Endo/Lysosomal-Escapable Lipid Nanoparticle Platforms for Enhancing mRNA Delivery in Cancer Therapy
Abstract
1. Introduction
2. The Fate of mRNA-Loaded LNP
2.1. Pathway of mRNA-Loaded LNP Internalization
2.2. Processing of mRNA-Loaded LNP in Endo-Lysosome System
2.3. The Impact of Endo-Lysosome Process on mRNA Delivery
2.4. Potential Strategies for Enhancing mRNA Escape from Endo-Lysosome System
3. Progress of LNPs for mRNA Endo/Lysosomal Escape
3.1. Cationic/Ionizable Lipid Molecular Engineering
3.2. Helper Lipid Innovations
3.3. Cholesterol Optimization Strategies
3.4. PEG Lipid Engineering
3.5. Surface Coating and Shape Management
3.6. Ancillary Enhancement Strategies
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Wang, N.; Ma, T.; Yu, B. Targeting Epigenetic Regulators to Overcome Drug Resistance in Cancers. Signal Transduct. Target. Ther. 2023, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Huayamares, S.G.; Loughrey, D.; Kim, H.; Dahlman, J.E.; Sorscher, E.J. Nucleic Acid-Based Drugs for Patients with Solid Tumours. Nat. Rev. Clin. Oncol. 2024, 21, 407–427. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA Vaccine Development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. mRNA-Based Therapeutics: Looking beyond COVID-19 Vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Wang, F.; Gan, X.; Zheng, T.; Chen, M.; Wei, L.; Chen, J.; Yu, C. CES1-Triggered Liver-Specific Cargo Release of CRISPR/Cas9 Elements by Cationic Triadic Copolymeric Nanoparticles Targeting Gene Editing of PCSK9 for Hyperlipidemia Amelioration. Adv. Sci. 2023, 10, e2300502. [Google Scholar] [CrossRef]
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer mRNA Vaccines: Clinical Advances and Future Opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 489–500. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical Advances and Ongoing Trials on mRNA Vaccines for Cancer Treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef]
- Berraondo, P.; Gomis, G.; Melero, I. The Liver as a Cytokine Factory Working on mRNA Blueprints for Cancer Immunotherapy. Cancer Cell 2024, 42, 502–504. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Y.; Solek, N.C.; Chen, J.; Gong, F.; Varley, A.J.; Golubovic, A.; Pan, A.; Dong, S.; Zheng, G.; et al. Tumor-Tailored Ionizable Lipid Nanoparticles Facilitate IL-12 Circular RNA Delivery for Enhanced Lung Cancer Immunotherapy. Adv. Mater. 2024, 36, e2400307. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Wagenaar, T.R.; Gieseke, F.; Bangari, D.S.; Callahan, M.; Cao, H.; Diekmann, J.; Diken, M.; Grunwitz, C.; Hebert, A.; et al. Local Delivery of mRNA-Encoded Cytokines Promotes Antitumor Immunity and Tumor Eradication across Multiple Preclinical Tumor Models. Sci. Transl. Med. 2021, 13, eabc7804. [Google Scholar] [CrossRef] [PubMed]
- Raab, M.; Kostova, I.; Peña-Llopis, S.; Fietz, D.; Kressin, M.; Aberoumandi, S.M.; Ullrich, E.; Becker, S.; Sanhaji, M.; Strebhardt, K. Rescue of P53 Functions by In Vitro-Transcribed mRNA Impedes the Growth of High-Grade Serous Ovarian Cancer. Cancer Commun. 2024, 44, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-X.; Wang, Y.; Ding, J.; Jiang, A.; Wang, J.; Yu, M.; Blake, S.; Liu, S.; Bieberich, C.J.; Farokhzad, O.C.; et al. Reactivation of the Tumor Suppressor PTEN by mRNA Nanoparticles Enhances Antitumor Immunity in Preclinical Models. Sci. Transl. Med. 2021, 13, eaba9772. [Google Scholar] [CrossRef]
- Katti, A.; Diaz, B.J.; Caragine, C.M.; Sanjana, N.E.; Dow, L.E. CRISPR in Cancer Biology and Therapy. Nat. Rev. Cancer 2022, 22, 259–279. [Google Scholar] [CrossRef]
- Wagner, D.L.; Fritsche, E.; Pulsipher, M.A.; Ahmed, N.; Hamieh, M.; Hegde, M.; Ruella, M.; Savoldo, B.; Shah, N.N.; Turtle, C.J.; et al. Immunogenicity of CAR T Cells in Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 379–393. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Verbeke, R.; Dewitte, H.; Lentacker, I.; Vermaelen, K.; Breckpot, K.; Van Lint, S. mRNA in Cancer Immunotherapy: Beyond a Source of Antigen. Mol. Cancer 2021, 20, 48. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Liu, H.; Zhou, X. Chemical Methods and Advanced Sequencing Technologies for Deciphering mRNA Modifications. Chem. Soc. Rev. 2021, 50, 13481–13497. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for Optimized mRNA Design Improves Stability and Immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef]
- McGee, J.E.; Kirsch, J.R.; Kenney, D.; Cerbo, F.; Chavez, E.C.; Shih, T.-Y.; Douam, F.; Wong, W.W.; Grinstaff, M.W. Complete Substitution with Modified Nucleotides in Self-Amplifying RNA Suppresses the Interferon Response and Increases Potency. Nat. Biotechnol. 2024, 43, 720–726. [Google Scholar] [CrossRef]
- Perkovic, M.; Gawletta, S.; Hempel, T.; Brill, S.; Nett, E.; Sahin, U.; Beissert, T. A Trans-Amplifying RNA Simplified to Essential Elements Is Highly Replicative and Robustly Immunogenic in Mice. Mol. Ther. 2023, 31, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.; Jiang, Y.; Kristensen, L.S.; Hansen, T.B.; Kjems, J. The Therapeutic Potential of Circular RNAs. Nat. Rev. Genet. 2025, 26, 230–244. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA Vaccine: A Potential Therapeutic Strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Ibba, M.L.; Ciccone, G.; Esposito, C.L.; Catuogno, S.; Giangrande, P.H. Advances in mRNA Non-Viral Delivery Approaches. Adv. Drug Deliv. Rev. 2021, 177, 113930. [Google Scholar] [CrossRef]
- Cullis, P.R.; Felgner, P.L. The 60-Year Evolution of Lipid Nanoparticles for Nucleic Acid Delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Sun, Y.; Yu, X.; Lee, S.M.; Cheng, Q.; Wei, T.; Gong, J.; Robinson, J.; Zhang, D.; et al. Preparation of Selective Organ-Targeting (SORT) Lipid Nanoparticles (LNPs) Using Multiple Technical Methods for Tissue-Specific mRNA Delivery. Nat. Protoc. 2023, 18, 265–291. [Google Scholar] [CrossRef]
- Lopes, C.; Cristóvão, J.; Silvério, V.; Lino, P.R.; Fonte, P. Microfluidic Production of mRNA-Loaded Lipid Nanoparticles for Vaccine Applications. Expert Opin. Drug Deliv. 2022, 19, 1381–1395. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle-mRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jozic, A.; Lin, Y.; Eygeris, Y.; Bloom, E.; Tan, X.; Acosta, C.; MacDonald, K.D.; Welsher, K.D.; Sahay, G. Engineering Lipid Nanoparticles for Enhanced Intracellular Delivery of mRNA through Inhalation. ACS Nano 2022, 16, 14792–14806. [Google Scholar] [CrossRef]
- Naslavsky, N.; Caplan, S. Advances and Challenges in Understanding Endosomal Sorting and Fission. FEBS J. 2023, 290, 4187–4195. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based Analysis of Lipid Nanoparticle-Mediated siRNA Delivery, Intracellular Trafficking and Endosomal Escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- van Hees, M.; Slott, S.; Hansen, A.H.; Kim, H.S.; Ji, H.P.; Astakhova, K. New Approaches to Moderate CRISPR-Cas9 Activity: Addressing Issues of Cellular Uptake and Endosomal Escape. Mol. Ther. 2022, 30, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Wagner, E. Strategies and Mechanisms for Endosomal Escape of Therapeutic Nucleic Acids. Curr. Opin. Chem. Biol. 2024, 81, 102506. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.; Gruenberg, J.; Marsh, M.; Wohlmann, J.; Jones, A.T.; Parton, R.G. Nanoparticle Entry into Cells; the Cell Biology Weak Link. Adv. Drug Deliv. Rev. 2022, 188, 114403. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Richards, D.M.; Endres, R.G. How Cells Engulf: A Review of Theoretical Approaches to Phagocytosis. Rep. Prog. Phys. 2017, 80, 126601. [Google Scholar] [CrossRef]
- Jia, X.; Yan, B.; Tian, X.; Liu, Q.; Jin, J.; Shi, J.; Hou, Y. CD47/SIRPα Pathway Mediates Cancer Immune Escape and Immunotherapy. Int. J. Biol. Sci. 2021, 17, 3281–3287. [Google Scholar] [CrossRef]
- Messerschmidt, C.; Hofmann, D.; Kroeger, A.; Landfester, K.; Mailänder, V.; Lieberwirth, I. On the Pathway of Cellular Uptake: New Insight into the Interaction between the Cell Membrane and Very Small Nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M. Macrophage Recognition of Crystals and Nanoparticles. Front. Immunol. 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.E.; Grinstein, S.; Freeman, S.A. SnapShot: Macropinocytosis. Cell 2017, 169, 766–766.e1. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Guo, H.; Edman, M.; Hamm-Alvarez, S.F. Application of Advances in Endocytosis and Membrane Trafficking to Drug Delivery. Adv. Drug Deliv. Rev. 2020, 157, 118–141. [Google Scholar] [CrossRef]
- Deng, H.; Dutta, P.; Liu, J. Stochastic Simulations of Nanoparticle Internalization through Transferrin Receptor Dependent Clathrin-Mediated Endocytosis. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2104–2111. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Cureton, D.K.; Massol, R.H.; Whelan, S.P.J.; Kirchhausen, T. The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis. PLoS Pathog. 2010, 6, e1001127. [Google Scholar] [CrossRef]
- Rességuier, J.; Levraud, J.-P.; Dal, N.K.; Fenaroli, F.; Primard, C.; Wohlmann, J.; Carron, G.; Griffiths, G.W.; Le Guellec, D.; Verrier, B. Biodistribution of Surfactant-Free Poly(Lactic-Acid) Nanoparticles and Uptake by Endothelial Cells and Phagocytes in Zebrafish: Evidence for Endothelium to Macrophage Transfer. J. Control. Release 2021, 331, 228–245. [Google Scholar] [CrossRef]
- Parton, R.G. Caveolae: Structure, Function, and Relationship to Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Qu, S.; Zhang, N.; Nie, K.; Wang, J.; Huang, Y.; Sui, D.; Yu, B.; Qin, M.; et al. Controllable Star Cationic Poly(Disulfide)s Achieve Genetically Cascade Catalytic Therapy by Delivering Bifunctional Fusion Plasmids. Adv. Mater. 2023, 35, e2307190. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, C.; Paunovska, K.; Schrader Echeverri, E.; Loughrey, D.; Da Silva Sanchez, A.J.; Ni, H.; Hatit, M.Z.C.; Lokugamage, M.P.; Kuzminich, Y.; Peck, H.E.; et al. Nanoparticle Single-Cell Multiomic Readouts Reveal That Cell Heterogeneity Influences Lipid Nanoparticle-Mediated Messenger RNA Delivery. Nat. Nanotechnol. 2022, 17, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, L.; Renzi, S.; Pirrottina, A.; Amenitsch, H.; De Lorenzi, V.; Pozzi, D.; Cardarelli, F.; Caracciolo, G. PEGylation-Dependent Cell Uptake of Lipid Nanoparticles Revealed by Spatiotemporal Correlation Spectroscopy. ACS Pharmacol. Transl. Sci. 2024, 7, 3004–3010. [Google Scholar] [CrossRef]
- Cui, L.; Hunter, M.R.; Sonzini, S.; Pereira, S.; Romanelli, S.M.; Liu, K.; Li, W.; Liang, L.; Yang, B.; Mahmoudi, N.; et al. Mechanistic Studies of an Automated Lipid Nanoparticle Reveal Critical Pharmaceutical Properties Associated with Enhanced mRNA Functional Delivery in Vitro and in Vivo. Small 2022, 18, e2105832. [Google Scholar] [CrossRef]
- Molina, F.M. Francisco Martín Gene Therapy Tools and Potential Applications; IntechOpen: London, UK, 2014. [Google Scholar]
- Sago, C.D.; Lokugamage, M.P.; Lando, G.N.; Djeddar, N.; Shah, N.N.; Syed, C.; Bryksin, A.V.; Dahlman, J.E. ; Lokugamage, M.P.; Lando, G.N.; Djeddar, N.; Shah, N.N.; Syed, C.; Bryksin, A.V.; Dahlman, J.E. Modifying a Commonly Expressed Endocytic Receptor Retargets Nanoparticles in Vivo. Nano Lett. 2018, 18, 7590–7600. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Querbes, W.; Alabi, C.; Eltoukhy, A.; Sarkar, S.; Zurenko, C.; Karagiannis, E.; Love, K.; Chen, D.; Zoncu, R.; et al. Efficiency of siRNA Delivery by Lipid Nanoparticles Is Limited by Endocytic Recycling. Nat. Biotechnol. 2013, 31, 653–658. [Google Scholar] [CrossRef]
- Paillard, A.; Hindré, F.; Vignes-Colombeix, C.; Benoit, J.-P.; Garcion, E. The Importance of Endo-Lysosomal Escape with Lipid Nanocapsules for Drug Subcellular Bioavailability. Biomaterials 2010, 31, 7542–7554. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Dammer, E.B.; Ren, R.-J.; Wang, G. The Endosomal-Lysosomal System: From Acidification and Cargo Sorting to Neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef]
- Naslavsky, N.; Caplan, S. The Enigmatic Endosome—Sorting the Ins and Outs of Endocytic Trafficking. J. Cell Sci. 2018, 131, jcs216499. [Google Scholar] [CrossRef]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome Maturation, Transport and Functions. Semin. Cell Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef]
- Lakadamyali, M.; Rust, M.J.; Zhuang, X. Ligands for Clathrin-Mediated Endocytosis Are Differentially Sorted into Distinct Populations of Early Endosomes. Cell 2006, 124, 997–1009. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, D.; He, X.; Huo, L.; Wang, J.; Zhang, X.; Wu, Y.; Zhang, R.; Gao, Y.; Kang, T. RAB22A Sorts Epithelial Growth Factor Receptor (EGFR) from Early Endosomes to Recycling Endosomes for Microvesicles Release. J. Extracell. Vesicles 2024, 13, e12494. [Google Scholar] [CrossRef] [PubMed]
- Ott, D.P.; Desai, S.; Solinger, J.A.; Kaech, A.; Spang, A. Coordination between ESCRT Function and Rab Conversion during Endosome Maturation. EMBO J. 2025, 44, 1574–1607. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, L.; Ma, Y.; Li, Y.; Li, H. Focus on the Morphogenesis, Fate and the Role in Tumor Progression of Multivesicular Bodies. Cell Commun. Signal. 2020, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Patak, J.; Zhang-James, Y.; Faraone, S.V. Endosomal System Genetics and Autism Spectrum Disorders: A Literature Review. Neurosci. Biobehav. Rev. 2016, 65, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Pamarthy, S.; Kulshrestha, A.; Katara, G.K.; Beaman, K.D. The Curious Case of Vacuolar ATPase: Regulation of Signaling Pathways. Mol. Cancer 2018, 17, 41. [Google Scholar] [CrossRef]
- Wartosch, L.; Stauber, T. A Role for Chloride Transport in Lysosomal Protein Degradation. Autophagy 2010, 6, 158–159. [Google Scholar] [CrossRef]
- Striepen, J.F.; Voeltz, G.K. Endosome Biogenesis Is Controlled by ER and the Cytoskeleton at Tripartite Junctions. Curr. Opin. Cell Biol. 2023, 80, 102155. [Google Scholar] [CrossRef]
- Poteryaev, D.; Datta, S.; Ackema, K.; Zerial, M.; Spang, A. Identification of the Switch in Early-to-Late Endosome Transition. Cell 2010, 141, 497–508. [Google Scholar] [CrossRef]
- Langemeyer, L.; Fröhlich, F.; Ungermann, C. Rab GTPase Function in Endosome and Lysosome Biogenesis. Trends Cell Biol. 2018, 28, 957–970. [Google Scholar] [CrossRef]
- Cullen, P.J.; Steinberg, F. To Degrade or Not to Degrade: Mechanisms and Significance of Endocytic Recycling. Nat. Rev. Mol. Cell Biol. 2018, 19, 679–696. [Google Scholar] [CrossRef]
- Vietri, M.; Radulovic, M.; Stenmark, H. The Many Functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef]
- Seaman, M.N.J. The Retromer Complex—Endosomal Protein Recycling and Beyond. J. Cell Sci. 2012, 125, 4693–4702. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between Endosomal Escape of LNP-mRNA and Loading into EVs for Transport to Other Cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef] [PubMed]
- Spadea, A.; Jackman, M.; Cui, L.; Pereira, S.; Lawrence, M.J.; Campbell, R.A.; Ashford, M. Nucleic Acid-Loaded Lipid Nanoparticle Interactions with Model Endosomal Membranes. ACS Appl. Mater. Interfaces 2022, 14, 30371–30384. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Paramasivam, P.; Franke, C.; Stöter, M.; Höijer, A.; Bartesaghi, S.; Sabirsh, A.; Lindfors, L.; Arteta, M.Y.; Dahlén, A.; Bak, A.; et al. Endosomal Escape of Delivered mRNA from Endosomal Recycling Tubules Visualized at the Nanoscale. J. Cell Biol. 2022, 221, e202110137. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, K.; Han, H.; Stenzel, M.; Yin, B.; Song, H.; Lawanprasert, A.; Nielsen, J.E.; Sharma, R.; Arogundade, O.H.; et al. Acid-Degradable Lipid Nanoparticles Enhance the Delivery of mRNA. Nat. Nanotechnol. 2024, 19, 1702–1711. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Li, M.; Rong, Y.; Chuang, Y.-S.; Peng, D.; Emr, S.D. Ubiquitin-Dependent Lysosomal Membrane Protein Sorting and Degradation. Mol. Cell 2015, 57, 467–478. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, W.; Han, Y.; Lee, W.-R.; Liou, J.; Jiang, Y. Lysosomal LAMP Proteins Regulate Lysosomal pH by Direct Inhibition of the TMEM175 Channel. Mol. Cell 2023, 83, 2524–2539.e7. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Perera, R.M. Lysosomes as Coordinators of Cellular Catabolism, Metabolic Signalling and Organ Physiology. Nat. Rev. Mol. Cell Biol. 2024, 25, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Perez, P.; Sun, X.; Chen, K.; Fatirkhorani, R.; Mammadova, J.; Wang, Z. MLKL Polymerization-Induced Lysosomal Membrane Permeabilization Promotes Necroptosis. Cell Death Differ. 2024, 31, 40–52. [Google Scholar] [CrossRef]
- Alvarez-Valadez, K.; Sauvat, A.; Diharce, J.; Leduc, M.; Stoll, G.; Guittat, L.; Lambertucci, F.; Paillet, J.; Motiño, O.; Ferret, L.; et al. Lysosomal Damage Due to Cholesterol Accumulation Triggers Immunogenic Cell Death. Autophagy 2024, 21, 934–956. [Google Scholar] [CrossRef]
- Meyer, H.; Kravic, B. The Endo-Lysosomal Damage Response. Annu. Rev. Biochem. 2024, 93, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, L.; Ye, Y.; Shan, Y.; Wan, C.; Wang, J.; Pei, D.; Shu, X.; Liu, J. SNX16 Regulates the Recycling of E-Cadherin through a Unique Mechanism of Coordinated Membrane and Cargo Binding. Structure 2017, 25, 1251–1263.e5. [Google Scholar] [CrossRef]
- Sapmaz, A.; Berlin, I.; Bos, E.; Wijdeven, R.H.; Janssen, H.; Konietzny, R.; Akkermans, J.J.; Erson-Bensan, A.E.; Koning, R.I.; Kessler, B.M.; et al. USP32 Regulates Late Endosomal Transport and Recycling through Deubiquitylation of Rab7. Nat. Commun. 2019, 10, 1454. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Marchitto, J.; Nguyen, T.D.T.; Marasini, R.; Celia, C.; Aryal, S. pH-Responsive Cationic Liposome for Endosomal Escape Mediated Drug Delivery. Colloids Surf. B Biointerfaces 2020, 188, 110804. [Google Scholar] [CrossRef]
- Mrksich, K.; Padilla, M.S.; Mitchell, M.J. Breaking the Final Barrier: Evolution of Cationic and Ionizable Lipid Structure in Lipid Nanoparticles to Escape the Endosome. Adv. Drug Deliv. Rev. 2024, 214, 115446. [Google Scholar] [CrossRef]
- Habrant, D.; Peuziat, P.; Colombani, T.; Dallet, L.; Gehin, J.; Goudeau, E.; Evrard, B.; Lambert, O.; Haudebourg, T.; Pitard, B. Design of Ionizable Lipids to Overcome the Limiting Step of Endosomal Escape: Application in the Intracellular Delivery of mRNA, DNA, and siRNA. J. Med. Chem. 2016, 59, 3046–3062. [Google Scholar] [CrossRef]
- Liu, Y.; He, F.; Chen, L.; Zhang, Y.; Zhang, H.; Xiao, J.; Meng, Q. Imidazolyl Lipids Enhanced LNP Endosomal Escape for Ferroptosis RNAi Treatment of Cancer. Small 2024, 20, e2402362. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Angelova, A.; Angelov, B.; Dyett, B.; Matthews, L.; Zhang, Y.; El Mohamad, M.; Cai, X.; Valimehr, S.; Drummond, C.J.; et al. Real-Time pH-Dependent Self-Assembly of Ionisable Lipids from COVID-19 Vaccines and in Situ Nucleic Acid Complexation. Angew. Chem. Int. Ed. Engl. 2023, 62, e202304977. [Google Scholar] [CrossRef]
- Aliakbarinodehi, N.; Niederkofler, S.; Emilsson, G.; Parkkila, P.; Olsén, E.; Jing, Y.; Sjöberg, M.; Agnarsson, B.; Lindfors, L.; Höök, F. Time-Resolved Inspection of Ionizable Lipid-Facilitated Lipid Nanoparticle Disintegration and Cargo Release at an Early Endosomal Membrane Mimic. ACS Nano 2024, 18, 22989–23000. [Google Scholar] [CrossRef]
- Goswami, R.; Jeon, T.; Nagaraj, H.; Zhai, S.; Rotello, V.M. Accessing Intracellular Targets through Nanocarrier-Mediated Cytosolic Protein Delivery. Trends Pharmacol. Sci. 2020, 41, 743–754. [Google Scholar] [CrossRef]
- Mo, Y.; Cheng, M.H.Y.; D’Elia, A.; Doran, K.; Ding, L.; Chen, J.; Cullis, P.R.; Zheng, G. Light-Activated siRNA Endosomal Release (LASER) by Porphyrin Lipid Nanoparticles. ACS Nano 2023, 17, 4688–4703. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, B.; Gao, W.; Li, L.; Li, T.; Sun, J.; Peng, X.; Li, X.; Wang, Z.; Wang, B.; et al. Enhanced Endosomal Escape by Photothermal Activation for Improved Small Interfering RNA Delivery and Antitumor Effect. Int. J. Nanomed. 2018, 13, 4333–4344. [Google Scholar] [CrossRef]
- Winkeljann, B.; Keul, D.C.; Merkel, O.M. Engineering Poly- and Micelleplexes for Nucleic Acid Delivery—A Reflection on Their Endosomal Escape. J. Control. Release 2023, 353, 518–534. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in Gene Therapy: Current Status and Clinical Applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Chen, K.; Zhu, R.; Zhang, Z.; Huang, H.; Qin, S.; Zheng, Q.; He, Z.; He, X.; Xiao, W.; et al. Manganese-Coordinated mRNA Vaccines with Enhanced mRNA Expression and Immunogenicity Induce Robust Immune Responses against SARS-CoV-2 Variants. Sci. Adv. 2022, 8, eabq3500. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef]
- Wei, Y.; He, T.; Bi, Q.; Yang, H.; Hu, X.; Jin, R.; Liang, H.; Zhu, Y.; Tong, R.; Nie, Y. A Cationic Lipid with Advanced Membrane Fusion Performance for pDNA and mRNA Delivery. J. Mater. Chem. B 2023, 11, 2095–2107. [Google Scholar] [CrossRef]
- Lee, S.M.; Cheng, Q.; Yu, X.; Liu, S.; Johnson, L.T.; Siegwart, D.J. A Systematic Study of Unsaturation in Lipid Nanoparticles Leads to Improved mRNA Transfection in Vivo. Angew. Chem. Int. Ed. Engl. 2021, 60, 5848–5853. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, H.; Li, Y.; Sun, S.; Gao, J.; Zhong, Y.; Sun, D.; Zhang, G. Metabolomics Revealed the Toxicity of Cationic Liposomes in HepG2 Cells Using UHPLC-Q-TOF/MS and Multivariate Data Analysis. Biomed. Chromatogr. 2017, 31, e4036. [Google Scholar] [CrossRef]
- Pushpa Ragini, S.; Dyett, B.P.; Sarkar, S.; Zhai, J.; White, J.F.; Banerjee, R.; Drummond, C.J.; Conn, C.E. A Systematic Study of the Effect of Lipid Architecture on Cytotoxicity and Cellular Uptake of Cationic Cubosomes. J. Colloid Interface Sci. 2024, 663, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Lin, J.; Huang, Y.; Li, L.; Delcassian, D.; Ge, Y.; Shi, Y.; Anderson, D.G. Synergistic Lipid Compositions for Albumin Receptor Mediated Delivery of mRNA to the Liver. Nat. Commun. 2020, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, Y.; Xue, W.; Liu, J.; Wang, Z.; Li, X. Multiple Tail Ionizable Lipids Improve in Vivo mRNA Delivery Efficiency with Biosafety. Int. J. Pharm. 2024, 667, 124868. [Google Scholar] [CrossRef]
- Settanni, G.; Brill, W.; Haas, H.; Schmid, F. pH-Dependent Behavior of Ionizable Cationic Lipids in mRNA-Carrying Lipoplexes Investigated by Molecular Dynamics Simulations. Macromol. Rapid Commun. 2022, 43, e2100683. [Google Scholar] [CrossRef]
- Dong, L.; Deng, X.; Li, Y.; Zhu, X.; Shu, M.; Chen, J.; Luo, H.; An, K.; Cheng, M.; Zhang, P.; et al. Stimuli-Responsive mRNA Vaccines to Induce Robust CD8+ T Cell Response via ROS-Mediated Innate Immunity Boosting. J. Am. Chem. Soc. 2024, 146, 19218–19228. [Google Scholar] [CrossRef]
- Álvarez-Benedicto, E.; Farbiak, L.; Márquez Ramírez, M.; Wang, X.; Johnson, L.T.; Mian, O.; Guerrero, E.D.; Siegwart, D.J. Optimization of Phospholipid Chemistry for Improved Lipid Nanoparticle (LNP) Delivery of Messenger RNA (mRNA). Biomater. Sci. 2022, 10, 549–559. [Google Scholar] [CrossRef]
- Sagi, A.; Mukthavaram, R.; Recatto, R.; Hong, H.; Davis, M.; Trelles, R.D.; El-Mecharrafie, N.; Acharya, G.; Gomez, A.; Leu, A.; et al. Efficacy Increase of Lipid Nanoparticles in Vivo by Inclusion of Bis(Monoacylglycerol)Phosphate. Nanomedicine 2022, 17, 1399–1410. [Google Scholar] [CrossRef]
- Iwakawa, K.; Sato, R.; Konaka, M.; Yamada, Y.; Harashima, H.; Sato, Y. Cubic Phase-Inducible Zwitterionic Phospholipids Improve the Functional Delivery of mRNA. Adv. Sci. 2025, 12, e2413016. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, M.; Lai, W.; Zhang, X.; Yang, B.; Chen, X.; Ni, Q. Activatable NIR-II Photothermal Lipid Nanoparticles for Improved Messenger RNA Delivery. Angew Chem. Int. Ed. Engl. 2023, 62, e202302676. [Google Scholar] [CrossRef]
- Wu, H.; Yu, M.; Miao, Y.; He, S.; Dai, Z.; Song, W.; Liu, Y.; Song, S.; Ahmad, E.; Wang, D.; et al. Cholesterol-Tuned Liposomal Membrane Rigidity Directs Tumor Penetration and Anti-Tumor Effect. Acta Pharm. Sin. B 2019, 9, 858–870. [Google Scholar] [CrossRef]
- Patel, S.K.; Billingsley, M.M.; Frazee, C.; Han, X.; Swingle, K.L.; Qin, J.; Alameh, M.-G.; Wang, K.; Weissman, D.; Mitchell, M.J. Hydroxycholesterol Substitution in Ionizable Lipid Nanoparticles for mRNA Delivery to T Cells. J. Control. Release 2022, 347, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating Endosomal Escape of Polymorphic Lipid Nanoparticles That Boost mRNA Delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.; Yang, Y.; Liu, X.; Jiang, Y. Corosolic Acid Derivative-Based Lipid Nanoparticles for Efficient RNA Delivery. J. Control. Release 2025, 378, 1–17. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, Y.; Guo, M.; Fan, N.; Chen, K.; Qin, S.; Xiao, W.; Zheng, Q.; Huang, H.; Wei, X.; et al. Sodium Alginate Coating Simultaneously Increases the Biosafety and Immunotherapeutic Activity of the Cationic mRNA Nanovaccine. Acta Pharm. Sin. B 2023, 13, 942–954. [Google Scholar] [CrossRef]
- Kong, W.; Wei, Y.; Dong, Z.; Liu, W.; Zhao, J.; Huang, Y.; Yang, J.; Wu, W.; He, H.; Qi, J. Role of Size, Surface Charge, and PEGylated Lipids of Lipid Nanoparticles (LNPs) on Intramuscular Delivery of mRNA. J. Nanobiotechnol. 2024, 22, 553. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, C.; Yi, X.; Han, J.; Wang, J.; Liu, F.; Ling, Q.; Li, H.; Gu, Z. Fluorinated Lipid Nanoparticles for Enhancing mRNA Delivery Efficiency. ACS Nano 2024, 18, 7825–7836. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Huang, J.; Shi, Y.; Luo, Z.; Zhang, J.; Guo, X.; Jiang, M.; Li, X.; Yin, H.; et al. Nonlysosomal Route of mRNA Delivery and Combining with Epigenetic Regulation Optimized Antitumor Immunoprophylactic Efficacy. Adv. Healthc. Mater. 2023, 12, e2202460. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Shen, M.; Pattipeiluhu, R.; Zhou, X.; Zhang, Y.; Bakkum, T.; Sharp, T.H.; Boyle, A.L.; Kros, A. Efficient mRNA Delivery Using Lipid Nanoparticles Modified with Fusogenic Coiled-Coil Peptides. Nanoscale 2023, 15, 15206–15218. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.; Sui, D.; Yang, Q.; Wang, T.; Xu, Z.; Li, X.; Gao, X.; Yan, X.; Liu, X.; et al. Simultaneous Dendritic Cells Targeting and Effective Endosomal Escape Enhance Sialic Acid-Modified mRNA Vaccine Efficacy and Reduce Side Effects. J. Control. Release 2023, 364, 529–545. [Google Scholar] [CrossRef]
- Zheng, L.; Bandara, S.R.; Tan, Z.; Leal, C. Lipid Nanoparticle Topology Regulates Endosomal Escape and Delivery of RNA to the Cytoplasm. Proc. Natl. Acad. Sci. USA 2023, 120, e2301067120. [Google Scholar] [CrossRef] [PubMed]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful Reprogramming of Cellular Protein Production through mRNA Delivered by Functionalized Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Douglas, C.J.; Zhang, S.; Seath, C.P.; Bao, H. Targeting Recycling Endosomes to Potentiate mRNA Lipid Nanoparticles. Nano Lett. 2024, 24, 5104–5109. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; DuRoss, A.; Sun, C.; Murphy-Benenato, K.E.; Mihai, C.; Almarsson, Ö.; Sahay, G. Boosting Intracellular Delivery of Lipid Nanoparticle-Encapsulated mRNA. Nano Lett. 2017, 17, 5711–5718. [Google Scholar] [CrossRef]
- Zhang, J.; Shrivastava, S.; Cleveland, R.O.; Rabbitts, T.H. Lipid-mRNA Nanoparticle Designed to Enhance Intracellular Delivery Mediated by Shock Waves. ACS Appl. Mater. Interfaces 2019, 11, 10481–10491. [Google Scholar] [CrossRef]
- Chen, J.; Patel, A.; Mir, M.; Hudock, M.R.; Pinezich, M.R.; Guenthart, B.; Bacchetta, M.; Vunjak-Novakovic, G.; Kim, J. Enhancing Cytoplasmic Expression of Exogenous mRNA through Dynamic Mechanical Stimulation. Adv. Healthc. Mater. 2025, 14, e2401918. [Google Scholar] [CrossRef]
- den Roover, S.; Aerts, J.L. Unveiling the Intricacies of Gene Delivery: Caveolae-Mediated Endocytosis Induces Efficient mRNA Delivery in Slow-Dividing Cells. Mol. Ther. Nucleic Acids 2023, 33, 545–547. [Google Scholar] [CrossRef]
- Mo, Y.; Keszei, A.F.A.; Kothari, S.; Liu, H.; Pan, A.; Kim, P.; Bu, J.; Kamanzi, A.; Dai, D.L.; Mazhab-Jafari, M.T.; et al. Lipid-siRNA Organization Modulates the Intracellular Dynamics of Lipid Nanoparticles. J. Am. Chem. Soc. 2025, 147, 10430–10445. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ukwattage, V.; Xiong, Y.; Such, G.K. Advancing Endosomal Escape of Polymeric Nanoparticles: Towards Improved Intracellular Delivery. Mater. Horiz. 2025, 12, 3622–3632. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Saltzman, W.M.; Piotrowski-Daspit, A.S. Escaping the Endosome: Assessing Cellular Trafficking Mechanisms of Non-Viral Vehicles. J. Control. Release 2021, 335, 465–480. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hersch, N.; Gerlach, S.; Dreissen, G.; Springer, R.; Merkel, R.; Csiszár, A.; Hoffmann, B. Complex Size and Surface Charge Determine Nucleic Acid Transfer by Fusogenic Liposomes. Int. J. Mol. Sci. 2020, 21, 2244. [Google Scholar] [CrossRef] [PubMed]
- Forster, J., III; Nandi, D.; Kulkarni, A. mRNA-Carrying Lipid Nanoparticles That Induce Lysosomal Rupture Activate NLRP3 Inflammasome and Reduce mRNA Transfection Efficiency. Biomater. Sci. 2022, 10, 5566–5582. [Google Scholar] [CrossRef]
- Liu, H.; Chen, M.Z.; Payne, T.; Porter, C.J.H.; Pouton, C.W.; Johnston, A.P.R. Beyond the Endosomal Bottleneck: Understanding the Efficiency of mRNA/LNP Delivery. Adv. Funct. Mater. 2024, 34, 2404510. [Google Scholar] [CrossRef]
- Ma, Y.; Fenton, O.S. An Efficacy and Mechanism Driven Study on the Impact of Hypoxia on Lipid Nanoparticle Mediated mRNA Delivery. J. Am. Chem. Soc. 2023, 145, 11375–11386. [Google Scholar] [CrossRef]
- Ma, Y.; Fenton, O.S. A Unified Strategy to Improve Lipid Nanoparticle Mediated mRNA Delivery Using Adenosine Triphosphate. J. Am. Chem. Soc. 2023, 145, 19800–19811. [Google Scholar] [CrossRef]

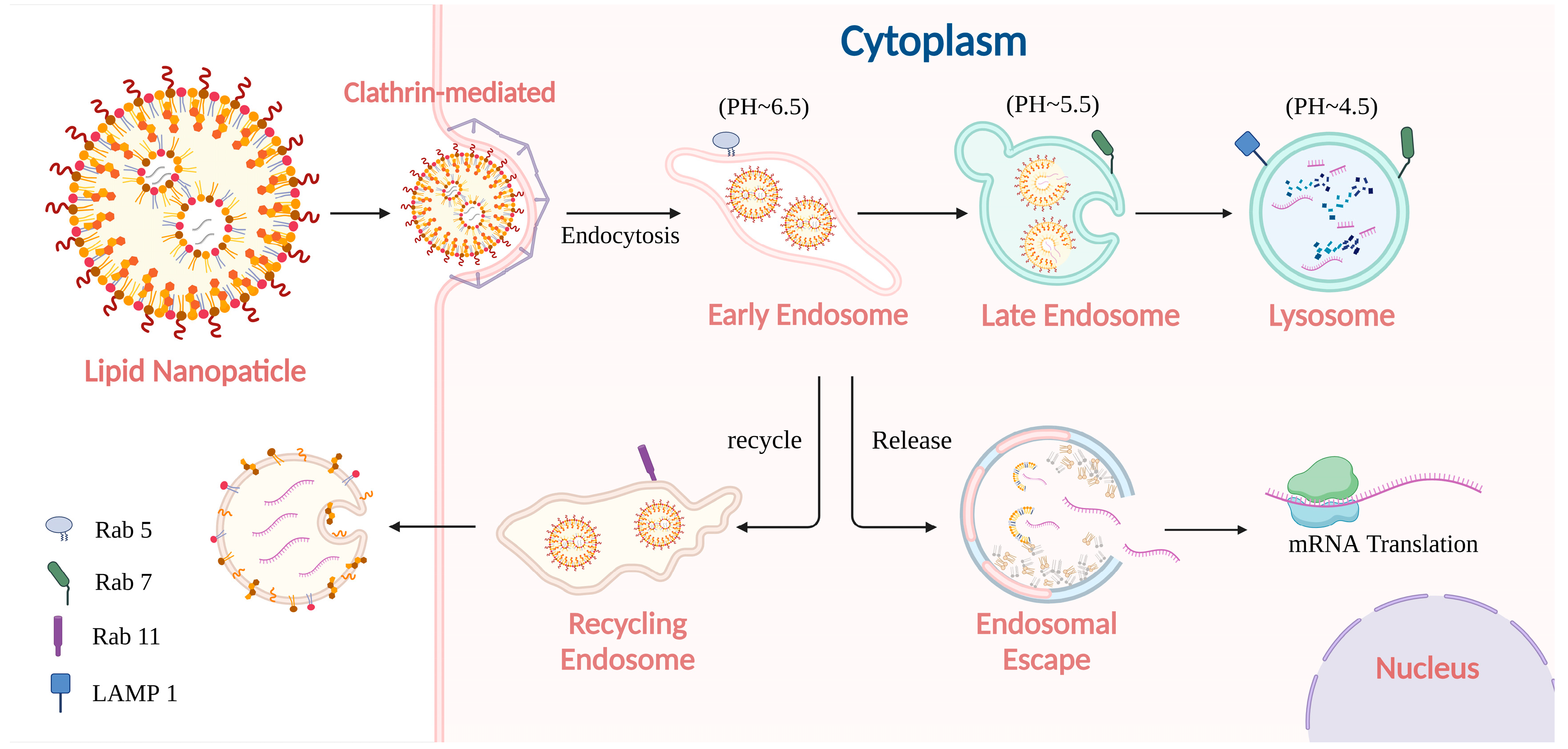
| Key Steps | Challenges | Strategies | References |
|---|---|---|---|
| Sorting of LNPs/mRNA within early endosomes. | A significant portion of internalized LNP/mRNA (~70% for siRNA) can be recycled back out of the cell via recycling endosomes, reducing the intracellular dose. | Inhibit or modulate endocytic recycling pathways: Block key proteins Target recycling machinery components Modulate retromer, SNX27, retriever, and WASH complexes. | [72,75,77,87,88] |
| Impairment of recycling pathways can disrupt cellular homeostasis. | Use interventions with caution, considering potential side effects on normal cellular functions. | [72] | |
| Release of mRNA from endosomes (early or Rab11-positive recycling endosomes) into the cytoplasm. | Low natural efficiency of endosomal escape for conventional LNPs. Most mRNA remains trapped and gets degraded. | Enhance LNP-endosomal membrane fusion: Utilize pH-responsive ionizable lipids (pKa ~6.2–6.5) that become cationic in acidic endosomes. Optimize LNP formulation (lipid composition, DSPC concentration). | [76,78,79,80,89,90,91,92,93,94] |
| Escape is influenced by endosomal properties (acidification, size, type). | Target escapes from favorable endosomal compartments Ensure endosomal acidification for ionizable lipid function. | [78] | |
| Potential release of mRNA from lysosomes into the cytoplasm. | Lysosomes exhibit substantial degradative functions. The induction of lysosomal membrane permeabilization (LMP) presents a potential risk of cytotoxicity and subsequent cell death. | Induce controlled LMP through photo-induced methods, photothermal techniques, and the proton sponge effect, such as those involving PEI and manganese. | [84,85,86,95,96,97,98,99,100] |
| Cellular mechanisms exist to repair lysosomal damage. | Formulate strategies that either minimally activate apoptotic pathways or utilize intrinsic cellular repair mechanisms. | [85,86] |
| Innovations | Mechanisms | Efficacy | References |
|---|---|---|---|
| Cationic amphiphilic lipids with systematically varied hydrophobic tail lengths, quantities, and degrees of unsaturation | Length of hydrophobic tails determines formation and stability; unsaturated hydrophobic tails enhance membrane fusion and fluidity. | Achieved efficient nucleic acid compaction, protection, and release; significantly influenced transgene expression. | [102] |
| Cationic lipid with an unsaturated citronellol tail (4A3-Cit) | Unique unsaturated structure promotes better disruption and fusion with endosomal membranes. | Demonstrated superior lipid fusion capabilities; enhanced fusion ability facilitates mRNA release from endosomes into the cytoplasm, improving overall efficacy. | [103] |
| Lipids with alkyne and ester groups incorporated into the DLin-MC3-DMAbackbone structure | Introduction of alkyne lipids significantly enhanced membrane fusion. | The release of mRNA is enhanced, leading to a synergistic improvement in the efficiency of mRNA delivery. | [106] |
| New identified ionizable lipid, U19 (from a series with two, three, and four tails featuring an imidazole head group) | Significant endo/lysosomal escape. | Significant endo/lysosomal escape, prolonged mRNA expression duration compared to ALC-0315. | [107] |
| Ionizable cationic lipids like DODMA | At low pH, DODMA interacts weakly with RNA, anchoring it near the lipid bilayer surface. As the pH increases, this interaction becomes repulsive, leading to the redistribution of RNA within the lipoplex. Additionally, DODMA enhances leaflet flipping at high pH to facilitate fusion with the endosomal membrane. | Alterations in pH facilitate endosomal escape, thereby enhancing mRNA delivery and therapeutic efficacy. | [108] |
| Esterase-responsive bivalent ionizable lipid LNPs | Designed to degrade rapidly in response to intracellular cues; rapid degradation of LNP in antigen-presenting cells, leading to efficient mRNA release. | Efficient mRNA release, robust antigen presentation; induced high magnitude of antigen-specific CD8+ T cells to infiltrate tumors and orchestrate innate and adaptive immunity to control tumor growth. | [109] |
| LNPs with systematically modified phospholipid component | Chemical nature of phospholipids can significantly influence mRNA delivery by promoting membrane fusion and facilitating endosomal escape; PE head groups are effective due to inherent fusogenic characteristics. | Significantly influence mRNA delivery, enhance endosomal escape. | [110] |
| LNPs incorporating bis (monoacylglycerol) phosphate (BMP) lipid | Enhanced endosomal membrane fusion. | More efficient delivery of mRNA to the cytosol. | [111] |
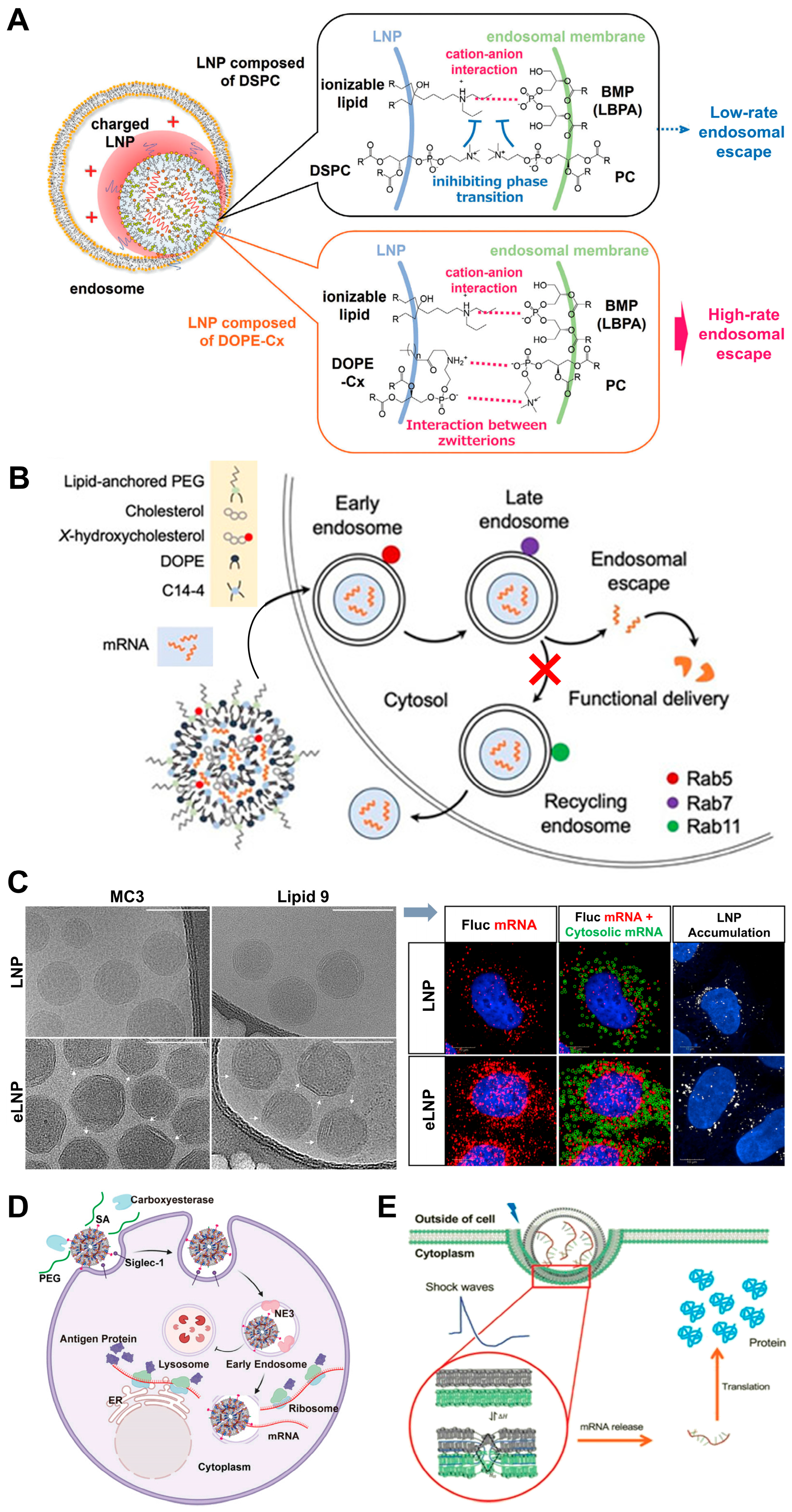
| Novel zwitterionic phospholipid (DOPE-Cx, e.g., DOPE-C8) by attaching hydrophobic moieties to the zwitterionic head group of DOPE | Alters orientation and interacts more effectively with phosphatidyl choline (PC); forms ion pairs with PC in the endosomal membrane to facilitate a phase transition from a lamellar phase to a non-lamellar cubic phase, promoting membrane fusion. | Enhances the efficiency of endosomal escape and improves the functional delivery of mRNA; DOPE-C8 readily induces cubic phases when mixed with PC. | [112] |
| NIR-II LNPs (incorporating Cy-lipid synthesized via nucleophilic substitution between a thiol lipid and NIR-II dye) | In acidic endosomes, protonated Cy-lipid activates NIR-II absorption, converting 1064 nm laser energy into heat, inducing morphological changes, disrupting the endosomal membrane, and enabling mRNA escape. | 3-fold increase in eGFP mRNA translation upon laser irradiation (cellular); 4.5-fold boost in liver luciferase mRNA translation efficiency (in-vivo); effectively enhances mRNA delivery and protein production. | [113] |
| LNPs containing 25% and 50% substitutions of 7α-hydroxycholesterol | Increased co-localization with acidic organelles (enhanced endosomal accumulation); reduces expression of Rab11, decreasing endosomal recycling and increasing the likelihood of endosomal escape by altering endosomal trafficking mechanisms. | Significantly enhances mRNA delivery to T cells. | [115] |
| LNPs with C-24 alkyl substitutions of cholesterol (e.g., β-sitosterol, LNP-Sito) | C-24 alkyl group introduces minor defects in the lipid bilayer organization, facilitating membrane destabilization and promoting fusion with endosomal membranes. LNP-Sito has polyhedral, faceted shapes, a more regular lamellar phase with tighter lipid/mRNA packing, and a high degree of multi-lamellarity (45% particles forming multiple lipid bilayers), allowing multiple fusion events. | Significantly outperformed traditional cholesterol-based LNPs in mRNA transfection, with up to 211-fold improvement in transfection efficiency; 10-fold increase in detectable late endosomal perturbation events; superior capability to escape from endosomal entrapment; enhanced gene delivery. | [77,116,117] |
| Cholesterol-free and corosolic acid-containing LNPs (CAxLNPs) | Great capacity for membrane fusion. | Significantly improved cellular uptake and endosomal escape; improved transfection efficiency. | [118] |
| SA@DOTAP-mRNA formulation (absence of cholesterol) | Absence of cholesterol allows for easier fusion of DOTAP molecules with the lysosomal membrane. | Enhanced escape efficiency. | [119] |
| LNPs containing DMG-PEG2k (with shorter acyl chain) | Faster shedding rate of DMG-PEG2k from the nanoparticle structure, facilitating quicker mRNA release and translation. | Superior endosomal escape compared to longer acyl chains (DSG-PEG2k and DSPE-PEG2k); higher transfection efficiency (in vivo); optimal transfection efficiency at 1.5 mol% PEGylated lipids. | [120] |
| Fluorinated modification of PEG-DSPE (FPD) in LNPs | FPD-LNPs escape from lysosomes and uniformly distribute in the cytoplasm within 4 h; accelerates the release of mRNA from endosomes. | Substantial improvement in mRNA expression efficiency in both tumor cells and primary DCs; increased efficiency of mRNA transfection and expression. | [121] |
| Acid-degradable PEG lipids (ADP-LNPs) synthesized with azido-acetal linker | Maintained high levels of PEGylation extracellularly (enhancing circulation/diffusion); rapid hydrolysis of PEG chains in endosomes upon endocytosis, promoting fusion with the endosomal membrane and facilitating endosomal escape. | Efficiently transfected cells with high PEG content (up to 40 mol%) where conventional LNPs were ineffective; enhanced endosomal escape confirmed. | [79] |
| Pardaxin-modified liposomes (P-Lipo) | Facilitates non-lysosomal delivery route, reducing lysosome degradation of cargos. | Reduced degradation of mRNA by lysosomes; significantly improved the transfection efficiency of mRNA in DCs compared with unmodified liposomes. | [122] |
| LNPs modified using coiled-coil lipopeptides | Formation of coiled-coil structures accelerates nucleic acid uptake; cellular uptake predominantly driven by membrane fusion, bypassing conventional endocytic pathways, leading to direct delivery into the cytosol. | Significantly enhanced mRNA expression; effectively circumvented the common issue of limited endosomal escape. | [123] |
| Sialic acid (SA)-modified mRNA loading LNPs | Promoted rapid uptake by DCs; enabled over 80% of the LNPs to escape from early endosomes within 2 h, avoiding entry into lysosomes (co-localization with lysosomes less than 10% vs. 40–60% for others). | Efficient endosomal escape crucial for successful mRNA translation, significantly enhancing target protein expression in DCs; superior antitumor efficacy compared to commercially formulated mRNA vaccines in tumor-bearing mouse models. | [124] |
| Sodium alginate (SA)-coated cationic mRNA LNPs (SA@DOTAP-mRNA) | Low co-localization rate with endo/lysosomes; neutralization of H+ ions by SA side chains allows uncharged hydrophobic side chains to insert into the hydrophobic part of the endosomal membrane, reducing endosomal stability and facilitating escape. | Efficient escape from the endosomal pathway; superior antitumor efficacy compared to conventional cationic liposome/mRNA complexes (tumor growth inhibition, established tumors regression). | [119] |
| Non-lamellar LNPs (e.g., bicontinuous cubic and inverse hexagonal structured LNPs, cuboplexes) | Enhanced fusogenicity with endosomal membranes; more readily fuse with endosomes. | Higher extent of endosomal escape, more efficient RNA delivery, and less endosomal entrapment compared to lamellar structured LNPs. | [125] |
| mRNA-loaded LNPs with disordered inverse hexagonal phase internal structure | Structure facilitates the release of mRNA from the LNP core, allowing it to diffuse out upon fusion with the endosomal membrane. | Optimizing size and surface characteristics can significantly enhance intracellular protein production; larger LNPs with a specific surface composition showed higher transfection efficacy. | [126] |
| LNP-mRNA (optimized by molar ratio of ionizable lipids to mRNA nucleotides) | A 1:1 molar ratio of ionizable lipids to mRNA nucleotides is optimal; at this ratio, the mRNA is neutrally charged by the ionizable lipids, facilitating its translocation across the negatively charged endosomal membrane. | Optimal for enabling mRNA to escape from the endosomal membrane into the cytoplasm. | [75] |
| mRNA-loaded LNPs combined with small molecules NAV2729 (NAV) and endosidin 5 (ES5) | NAV inhibits ARF6-dependent endocytic recycling; ES5 disrupts the interaction of Annexin A6 (ANXA6) with lipids; both facilitate mRNA release by targeting recycling endosomes without accelerating LNP uptake. | Markedly augment the delivery efficiency of mRNA by LNPs; potentiate mRNA-loaded LNPs delivery. | [127] |
| LNP-mediated mRNA delivery combined with leukotriene antagonists (e.g., MK-571) | Leukotriene antagonists modulate the mTOR signaling pathway. | Significantly boosted intracellular mRNA delivery both in vitro and in vivo. | [128] |
| Acoustically responsive fusogenic LNP | Features two phase transitions near physiological conditions to overcome the energy barrier for fusion; shock wave promotes phase transitions and fusion. | Enables efficient mRNA endosomal escape and protein expression; significantly increasing mRNA expression; enhanced delivery when transfection occurred in the presence of acoustic shock waves. | [129] |
| LNP-mediated mRNA delivery enhanced by mechanical oscillation | Mechanical oscillation at a frequency of 65 Hz significantly enhances the endosomal escape by promoting the fusion of oppositely charged LNPs; enhanced fusion facilitates the release of mRNA from endosomes into the cytoplasm. | Significantly enhanced endosomal escape; improved transfection efficiency; does not compromise cell viability or cause significant damage to cellular structures. | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, R.; Xie, Y.; Qin, X.; Zhou, Y.; Xu, C. Endo/Lysosomal-Escapable Lipid Nanoparticle Platforms for Enhancing mRNA Delivery in Cancer Therapy. Pharmaceutics 2025, 17, 803. https://doi.org/10.3390/pharmaceutics17070803
Wang J, Chen R, Xie Y, Qin X, Zhou Y, Xu C. Endo/Lysosomal-Escapable Lipid Nanoparticle Platforms for Enhancing mRNA Delivery in Cancer Therapy. Pharmaceutics. 2025; 17(7):803. https://doi.org/10.3390/pharmaceutics17070803
Chicago/Turabian StyleWang, Jiapeng, Renjie Chen, Yongyi Xie, Xuanting Qin, You Zhou, and Chuanshan Xu. 2025. "Endo/Lysosomal-Escapable Lipid Nanoparticle Platforms for Enhancing mRNA Delivery in Cancer Therapy" Pharmaceutics 17, no. 7: 803. https://doi.org/10.3390/pharmaceutics17070803
APA StyleWang, J., Chen, R., Xie, Y., Qin, X., Zhou, Y., & Xu, C. (2025). Endo/Lysosomal-Escapable Lipid Nanoparticle Platforms for Enhancing mRNA Delivery in Cancer Therapy. Pharmaceutics, 17(7), 803. https://doi.org/10.3390/pharmaceutics17070803






