Design and Activity Evaluation of Berberine-Loaded Dual pH and Enzyme-Sensitive Colon-Targeting Microparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Berberine Chitosan Nanoparticles (BBR-CS NPs)
2.3. Preparation of Berberine Microparticles (BBR-ES MPs)
2.4. Characterization of BBR-CS NPs and BBR-ES MPs
2.5. Detection of Encapsulation Rate and Drug Loading
2.6. In Vivo Targeting of Berberine Particles
2.7. The Therapeutic Effect of Berberine Microparticles on UC
2.7.1. Animal Grouping
2.7.2. Establishment and Administration of UC Model
2.7.3. Disease Activity Index (DAI) Evaluation
2.7.4. Animal Sacrifice and Tissue Preservation
2.7.5. Colon Length Measurement
2.7.6. Histopathological Examination
2.7.7. Myeloperoxidase (MPO) Activity Assay
2.7.8. Inflammatory Cytokine Analysis
2.8. Impact of Berberine Microparticles on the Gut Microbiota in UC Mice
2.8.1. Fecal Sample Collection and Sequencing
2.8.2. Bioinformatics and Taxonomic Profiling
2.8.3. Alpha Diversity Analysis
2.8.4. Beta Diversity Analysis
2.8.5. Differential Species and Biomarker Identification
2.9. Statistical Analysis
3. Results
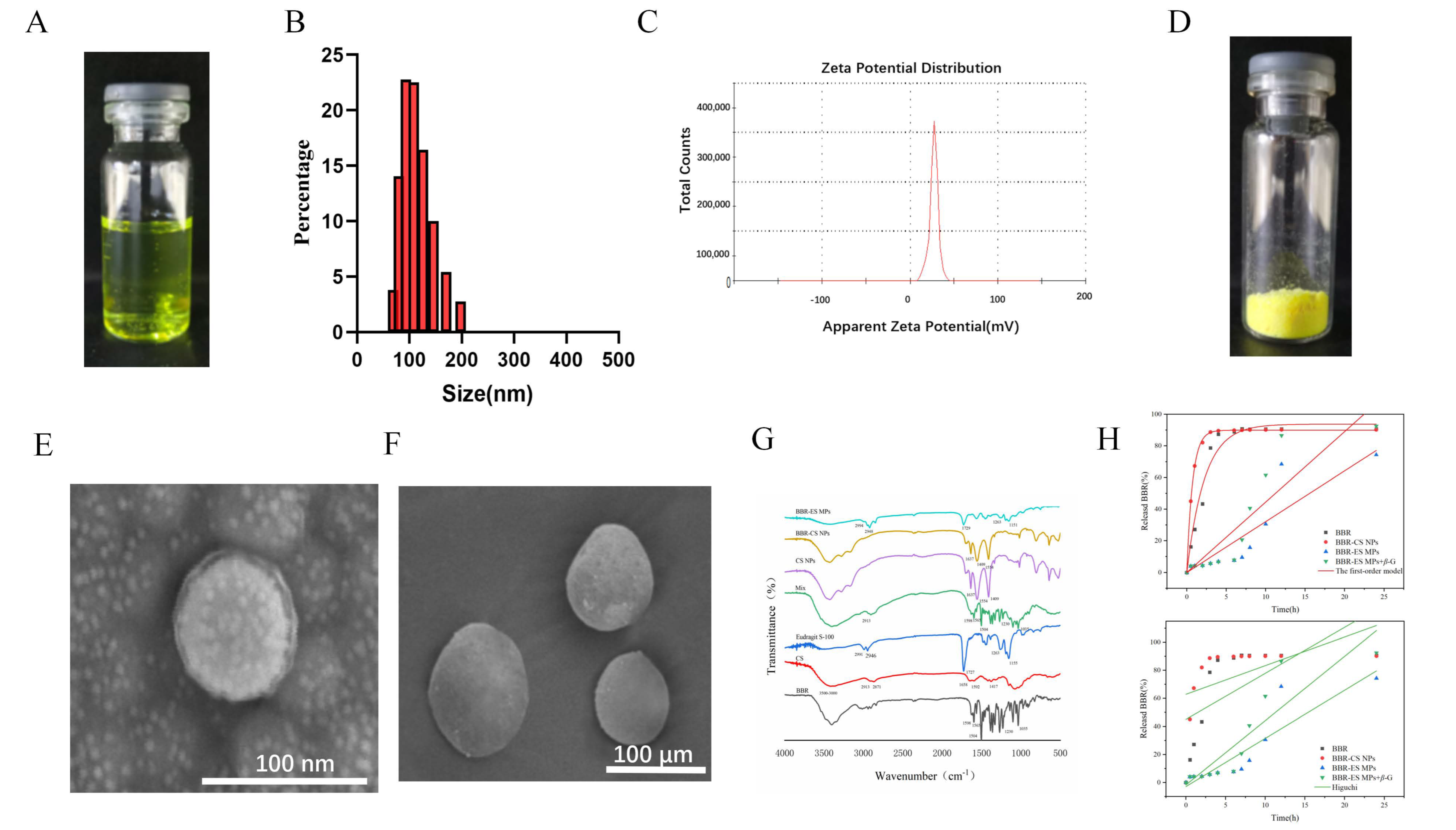
3.1. Synthesis and Characterization of BBR-CS NPs
3.2. Synthesis and Characterization of BBR-ES MPs
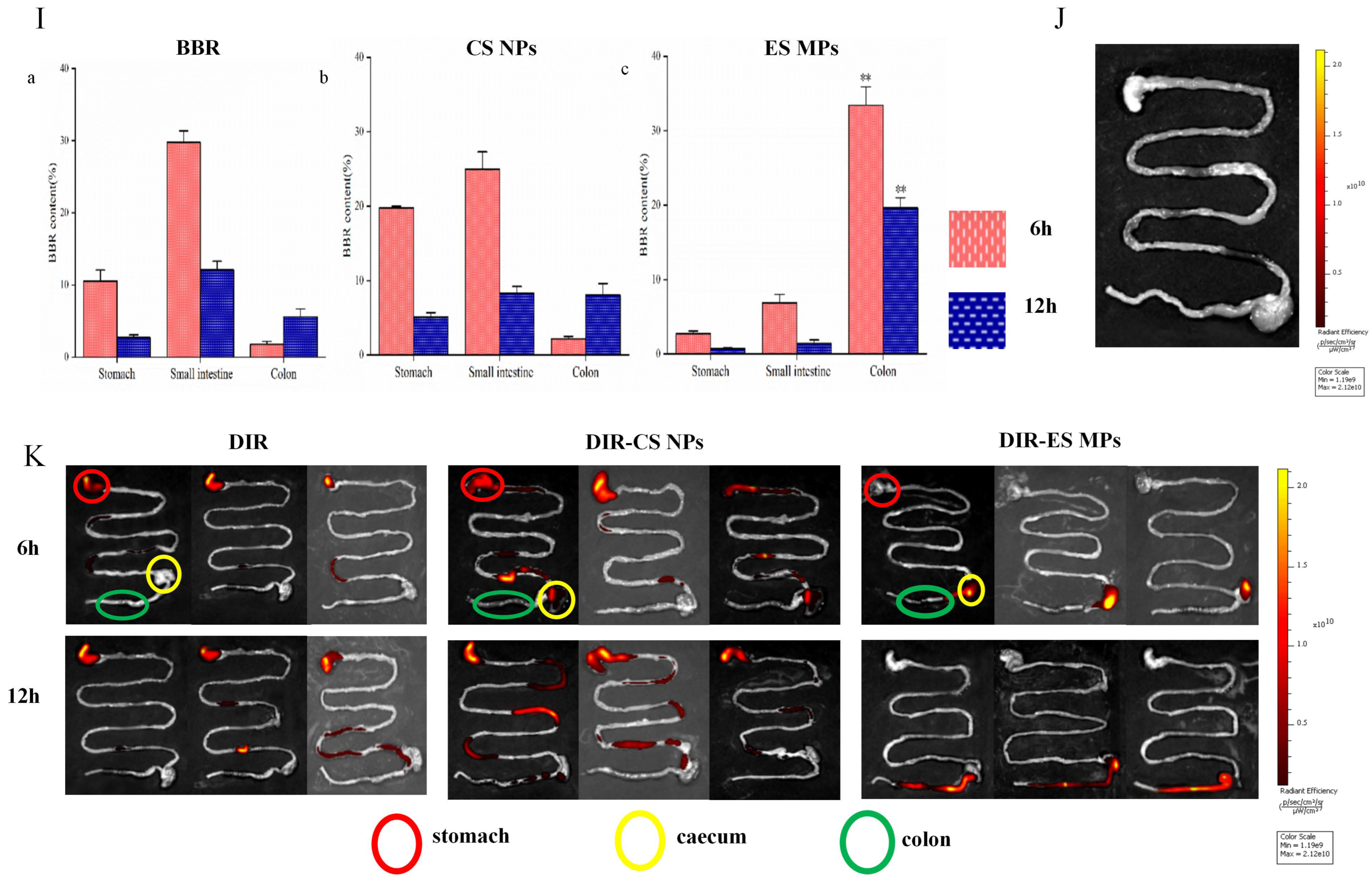
3.3. Tissue Distribution Test Results
3.4. Therapeutic Efficacy of Berberine Microparticles in UC Mice
3.4.1. Body Weight Changes
3.4.2. Disease Activity Index (DAI) Scores
3.4.3. Measurement Results of Colon Length
3.4.4. Histopathological Analysis
3.4.5. Myeloperoxidase (MPO) Activity
3.4.6. Inflammatory Cytokine Levels
3.5. Effects of Berberine Microparticles on the Gut Microbiota in UC Mice
3.5.1. Bioinformatic and Taxonomic Composition Analysis
Phylum-Level Composition
Genus-Level Composition
Class-Level Composition
3.5.2. The Results of Alpha Diversity Analysis
Alpha Diversity Indices
Rank-Abundance Curves
3.5.3. The Results of Beta Diversity Analysis
PCoA and NMDS
3.5.4. Differential Species and Biomarker Analysis
ASV/OTU Venn Diagram
Heatmap of Genus-Level Composition
OPLS-DA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| BBR-CS NPs | Berberine-loaded chitosan nanoparticles |
| BBR-ES MPs | Berberine Eudragit S-100 microparticles |
References
- Zhang, T.; Zhu, G.; Lu, B.; Peng, Q. Oral Nano-Delivery Systems for Colon Targeting Therapy. Pharm. Nanotechnol. 2017, 5, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Tian, L.; Zhao, Z.; Chen, S.; Zhao, Q.; Hong, J.; Xie, Y.; Zhou, N.; Fu, Y. The sinomenine enteric-coated microspheres suppressed the TLR/NF-κB signaling in DSS-induced experimental colitis. Int. Immunopharmacol. 2017, 50, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, Y.; Deng, D.; Su, S.; Li, S.; Xiang, L.; Hu, Y.; Wang, P.; Meng, X. Coptisine from Coptis chinensis exerts diverse beneficial properties: A concise review. J. Cell. Mol. Med. 2019, 23, 7946–7960. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yi, Y.; Yong, J.; Sun, J.; Song, Z.; Li, D.; Li, Y. Inhibitory effect of berberine hydrochloride against Candida albicans and the role of the HOG-MAPK pathway. J. Antibiot. 2021, 74, 807–816. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.-J.; et al. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef]
- Xia, L.M.; Luo, M.H. Study progress of berberine for treating cardiovascular disease. Chronic Dis. Transl. Med. 2016, 1, 231–235. [Google Scholar] [CrossRef]
- Wang, Z.; Dan, W.; Zhang, N.; Fang, J.; Yang, Y. Colorectal cancer and gut microbiota studies in China. Gut Microbes 2023, 15, 2236364. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, W.; Li, Y.; Xian, X.; Zhao, Z.; Liu, C.; Zou, J.; Li, J.; Fang, X.; Zhu, Y. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem. Pharmacol. 2020, 174, 113776. [Google Scholar] [CrossRef]
- Sun, R.; Kong, B.; Yang, N.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Feng, S.; Fei, F.; Huang, J.; et al. The Hypoglycemic Effect of Berberine and Berberrubine Involves Modulation of Intestinal Farnesoid X Receptor Signaling Pathway and Inhibition of Hepatic Gluconeogenesis. Drug Metab. Dispos. 2021, 49, 276–286. [Google Scholar] [CrossRef]
- Maroni, A.; Moutaharrik, S.; Zema, L.; Gazzaniga, A. Enteric coatings for colonic drug delivery: State of the art. Expert Opin. Drug Deliv. 2017, 14, 1027–1029. [Google Scholar] [CrossRef]
- McCoubrey, L.E.; Favaron, A.; Awad, A.; Orlu, M.; Gaisford, S.; Basit, A.W. Colonic drug delivery: Formulating the next generation of colon-targeted therapeutics. J. Control. Release 2023, 353, 1107–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.-S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Fahmy, U.A.; Ahmed, O.A.A.; Caruso, G.; Caraci, F.; Asfour, H.Z.; Bakhrebah, M.A.; N. Alomary, M.; Abdulaal, W.H.; Okbazghi, S.Z.; et al. Chitosan Coated Microparticles Enhance Simvastatin Colon Targeting and Pro-Apoptotic Activity. Mar. Drugs 2020, 18, 226. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Wang, Y.; Hao, W.; Yuan, Q.; Zhou, H.; Gao, C.; Wang, Y.; Wu, X.; Wang, S. Targeted delivery of Chinese herb pair-based berberine/tannin acid self-assemblies for the treatment of ulcerative colitis. J. Adv. Res. 2022, 40, 263–276. [Google Scholar] [CrossRef]
- Souto, E.B.; da Ana, R.; Souto, S.B.; Zielińska, A.; Marques, C.; Andrade, L.N.; Horbańczuk, O.K.; Atanasov, A.G.; Lucarini, M.; Durazzo, A.; et al. In Vitro Characterization, Modelling, and Antioxidant Properties of Polyphenon-60 from Green Tea in Eudragit S100-2 Chitosan Microspheres. Nutrients 2020, 12, 967. [Google Scholar] [CrossRef]
- Hu, R.; Chen, X.; Li, Z.; Zhao, G.; Ding, L.; Chen, L.; Dai, C.; Chen, Y.; Zhang, B. Liquid Nanoparticles for Nanocatalytic Cancer Therapy. Adv. Mater. 2023, 35, e2306469. [Google Scholar] [CrossRef]
- Yan, Y.-X.; Shao, M.-J.; Qi, Q.; Xu, Y.-S.; Yang, X.-Q.; Zhu, F.-H.; He, S.-J.; He, P.-L.; Feng, C.-L.; Wu, Y.-W.; et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol. Sin. 2018, 39, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, J.; Wang, Y.; Huang, J.; Yang, X.; Ma, J.; Liu, Z.; Wang, F.; Tang, X. Modified Gegen Qinlian decoction ameliorates DSS-induced chronic colitis in mice by restoring the intestinal mucus barrier and inhibiting the activation of γδT17 cells. Phytomedicine 2023, 111, 154660. [Google Scholar] [CrossRef]
- Trentini, A.; Rosta, V.; Spadaro, S.; Bellini, T.; Rizzo, P.; Sega, F.V.D.; Passaro, A.; Zuliani, G.; Gentili, V.; Campo, G.; et al. Development, optimization and validation of an absolute specific assay for active myeloperoxidase (MPO) and its application in a clinical context: Role of MPO specific activity in coronary artery disease. Clin. Chem. Lab. Med. 2020, 58, 1749–1758. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Xie, Y.; Yang, Q.; Luo, H.; Jia, P.; Shi, Z.; Wang, S.; Zheng, X. Feiyangchangweiyan capsule protects against ulcerative colitis in mice by modulating the OSM/OSMR pathway and improving gut microbiota. Phytomedicine 2021, 80, 153372. [Google Scholar] [CrossRef]
- Wang, J.-L.; Han, X.; Li, J.-X.; Shi, R.; Liu, L.-L.; Wang, K.; Liao, Y.-T.; Jiang, H.; Zhang, Y.; Hu, J.-C.; et al. Differential analysis of intestinal microbiota and metabolites in mice with dextran sulfate sodium-induced colitis. World J. Gastroenterol. 2022, 28, 6109–6130. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.F.; Chung, C.C.; Roberson, D.; Mitchell, J.; Zhang, X.; Im, K.M.; He, J.; Chanock, S.J.; Yeager, M.; Dean, M. The new sequencer on the block: Comparison of Life Technology’s Proton sequencer to an Illumina HiSeq for whole-exome sequencing. Hum. Genet. 2013, 132, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, F.M.; Jiménez-Alfaro, B.; Jandt, U.; Chytrý, M.; Field, R.; Kessler, M.; Lenoir, J.; Schrodt, F.; Wiser, S.K.; Khan, M.A.S.A.; et al. Global patterns of vascular plant alpha diversity. Nat. Commun. 2022, 13, 4683. [Google Scholar] [CrossRef]
- Godsoe, W.; Bellingham, P.J.; Moltchanova, E. Disentangling Niche Theory and Beta Diversity Change. Am. Nat. 2022, 199, 510–522. [Google Scholar] [CrossRef]
- Mohanbhai, S.J.; Sardoiwala, M.N.; Gupta, S.; Shrimali, N.; Choudhury, S.R.; Sharma, S.S.; Guchhait, P.; Karmakar, S. Colon targeted chitosan-melatonin nanotherapy for preclinical Inflammatory Bowel Disease. Biomater. Adv. 2022, 136, 212796. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, C.; Lu, H.; Feng, C.; He, P.; Yang, X.; Xiang, C.; Zuo, J.; Tang, W. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm. Sin. B 2020, 10, 447–461. [Google Scholar] [CrossRef]
- Tavakoli, P.; Vollmer-Conna, U.; Hadzi-Pavlovic, D.; Grimm, M.C. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021, 42, 1603990. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Tang, B.; Wang, F.-C.; Tang, L.; Lei, Y.-Y.; Luo, Y.; Huang, S.-J.; Yang, M.; Wu, L.-Y.; Wang, W.; et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020, 10, 5225–5241. [Google Scholar] [CrossRef]


| Score | Loss of Weight (%) | Stool Property | Archorrhagia |
|---|---|---|---|
| 0 | 0 | Normal | Normal |
| 1 | 1~5 | ||
| 2 | 6~10 | Semi-loose stools | Hyporrhea |
| 3 | 11~15 | ||
| 4 | >15 | Loose stools | Severepostpartumbleeding |
| First-Order Model | HIGUCHI | |||||
|---|---|---|---|---|---|---|
| Q = Qm(1 - e−kt) | Q = Kt1/2 + b | |||||
| Qm | k | R2 | k | b | R2 | |
| bbr | 93.80078 | 0.44329 | 0.96671 | 6.56734 | 45.0763 | 0.35324 |
| bbr-cs nps | 89.95636 | 1.36394 | 0.99938 | 4.08993 | 62.91878 | 0.17352 |
| bbr-es mps | 50,737.76517 | 6.34 × 10−5 | 0.79152 | 6.87697 | −2.8994 | 0.79922 |
| bbr-es mps + β-g | −53,502.00682 | −8.32 × 10−5 | 0.80291 | 9.17465 | −1.70147 | 0.82228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Chai, X.; Zeng, X.; Wang, Q.; Ling, Y.; Wang, L.; Su, J. Design and Activity Evaluation of Berberine-Loaded Dual pH and Enzyme-Sensitive Colon-Targeting Microparticles. Pharmaceutics 2025, 17, 778. https://doi.org/10.3390/pharmaceutics17060778
Sun J, Chai X, Zeng X, Wang Q, Ling Y, Wang L, Su J. Design and Activity Evaluation of Berberine-Loaded Dual pH and Enzyme-Sensitive Colon-Targeting Microparticles. Pharmaceutics. 2025; 17(6):778. https://doi.org/10.3390/pharmaceutics17060778
Chicago/Turabian StyleSun, Jingqi, Xinlong Chai, Xiwen Zeng, Qingwei Wang, Yanwen Ling, Lihong Wang, and Jin Su. 2025. "Design and Activity Evaluation of Berberine-Loaded Dual pH and Enzyme-Sensitive Colon-Targeting Microparticles" Pharmaceutics 17, no. 6: 778. https://doi.org/10.3390/pharmaceutics17060778
APA StyleSun, J., Chai, X., Zeng, X., Wang, Q., Ling, Y., Wang, L., & Su, J. (2025). Design and Activity Evaluation of Berberine-Loaded Dual pH and Enzyme-Sensitive Colon-Targeting Microparticles. Pharmaceutics, 17(6), 778. https://doi.org/10.3390/pharmaceutics17060778






