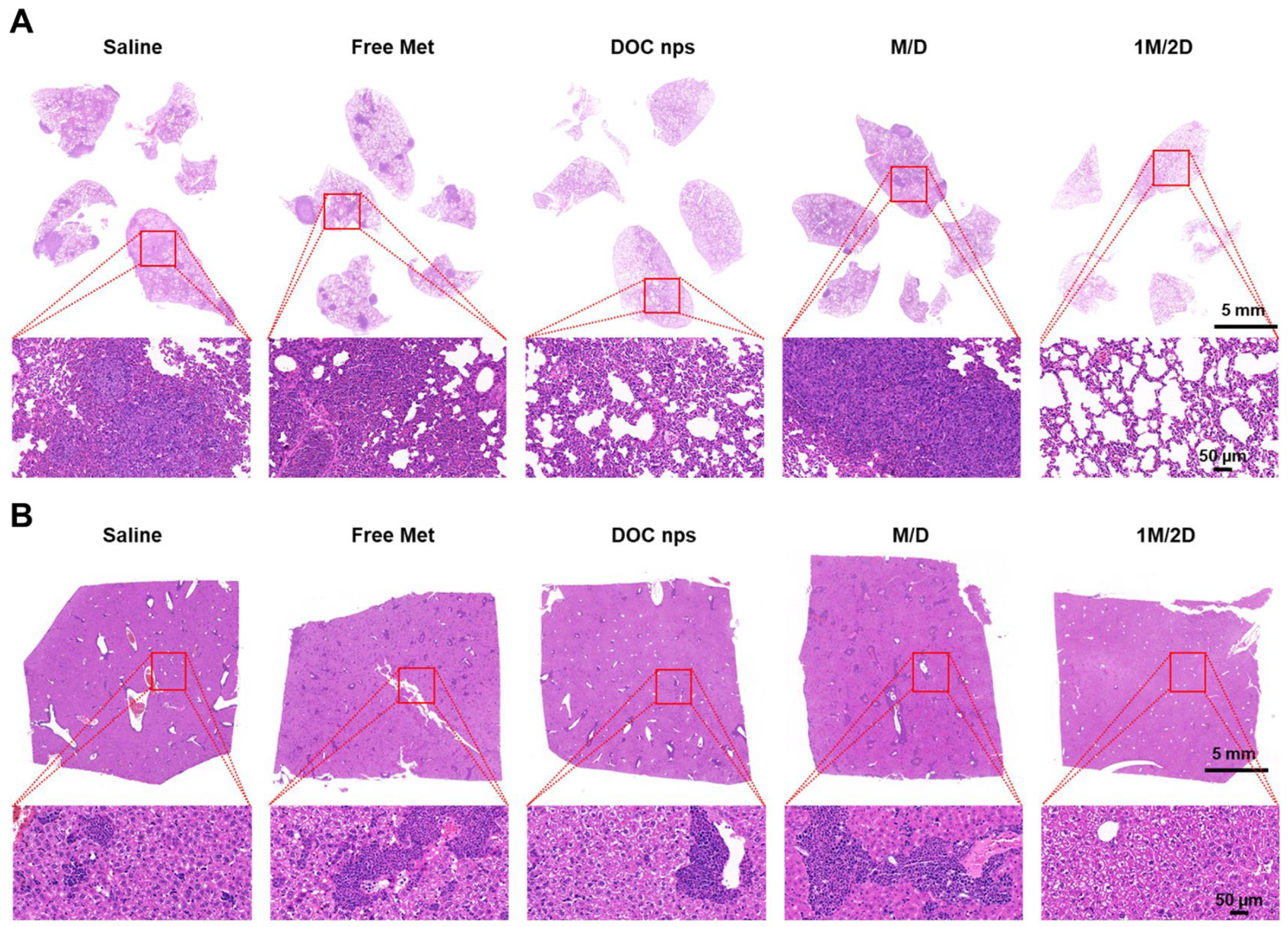
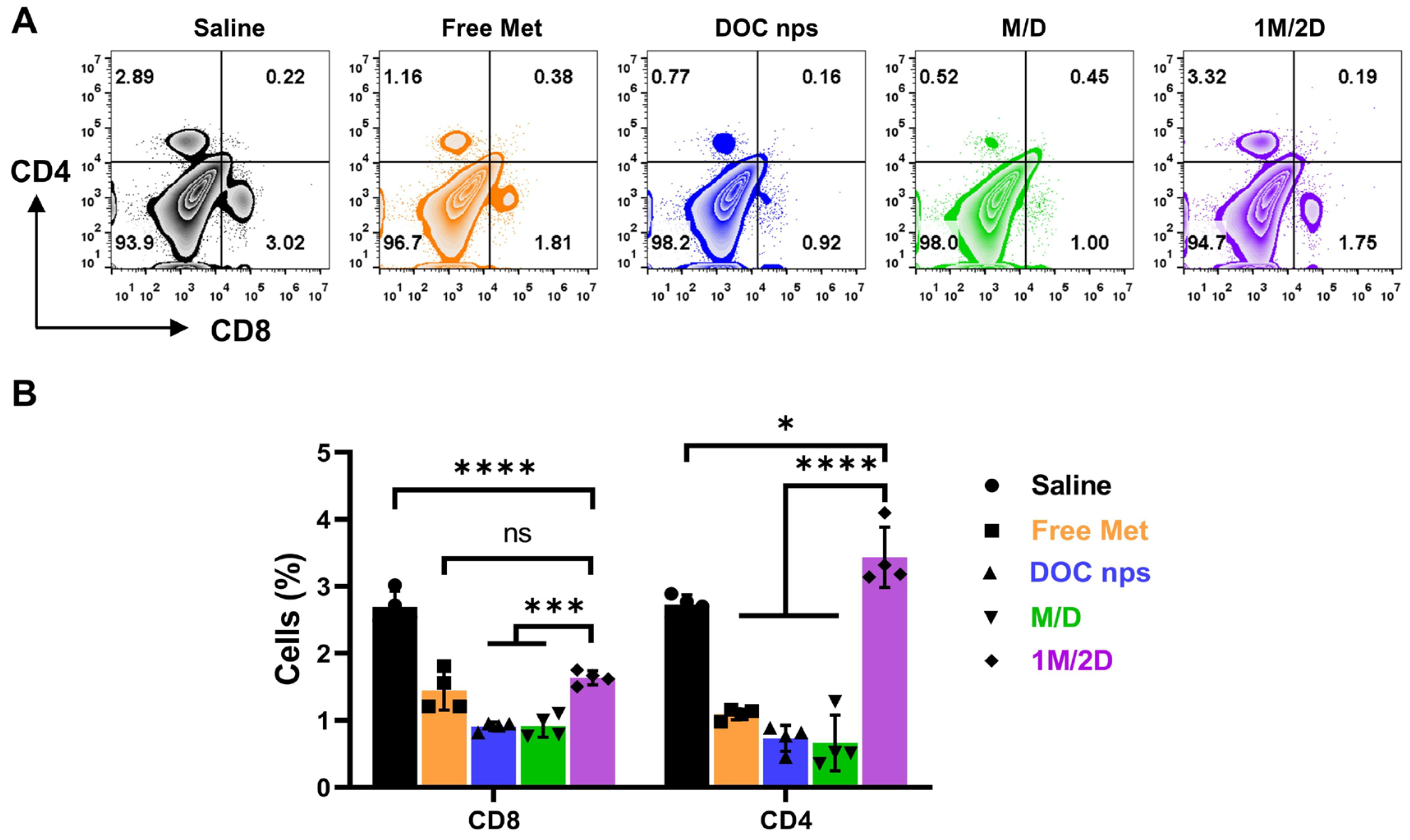
3.3. The In Vivo Therapeutic Effects of DOC nps with Different i.v. Administration Sequences of Metformin
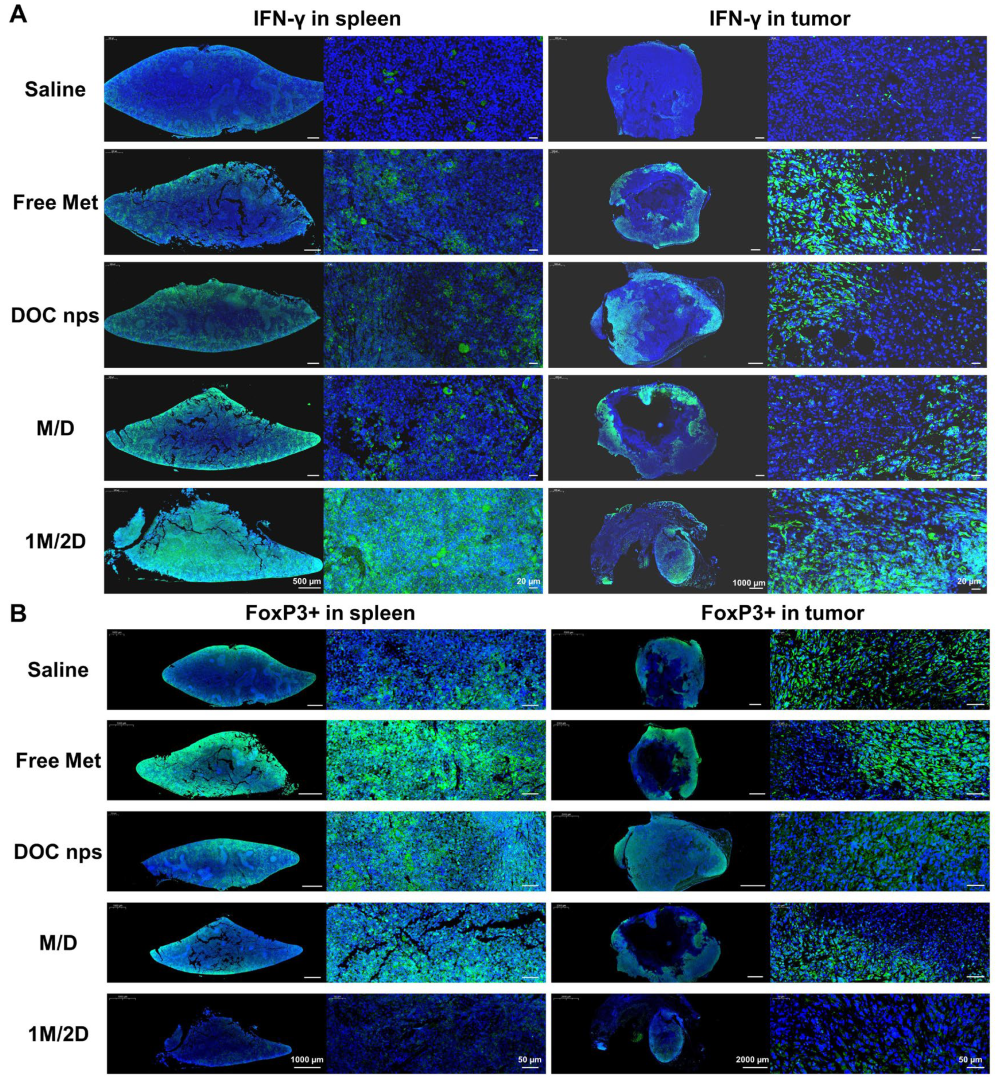
3.4. The In Vivo Therapeutic Effects of DOC nps with Different Intra-Tumoral Administration Sequences of Metformin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, S.M. Chemotherapy: Updates and new perspectives. Oncologist 2011, 16 (Suppl. S1), 30–39. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Theijse, R.T.; Seelen, L.W.F.; Groot Koerkamp, B.; van Eijck, C.H.J.; Wolfgang, C.L.; van Tienhoven, G.; van Santvoort, H.C.; Molenaar, I.Q.; Wilmink, J.W.; et al. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Pirker, R. Chemotherapy remains a cornerstone in the treatment of nonsmall cell lung cancer. Curr. Opin. Oncol. 2020, 32, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer chemotherapy: Insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef]
- Tamargo, J.; Caballero, R.; Delpón, E. Cancer Chemotherapy and Cardiac Arrhythmias: A Review. Drug Saf. 2015, 38, 129–152. [Google Scholar] [CrossRef]
- Zhou, J.; Giannakakou, P. Targeting microtubules for cancer chemotherapy. Curr. Med. Chem. Anticancer. Agents 2005, 5, 65–71. [Google Scholar] [CrossRef]
- Han, Y.; Smith, M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013, 4, 156. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Tan, P.; Wang, Y.; Pan, X.; Lin, T.; Jiang, Y.; Wang, B.; Xu, H.; Wang, Y.; et al. Acquired semi-squamatization during chemotherapy suggests differentiation as a therapeutic strategy for bladder cancer. Cancer Cell 2022, 40, 1044–1059 e8. [Google Scholar] [CrossRef]
- Bokil, A.; Sancho, P. Mitochondrial determinants of chemoresistance. Cancer Drug Resist. 2019, 2, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Yoo, H.C.; Ahn, E.; Luo, E.; Kim, Y.; Sung, Y.; Yu, Y.C.; Kim, K.; Min, D.S.; Lee, H.S.; et al. Enhanced Glutaminolysis Drives Hypoxia-Induced Chemoresistance in Pancreatic Cancer. Cancer Res. 2023, 83, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Yeldag, G.; Rice, A.; Del Rio Hernandez, A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Zhao, F.X.; Ma, X.F.; Shen, G.S.; Ren, D.F.; Zheng, F.C.; Du, F.; Wang, Z.Y.; Ahmad, R.; Yuan, X.Y.; et al. A comparison between triplet and doublet chemotherapy in improving the survival of patients with advanced gastric cancer: A systematic review and meta-analysis. BMC Cancer 2019, 19, 1125. [Google Scholar] [CrossRef]
- Tam, C.; Rao, S.; Waye, M.M.Y.; Ng, T.B.; Wang, C.C. Autophagy signals orchestrate chemoresistance of gynecological cancers. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188525. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Sampaio-Ribeiro, G.; Gomes, C.M.F. Chemoresistance-Related Stem Cell Signaling in Osteosarcoma and Its Plausible Contribution to Poor Therapeutic Response: A Discussion That Still Matters. Int. J. Mol. Sci. 2022, 23, 11416. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Panaampon, J.; Zhou, Y.B.; Saengboonmee, C. Metformin as a booster of cancer immunotherapy. Int. Immunopharmacol. 2023, 121, 110528. [Google Scholar] [CrossRef]
- Georgopoulos, N.S.; Tolia, M.; Mauri, D.; Kamposioras, K.; Charalampakis, N.; Tsoukalas, N.; Gkantaifi, A. Metformin: A Promising Radiosensitizer in Neoadjuvant Rectal Cancer Treatment. Rev. Recent. Clin. Tria 2023, 18, 172–180. [Google Scholar] [CrossRef]
- Mu, W.; Jiang, Y.Y.; Liang, G.Q.; Feng, Y.; Qu, F.L. Metformin: A Promising Antidiabetic Medication for Cancer Treatment. Curr. Drug Targets 2023, 24, 41–54. [Google Scholar] [CrossRef]
- Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. Int. J. Mol. Sci. 2024, 25, 4083. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Chu, W.; Ashamalla, S.; Fenech, D.; Berry, S.; Kiss, A.; Koritzinsky, M. Metformin with neoadjuvant chemoradiation to improve pathologic response in rectal cancer: A pilot phase I/II trial. Clin. Transl. Radiat. Oncol. 2021, 30, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Huang, Y.C.; Chen, H.J.; Chiu, H.C.; Huang, Y.J.; Wo, T.Y.; Weng, S.H.; Lin, Y.W. Metformin-mediated downregulation of p38 mitogen-activated protein kinase-dependent excision repair cross-complementing 1 decreases DNA repair capacity and sensitizes human lung cancer cells to paclitaxel. Biochem. Pharmacol. 2013, 85, 583–594. [Google Scholar] [CrossRef]
- Abo-Elmatty, D.M.; Ahmed, E.A.; Tawfik, M.K.; Helmy, S.A. Metformin enhancing the antitumor efficacy of carboplatin against Ehrlich solid carcinoma grown in diabetic mice: Effect on IGF-1 and tumoral expression of IGF-1 receptors. Int. Immunopharmacol. 2017, 44, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.B.; Yan, Z.X.; Zhao, W.L.; Mi, J.Q.; Li, J.M.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Canc Res. 2018, 37, 63. [Google Scholar] [CrossRef]
- Ko, E.; Baek, S.; Kim, J.; Park, D.; Lee, Y. Antitumor Activity of Combination Therapy with Metformin and Trametinib in Non-Small Cell Lung Cancer Cells. Dev. Reprod. 2020, 24, 113–123. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Cong, S.; Zhang, F. Insulin-Like Growth Factor-1 Promotes Human Uterine Leiomyoma Cell Proliferation via PI3K/AKT/mTOR Pathway. Cells Tissues Organs 2023, 212, 194–202. [Google Scholar] [CrossRef]
- Yang, S.P.; Su, Q.; Zhang, Y.R.; Sun, Y.; Chai, Y.R. Metformin ameliorates thymus degeneration of mice by regulating mitochondrial function. Int. Immunopharmacol. 2022, 108, 108744. [Google Scholar] [CrossRef]
- Zaidi, S.; Gandhi, J.; Joshi, G.; Smith, N.L.; Khan, S.A. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 351–361. [Google Scholar] [CrossRef]
- Peng, T.; Shao, X.; Song, W.; Xu, W.; Xiong, W.; He, Y.; Ding, Y.; Huang, Y. Intratumoral lactic acid neutralization strategy for boosting chemoimmunotherapy using liposomal sodium bicarbonate. Sci. Bull. 2024, 69, 3936–3948. [Google Scholar] [CrossRef]
- Kolosenko, I.; Avnet, S.; Baldini, N.; Viklund, J.; De Milito, A. Therapeutic implications of tumor interstitial acidification. Semin. Cancer Biol. 2017, 43, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, H.; Chen, N.; Zhang, C.; Guo, X. Metformin protects cardiomyocytes against oxygen-glucose deprivation injury by promoting autophagic flux through AMPK pathway. J. Drug Target. 2021, 29, 551–561. [Google Scholar] [CrossRef]
- Wu, Z.; Bai, Y.; Qi, Y.; Chang, C.; Jiao, Y.; Bai, Y.; Guo, Z. Metformin ameliorates ferroptosis in cardiac ischemia and reperfusion by reducing NOX4 expression via promoting AMPKalpha. Pharm. Biol. 2023, 61, 886–896. [Google Scholar] [CrossRef]
- Sebestyén, A.; Dankó, T.; Sztankovics, D.; Moldvai, D.; Raffay, R.; Cervi, C.; Krencz, I.; Zsiros, V.; Jeney, A.; Petovari, G. The role of metabolic ecosystem in cancer progression—Metabolic plasticity and mTOR hyperactivity in tumor tissues. Cancer Metast Rev. 2021, 40, 989–1033. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef]
- Turpin, R.; Liu, R.X.; Munne, P.M.; Peura, A.; Rannikko, J.H.; Philips, G.; Boeckx, B.; Salmelin, N.; Hurskainen, E.; Suleymanova, I.; et al. Respiratory complex I regulates dendritic cell maturation in explant model of human tumor immune microenvironment. J. Immunother. Cancer 2024, 12, e008053. [Google Scholar] [CrossRef]
- Luo, L.H.; Li, X.; Zhang, J.L.; Zhu, C.Q.; Jiang, M.S.; Luo, Z.Y.; Qin, B.; Wang, Y.Q.; Chen, B.; Du, Y.Z.; et al. Enhanced immune memory through a constant photothermal-metabolism regulation for cancer prevention and treatment. Biomaterials 2021, 270, 120678. [Google Scholar] [CrossRef]
- Luo, L.H.; Qin, B.; Jiang, M.S.; Xie, L.; Luo, Z.Y.; Guo, X.M.; Zhang, J.L.; Li, X.; Zhu, C.Q.; Du, Y.Z.; et al. Regulating immune memory and reversing tumor thermotolerance through a step-by-step starving-photothermal therapy. J. Nanobiotechnol. 2021, 19, 297. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Luo, L.H.; Jiang, X.D.; Jiang, M.S.; Luo, Z.Y.; Li, X.; Qiu, W.G.; Jin, Z.L.; Shen, T.X.; Li, C.L.; et al. Selective Intratumoral Drug Release and Simultaneous Inhibition of Oxidative Stress by a Highly Reductive Nanosystem and Its Application as an Anti-tumor Agent. Theranostics 2020, 10, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Q.; Shi, Y.Y.; Li, Q.P.; Luo, L.H.; Li, X.; Luo, Z.Y.; Lu, Y.C.; Zhang, J.L.; Jiang, M.S.; Qin, B.; et al. Rational administration sequencing of immunochemotherapy elicits powerful anti-tumor effect. J. Control Release 2022, 341, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. Int. J. Mol. Sci. 2022, 23, 12893. [Google Scholar] [CrossRef] [PubMed]
- He, K.C.; Li, Z.T.Y.; Ye, K.; Zhou, Y.H.; Yan, M.B.; Qi, H.; Hu, H.T.; Dai, Y.B.; Tang, Y.X. Novel sequential therapy with metformin enhances the effects of cisplatin in testicular germ cell tumours via YAP1 signalling. Cancer Cell Int. 2022, 22, 113. [Google Scholar] [CrossRef]
- Marullo, R.; Castro, M.; Yomtoubian, S.; Calvo-Vidal, M.N.; Revuelta, M.V.; Krumsiek, J.; Cho, A.; Morgado, P.C.; Yang, S.; Medina, V.; et al. The metabolic adaptation evoked by arginine enhances the effect of radiation in brain metastases. Sci. Adv. 2021, 7, eabg1964. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Gong, C.A.; Yu, X.Y.; Zhang, W.; Han, L.; Wang, R.; Wang, Y.J.; Gao, S.; Yuan, Y.F. Regulating the immunosuppressive tumor microenvironment to enhance breast cancer immunotherapy using pH-responsive hybrid membrane-coated nanoparticles. J. Nanobiotechnol. 2021, 19, 58. [Google Scholar] [CrossRef]
- Wei, Z.H.; Zhang, X.Q.; Yong, T.Y.; Bie, N.N.; Zhan, G.T.; Li, X.; Liang, Q.L.; Li, J.Y.; Yu, J.J.; Huang, G.; et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat. Commun. 2021, 12, 440. [Google Scholar] [CrossRef]
- Liu, B.; Liang, B.B.; Cao, W.D.; Su, X.X.; Cao, Q.; Mao, Z.W. Platinum-Metformin Conjugates Acting as Promising PD-L1 Inhibitors through the AMP-Activated Protein Kinase Mediated Lysosomal Degradation Pathway. Angew. Chem. Int. Ed. 2024, 136, e202410586. [Google Scholar] [CrossRef]
- Xiong, W.; Qi, L.; Jiang, N.; Zhao, Q.; Chen, L.X.; Jiang, X.; Li, Y.; Zhou, Z.G.; Shen, J.L. Metformin Liposome-Mediated PD-L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl. Mater. Inter. 2021, 13, 8026–8041. [Google Scholar] [CrossRef]
- Canale, F.P.; Basso, C.; Antonini, G.; Perotti, M.; Li, N.; Sokolovska, A.; Neumann, J.; James, M.J.; Geiger, S.; Jin, W.J.; et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021, 598, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Guo, X.; Lu, Y.; Liu, X.; Jiang, M.; Li, X.; Qin, B.; Luo, Z.; Liu, H.; et al. Arginine Supplementation Targeting Tumor-Killing Immune Cells Reconstructs the Tumor Microenvironment and Enhances the Antitumor Immune Response. ACS Nano 2022, 16, 12964–12978. [Google Scholar] [CrossRef] [PubMed]
- Halma, M.T.J.; Tuszynski, J.A.; Marik, P.E. Cancer Metabolism as a Therapeutic Target and Review of Interventions. Nutrients 2023, 15, 4245. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Akhter, M.S.; Uppal, P. Toxicity of Metformin and Hypoglycemic Therapies. Adv. Chronic Kidney D 2020, 27, 18–30. [Google Scholar] [CrossRef]
- Wang, G.S.; Hoyte, C. Review of Biguanide (Metformin) Toxicity. J. Intensive Care Med. 2019, 34, 863–876. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Mao, J.; Hu, Y.; Huang, X.; You, J.; Luo, L. Metformin-Sensitized Chemotherapy of Docetaxel Nanoemulsions Based on a Sequential Administration. Pharmaceutics 2025, 17, 812. https://doi.org/10.3390/pharmaceutics17070812
Zhang J, Mao J, Hu Y, Huang X, You J, Luo L. Metformin-Sensitized Chemotherapy of Docetaxel Nanoemulsions Based on a Sequential Administration. Pharmaceutics. 2025; 17(7):812. https://doi.org/10.3390/pharmaceutics17070812
Chicago/Turabian StyleZhang, Junlei, Jiapeng Mao, Yilong Hu, Xingze Huang, Jian You, and Lihua Luo. 2025. "Metformin-Sensitized Chemotherapy of Docetaxel Nanoemulsions Based on a Sequential Administration" Pharmaceutics 17, no. 7: 812. https://doi.org/10.3390/pharmaceutics17070812
APA StyleZhang, J., Mao, J., Hu, Y., Huang, X., You, J., & Luo, L. (2025). Metformin-Sensitized Chemotherapy of Docetaxel Nanoemulsions Based on a Sequential Administration. Pharmaceutics, 17(7), 812. https://doi.org/10.3390/pharmaceutics17070812






