Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation for Physicochemical Characterization
2.3. Synthetic Procedures
2.3.1. Synthesis of the Glycolipid
2.3.2. Preparation of the GlycoZIF and ZIF Control Particles
2.4. In Vitro and Ex Vivo Protocols
2.4.1. Cell Cultures
2.4.2. Cell Viability
2.4.3. Cell Internalization of GlycoZIF and ZIF Particles
2.4.4. Analysis of Nitrites (NO2−)
2.4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.4.6. Western Blot Analysis
2.4.7. Retinal Explant Cultures
2.4.8. Retinal Explants Immunofluorescence Analysis
2.4.9. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of GlycoZIF Nanoparticles
3.2. Cytotoxicity and Cellular Uptake of GlycoZIF Nanoparticles
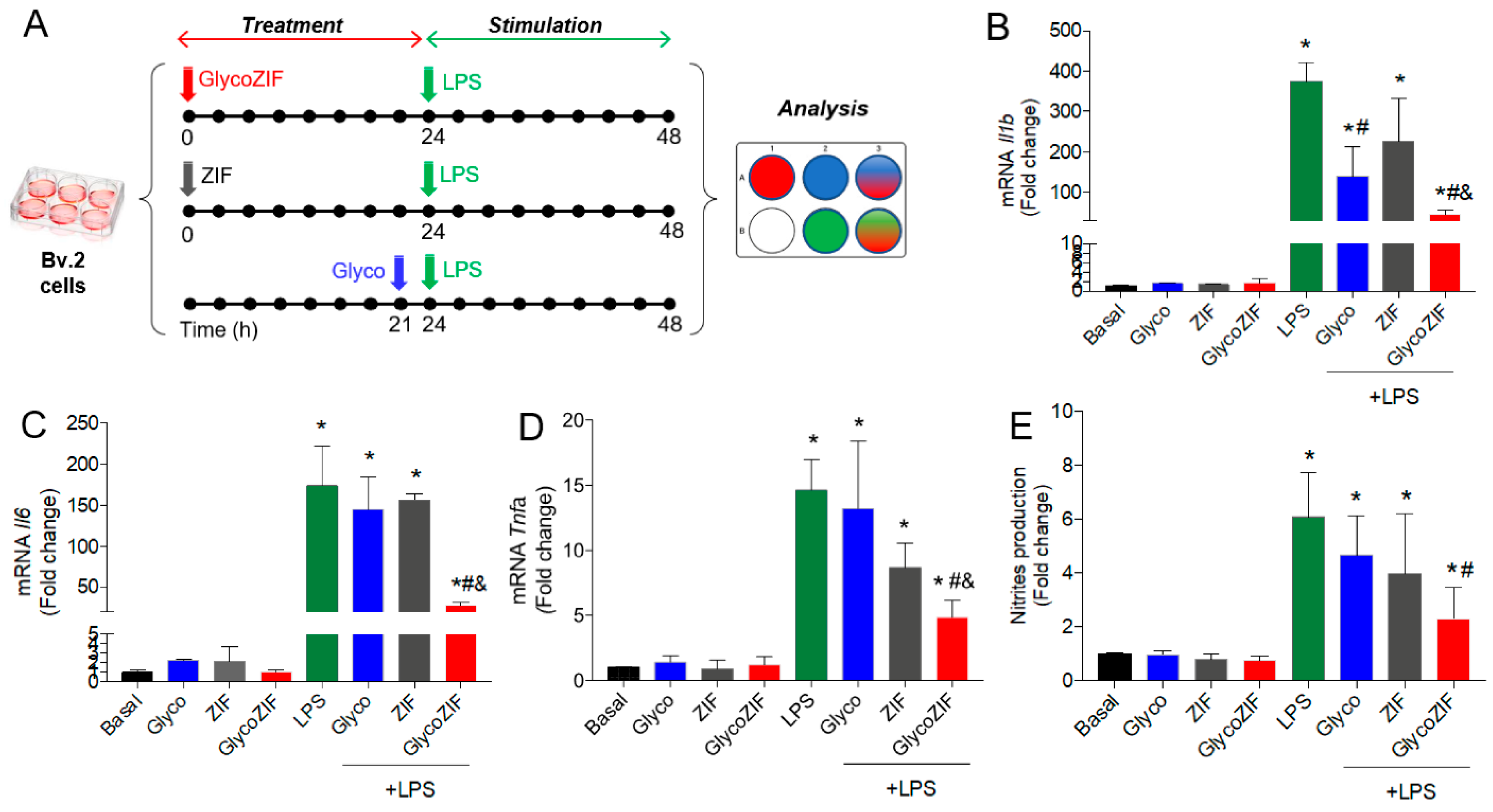
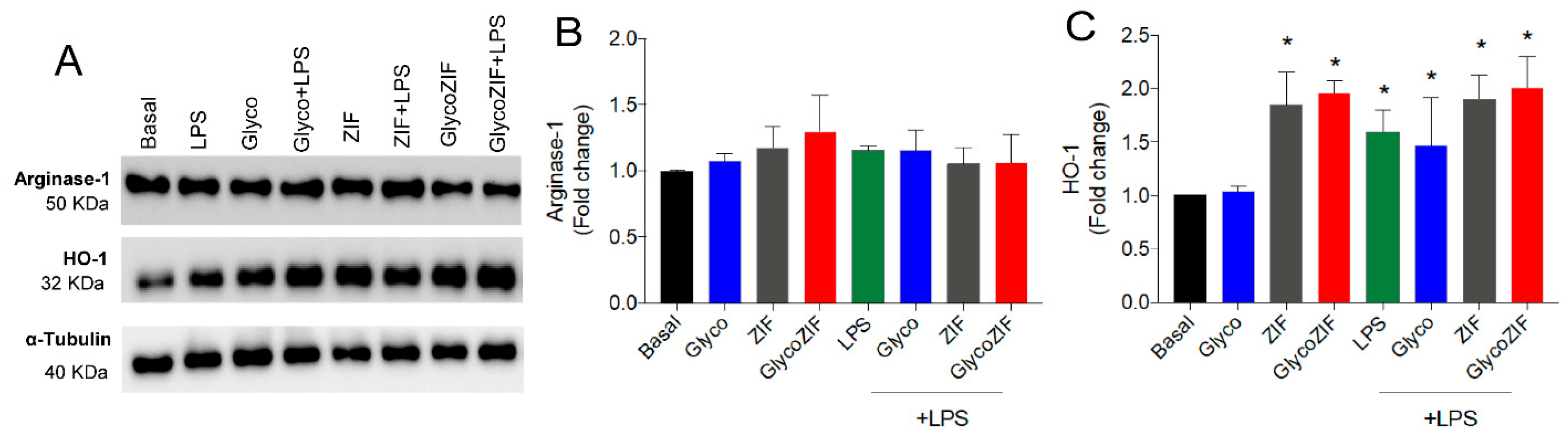
3.3. Protective Effect of GlycoZIF Against LPS-Induced Proinflammatory Mediators on Bv.2 Microglial Cells
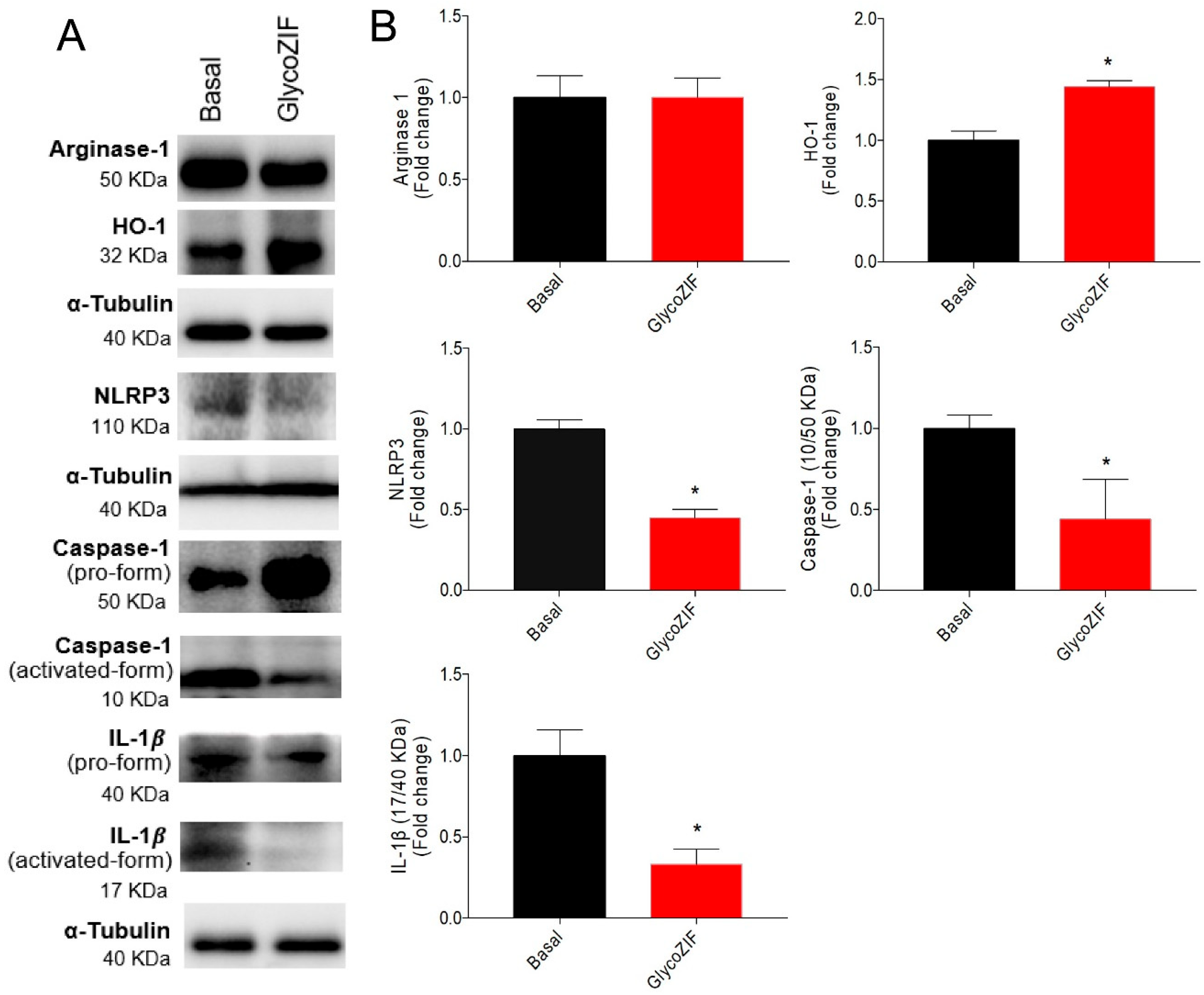
3.4. GlycoZIF Regulates NLRP3 Inflammasome Complex Activity
3.5. GlycoZIF Modulates Neuroinflammation in Retinal Explants from BB Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARVO | Association for research in vision and ophthalmology |
| BB | Bio-Breeding |
| DDS | Drug delivery system |
| dh | Hydrodynamic diameter |
| DM | Diabetes mellitus |
| DR | Diabetic retinopathy |
| DSO2-ONJ | (1R)-1-Dodecylsulfonyl-5N,6O-oxomethylidenenojirimycin |
| EE | Encapsulation efficiency |
| FA@GlycoZIF | GlycoZIF labeled with fluoresceinamine |
| FA@ZIF | Control ZIF labeled |
| FA | Fluorescent cargo |
| FDA | Food and Drug Administration |
| FELASA | Federation of European Laboratory Animal Science Associations |
| GFAP | Glial fibrillary acidic protein |
| HLPC | High-performance liquid chromatography |
| HmIM | 2-Methylimidazole |
| HO-1 | Heme-oxygenase-1 |
| IBA-1+ | Ionized calcium-binding adaptor molecule-1 |
| LC | Loading capacity |
| MAPK | Mitogen-activated protein kinase |
| MeOH | Methanol |
| MFI | Mean fluorescence intensity per cell |
| MOFs | Metal–Organic Frameworks |
| NJ | Nojirimycin |
| ROS | Reactive oxygen species |
| sp2-IGLs | sp2-Iminoglycolipids |
| T1DM | Type 1 DM |
| Th1 | T-helper 1 |
| T-PBS | Tween-20-PBS |
| VEGF | Vascular endothelial growth factor |
| WT | Wild type |
| ZIF | Zeolitic-Imidazolate Framework |
| ZIF-8 | Zeolitic Imidazolate Framework-8 |
References
- Zhang, Y.; Zhou, A. Macrophage activation contributes to diabetic retinopathy. J. Mol. Med. 2024, 102, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, G.T.; Zhang, J.F. Inflammation in diabetic retinopathy: Possible roles in pathogenesis and potential implications for therapy. Neural Regen. Res. 2023, 18, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The role of inflammation in immune system of diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Front. Immunol. 2022, 13, 1055087. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; García-Moreno, M.I.; García Fernández, J.M.; Ortiz Mellet, C. sp2-Iminosugars as chemical mimics for glycodrug design. In Small Molecule Drug Discovery: Methods, Molecules and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–224. [Google Scholar] [CrossRef]
- Padilla-Pérez, M.C.; Sánchez-Fernández, E.M.; González-Bakker, A.; Puerta, A.; Padrón, J.M.; Martín-Loro, F.; Arroba, A.I.; García Fernández, J.M.; Ortiz Mellet, C. Fluoro-labelled sp2-iminoglycolipids with immunomodulatory properties. Eur. J. Med. Chem. 2023, 255, 115390. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; García-Hernández, R.; Gamarro, F.; Arroba, A.I.; Aguilar-Diosdado, M.; Padrón, J.M.; García Fernández, J.M.; Ortiz Mellet, C. Synthesis of sp2-Iminosugar Selenoglycolipids as Multitarget Drug Candidates with Antiproliferative, Leishmanicidal and Anti-Inflammatory Properties. Molecules 2021, 26, 7501. [Google Scholar] [CrossRef]
- Cano-Cano, F.; Alcalde-Estévez, E.; Gómez-Jaramillo, L.; Iturregui, M.; Sánchez-Fernández, E.M.; García Fernández, J.M.; Ortiz Mellet, C.; Campos-Caro, A.; López-Tinoco, C.; Aguilar-Diosdado, M.; et al. Anti-Inflammatory (M2) Response Is Induced by a sp2-Iminosugar Glycolipid Sulfoxide in Diabetic Retinopathy. Front. Immunol. 2021, 12, 632132. [Google Scholar] [CrossRef]
- Gómez-Jaramillo, L.; Cano-Cano, F.; Sánchez-Fernández, E.M.; Ortiz Mellet, C.; García-Fernández, J.M.; Alcalá, M.; Álvarez-Gallego, F.; Iturregui, M.; González-Montelongo, M.d.C.; Campos-Caro, A.; et al. Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ. Int. J. Mol. Sci. 2022, 23, 8450. [Google Scholar] [CrossRef]
- Alcalde-Estévez, E.; Arroba, A.I.; Sánchez-Fernández, E.M.; Ortiz Mellet, C.; García Fernández, J.M.; Masgrau, L.; Valverde, Á.M. The sp2-iminosugar glycolipid 1-dodecylsulfonyl-5N,6O-oxomethylidenenojirimycin (DSO2-ONJ) as selective anti-inflammatory agent by modulation of hemeoxygenase-1 in Bv.2 microglial cells and retinal explants. Food Chem. Toxicol. 2018, 111, 454–466. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-based ocular drug delivery systems: Recent advances and future prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Sabouni, R.; Husseini, G.A. Biomedical Applications of Metal-Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials 2022, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Gandara-Loe, J.; Ortuño-Lizarán, I.; Fernández-Sanchez, L.; Alió, J.L.; Cuenca, N.; Vega-Estrada, A.; Silvestre-Albero, J. Metal-Organic Frameworks as Drug Delivery Platforms for Ocular Therapeutics. ACS Appl. Mater. Interfaces 2019, 11, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Jassal, A.K. Recent advances in MOFs-based nanocomposites for treatment of retinopathy or retina-related biomedical applications. In Advances in Nano and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 467–486. [Google Scholar] [CrossRef]
- Amadio, M.; Pascale, A.; Cupri, S.; Pignatello, R.; Osera, C.; D’Agata, V.; D’Amico, A.G.; Leggio, G.M.; Ruozi, B.; Govoni, S.; et al. Nanosystems based on siRNA silencing HuR expression counteract diabetic retinopathy in rat. Pharmacol. Res. 2016, 111, 713–720. [Google Scholar] [CrossRef]
- Stark, A.K.; Penn, J.S. Prostanoid signaling in retinal cells elicits inflammatory responses relevant to early-stage diabetic retinopathy. J. Neuroinflamm. 2024, 21, 329. [Google Scholar] [CrossRef]
- Guerrero, F.; Carmona, A.; Vidal, V.; Franco, A.; Martín-Malo, A.; Sánchez-Fernández, E.M.; Carrillo-Carrión, C. A selenoureido-iminoglycolipid transported by zeolitic-imidazolate framework nanoparticles: A novel antioxidant therapeutic approach. Nanoscale Horiz. 2023, 8, 1700–1710. [Google Scholar] [CrossRef]
- Rodríguez-Marín, R.; Balestra, S.R.G.; Hamad, S.; Sánchez-Fernández, E.M.; Carrillo-Carrión, C. Engineering nanoscale glyco-zeolitic-imidazolate frameworks: Insights into the mechanism of formation. Mater. Today Chem. 2025, 44, 102546. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Haddad, S.; Marshall, R.J.; Lázaro, I.A.; Boix, G.; Imaz, I.; Maspoch, D.; Forgan, R.S.; Fairen-Jimenez, D. Tuning the Endocytosis Mechanism of Zr-Based Metal-Organic Frameworks through Linker Functionalization. ACS Appl. Mater. Interfaces 2017, 9, 35516–35525. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Guillen, M.I.; Ferrandiz, M.L.; Megías, J.; Motterlini, R. Carbon Monoxide-Releasing Molecules: A Pharmacological Expedient to Counteract Inflammation. Curr. Pharm. Des. 2008, 14, 465–472. [Google Scholar] [CrossRef]
- Megías, J.; Guillén, M.I.; Clérigues, V.; Rojo, A.I.; Cuadrado, A.; Castejón, M.A.; Gomar, F.; Alcaraz, M.J. Heme oxygenase-1 induction modulates microsomal prostaglandin E synthase-1 expression and prostaglandin E2 production in osteoarthritic chondrocytes. Biochem. Pharmacol. 2009, 77, 1806–1813. [Google Scholar] [CrossRef]
- Zhang, J.J.; Ni, P.; Song, Y.; Gao, M.J.; Guo, X.Y.; Zhao, B.Q. Effective protective mechanisms of HO-1 in diabetic complications: A narrative review. Cell Death Discov. 2024, 10, 433. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, K.; Liu, J.; Tian, Y.; Li, H.; Wang, H.; Lin, Z.; Qiu, M.; Tang, B. A ZIF-8-based multifunctional intelligent drug release system for chronic osteomyelitis. Colloids Surf. B Biointerfaces 2022, 212, 112354. [Google Scholar] [CrossRef] [PubMed]
- Masoudifar, R.; Pouyanfar, N.; Liu, D.; Ahmadi, M.; Landi, B.; Akbari, M.; Moayeri-Jolandan, S.; Ghorbani-Bidkorpeh, F.; Asadian, E.; Shahbazi, M.-A. Surface engineered metal-organic frameworks as active targeting nanomedicines for mono- and multi-therapy. Appl. Mater. Today 2022, 29, 101646. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Zhu, Z.; Zhang, X.; Wang, J. Advances in Immunomodulatory MOFs for Biomedical Applications. Small 2024, 20, e2307299. [Google Scholar] [CrossRef]
- Hidalgo, T.; Simón-Vázquez, R.; González-Fernández, A.; Horcajada, P. Cracking the immune fingerprint of metal-organic frameworks. Chem. Sci. 2022, 13, 934–944. [Google Scholar] [CrossRef]
- Lang, Y.; Chu, F.; Shen, D.; Zhang, W.; Zheng, C.; Zhu, J.; Cui, L. Role of inflammasomes in neuroimmune and neurodegenerative diseases: A systematic review. Mediat. Inflamm. 2018, 2018, 1549549. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Bartok, E.; Rieger, A.; Franchi, L.; Núñez, G.; Hornung, V. Cutting Edge: Reactive Oxygen Species Inhibitors Block Priming, but Not Activation, of the NLRP3 Inflammasome. J. Immunol. 2011, 187, 613–617. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, D.A.; Gardner, T.W. Altered Expression of Retinal Occludin and Glial Fibrillary Acidic Protein in Experimental Diabetes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Baleriola, J.; Suárez, T.; De La Rosa, E.J. DNA-PK promotes the survival of young neurons in the embryonic mouse retina. Cell Death Differ. 2010, 17, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Alcalde-Estevez, E.; García-Ramírez, M.; Cazzoni, D.; de la Villa, P.; Sánchez-Fernández, E.M.; Ortiz Mellet, C.; García Fernández, J.M.; Hernández, C.; Simó, R.; et al. Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1663–1674. [Google Scholar] [CrossRef]
- Mosher, K.I.; Wyss-Coray, T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 594–604. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Paredes, E.; Martín-Loro, F.; Rodríguez-Marín, R.; Gómez-Jaramillo, L.; Sánchez-Fernández, E.M.; Carrillo-Carrión, C.; Arroba, A.I. Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks. Pharmaceutics 2025, 17, 791. https://doi.org/10.3390/pharmaceutics17060791
Díaz-Paredes E, Martín-Loro F, Rodríguez-Marín R, Gómez-Jaramillo L, Sánchez-Fernández EM, Carrillo-Carrión C, Arroba AI. Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks. Pharmaceutics. 2025; 17(6):791. https://doi.org/10.3390/pharmaceutics17060791
Chicago/Turabian StyleDíaz-Paredes, Elena, Francisco Martín-Loro, Rocío Rodríguez-Marín, Laura Gómez-Jaramillo, Elena M. Sánchez-Fernández, Carolina Carrillo-Carrión, and Ana I. Arroba. 2025. "Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks" Pharmaceutics 17, no. 6: 791. https://doi.org/10.3390/pharmaceutics17060791
APA StyleDíaz-Paredes, E., Martín-Loro, F., Rodríguez-Marín, R., Gómez-Jaramillo, L., Sánchez-Fernández, E. M., Carrillo-Carrión, C., & Arroba, A. I. (2025). Exploring a Novel Anti-Inflammatory Therapy for Diabetic Retinopathy Based on Glyco-Zeolitic-Imidazolate Frameworks. Pharmaceutics, 17(6), 791. https://doi.org/10.3390/pharmaceutics17060791







