Sivelestat-Loaded Neutrophil-Membrane-Coated Antioxidative Nanoparticles for Targeted Endothelial Protection in Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Cells, and Animals
2.2. Animal Model
2.3. Extraction of the Neutrophil Membrane
2.4. Preparation of Siv@NMLs
2.5. Characterization of Siv@NMs
2.6. Cell Proliferation Activity and Hemocompatibility of Siv@NMs
2.7. Fluorescent Observation of Siv@NMs
2.8. H&E Staining
2.9. TUNEL Assay
2.10. JC-1 Assay
2.11. ROS Detection
2.12. ELISA
2.13. Western Blotting
2.14. Statistics Analysis
3. Results
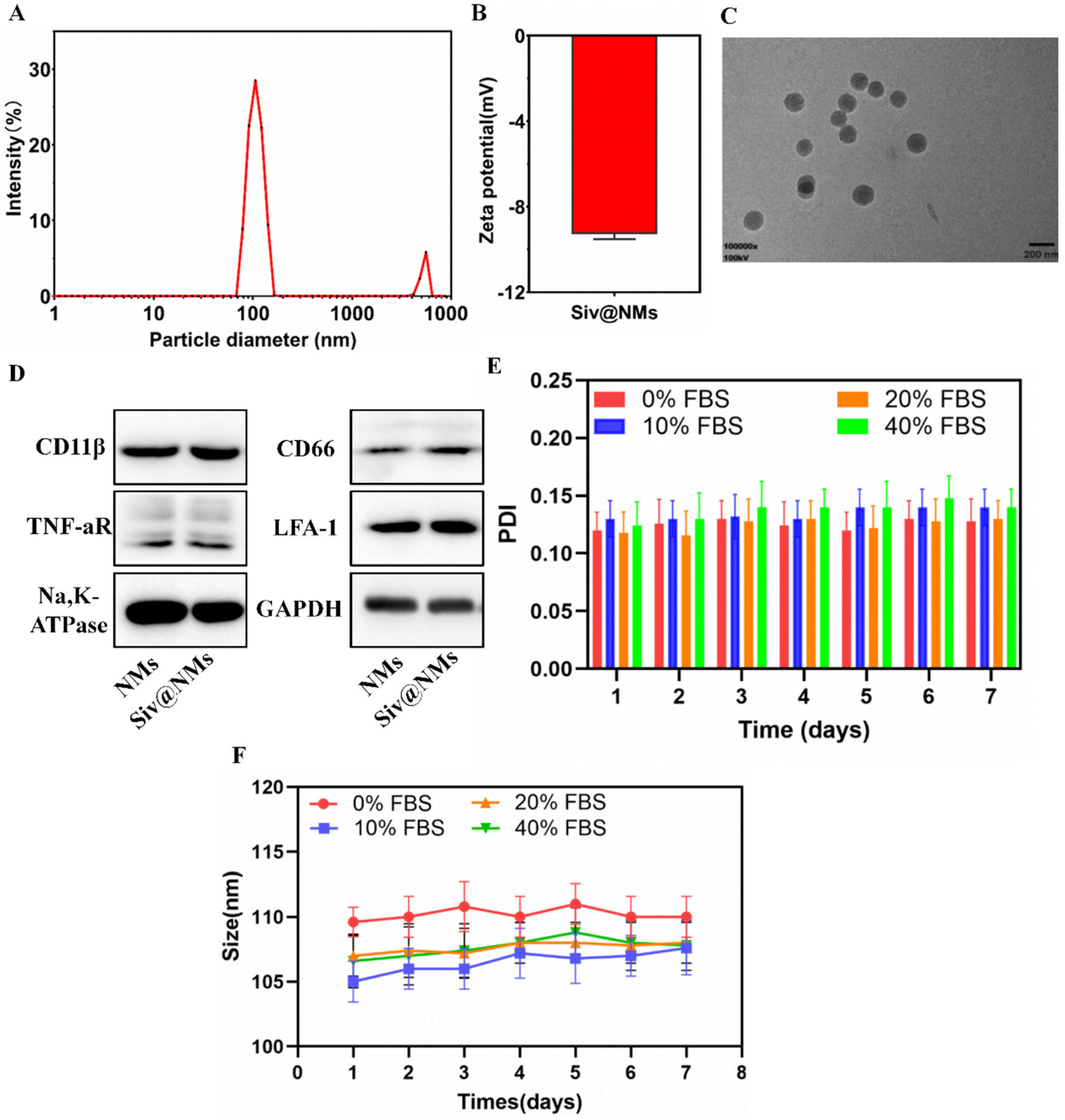
3.1. Characterization of Siv@NMs
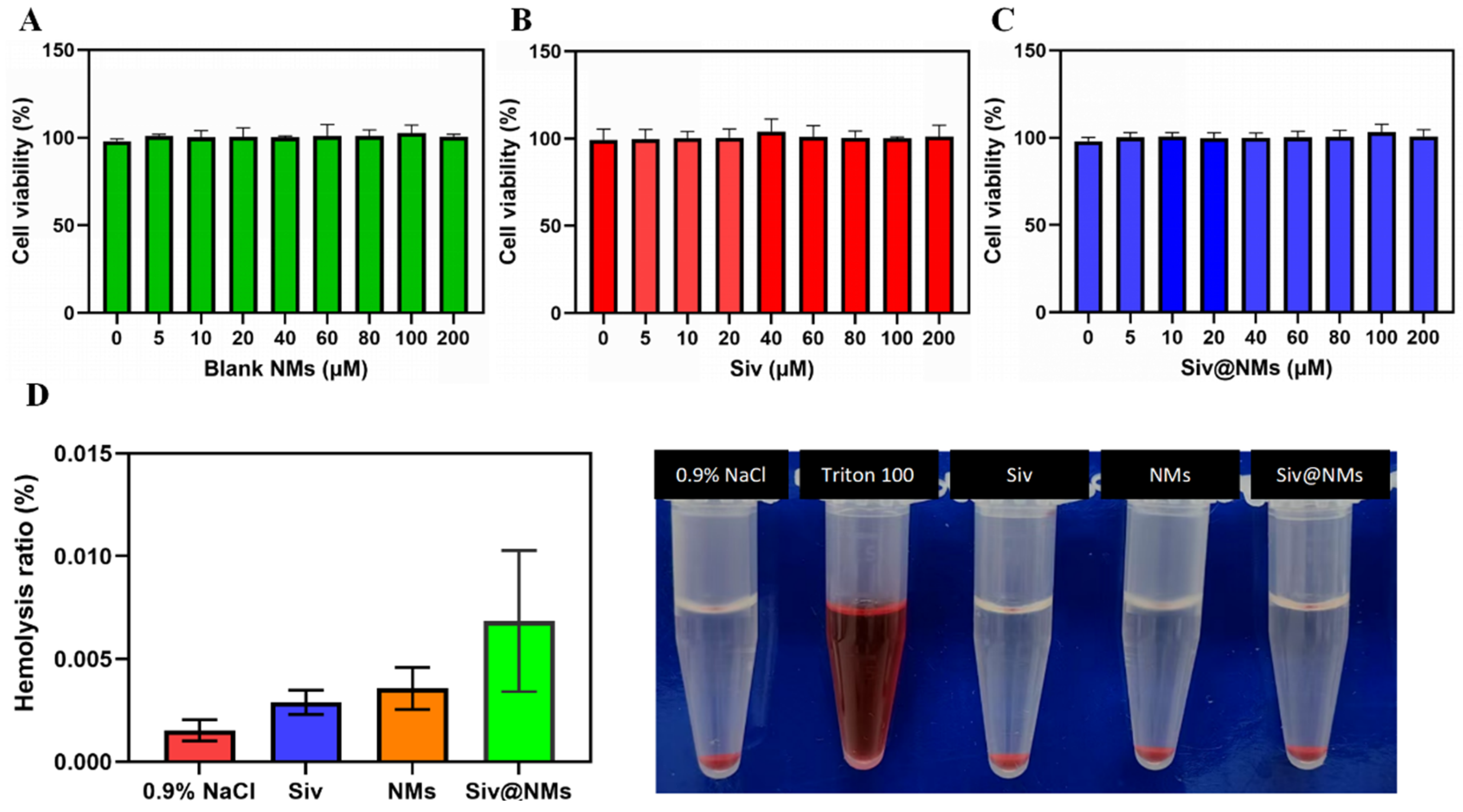
3.2. Biocompatibility of Siv@NMs
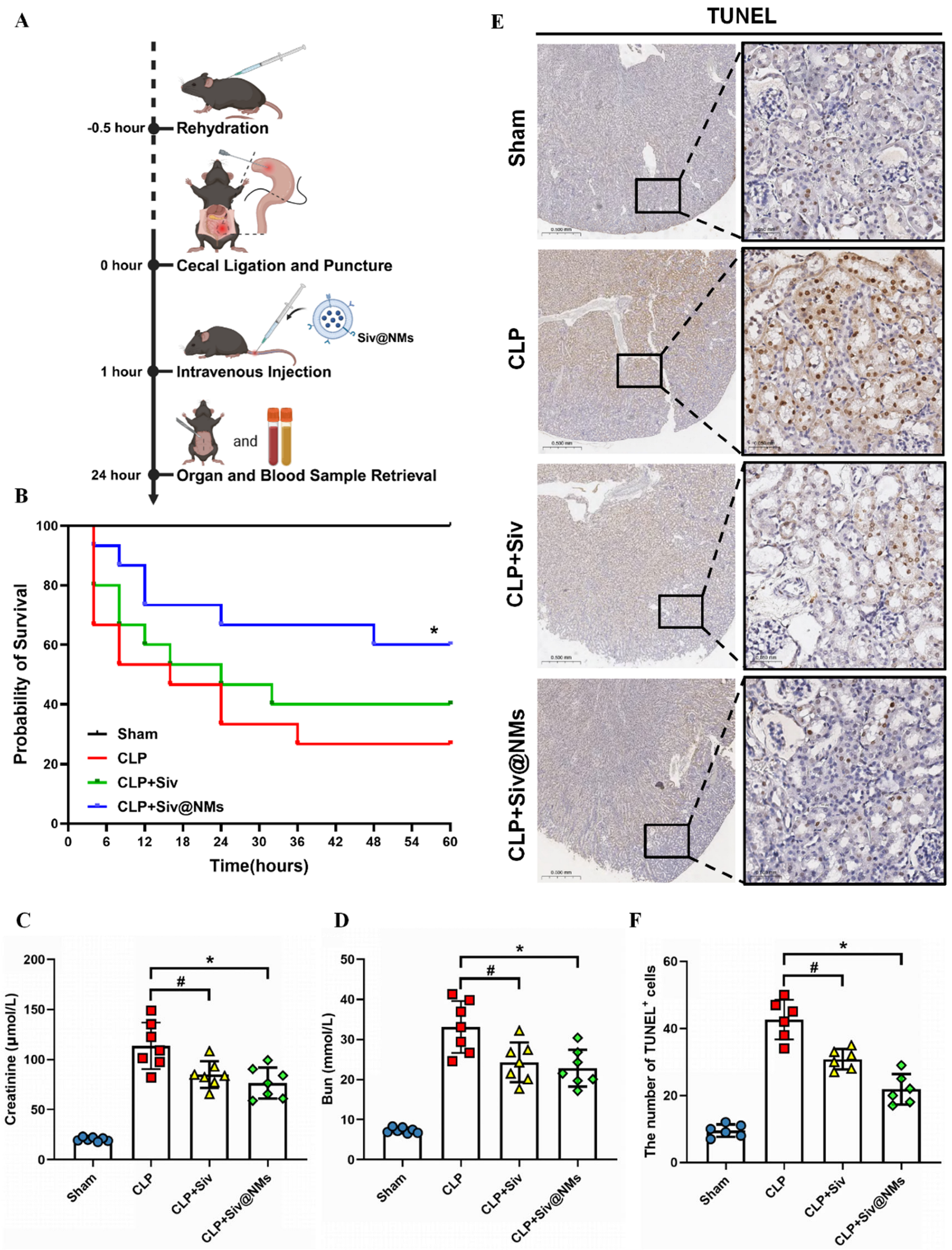
3.3. Therapeutic Effects of Siv@NMs in Sepsis
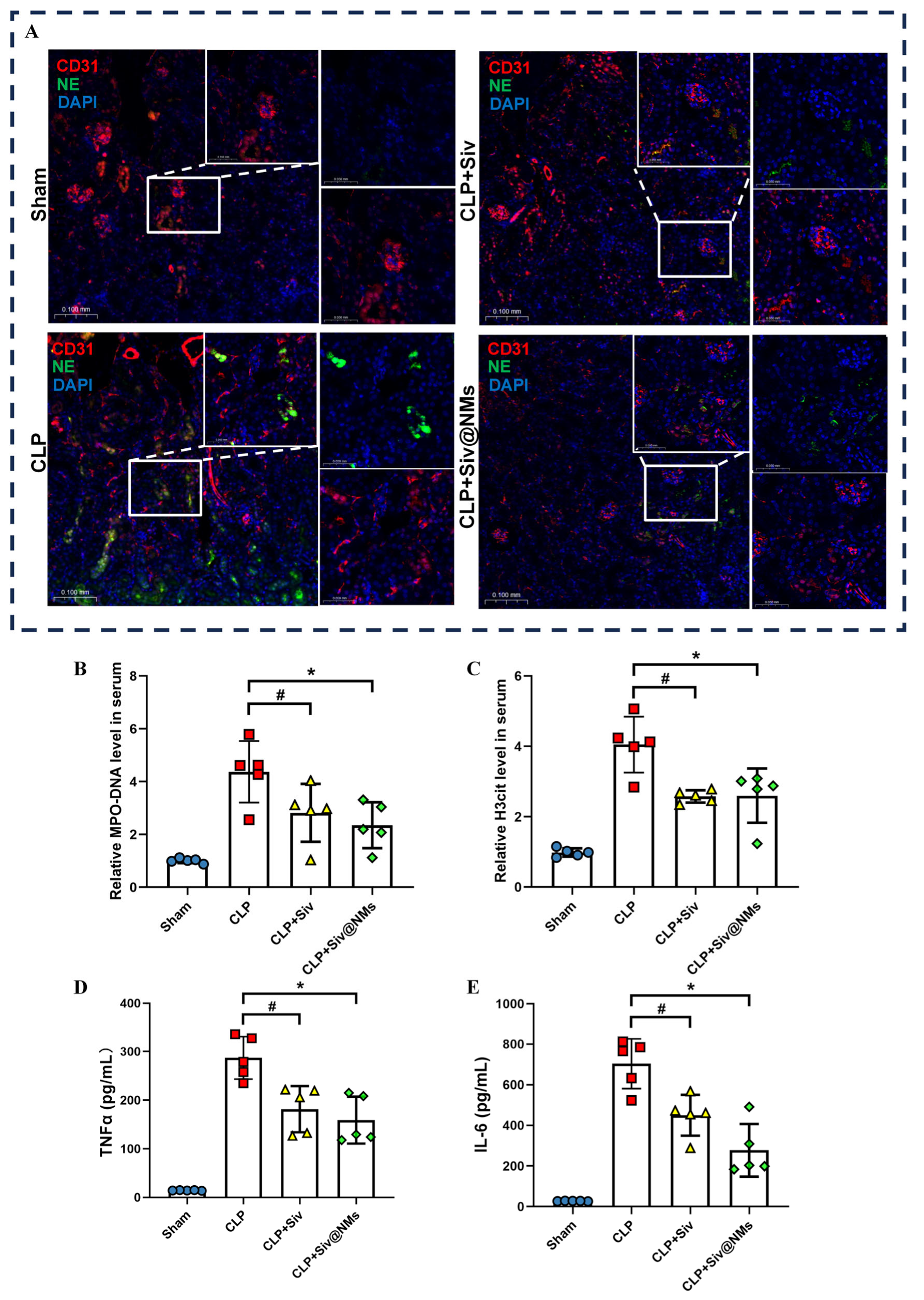
3.4. Therapeutic Effects of Siv@NMs on Renal NET Formation in Septic Conditions
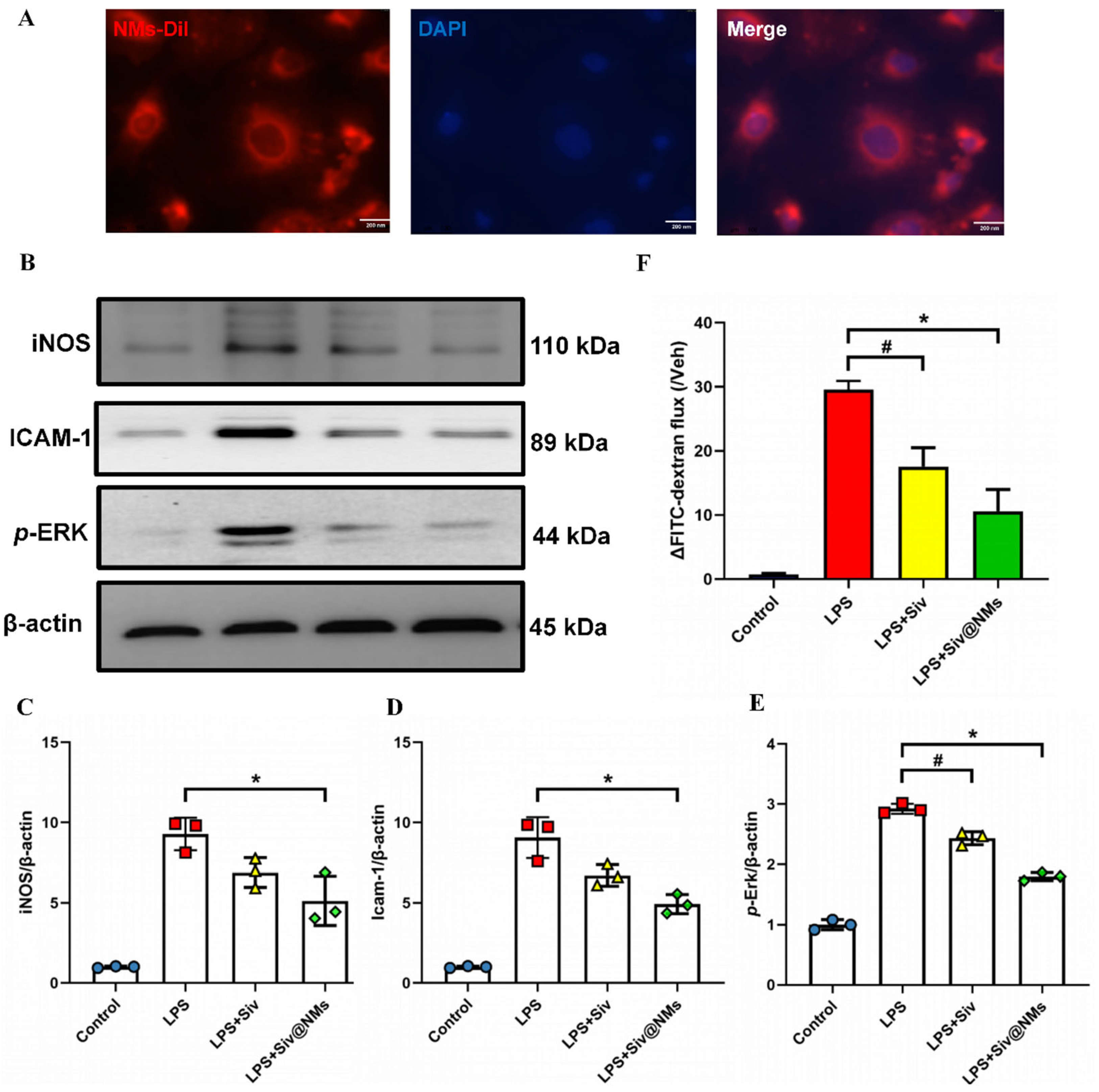
3.5. Therapeutic Effects of Siv@NMs on Endothelial Dysfunction
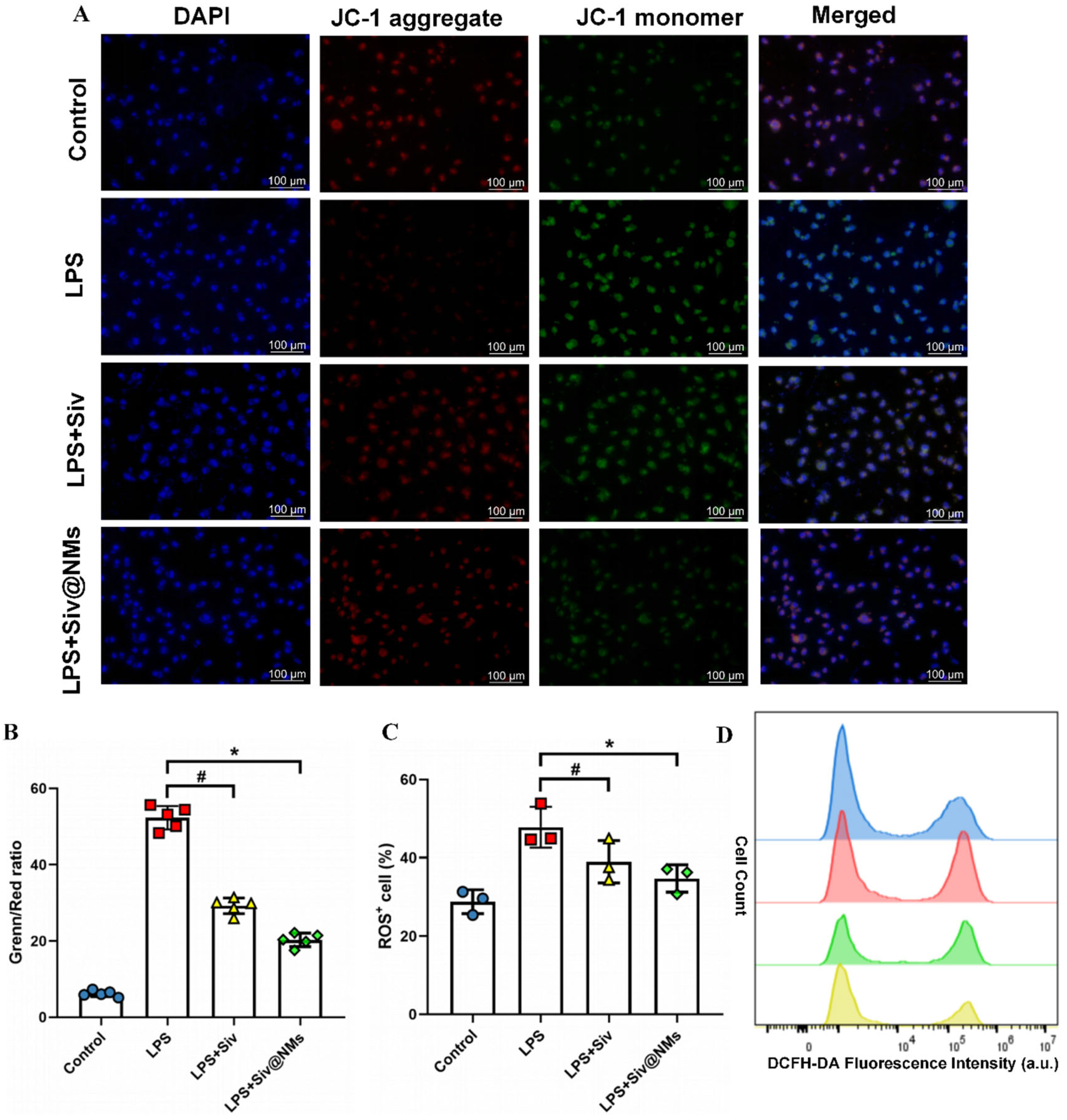
3.6. Therapeutic Effects of Siv@NMs on Mitochondrial Dysfunction and Oxidative Stress in Endothelial Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef] [PubMed]
- Lelubre, C.; Vincent, J.L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 2018, 14, 417–427. [Google Scholar] [CrossRef]
- Girardis, M.; David, S.; Ferrer, R.; Helms, J.; Juffermans, N.P.; Martin-Loeches, I.; Povoa, P.; Russell, L.; Shankar-Hari, M.; Iba, T.; et al. Understanding, assessing and treating immune, endothelial and haemostasis dysfunctions in bacterial sepsis. Intensive Care Med. 2024, 50, 1580–1592. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Machireddy, N.; Evans, C.E.; Trewartha, S.D.; Hu, G.; Fang, Y.; Mutlu, G.M.; Wu, D.; Zhao, Y.Y. Endothelial FoxM1 reactivates aging-impaired endothelial regeneration for vascular repair and resolution of inflammatory lung injury. Sci. Transl. Med. 2023, 15, eabm5755. [Google Scholar] [CrossRef]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Wei, J.; Mo, H.; Zhang, Y.; Deng, W.; Zheng, S.; Mao, H.; Ji, Y.; Jiang, H.; Zhu, Y. Evolutionary trend analysis and knowledge structure mapping of endothelial dysfunction in sepsis: A bibliometrics study. World J. Emerg. Med. 2024, 15, 386–396. [Google Scholar] [CrossRef]
- Wang, C.L.; Wang, Y.; Jiang, Q.L.; Zeng, Y.; Yao, Q.P.; Liu, X.; Li, T.; Jiang, J. DNase I and Sivelestat Ameliorate Experimental Hindlimb Ischemia-Reperfusion Injury by Eliminating Neutrophil Extracellular Traps. J. Inflamm. Res. 2023, 16, 707–721. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, Y.; Yuan, M.; Liu, Y.; Song, Y.; Shen, L.; Xiao, Y.; Wang, X.; Wang, D.; Liu, L.; et al. Sivelestat sodium protects against renal ischemia/reperfusion injury by reduction of NETs formation. Arch. Biochem. Biophys. 2025, 765, 110318. [Google Scholar] [CrossRef]
- Okeke, E.B.; Louttit, C.; Fry, C.; Najafabadi, A.H.; Han, K.; Nemzek, J.; Moon, J.J. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 2020, 238, 119836. [Google Scholar] [CrossRef]
- Zhou, P.; Li, T.; Jin, J.; Liu, Y.; Li, B.; Sun, Q.; Tian, J.; Zhao, H.; Liu, Z.; Ma, S.; et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicine 2020, 53, 102671. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Fernandes, C.; Rebordao, M.R.; Szostek-Mioduchowska, A.; Lukasik, K.; Gawronska-Kozak, B.; Telo da Gama, L.; Skarzynski, D.J.; Ferreira-Dias, G. The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium. Animals 2020, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Dong, L.; Gao, S.; Zhang, J.; Feng, Y.; Gu, L.; Yang, J.; Liu, X.; Wang, Y.; Mao, Z.; et al. Safety, tolerability, pharmacokinetics and neutrophil elastase inhibitory effects of Sivelestat: A randomized, double-blind, placebo-controlled single- and multiple-dose escalation study in Chinese healthy subjects. Eur. J. Pharm. Sci. 2024, 195, 106723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, H.; Li, Z.; Zhang, P.; Pan, L.; Wang, L.; Mao, J.; Li, F.; Zhang, L. Population pharmacokinetics of sivelestat in Chinese patients with severe pneumonia. Fundam. Clin. Pharmacol. 2025, 39, e70001. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Yang, N.; Li, M.; Wu, L.; Song, Y.; Yu, S.; Wan, Y.; Cheng, W.; Yang, B.; Mou, X.; Yu, H.; et al. Peptide-anchored neutrophil membrane-coated biomimetic nanodrug for targeted treatment of rheumatoid arthritis. J. Nanobiotechnol. 2023, 21, 13. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, C.; Feng, J.; Long, H.; Wang, Y.; Wang, P.; Hu, C.; Yue, Y.; Zhang, C.; Liu, Z.; et al. Anti-inflammatory mechanisms of neutrophil membrane-coated nanoparticles without drug loading. J. Control Release 2024, 369, 12–24. [Google Scholar] [CrossRef]
- Wu, B.; Lin, L.; Zhou, F.; Wang, X. Precise engineering of neutrophil membrane coated with polymeric nanoparticles concurrently absorbing of proinflammatory cytokines and endotoxins for management of sepsis. Bioprocess. Biosyst. Eng. 2020, 43, 2065–2074. [Google Scholar] [CrossRef]
- Nepal, S.; Tiruppathi, C.; Tsukasaki, Y.; Farahany, J.; Mittal, M.; Rehman, J.; Prockop, D.J.; Malik, A.B. STAT6 induces expression of Gas6 in macrophages to clear apoptotic neutrophils and resolve inflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 16513–16518. [Google Scholar] [CrossRef]
- Li, J.; Ke, W.; Wang, L.; Huang, M.; Yin, W.; Zhang, P.; Chen, Q.; Ge, Z. Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation-chemotherapy. J. Control Release 2016, 225, 64–74. [Google Scholar] [CrossRef]
- Jiang, C.; Shi, Q.; Yang, J.; Ren, H.; Zhang, L.; Chen, S.; Si, J.; Liu, Y.; Sha, D.; Xu, B.; et al. Ceria nanozyme coordination with curcumin for treatment of sepsis-induced cardiac injury by inhibiting ferroptosis and inflammation. J. Adv. Res. 2024, 63, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Li, X.; Zhang, J.; Shi, P.; Cao, Y.; Zhuang, Y.; Tong, L. Neutrophil membrane-coated taurine nanoparticles protect against hepatic ischemia-reperfusion injury. Eur. J. Pharmacol. 2023, 949, 175712. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Dirisala, A.; Guo, H.; Liu, X.; Kobayashi, S.; Kinoh, H.; Anada, T.; Tanaka, M.; Kataoka, K.; Li, J. Engineering durable antioxidative nanoreactors as synthetic organelles for autoregulatory cellular protection against oxidative stress. J. Control Release 2025, 382, 113683. [Google Scholar] [CrossRef]
- Zhan, Z.; Chai, L.; Yang, H.; Dai, Y.; Wei, Z.; Wang, D.; Lv, Y. Endoplasmic Reticulum Peroxynitrite Fluctuations in Hypoxia-Induced Endothelial Injury and Sepsis with a Two-Photon Fluorescence Probe. Anal. Chem. 2023, 95, 5585–5593. [Google Scholar] [CrossRef]
- Lin, S.P.; Wei, J.X.; Hu, J.S.; Bu, J.Y.; Zhu, L.D.; Li, Q.; Liao, H.J.; Lin, P.Y.; Ye, S.; Chen, S.Q.; et al. Artemisinin improves neurocognitive deficits associated with sepsis by activating the AMPK axis in microglia. Acta Pharmacol. Sin. 2021, 42, 1069–1079. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Mao, M.; Bing, H.; Sun, L.; Xu, W.; Tian, W.; Xia, Z.; Jin, X.; Chu, Q. Sivelestat Sodium Alleviates Ischemia-Reperfusion-Induced Acute Kidney Injury via Suppressing TLR4/Myd88/NF-kappaB Signaling Pathway in Mice. Drug Des. Dev. Ther. 2024, 18, 4449–4458. [Google Scholar] [CrossRef]
- Nie, H.; Xiong, Q.; Lan, G.; Song, C.; Yu, X.; Chen, L.; Wang, D.; Ren, T.; Chen, Z.; Liu, X.; et al. Sivelestat Alleviates Atherosclerosis by Improving Intestinal Barrier Function and Reducing Endotoxemia. Front. Pharmacol. 2022, 13, 838688. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; ZhuGe, D.L.; Tong, M.Q.; Lin, M.T.; Zheng, Y.W.; Jiang, X.; Yang, W.G.; Yao, Q.; Xiang, Q.; Li, X.K.; et al. Ulcerative colitis-specific delivery of keratinocyte growth factor by neutrophils-simulated liposomes facilitates the morphologic and functional recovery of the damaged colon through alleviating the inflammation. J. Control Release 2019, 299, 90–106. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, H.; Long, J.; Lu, Z.; Chun, C.; Li, X. Neutrophil membrane-coated therapeutic liposomes for targeted treatment in acute lung injury. Int. J. Pharm. 2022, 624, 121971. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Shi, C.; Zhao, X.; Gao, L.; Guo, F.; Han, M.; Yang, Z.; Zhang, J.; Tang, C.; et al. Cell membrane derived liposomes loaded with DNase I target neutrophil extracellular traps which inhibits colorectal cancer liver metastases. J. Control Release 2023, 357, 620–629. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Xu, L.; Tu, F.; Rui, X.; Zhang, L.; Yan, Z.; Liu, Y.; Hu, R. Neutrophil membrane-coated nanoparticles exhibit increased antimicrobial activities in an anti-microbial resistant K. pneumonia infection model. Nanomedicine 2023, 48, 102640. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Junho, C.V.C.; Bernhard, S.M.; Schindewolf, M.; Noels, H.; Doring, Y. NETs-Induced Thrombosis Impacts on Cardiovascular and Chronic Kidney Disease. Circ. Res. 2023, 132, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, Y.; Shi, Y.; Zhang, J.; Liu, X.; Liu, Z.; Lv, J.; Leng, Y. The emerging role of neutrophilic extracellular traps in intestinal disease. Gut Pathog. 2022, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Privratsky, J.R.; Ide, S.; Chen, Y.; Kitai, H.; Ren, J.; Fradin, H.; Lu, X.; Souma, T.; Crowley, S.D. A macrophage-endothelial immunoregulatory axis ameliorates septic acute kidney injury. Kidney Int. 2023, 103, 514–528. [Google Scholar] [CrossRef]
- Ushiyama, A.; Kataoka, H.; Iijima, T. Glycocalyx and its involvement in clinical pathophysiologies. J. Intensive Care 2016, 4, 59. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; De Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef]
- Fox, E.D.; Heffernan, D.S.; Cioffi, W.G.; Reichner, J.S. Neutrophils from critically ill septic patients mediate profound loss of endothelial barrier integrity. Crit. Care 2013, 17, R226. [Google Scholar] [CrossRef]
- Wen, L.; Marki, A.; Roy, P.; McArdle, S.; Sun, H.; Fan, Z.; Gingras, A.R.; Ginsberg, M.H.; Ley, K. Kindlin-3 recruitment to the plasma membrane precedes high-affinity beta2-integrin and neutrophil arrest from rolling. Blood 2021, 137, 29–38. [Google Scholar] [CrossRef]
- Butler, L.M.; Khan, S.; Ed Rainger, G.; Nash, G.B. Effects of endothelial basement membrane on neutrophil adhesion and migration. Cell. Immunol. 2008, 251, 56–61. [Google Scholar] [CrossRef]
- Liu, L.; Bai, X.; Martikainen, M.V.; Karlund, A.; Roponen, M.; Xu, W.; Hu, G.; Tasciotti, E.; Lehto, V.P. Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nat. Commun. 2021, 12, 5726. [Google Scholar] [CrossRef]
- Kumar, H.; Choi, H.; Jo, M.J.; Joshi, H.P.; Muttigi, M.; Bonanomi, D.; Kim, S.B.; Ban, E.; Kim, A.; Lee, S.H.; et al. Neutrophil elastase inhibition effectively rescued angiopoietin-1 decrease and inhibits glial scar after spinal cord injury. Acta Neuropathol. Commun. 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef] [PubMed]
- Khakpour, S.; Wilhelmsen, K.; Hellman, J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun. 2015, 21, 827–846. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, J.; Zheng, D.; Su, L.; Peng, T.; Tang, J. Protective role of endothelial calpain knockout in lipopolysaccharide-induced acute kidney injury via attenuation of the p38-iNOS pathway and NO/ROS production. Exp. Mol. Med. 2020, 52, 702–712. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Webber, R.J.; Sweet, R.M.; Webber, D.S. Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations. Int. J. Mol. Sci. 2021, 22, 13371. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Fernando, S.M.; Rochwerg, B.; Seely, A.J.E. Clinical implications of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). CMAJ 2018, 190, E1058–E1059. [Google Scholar] [CrossRef]
- Lopes-Pires, M.E.; Frade-Guanaes, J.O.; Quinlan, G.J. Clotting Dysfunction in Sepsis: A Role for ROS and Potential for Therapeutic Intervention. Antioxidants 2021, 11, 88. [Google Scholar] [CrossRef]
- Teixeira, R.B.; Pfeiffer, M.; Zhang, P.; Shafique, E.; Rayta, B.; Karbasiafshar, C.; Ahsan, N.; Sellke, F.W.; Abid, M.R. Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction. Basic. Res. Cardiol. 2023, 118, 3. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, C.; Di, T.; Chen, L.; Zhao, W.; Wei, L.; Zhou, S.; Wu, X.; Wang, G.; Zhang, Y. Salvianolic acid B alleviates diabetic endothelial and mitochondrial dysfunction by down-regulating apoptosis and mitophagy of endothelial cells. Bioengineered 2022, 13, 3486–3502. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.G.; Wenceslau, C.F.; Goulopoulou, S.; Ogbi, S.; Baban, B.; Sullivan, J.C.; Matsumoto, T.; Webb, R.C. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc. Res. 2015, 107, 119–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Zhong, A.; Zhang, Y.; Deng, E.; Mo, H.; Zhao, H.; Huang, J.; Peng, H.; Zhang, K.; Chen, X.; et al. Sivelestat-Loaded Neutrophil-Membrane-Coated Antioxidative Nanoparticles for Targeted Endothelial Protection in Sepsis. Pharmaceutics 2025, 17, 766. https://doi.org/10.3390/pharmaceutics17060766
Wei J, Zhong A, Zhang Y, Deng E, Mo H, Zhao H, Huang J, Peng H, Zhang K, Chen X, et al. Sivelestat-Loaded Neutrophil-Membrane-Coated Antioxidative Nanoparticles for Targeted Endothelial Protection in Sepsis. Pharmaceutics. 2025; 17(6):766. https://doi.org/10.3390/pharmaceutics17060766
Chicago/Turabian StyleWei, Juexian, Aijia Zhong, Yuting Zhang, Ehua Deng, Hengzong Mo, Hongyu Zhao, Jiayu Huang, Huaidong Peng, Kaiyin Zhang, Xiaohui Chen, and et al. 2025. "Sivelestat-Loaded Neutrophil-Membrane-Coated Antioxidative Nanoparticles for Targeted Endothelial Protection in Sepsis" Pharmaceutics 17, no. 6: 766. https://doi.org/10.3390/pharmaceutics17060766
APA StyleWei, J., Zhong, A., Zhang, Y., Deng, E., Mo, H., Zhao, H., Huang, J., Peng, H., Zhang, K., Chen, X., Mao, H., Chen, Y., & Zhu, Y. (2025). Sivelestat-Loaded Neutrophil-Membrane-Coated Antioxidative Nanoparticles for Targeted Endothelial Protection in Sepsis. Pharmaceutics, 17(6), 766. https://doi.org/10.3390/pharmaceutics17060766






