Development of Ethosomes for the Topical Treatment of Androgenic Alopecia: Ethanol Effect on Dutasteride Targeting to the Hair Follicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethosome Preparation
2.3. Characterization of DUT-Loaded Ethosomes
2.3.1. Ethosomes’ Size and Zeta Potential
2.3.2. Determination of DUT Content
2.3.3. Morphological Analyses
2.3.4. Viscosity
2.4. Drug Release
2.5. In Vitro Skin Penetration Tests
2.6. Analytical Method
2.7. Statistical Analysis
3. Results and Discussion
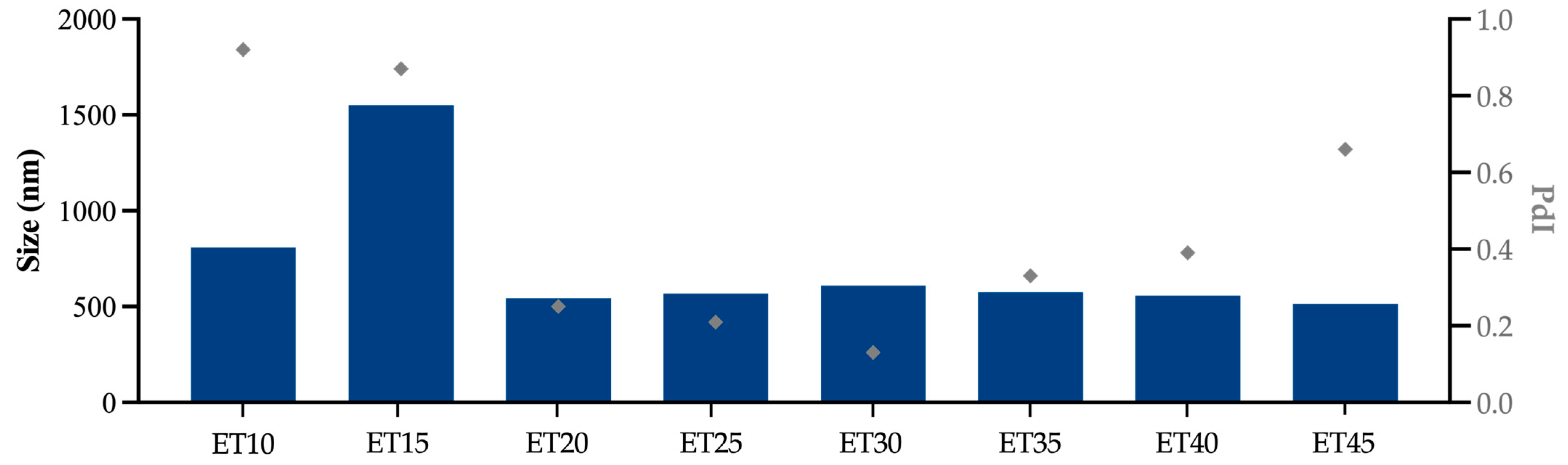
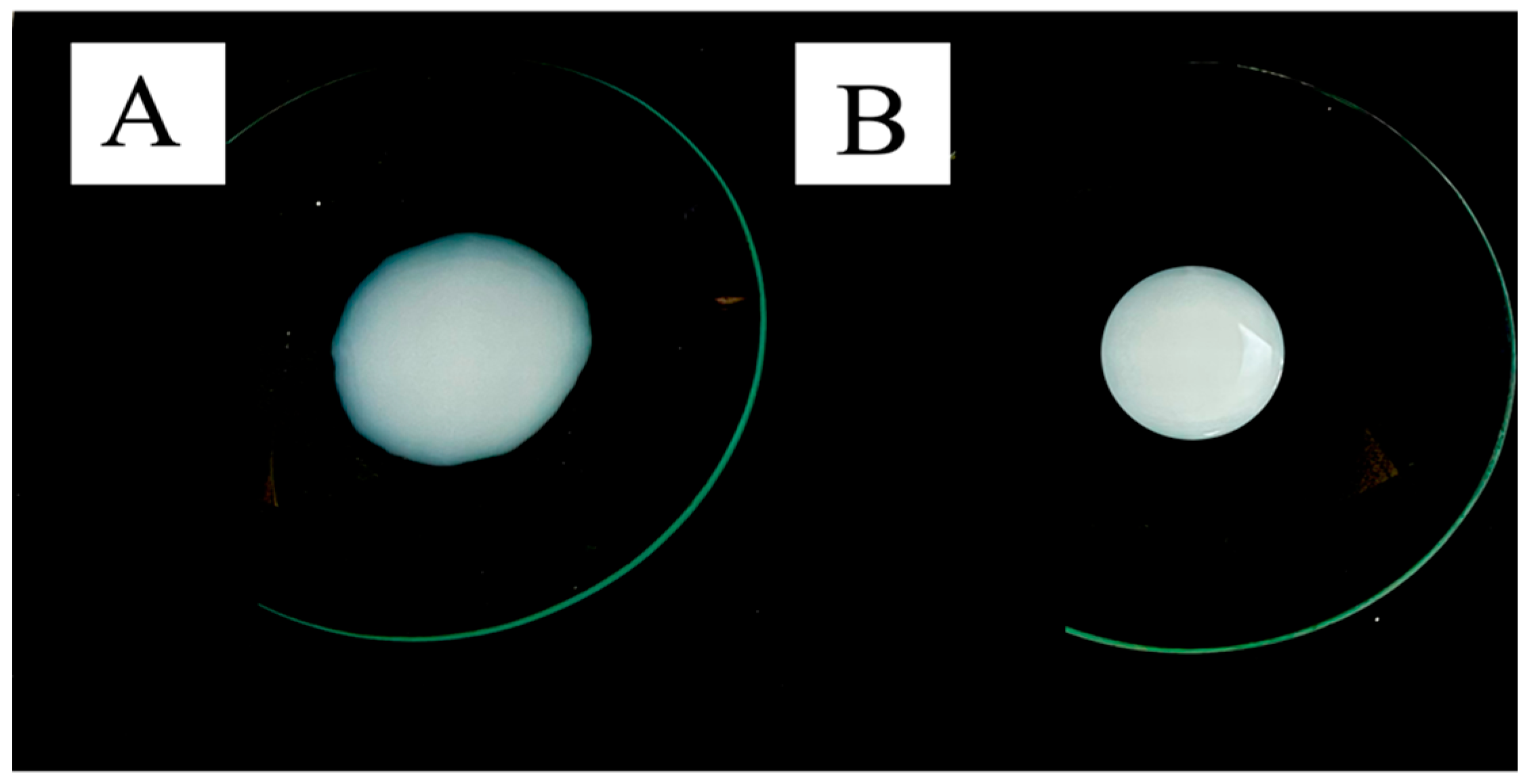
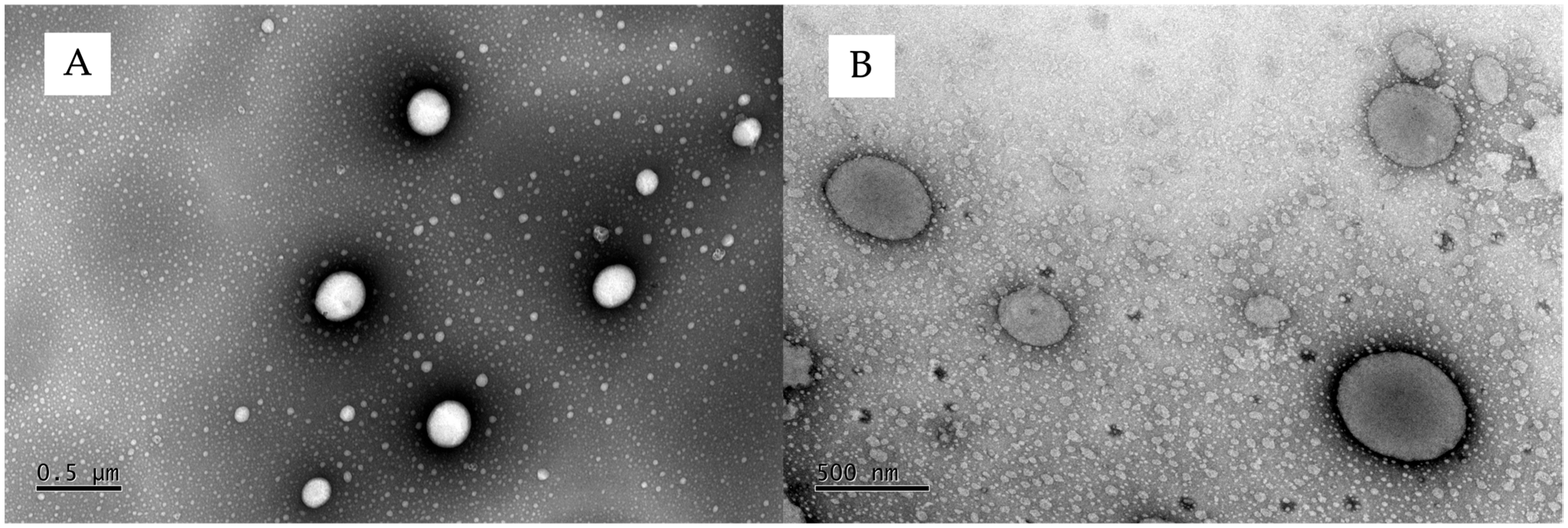
3.1. Ethosome Characterization
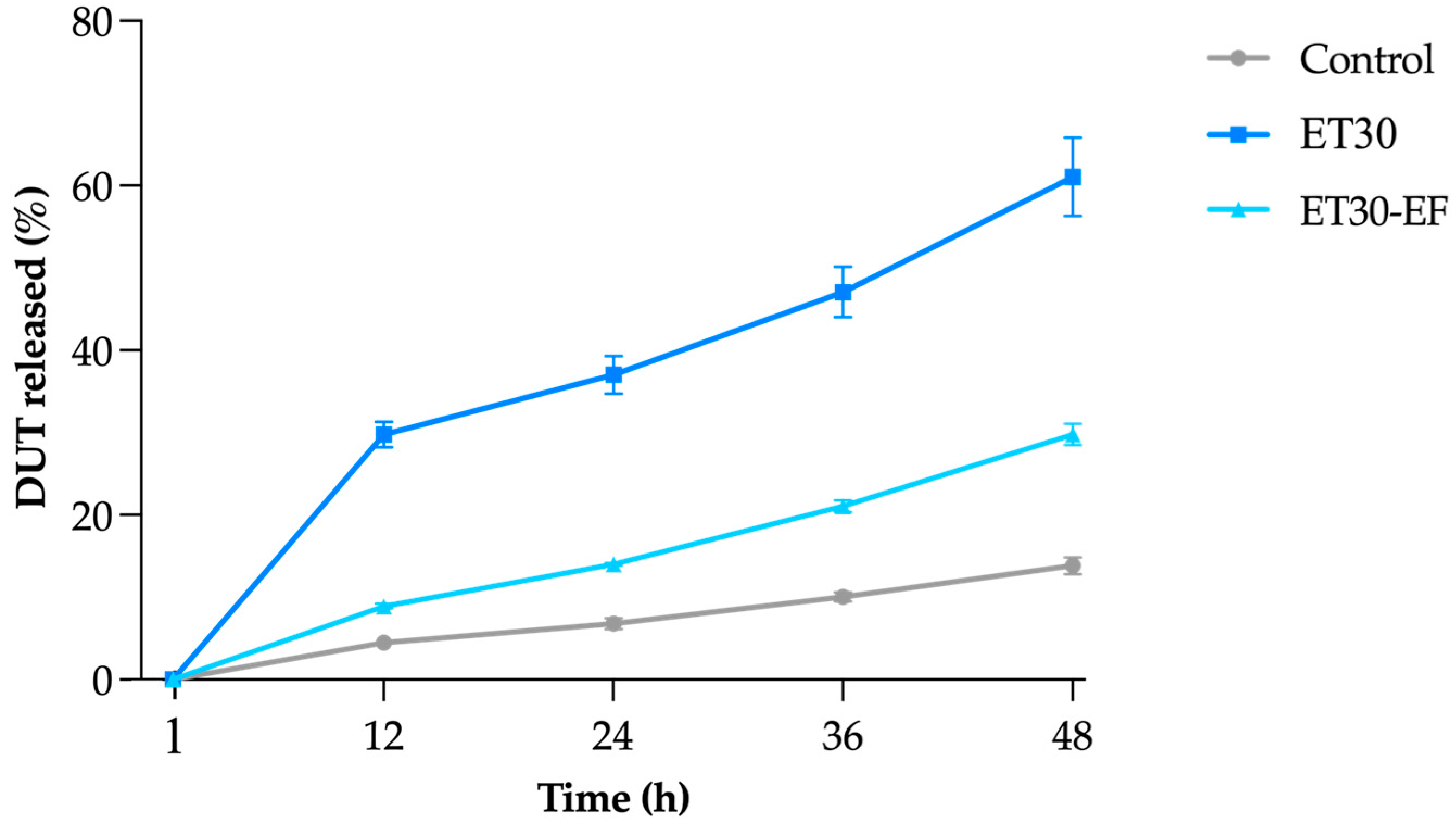
3.2. In Vitro Drug Release
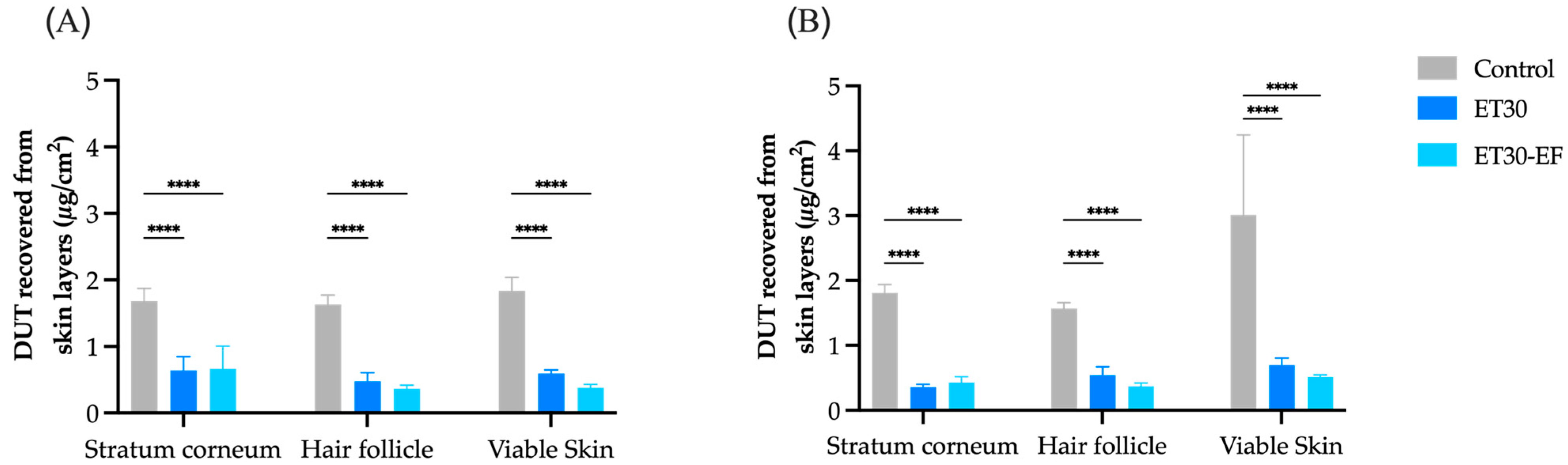
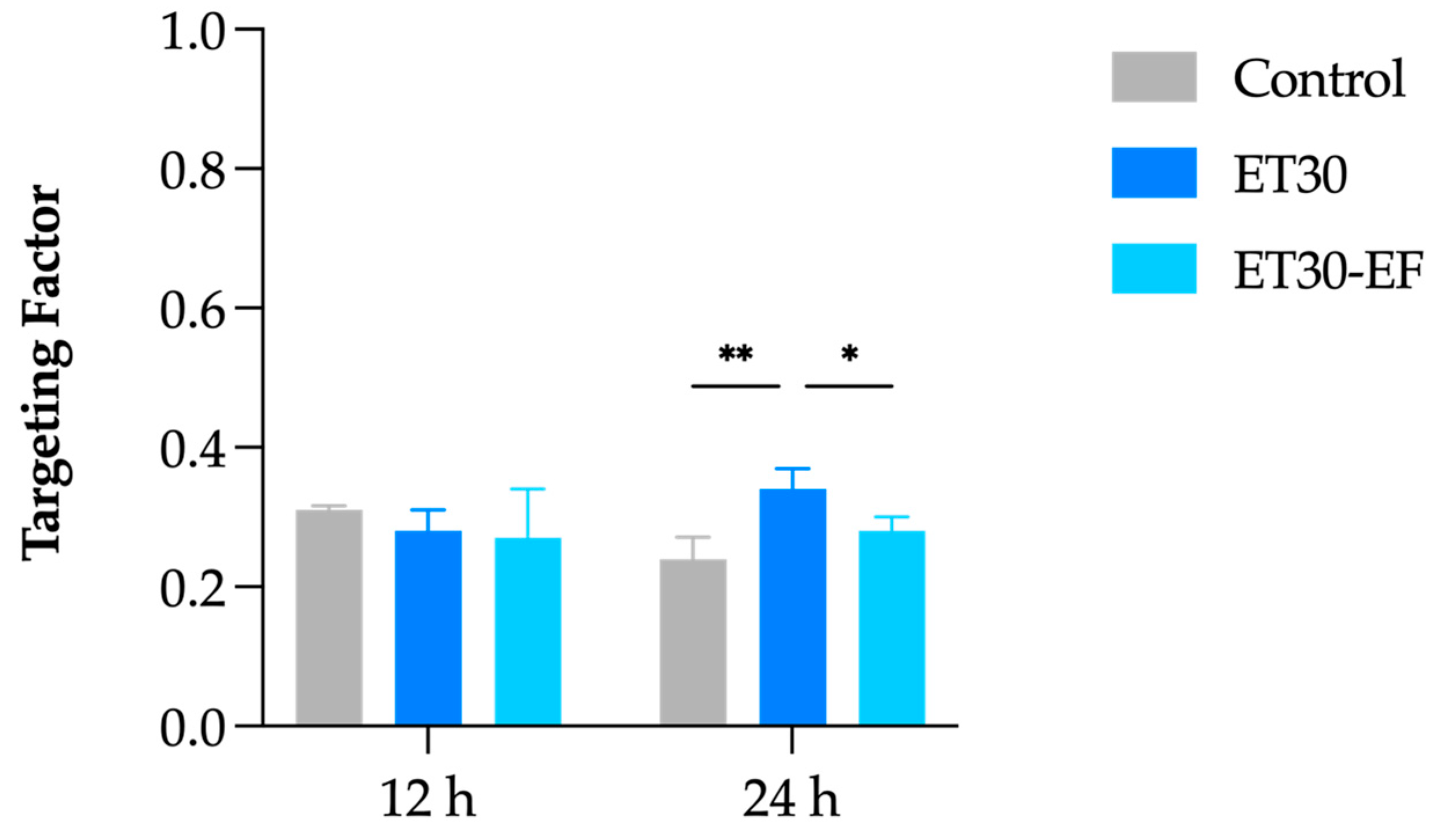
3.3. In Vitro Skin Penetration Experiment and Follicular Targeting Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, C.H.; Sood, T.; Zito, P.M. Androgenetic Alopecia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK430924/ (accessed on 3 April 2025).
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aso, T.; Ono, J.; Hosoi, R.; Kaneko, T. Androgenetic Alopecia Treatment in Asian Men. J. Clin. Aesthet. Dermatol. 2018, 11, 32–35. [Google Scholar]
- de Lacharrière, O.; Deloche, C.; Misciali, C.; Piraccini, B.M.; Vincenzi, C.; Bastien, P.; Tardy, I.; Bernard, B.A.; Tosti, A. Hair Diameter Diversity: A Clinical Sign Reflecting the Follicle Miniaturization. Arch. Dermatol. 2001, 137, 641–646. [Google Scholar]
- Katzer, T.; Leite Junior, A.; Beck, R.; Da Silva, C. Physiopathology and current treatments of androgenetic alopecia: Going beyond androgens and anti-androgens. Dermatol. Ther. 2019, 32, e13059. [Google Scholar] [CrossRef] [PubMed]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5 Alpha-Reductase Isozyme Family: A Review of Basic Biology and Their Role in Human Diseases. Adv. Urol. 2012, 2012, 530121. [Google Scholar] [CrossRef]
- Bayne, E.K.; Flanagan, J.; Einstein, M.; Ayala, J.; Chang, B.; Azzolina, B.; Whiting, D.A.; Mumford, R.A.; Thiboutot, D.; Singer, I.I.; et al. Immunohistochemical localization of types 1 and 2 5α-reductase in human scalp: 5α-reductase types in human scalp. Br. J. Dermatol. 1999, 141, 481–491. [Google Scholar] [CrossRef]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Ghonemy, S.; Alarawi, A.; Bessar, H. Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: A trichoscopic evaluation. J. Dermatol. Treat. 2021, 32, 236–241. [Google Scholar] [CrossRef]
- Rietschel, R.L.; Duncan, S.H. Safety and efficacy of topical minoxidil in the management of androgenetic alopecia. J. Am. Acad. Dermatol. 1987, 16, 677–685. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kim, S.; Lee, S.Y.; Lee, H.; Han, D.W.; Yang, S.Y.; Kim, K.S. Transdermal delivery of Minoxidil using HA-PLGA nanoparticles for the treatment in alopecia. Biomater. Res. 2019, 23, 16. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Irwig, M.S. Persistent Sexual Side Effects of Finasteride: Could They Be Permanent? J. Sex. Med. 2012, 9, 2927–2932. [Google Scholar] [CrossRef]
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair Loss: Common Causes and Treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar]
- Van Zuuren, E.J.; Fedorowicz, Z. Interventions for Female Pattern Hair Loss. JAMA Dermatol. 2017, 153, 329. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, G.; Cauci, S.; Mazzon, G.; Trombetta, C. An observational retrospective evaluation of 79 young men with long-term adverse effects after use of finasteride against androgenetic alopecia. Andrology 2016, 4, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ganzer, C.A.; Jacobs, A.R. Emotional Consequences of Finasteride: Fool’s Gold. Am. J. Mens. Health 2018, 12, 90–95. [Google Scholar] [CrossRef]
- Hirshburg, J.M.; Kelsey, P.A.; Therrien, C.A.; Gavino, A.C.; Reichenberg, J.S. Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. J. Clin. Aesthet. Dermatol. 2016, 9, 56–62. [Google Scholar]
- Irwig, M.S.; Kolukula, S. Persistent Sexual Side Effects of Finasteride for Male Pattern Hair Loss. J. Sex. Med. 2011, 8, 1747–1753. [Google Scholar] [CrossRef]
- Ali, A.K.; Heran, B.S.; Etminan, M. Persistent Sexual Dysfunction and Suicidal Ideation in Young Men Treated with Low-Dose Finasteride: A Pharmacovigilance Study. Pharmacotherapy 2015, 35, 687–695. [Google Scholar] [CrossRef]
- Irwig, M.S. Finasteride and Suicide: A Postmarketing Case Series. Dermatology 2020, 236, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Busanello, E.B.; Turcatel, E. Androgenic alopecia and dutasteride in hair mesotherapy: A short review. Our Dermatol. Online 2018, 9, 75–79. [Google Scholar] [CrossRef][Green Version]
- Clark, R.V.; Hermann, D.J.; Cunningham, G.R.; Wilson, T.H.; Morrill, B.B.; Hobbs, S. Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. J. Clin. Endocrinol. Metab. 2004, 89, 2179–2184. [Google Scholar] [CrossRef]
- Arif, T.; Dorjay, K.; Adil, M.; Sami, M. Dutasteride in Androgenetic Alopecia: An Update. Curr. Clin. Pharmacol. 2017, 12, 31–35. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, S.; Gao, Z.; Wu, J.; Ma, J.; Cui, Y. The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: A systematic review and meta-analysis. Clin. Interv. Aging 2019, 14, 399–406. [Google Scholar] [CrossRef]
- Andrade, J.F.M.; Verbinnen, A.; Bakst, A.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. An update on nanocarriers for follicular-targeted drug delivery for androgenetic alopecia topical treatment. Expert Opin. Drug Deliv. 2025, 22, 367–381. [Google Scholar] [CrossRef]
- Chen, X.; Yan, P.; Zhang, W.; He, X.; Jiang, R.; Li, Y.; Sun, J.; Jiang, J. Bioengineered polyester nanoparticles for the synergistic treatment of androgenic alopecia via the suppression of 5α-reductase and knockdown of androgen receptor. Front. Bioeng. Biotechnol. 2022, 10, 1033987. [Google Scholar] [CrossRef]
- Ushirobira, C.Y.; Afiune, L.A.F.; Pereira, M.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Dutasteride nanocapsules for hair follicle targeting: Effect of chitosan-coating and physical stimulus. Int. J. Biol. Macromol. 2020, 151, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Afiune, L.A.F.; Ushirobira, C.Y.; Barbosa, D.P.P.; de Souza, P.E.N.; Leles, M.I.G.; Cunha-Filho, M.; Gelfuso, G.M.; Soler, M.A.G.; Gratieri, T. Novel iron oxide nanocarriers loading finasteride or dutasteride: Enhanced skin penetration for topical treatment of alopecia. Int. J. Pharm. 2020, 587, 119709. [Google Scholar] [CrossRef]
- Andrade, J.F.M.; Matos, B.N.; Rocho, R.V.; Barbalho, G.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Evaluation of Dutasteride-Loaded Liposomes and Transfersomes for Follicular-Targeting for Androgenic Alopecia Topical Treatment. Pharmaceutics 2024, 16, 1524. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Abdul Karim Khan, N.; Abou Assi, R.; Ali Khan, A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. IJN 2016, 2279. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010L0063 (accessed on 27 May 2025).
- National Research Guide. Guide for the Care and Use of Laboratory Animals: Eighth Edition; National Academies Press: Washington, DC, USA, 2011; p. 12910. Available online: http://www.nap.edu/catalog/12910 (accessed on 27 May 2025).
- Agência Navional de Vigilância Sanitária. ANVISA. Resolução da Diretoria Colegiada (RDC) n 35, de 7 de agosto de 2015. [Internet]. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/concea/arquivos/pdf/legislacao/resolucao-rdc-no-35-de-7-de-agosto-de-2015-anvisa.pdf/view&ved=2ahUKEwjr1JnP48aNAxXmKLkGHdfNAOAQFnoECBcQAQ&usg=AOvVaw0tiAIakzDseRAGsBYfIjjk (accessed on 27 May 2025).
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.H.S.; Da Silva, P.B.; Szlachetka, I.O.; Da Silva, S.W.; Fonseca-Santos, B.; Chorilli, M.; Ganassin, R.; De Oliveira, G.R.T.; Da Rocha, M.C.O.; Fernandes, R.P.; et al. The influence of NLC composition on curcumin loading under a physicochemical perspective and in vitro evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125070. [Google Scholar] [CrossRef]
- Zuo, J.; Gao, Y.; Bou-Chacra, N.; Löbenberg, R. Evaluation of the DDSolver Software Applications. BioMed Res. Int. 2014, 2014, 204925. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Santos, G.A.; Angelo, T.; Andrade, L.M.; Silva, S.M.M.; Magalhães, P.O.; Cunha-Filho, M.; Gelfuso, G.M.; Taveira, S.F.; Gratieri, T. The role of formulation and follicular pathway in voriconazole cutaneous delivery from liposomes and nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2018, 170, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Raj, P.M.; Ram, A. Nanosized ethanol based malleable liposomes of cytarabine to accentuate transdermal delivery: Formulation optimization, in vitro skin permeation and in vivo bioavailability. Artif. Cells Nanomed. Biotechnol. 2018, 46, 951–963. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.-M.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- Martins Andrade, J.F.; Weiss, A.-V.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T.; Schneider, M. Effect of gelatin nanoparticles’ size and charge on iontophoretic targeted deposition to the hair follicles. Int. J. Pharm. 2024, 667, 124906. [Google Scholar] [CrossRef]
- Pang, X.; Han, S.; Zheng, K.; Jiang, L.; Wang, J.; Qian, S. Cellulose nanocrystal-stabilized Pickering emulsion gels as vehicles for follicular delivery of minoxidil. Int. J. Biol. Macromol. 2024, 277, 134297. [Google Scholar] [CrossRef]
- Tampucci, S.; Paganini, V.; Burgalassi, S.; Chetoni, P.; Monti, D. Nanostructured Drug Delivery Systems for Targeting 5-α-Reductase Inhibitors to the Hair Follicle. Pharmaceutics 2022, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Y.; Liu, Z.; Guo, T.; Ai, X.; He, Y.; Hou, X.; Feng, N. Synergistic treatment of androgenetic alopecia with follicular co-delivery of minoxidil and cedrol in metal–organic frameworks stabilized by covalently cross-linked cyclodextrins. Int. J. Pharm. 2024, 654, 123948. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40nm, but not 750 or 1,500nm, Nanoparticles Enter Epidermal CD1a+ Cells after Transcutaneous Application on Human Skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef]
- Mahe, B.; Vogt, A.; Liard, C.; Duffy, D.; Abadie, V.; Bonduelle, O.; Boissonnas, A.; Sterry, W.; Verrier, B.; Blume-Peytavi, U.; et al. Nanoparticle-Based Targeting of Vaccine Compounds to Skin Antigen-Presenting Cells By Hair Follicles and their Transport in Mice. J. Investig. Dermatol. 2009, 129, 1156–1164. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef]
- Al-Suwayeh, S.A.; Taha, E.I.; Al-Qahtani, F.M.; Ahmed, M.O.; Badran, M.M. Evaluation of skin permeation and analgesic activity effects of carbopol lornoxicam topical gels containing penetration enhancer. Sci. World J. 2014, 2014, 127495. [Google Scholar] [CrossRef]
- Siemiradzka, W.; Dolińska, B.; Ryszka, F. Influence of Concentration on Release and Permeation Process of Model Peptide Substance-Corticotropin-From Semisolid Formulations. Molecules 2020, 25, 2767. [Google Scholar] [CrossRef] [PubMed]
- Mulye, N.V.; Turco, S.J. A Simple Model Based on First Order Kinetics to Explain Release of Highly Water Soluble Drugs from Porous Dicalcium Phosphate Dihydrate Matrices. Drug Dev. Ind. Pharm. 1995, 21, 943–953. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Klech, C.M.; Simonelli, A.P. Examination of the moving boundaries associated with non-fickian water swelling of glassy gelatin beads: Effect of solution pH. J. Membr. Sci. 1989, 43, 87–101. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Takayama, K.; Maitani, Y.; Machida, Y.; Nagai, T. Effect of ethanol on skin permeation of nonionized and ionized diclofenac. Int. J. Pharm. 1993, 89, 191–198. [Google Scholar] [CrossRef]
- Bommannan, D.; Potts, R.O.; Guy, R.H. Examination of the effect of ethanol on human stratum corneum in vivo using infrared spectroscopy. J. Control Release 1991, 16, 299–304. [Google Scholar] [CrossRef]
- Heard, C.M.; Kung, D.; Thomas, C.P. Skin penetration enhancement of mefenamic acid by ethanol and 1,8-cineole can be explained by the ‘pull’ effect. Int. J. Pharm. 2006, 321, 167–170. [Google Scholar] [CrossRef]
- Heard, C.M.; Screen, C. Probing the permeation enhancement of mefenamic acid by ethanol across full-thickness skin, heat-separated epidermal membrane and heat-separated dermal membrane. Int. J. Pharm. 2008, 349, 323–325. [Google Scholar] [CrossRef]
- Esposito, E.; Calderan, L.; Galvan, A.; Cappellozza, E.; Drechsler, M.; Mariani, P.; Pepe, A.; Sguizzato, M.; Vigato, E.; Dalla Pozza, E.; et al. Ex Vivo Evaluation of Ethosomes and Transethosomes Applied on Human Skin: A Comparative Study. IJMS 2022, 23, 15112. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lv, H.; Han, G.; Ma, K. Ethosomes Loaded with Cryptotanshinone for Acne Treatment through Topical Gel Formulation. PLoS ONE 2016, 11, e0159967. [Google Scholar] [CrossRef]
- Kumar Sarwa, K.; Rudrapal, M.; Mazumder, B. Topical ethosomal capsaicin attenuates edema and nociception in arthritic rats. Drug Deliv. 2015, 22, 1043–1052. [Google Scholar] [CrossRef]
- Hallan, S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef]
- Ferrara, F.; Benedusi, M.; Cervellati, F.; Sguizzato, M.; Montesi, L.; Bondi, A.; Drechsler, M.; Pula, W.; Valacchi, G.; Esposito, E. Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation. IJMS 2022, 23, 8756. [Google Scholar] [CrossRef]
- Sicurella, M.; Pula, W.; Musiał, K.; Cieślik-Boczula, K.; Sguizzato, M.; Bondi, A.; Drechsler, M.; Montesi, L.; Esposito, E.; Marconi, P. Ethosomal Gel for Topical Administration of Dimethyl Fumarate in the Treatment of HSV-1 Infections. IJMS 2023, 24, 4133. [Google Scholar] [CrossRef] [PubMed]
- Brain, K.; Walters, K.A.; Watkinson, A.C. Methods for studying percutaneous absorption. In Dermatological and Transdermal Formulations; CRC Press: Boca Raton, FL, USA, 2002; pp. 197–270. ISBN 978-0-429-16432-3. [Google Scholar]
- Keam, S.J.; Scott, L.J. Dutasteride: A Review of its Use in the Management of Prostate Disorders. Drugs 2008, 68, 463–485. [Google Scholar] [CrossRef] [PubMed]
| Ethosomes | DUT 17 mM | PC 200 mM | Purified Water |
|---|---|---|---|
| ET10 | 0.30 mL | 0.70 mL | 9.0 mL |
| ET15 | 0.30 mL | 1.20 mL | 8.5 mL |
| ET20 | 0.30 mL | 1.70 mL | 8.0 mL |
| ET25 | 0.30 mL | 2.20 mL | 7.5 mL |
| ET30 | 0.30 mL | 2.70 mL | 7.0 mL |
| ET35 | 0.30 mL | 3.20 mL | 6.5 mL |
| ET40 | 0.30 mL | 3.70 mL | 6.0 mL |
| ET45 | 0.30 mL | 4.20 mL | 5.5 mL |
| Ethosomes | Hydrodynamic Size (nm) | PdI | Zeta Potential (mV) | DUT Dose (mg/mL) |
|---|---|---|---|---|
| ET30 | 604.8 ± 43.3 | 0.13 ± 0.09 | 2.2 ± 0.2 | 0.30 ± 0.01 |
| ET30-EF | 581.7 ± 20.5 | 0.05 ± 0.07 | 2.7 ± 0.1 | 0.29 ± 0.01 |
| Samples | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| MSC ± SD | R2 ± SD | MSC ± SD | R2 ± SD | MSC ± SD | R2 ± SD | MSC ± SD | R2 ± SD | |
| Control | 2.03 ± 0.1 a | 0.91 ± 0.0 a | 1.94 ± 0.1 a | 0.90 ± 0.1 a | 0.35 ± 0.1 a | 0.52 ± 0.0 a | 2.41 ± 0.4 a | 0.94 ± 0.0 a |
| ET30 | 1.88 ± 0.1 a | 0.89 ± 0.0 a | 2.64 ± 0.2 b | 0.95 ± 2.6 a | 1.41 ± 0.1 b | 0.83 ± 0.0 a | 0.84 ± 0.1 b | 0.71 ± 0.0 a |
| ET30-EF | 1.92 ± 0.0 a | 0.90 ± 0.0 a | 1.69 ± 0.0 a | 0.88 ± 0.0 a | 0.26 ± 0.0 a | 0.48 ± 0.0 a | 2.25 ± 0.1 a | 0.93 ± 0.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, J.F.M.; Rocho, R.V.; Matos, B.N.; Barbalho, G.N.; Nunes, K.M.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Development of Ethosomes for the Topical Treatment of Androgenic Alopecia: Ethanol Effect on Dutasteride Targeting to the Hair Follicles. Pharmaceutics 2025, 17, 786. https://doi.org/10.3390/pharmaceutics17060786
Andrade JFM, Rocho RV, Matos BN, Barbalho GN, Nunes KM, Cunha-Filho M, Gelfuso GM, Gratieri T. Development of Ethosomes for the Topical Treatment of Androgenic Alopecia: Ethanol Effect on Dutasteride Targeting to the Hair Follicles. Pharmaceutics. 2025; 17(6):786. https://doi.org/10.3390/pharmaceutics17060786
Chicago/Turabian StyleAndrade, Jayanaraian F. M., Rafael V. Rocho, Breno N. Matos, Geisa N. Barbalho, Kariane M. Nunes, Marcilio Cunha-Filho, Guilherme M. Gelfuso, and Tais Gratieri. 2025. "Development of Ethosomes for the Topical Treatment of Androgenic Alopecia: Ethanol Effect on Dutasteride Targeting to the Hair Follicles" Pharmaceutics 17, no. 6: 786. https://doi.org/10.3390/pharmaceutics17060786
APA StyleAndrade, J. F. M., Rocho, R. V., Matos, B. N., Barbalho, G. N., Nunes, K. M., Cunha-Filho, M., Gelfuso, G. M., & Gratieri, T. (2025). Development of Ethosomes for the Topical Treatment of Androgenic Alopecia: Ethanol Effect on Dutasteride Targeting to the Hair Follicles. Pharmaceutics, 17(6), 786. https://doi.org/10.3390/pharmaceutics17060786





