Development and Optimization of Grape Skin Extract-Loaded Gelatin–Alginate Hydrogels: Assessment of Antioxidant and Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Skin Extract Preparation
2.2. Determination of Total Phenolic Content in Grape Skin Extract
2.3. In Vitro Antioxidant Potential Assessment of Grape Skin Extract
2.3.1. DPPH Radical Scavenging Assay
2.3.2. ABTS Radical Cation Decolorization Assay
2.3.3. Ferric Reducing Antioxidant Power Assay
2.4. HPLC Analysis of Grape Skin Extract
2.5. Synthesis of Hydrogels
2.6. Hydrogel Characterization
2.6.1. Equilibrium Swelling Degree
2.6.2. Scanning Electron Microscopy
2.6.3. Mechanical Properties Evaluation
2.6.4. FTIR Analysis
2.6.5. In Vitro Extract Release Study
2.6.6. Analysis of the Kinetics of Drug Release
2.7. In Vitro Biocompatibility Testing of Hydrogels Based on Sustainable Grape Skin Extract
2.7.1. Cell Culture
2.7.2. Hydrogel Extract Preparation
2.7.3. Extract Cytotoxicity Test
2.7.4. Direct Cytotoxicity Test
2.7.5. MTT Assay
2.7.6. Confluency and Morphology Analysis
2.7.7. Cell Viability Staining
2.8. In Vitro Antimicrobial Testing of Hydrogels Loaded with Sustainable Grape Skin Extract
2.8.1. Direct Contact Antimicrobial Test
2.8.2. Live/Dead Staining
2.9. Antioxidant Properties of Hydrogels Loaded with Sustainable Grape Skin Extract
2.9.1. Sample Preparation
2.9.2. TPC
2.9.3. DPPH Radical Scavenging Assay
2.9.4. ABTS Radical Cation Decolorization Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield, Total Phenolic Content, and Antioxidant Activity of Grape Skin Extract
3.2. HPLC Analysis of Grape Skin Extract
3.3. Physico-Chemical Characterization of Hydrogels Loaded with Sustainable Grape Skin Extract
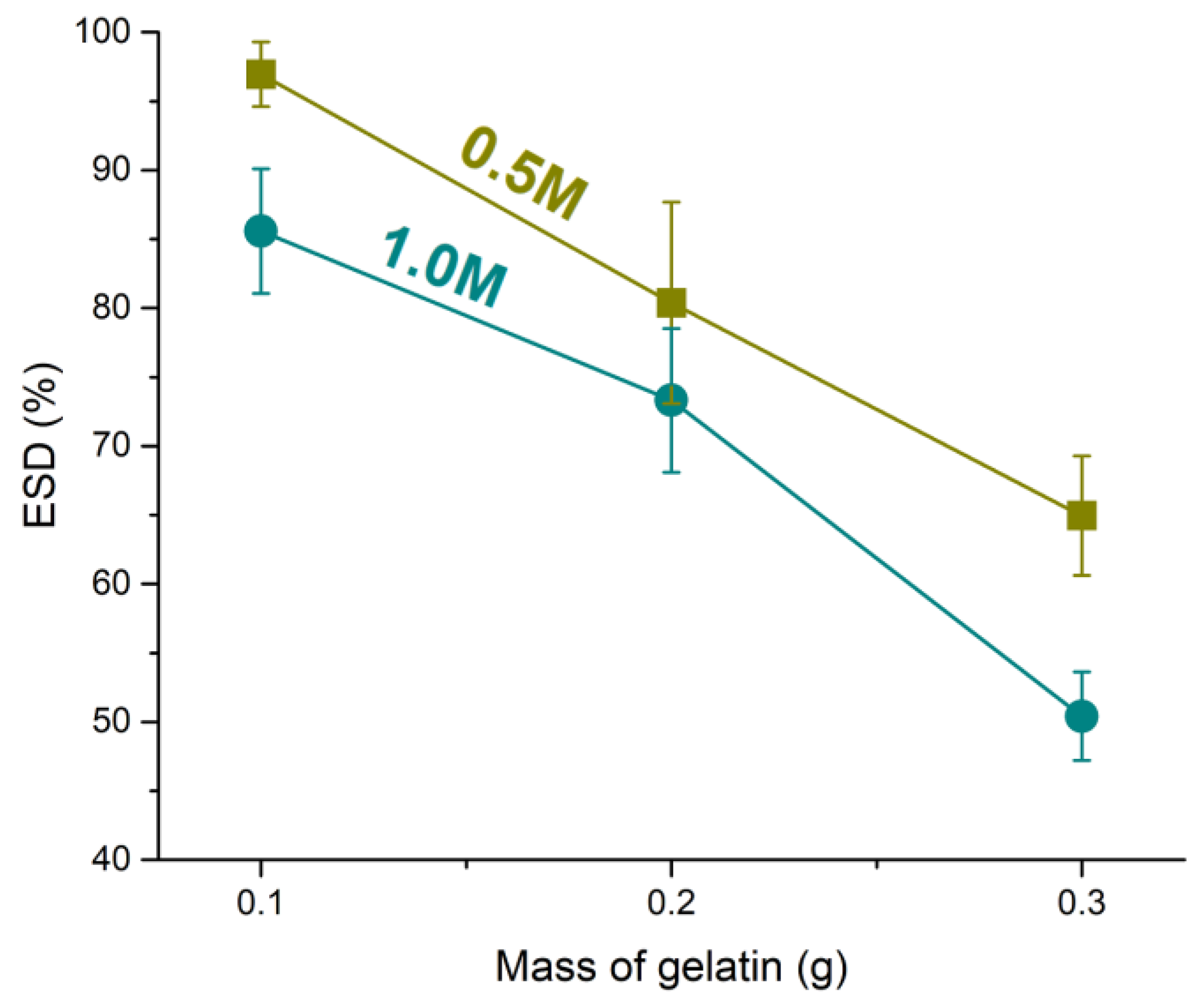
3.3.1. Swelling Properties
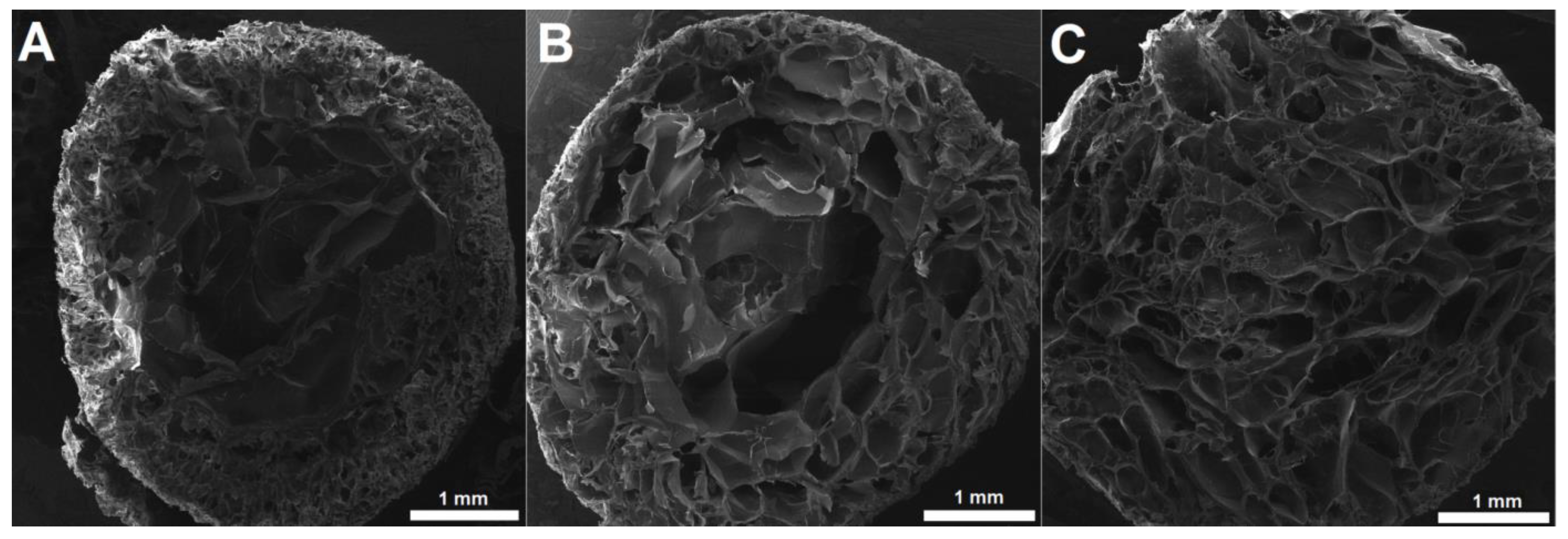
3.3.2. Microstructure
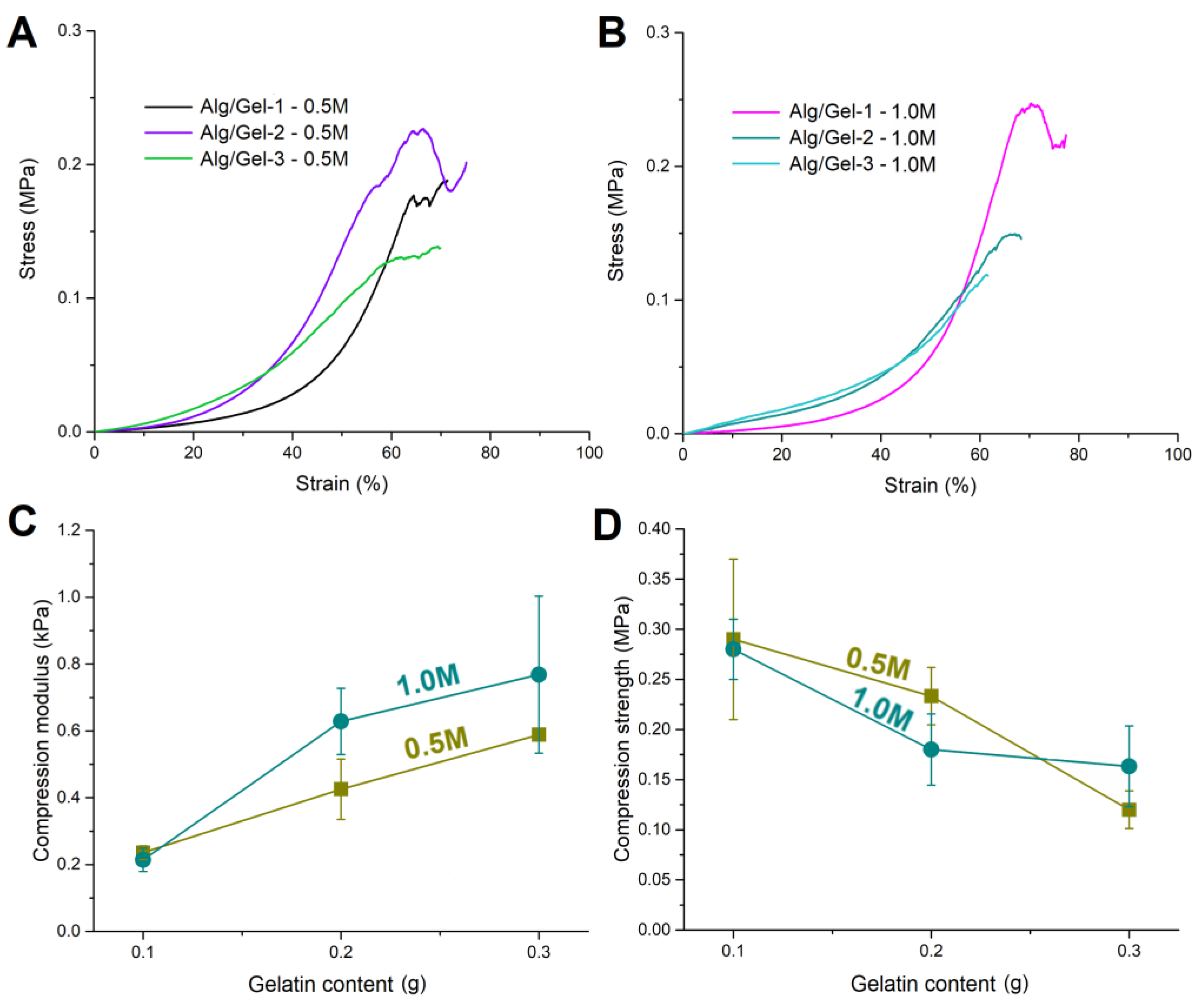
3.3.3. Mechanical Properties
3.3.4. FTIR Analysis
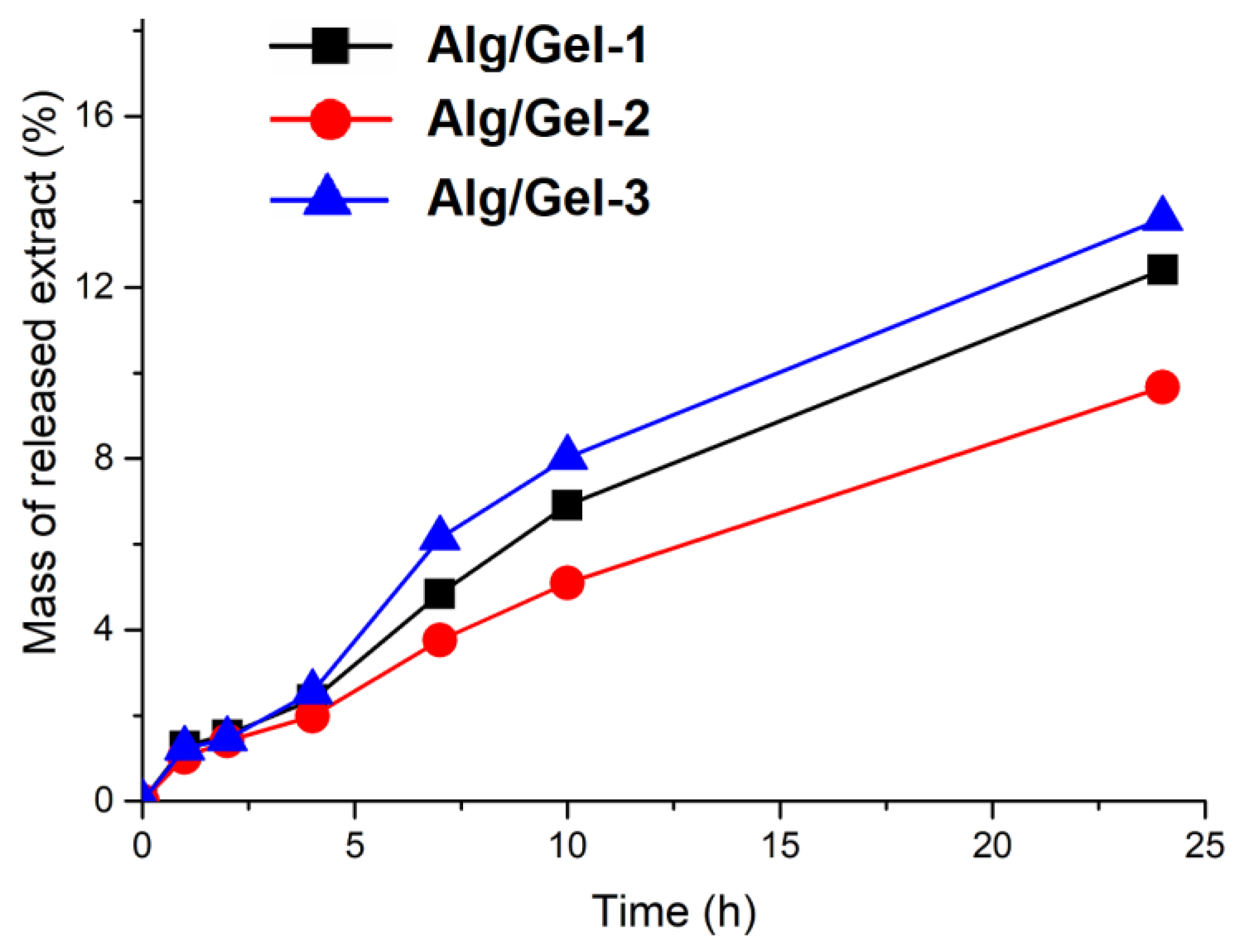
3.3.5. In Vitro Extract Release from Hydrogels
3.3.6. Analysis of the Kinetics of Drug Release
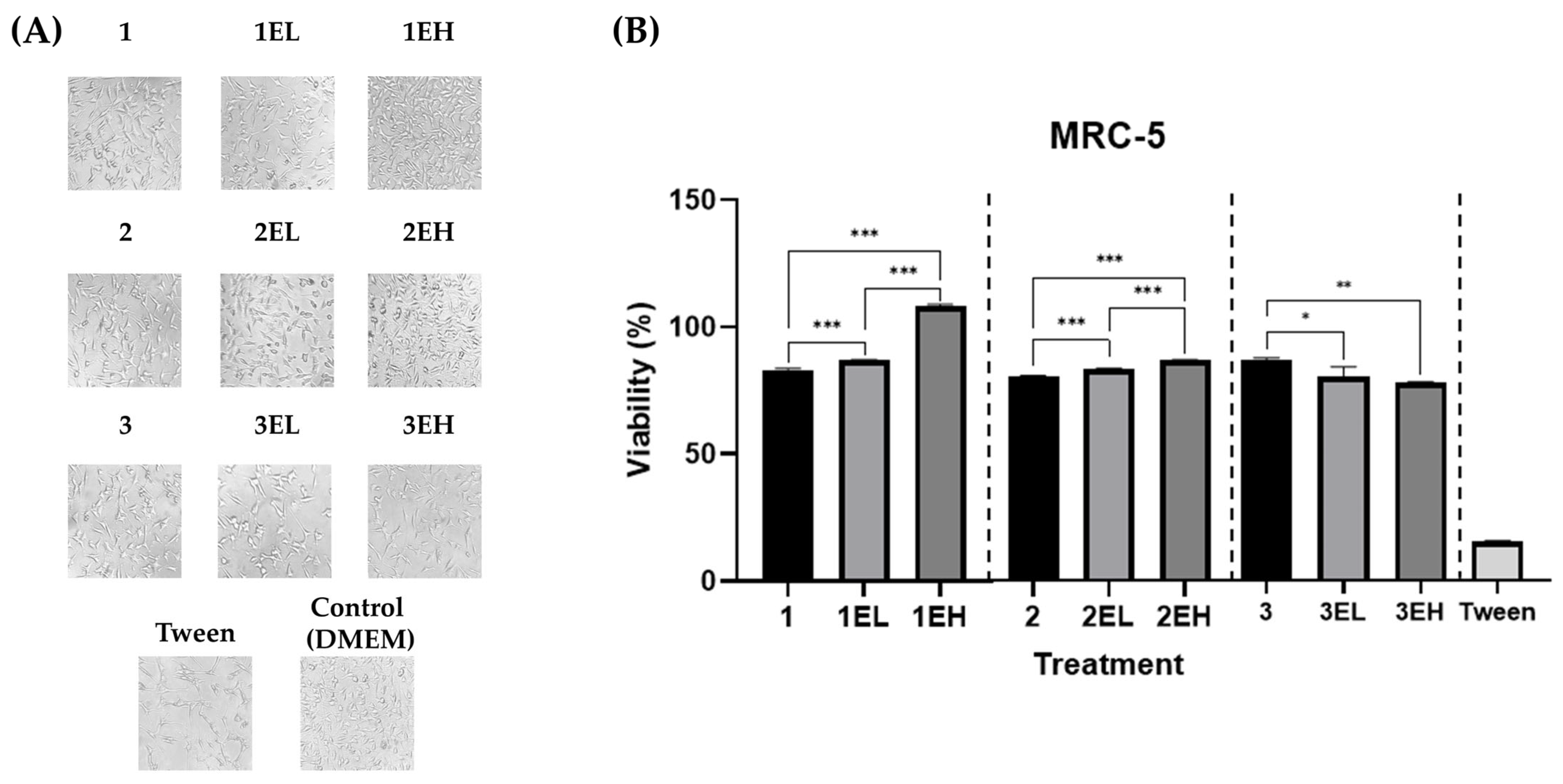
3.4. Biocompatibility of Hydrogels Loaded with Sustainable Grape Skin Extract
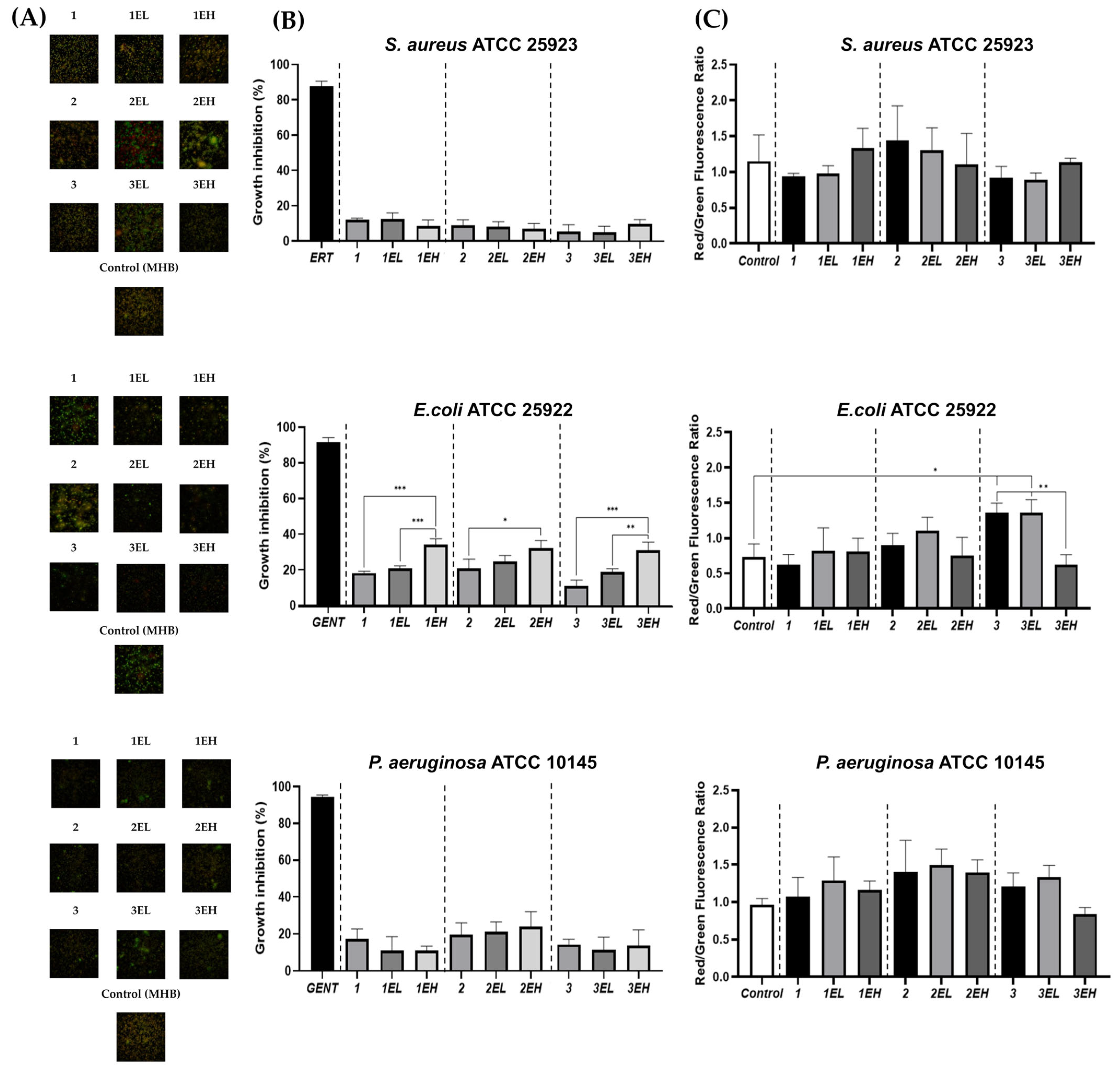
3.5. Antimicrobial Activity of Hydrogels Loaded with Sustainable Grape Skin Extract
3.6. Antioxidant Activity of Hydrogels Loaded with Sustainable Grape Skin Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Zhang, X.; Wang, Y.; Chen, W.; Zhu, Z.; Wang, S. Hydrogels and Hydrogel-Based Drug Delivery Systems for Promoting Refractory Wound Healing: Applications and Prospects. Int. J. Biol. Macromol. 2025, 285, 138098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B. Hydrogels for wound dressings: Applications in burn treatment and skin regeneration. Biomolecules 2023, 13, 133. [Google Scholar] [CrossRef]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.-B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Maikovych, O.; Pasetto, P.; Nosova, N.; Kudina, O.; Ostapiv, D.; Samaryk, V.; Varvarenko, S. Functional Properties of Gelatin–Alginate Hydrogels for Use in Chronic Wound Healing Applications. Gels 2025, 11, 174. [Google Scholar] [CrossRef]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C 2020, 113, 110957. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.; Yu, C.; Huang, X.; Zhang, X. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 129, 109609. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-printed gelatin-alginate hydrogel dressings for burn wound healing: A comprehensive study. Int. J. Bioprint. 2022, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yu, B.; Zhu, Z.; Yang, Z.; Shao, W. Synthesis of antibacterial gelatin/sodium alginate sponges and their antibacterial activity. Polymers 2020, 12, 1926. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.D.; Madureira, J.; Margaça, F.M.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Cebova, M.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Nedomova, S.; Baron, M.; Sochor, J. Health effects of grape seed and skin extracts and their influence on biochemical markers. Molecules 2020, 25, 5311. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S.; et al. Antioxidant and wound healing potential of Vitis vinifera seeds supported by phytochemical characterization and docking studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Avdović, E.; Antonijević, M.; Simijonović, D.; Grujović, M.; Marković, K.; Milenković, D.; Ćirić, A.; Marković, Z. Ultrasound-assisted extraction of bioactive phenolics from Marselan and Shiraz grape skins: A step toward circular economy in food and pharmaceutical industry. LWT 2025, 211, 117817. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Niño-Medina, G.; Martínez-Ávila, G.C. Phenolic compounds recovery from grape fruit and by-products: An overview of extraction methods. Grape Wine Biotechnol. 2016, 5, 104–123. [Google Scholar] [CrossRef]
- Marcellin-Gros, R.; Hévin, S.; Chevalley, C.; Boccard, J.; Hofstetter, V.; Gindro, K.; Wolfender, J.-L.; Kehrli, P. An advanced metabolomic approach on grape skins untangles cultivar preferences by Drosophila suzukii for oviposition. Front. Plant Sci. 2024, 15, 1435943. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 107–115. [Google Scholar]
- Irshad, A.; Jawad, R.; Mushtaq, Q.; Spalletta, A.; Martin, P.; Ishtiaq, U. Determination of antibacterial and antioxidant potential of organic crude extracts from Malus domestica, Cinnamomum verum and Trachyspermum ammi. Sci. Rep. 2025, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Antonijević, M.R.; Simijonović, D.M.; Avdović, E.H.; Ćirić, A.; Petrović, Z.D.; Marković, J.D.; Stepanić, V.; Marković, Z.S. Green one-pot synthesis of coumarin-hydroxybenzohydrazide hybrids and their antioxidant potency. Antioxidants 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Mavric-Scholze, E.; Simijonović, D.; Avdović, E.; Milenković, D.; Šaćirović, S.; Ćirić, A.; Marković, Z. Comparative analysis of antioxidant activity and content of (poly)phenolic compounds in Cabernet Sauvignon and Merlot wines of Slovenian and Serbian vineyards. Food Chem. X 2024, 25, 102108. [Google Scholar] [CrossRef] [PubMed]
- Karaman Ersoy, Ş.; Vural, T.; Sözgen Başkan, K.; Tütem, E. Comparison of total antioxidant capacities and phenolic constituents of grapes cultivated in Turkey for wine production. Anal. Lett. 2024, 58, 1464–1478. [Google Scholar] [CrossRef]
- Pilipenko, N.; Gonçalves, O.H.; Bona, E.; Fernandes, I.P.; Pinto, J.A.; Sorita, G.D.; Leimann, F.V.; Barreiro, M.F. Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 2019, 223, 115035. [Google Scholar] [CrossRef] [PubMed]
- Rayyan, M.; Terkawi, T.; Abdo, H.; Abdel Azim, D.; Khalaf, A.; AlKhouli, Z.; Meziad, M.; Alshamma’a, M.; Abu Naim, H. Efficacy of grape seed extract gel in the treatment of chronic periodontitis: A randomized clinical study. J. Investig. Clin. Dent. 2018, 9, e12318. [Google Scholar] [CrossRef]
- Kawarkhe, P.R.; Deshmane, S.V.; Biyani, K.R. Formulation and evaluation of antioxidant face cream containing raspberry fruit and grape seeds extract. Res. J. Top. Cosmet. Sci. 2016, 7, 73–78. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Ugrinovic, V.; Markovic, M.; Bozic, B.; Panic, V.; Veljovic, D. Physically crosslinked poly(methacrylic acid)/gelatin hydrogels with excellent fatigue resistance and shape memory properties. Gels 2024, 10, 444. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices. Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Baskić, D.; Popović, S.; Ristić, P.; Arsenijević, N.N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006, 30, 924–932. [Google Scholar] [CrossRef]
- Lazovic, A.; Markovic, B.S.; Corovic, I.; Markovic, T.; Andjelkovic, M.; Stojanovic, B.; Jovanovic, I.; Mitrovic, M. Unlocking the molecular mechanisms of anticancer and immunomodulatory potentials of cariprazine in triple negative breast cancer. Biomed. Pharmacother. 2025, 184, 117931. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Saranya, S.; Mukherjee, A.; Chandrasekaran, N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: Investigation of its bactericidal activity. J. Nanosci. Nanotechnol. 2013, 13, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Acharya, K. Simplified methods for microtiter based analysis of in vitro antioxidant activity. Asian J. Pharm. 2017, 11, 327–335. [Google Scholar]
- Ramírez-García, O.; Salinas-Moreno, Y.; Santillán-Fernández, A.; Sumaya-Martínez, M.T. Screening antioxidant capacity of Mexican maize (Zea mays L.) landraces with colored grain using ABTS, DPPH and FRAP methods. Cereal Res. Commun. 2022, 50, 1075–1083. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Naiker, M. Microplate methods for measuring phenolic content and antioxidant capacity in chickpea: Impact of shaking. Eng. Proc. 2023, 48, 57. [Google Scholar]
- Bradic, J.; Petrovic, A.; Nikolic, M.; Nedeljkovic, N.; Andjic, M.; Baljak, J.; Jakovljevic, V.; Kocovic, A.; Tadic, V.; Stojanovic, A.; et al. Potentilla tormentilla extract loaded gel: Formulation, in vivo and in silico evaluation of anti-inflammatory properties. Int. J. Mol. Sci. 2024, 25, 9389. [Google Scholar] [CrossRef]
- Locatelli, C.; Filippin-Monteiro, F.B.; Centa, A.; Creczinsky-Pasa, T.B. Antioxidant, antitumoral and anti-inflammatory activities of gallic acid. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications, 4th ed.; Nova Publishers: New York, NY, USA, 2013; pp. 1–23. [Google Scholar]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, G.; Witczak, M.; Kuterasiński, Ł. Structure effects on swelling properties of hydrogels based on sodium alginate and acrylic polymers. Molecules 2024, 29, 1937. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Bartniak, M.; Waśko, J.; Kolesińska, B.; Grabarczyk, J.; Bociaga, D. The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study. Gels 2024, 10, 491. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A review on the adaption of alginate-gelatin hydrogels for 3D cultures and bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.; Baldy-Chudzik, K.; Mazurek-Popczyk, J.; Zaręba, Ł.; Klekiel, T.; Będziński, R. Changes in the mechanical properties of alginate-gelatin hydrogels with the addition of Pygeum africanum with potential application in urology. Int. J. Mol. Sci. 2022, 23, 10324. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Kaźmierski, Ł.; Bartniak, M.; Bajek, A. Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink. Polymers 2024, 16, 2560. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-based wound dressing materials loaded with bioactive agents: Potential materials for the treatment of diabetic wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.; Song, W.; Yu, B.; Liu, H. Rational design in functional hydrogels towards biotherapeutics. Mater. Des. 2022, 223, 111086. [Google Scholar] [CrossRef]
- Hu, Q.; Nie, Y.; Xiang, J.; Xie, J.; Si, H.; Li, D.; Zhang, S.; Li, M.; Huang, S. Injectable sodium alginate hydrogel loaded with plant polyphenol-functionalized silver nanoparticles for bacteria-infected wound healing. Int. J. Biol. Macromol. 2023, 234, 123691. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Jiang, F.; Li, Q.; McDowell, A.; Li, Y.; Wang, Y.; Liu, S.; Zhang, C.; Pan, X. Multifunctional polysaccharide/metal/polyphenol double-crosslinked hydrogel for infected wound. Carbohydr. Polym. 2024, 332, 121912. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A promising avenue in therapeutic solutions for wound care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Karimi Hajishoreh, N.; Dadashpour, M.; Akbarzadeh, A. Preparation and in vitro evaluation of biological agents based on Zinc-laponite-curcumin incorporated in alginate hydrogel. J. Biol. Eng. 2023, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Lok, B.; Adam, M.A.; Kamal, L.Z.M.; Chukwudi, N.A.; Sandai, R.; Sandai, D. The assimilation of different carbon sources in Candida albicans: Fitness and pathogenicity. Med. Mycol. 2021, 59, 115–125. [Google Scholar] [CrossRef]
- Silao, F.G.S.; Ljungdahl, P.O. Amino acid sensing and assimilation by the fungal pathogen Candida albicans in the human host. Pathogens 2022, 11, 5. [Google Scholar] [CrossRef]
- Alves, C.T.; Ferreira, I.C.; Barros, L.; Silva, S.; Azeredo, J.; Henriques, M. Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 2014, 9, 139–146. [Google Scholar] [CrossRef]
- Martins-Santana, L.; Rezende, C.P.; Rossi, A.; Martinez-Rossi, N.M.; Almeida, F. Addressing Microbial Resistance Worldwide: Challenges over Controlling Life-Threatening Fungal Infections. Pathogens 2023, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.G.; Castillo, A.; Villarino, R.A.; Rama, J.L.; Abril, A.G.; De Miguel, T. Study of the antibacterial activity of rich polyphenolic extracts obtained from Cytisus scoparius against foodborne pathogens. Antibiotics 2023, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, Y.; Chen, H.; Su, Y.; Cheng, Y.; Xu, J.; Sun, H.; Song, K. A 3D bioprinted antibacterial hydrogel dressing of gelatin/sodium alginate loaded with ciprofloxacin hydrochloride. Biotechnol. J. 2024, 19, e2400209. [Google Scholar] [CrossRef] [PubMed]
- Andjic, M.; Bradic, J.; Kocovic, A.; Simic, M.; Krstonosic, V.; Capo, I.; Jakovljevic, V.; Lazarevic, N. Immortelle essential-oil-enriched hydrogel for diabetic wound repair: Development, characterization, and in vivo efficacy assessment. Pharmaceutics 2024, 16, 1309. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ouyang, Y.; Zhao, T.; Wang, Z.; Yan, Q.; Qian, Q.; Wang, W.; Wang, S. Antioxidant hydrogels: Antioxidant mechanisms, design strategies, and applications in the treatment of oxidative stress-related diseases. Adv. Healthc. Mater. 2024, 13, e2303817. [Google Scholar] [CrossRef]


| Formulations | Alg/Gel-1 | Alg/Gel-2 | Alg/Gel-3 |
|---|---|---|---|
| Alginate | 0.3 g | 0.3 g | 0.3 g |
| Gelatin | 0.1 g | 0.2 g | 0.3 g |
| Investigated Extract and Standards | Yield (%) | DPPH SC50 (µg/mL) | ABTS SC50 (µg/mL) | FRAP (A700nm) | TPC (mg GAE/g E) |
|---|---|---|---|---|---|
| E | 7.8 | 34.0 ± 0.6 | 17.0 ± 0.6 | 0.1864 ± 0.0049 | 7.9 ± 0.2 |
| NDGA | / | 0.5 ± 0.1 | ND | ND | / |
| Quercetin | / | 1.9 ± 0.1 | ND | ND | / |
| Ascorbic acid | / | 18.0 ± 0.1 | 31.1 ± 0.1 | 0.1249 ± 0.0022 | / |
| Sample | k | n | R2 |
|---|---|---|---|
| Alg/Gel-1 | 1.02 | 0.79 | 0.99 |
| Alg/Gel-2 | 0.81 | 0.78 | 0.99 |
| Alg/Gel-3 | 1.24 | 0.76 | 0.98 |
| InvestigatedHydrogels and Standards | DPPHSC50 (µg/mL) | ABTSSC50 (µg/mL) | TPC(µg GAE/g E) |
|---|---|---|---|
| 1 | 332.16 ± 33.49 a | 298.70 ± 29.93 a | 4.41 ± 0.01 |
| 1EL | 279.16 ± 29.09 a | 279.05 ± 26.33 a | 108.45 ± 13.35 * |
| 1EH | 200.71 ± 21.93 a*# | 169.86 ± 19.91a*# | 233.01 ± 23.24 *# |
| 2 | 341.73 ± 32.55 a | 297.99 ± 28.04 a | 4.46 ± 0.01 |
| 2EL | 281.46 ± 29.46 a | 249.47 ± 23.77 a | 115.10 ± 10.12 * |
| 2EH | 201.13 ± 21.25 a*# | 168.47 ± 18.80 a*# | 234.29 ± 20.16 *# |
| 3 | 348.91 ± 33.73 a | 296.89 ± 28.36 a | 4.24 ± 0.019 |
| 3EL | 288.65 ± 29.77 a | 223.67 ± 26.72 a | 117.02 ± 12.93 * |
| 3EH | 215.18 ± 21.78 a*# | 153.99 ± 19.09 a*# | 243.12 ± 21.90 *# |
| Trolox | 5.03 ± 0.32 | 6.90 ± 0.69 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradic, J.; Petrovic, A.; Kocovic, A.; Ugrinovic, V.; Popovic, S.; Ciric, A.; Markovic, Z.; Avdovic, E. Development and Optimization of Grape Skin Extract-Loaded Gelatin–Alginate Hydrogels: Assessment of Antioxidant and Antimicrobial Properties. Pharmaceutics 2025, 17, 790. https://doi.org/10.3390/pharmaceutics17060790
Bradic J, Petrovic A, Kocovic A, Ugrinovic V, Popovic S, Ciric A, Markovic Z, Avdovic E. Development and Optimization of Grape Skin Extract-Loaded Gelatin–Alginate Hydrogels: Assessment of Antioxidant and Antimicrobial Properties. Pharmaceutics. 2025; 17(6):790. https://doi.org/10.3390/pharmaceutics17060790
Chicago/Turabian StyleBradic, Jovana, Anica Petrovic, Aleksandar Kocovic, Vukasin Ugrinovic, Suzana Popovic, Andrija Ciric, Zoran Markovic, and Edina Avdovic. 2025. "Development and Optimization of Grape Skin Extract-Loaded Gelatin–Alginate Hydrogels: Assessment of Antioxidant and Antimicrobial Properties" Pharmaceutics 17, no. 6: 790. https://doi.org/10.3390/pharmaceutics17060790
APA StyleBradic, J., Petrovic, A., Kocovic, A., Ugrinovic, V., Popovic, S., Ciric, A., Markovic, Z., & Avdovic, E. (2025). Development and Optimization of Grape Skin Extract-Loaded Gelatin–Alginate Hydrogels: Assessment of Antioxidant and Antimicrobial Properties. Pharmaceutics, 17(6), 790. https://doi.org/10.3390/pharmaceutics17060790







