Advances in Transdermal Drug Delivery Systems and Clinical Applications in Inflammatory Skin Diseases
Abstract
1. Introduction
2. Barriers to Drug Delivery to Local Skin
3. Development and Evolution of TDDSs
3.1. Sonophoresis
3.2. Iontophoresis
3.3. Chemical Penetration Enhancer
3.4. Electroporation
3.5. Microneedling
3.5.1. Solid Microneedles
3.5.2. Dissolved Microneedles
3.5.3. Hydrogel Microneedles
3.5.4. Coated Microneedles
3.5.5. Hollow Microneedles
3.6. Transdermal Drug Delivery Using Nanocarriers
3.6.1. Liposomes
3.6.2. Lipid Nanoparticles
3.6.3. Bicelles
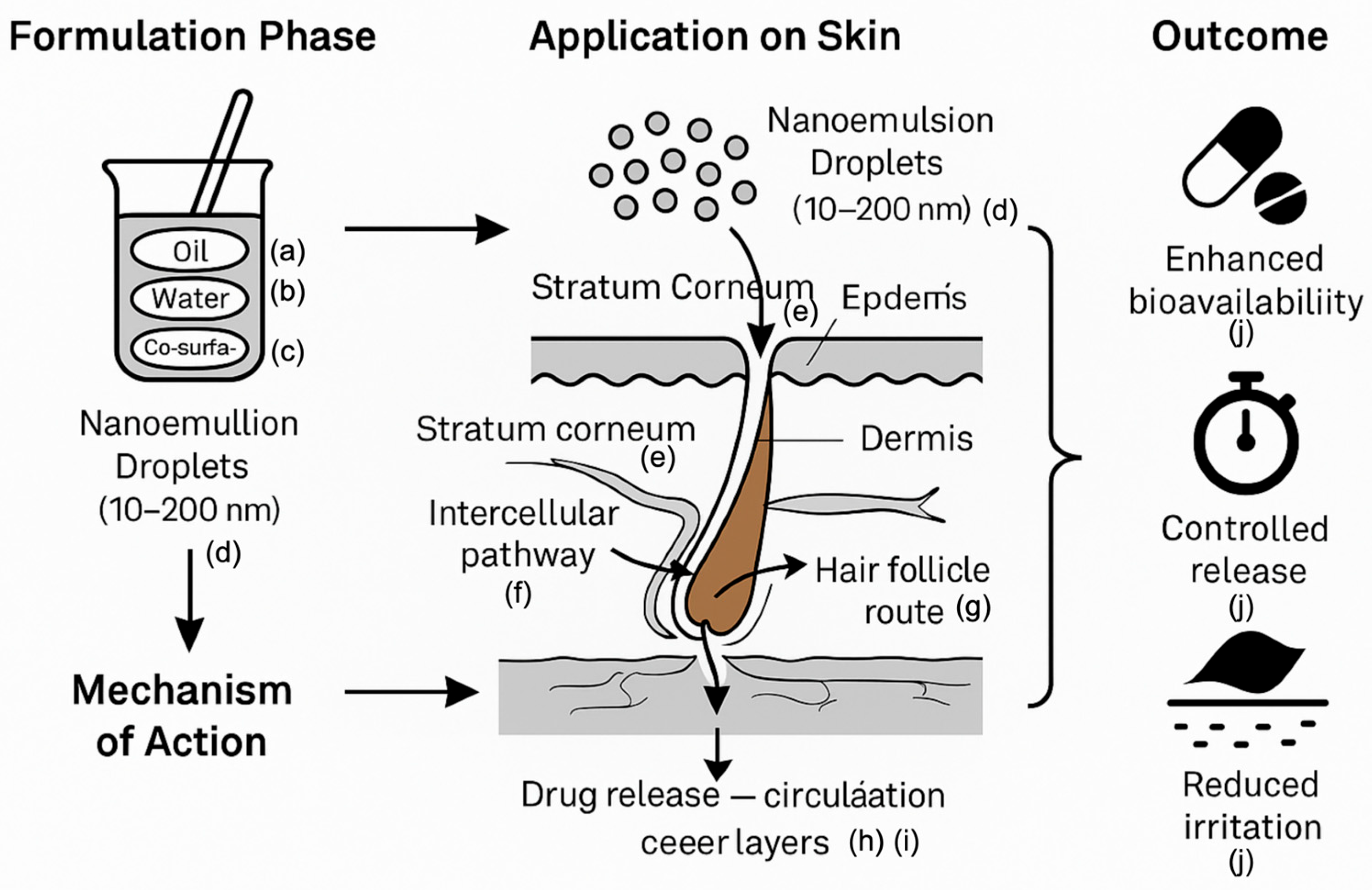
3.6.4. Nanoemulsions
4. Application of Transdermal Drug Delivery Systems in Inflammatory Skin Diseases
4.1. Overview of Inflammatory Skin Diseases
4.2. Application of Transdermal Drug Delivery System in Inflammatory Skin Diseases
4.3. Limitations of Transdermal Drug Delivery System Under Inflammatory Conditions
5. Limitations and Future Prospects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magnusson, B.M.; Walters, K.A.; Roberts, M.S. Veterinary Drug Delivery: Potential for Skin Penetration Enhancement. Adv. Drug Deliv. Rev. 2001, 50, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced Nanocarrier- and Microneedle-Based Transdermal Drug Delivery Strategies for Skin Diseases Treatment. Theranostics 2022, 12, 3372–3406. [Google Scholar] [CrossRef] [PubMed]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming Skin Barriers through Advanced Transdermal Drug Delivery Approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent Advances in Polymeric Transdermal Drug Delivery Systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin Permeabilization for Transdermal Drug Delivery: Recent Advances and Future Prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef]
- Tan, J.K.L.; Bhate, K. A Global Perspective on the Epidemiology of Acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef]
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef]
- Pickett, K.; Loveman, E.; Kalita, N.; Frampton, G.K.; Jones, J. Educational Interventions to Improve Quality of Life in People with Chronic Inflammatory Skin Diseases: Systematic Reviews of Clinical Effectiveness and Cost-Effectiveness. Health Technol. Assess. 2015, 19, 1–176. [Google Scholar] [CrossRef]
- García-Solano, J.; López-Avila, A.; Acosta, J.; Pérez-Guillermo, M. Diagnostic Cost-Effectiveness of the Skin Biopsy in Inflammatory Diseases of the Skin. Actas Dermosifiliogr. 2005, 96, 92–97. [Google Scholar] [CrossRef]
- Guo, J.-W.; Jee, S.-H. Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int. J. Mol. Sci. 2021, 22, 6078. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Banga, A.K. Advanced Transdermal Drug Delivery System: A Comprehensive Review of Microneedle Technologies, Novel Designs, Diverse Applications, and Critical Challenges. Int. J. Pharm. 2025, 670, 125118. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-Mediated Transdermal Drug Delivery for Treating Diverse Skin Diseases. Acta Biomater. 2021, 121, 119–133. [Google Scholar] [CrossRef]
- Qindeel, M.; Ullah, M.H.; Fakhar-Ud-Din; Ahmed, N.; Rehman, A.U. Recent Trends, Challenges and Future Outlook of Transdermal Drug Delivery Systems for Rheumatoid Arthritis Therapy. J. Control. Release 2020, 327, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xiao, W.; Xu, K.; He, Y.; Miao, X.; Dong, Y.; Sun, L. Potential Strategy of Microneedle-Based Transdermal Drug Delivery System for Effective Management of Skin-Related Immune Disorders. Eur. J. Pharm. Biopharm. 2024, 195, 114148. [Google Scholar] [CrossRef]
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal Patches: Design and Current Approaches to Painless Drug Delivery. Acta Pharm. 2019, 69, 197–215. [Google Scholar] [CrossRef]
- Wong, W.F.; Ang, K.P.; Sethi, G.; Looi, C.Y. Recent Advancement of Medical Patch for Transdermal Drug Delivery. Medicina 2023, 59, 778. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential Oils and Their Constituents as Skin Penetration Enhancer for Transdermal Drug Delivery: A Review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Bajza, Á.; Kocsis, D.; Berezvai, O.; Laki, A.J.; Lukács, B.; Imre, T.; Iván, K.; Szabó, P.; Erdő, F. Verification of P-Glycoprotein Function at the Dermal Barrier in Diffusion Cells and Dynamic “Skin-on-a-Chip” Microfluidic Device. Pharmaceutics 2020, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Mardhiah Adib, Z.; Ghanbarzadeh, S.; Kouhsoltani, M.; Yari Khosroshahi, A.; Hamishehkar, H. The Effect of Particle Size on the Deposition of Solid Lipid Nanoparticles in Different Skin Layers: A Histological Study. Adv. Pharm. Bull. 2016, 6, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Meinke, M.C.; Lange-Asschenfeldt, B.; Antoniou, C.; Mak, W.C.; Renneberg, R.; Sterry, W.; Patzelt, A. Drug Delivery with Topically Applied Nanoparticles: Science Fiction or Reality? Skin Pharmacol. Physiol. 2013, 26, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and Microparticles for Skin Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Supe, S.; Takudage, P. Methods for Evaluating Penetration of Drug into the Skin: A Review. Skin Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Honari, G. Skin Structure and Function. In Sensitive Skin Syndrome; Honari, G., Andersen, R.M., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 16–22. ISBN 978-1-315-12104-8. [Google Scholar]
- Kitaoka, M.; Wakabayashi, R.; Kamiya, N.; Goto, M. Solid-in-oil Nanodispersions for Transdermal Drug Delivery Systems. Biotechnol. J. 2016, 11, 1375–1385. [Google Scholar] [CrossRef]
- Danso, M.O.; Berkers, T.; Mieremet, A.; Hausil, F.; Bouwstra, J.A. An Ex Vivo Human Skin Model for Studying Skin Barrier Repair. Exp. Dermatol. 2015, 24, 48–54. [Google Scholar] [CrossRef]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef]
- Kim, T.I.; Park, H.-J.; Won, Y.-Y.; Choi, H.; Jeong, K.-H.; Sung, J.-Y.; Shin, M.K. Basement Membrane Status Is Intact in Urticarial Dermatitis vs. Adult-Onset Atopic Dermatitis. Ann. Dermatol. 2018, 30, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ghosh, P.; Li, S.K.; Newman, B.; Kasting, G.B.; Raney, S.G. Heat Effects on Drug Delivery across Human Skin. Expert Opin. Drug Deliv. 2016, 13, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Shomaker, T.S.; Zhang, J.; Ashburn, M.A. Assessing the Impact of Heat on the Systemic Delivery of Fentanyl through the Transdermal Fentanyl Delivery System. Pain Med. 2000, 1, 225–230. [Google Scholar] [CrossRef]
- Petersen, K.K.; Rousing, M.L.; Jensen, C.; Arendt-Nielsen, L.; Gazerani, P. Effect of Local Controlled Heat on Transdermal Delivery of Nicotine. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 236–242. [Google Scholar]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal Drug Delivery: Overcoming the Skin’s Barrier Function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Stefanov, S.R.; Andonova, V.Y. Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders. Pharmaceuticals 2021, 14, 1083. [Google Scholar] [CrossRef]
- Kushwaha, R.; Palei, N.N. Transdermal Drug Delivery Systems: Different Generations and Dermatokinetic Assessment of Drug Concentration in Skin. Pharm. Med. 2024, 38, 407–427. [Google Scholar] [CrossRef]
- Joshi, N.; Azizi Machekposhti, S.; Narayan, R.J. Evolution of Transdermal Drug Delivery Devices and Novel Microneedle Technologies: A Historical Perspective and Review. JID Innov. 2023, 3, 100225. [Google Scholar] [CrossRef]
- Uchida, N.; Yanagi, M.; Hamada, H. Physical Enhancement? Nanocarrier? Current Progress in Transdermal Drug Delivery. Nanomaterials 2021, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Janet, A.T.; Michael, J.T. Glucose Monitoring by Reverse Iontophoresis. Diabetes Metab. Res. Rev. 2002, 18, S49–S53. [Google Scholar] [CrossRef]
- Lavon, I.; Kost, J. Ultrasound and Transdermal Drug Delivery. Drug Discov. Today 2004, 9, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Vranić, E. Sonophoresis-Mechanisms and Application. Bosn. J. Basic Med. Sci. 2008, 4, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, H.; Seo, J.; Lee, S. Sonophoresis in Transdermal Drug Deliverys. Ultrasonics 2014, 54, 56–65. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, Z. Advances in Biological Application of and Research on Low-Frequency Ultrasound. Ultrasound Med. Biol. 2021, 47, 2839–2852. [Google Scholar] [CrossRef]
- Katz, N.P.; Shapiro, D.E.; Herrmann, T.E.; Kost, J.; Custer, L.M. Rapid Onset of Cutaneous Anesthesia with EMLA Cream after Pretreatment with a New Ultrasound-Emitting Device. Anesth. Analg. 2004, 98, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Alsalhi, W.; Alalola, A.; Randolph, M.; Gwillim, E.; Tosti, A. Novel Drug Delivery Approaches for the Management of Hair Loss. Expert Opin. Drug Deliv. 2020, 17, 287–295. [Google Scholar] [CrossRef]
- Marathe, D.; Bhuvanashree, V.S.; Mehta, C.H.; T., A.; Nayak, U.Y. Low-frequency Sonophoresis: A Promising Strategy for Enhanced Transdermal Delivery. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 1247450. [Google Scholar] [CrossRef]
- Bhatia, K.S.; Gao, S.; Freeman, T.P.; Singh, J. Effect of Penetration Enhancers and Iontophoresis on the Ultrastructure and Cholecystokinin-8 Permeability through Porcine Skin. J. Pharm. Sci. 1997, 86, 1011–1015. [Google Scholar] [CrossRef]
- Delgado-Charro, M.B.; Guy, R.H. Iontophoresis of Peptides. In Electronically Controlled Drug Delivery; Berner, B., Dinh, S.M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 129–158. ISBN 978-0-429-26283-8. [Google Scholar]
- Masada, T.; Higuchi, W.; Srinivasan, V.; Rohr, U.; Fox, J.; Behl, C.; Pons, S. Examination of Iontophoretic Transport of Ionic Drugs across Skin: Baseline Studies with the Four-Electrode System. Int. J. Pharm. 1989, 49, 57–62. [Google Scholar] [CrossRef]
- Dixit, N.; Bali, V.; Baboota, S.; Ahuja, A.; Ali, J. Iontophoresis—An Approach for Controlled Drug Delivery: A Review. Curr. Drug Deliv. 2007, 4, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Quan, P.; Li, S.; Liu, C.; Zhao, Y.; Zhao, Y.; Fang, L. Time Dependence of the Enhancement Effect of Chemical Enhancers: Molecular Mechanisms of Enhancing Kinetics. J. Control. Release 2017, 248, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.C.; Kearney, M.-C.; Quinn, H.L.; Courtenay, A.J.; Donnelly, R.F. Future of the Transdermal Drug Delivery Market—Have We Barely Touched the Surface? Expert Opin. Drug Deliv. 2016, 13, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of Penetration Potential of pH Responsive Double Walled Biodegradable Nanogels Coated with Eucalyptus Oil for the Controlled Delivery of 5-Fluorouracil: In Vitro and Ex Vivo Studies. J. Control. Release 2017, 253, 122–136. [Google Scholar] [CrossRef]
- Vitorino, C.; Almeida, J.; Gonçalves, L.M.; Almeida, A.J.; Sousa, J.J.; Pais, A.A.C.C. Co-Encapsulating Nanostructured Lipid Carriers for Transdermal Application: From Experimental Design to the Molecular Detail. J. Control. Release 2013, 167, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.; Yang, X.; Zhai, G. Breaking the Skin Barrier: Achievements and Future Directions. Curr. Pharm. Des. 2015, 21, 2713–2724. [Google Scholar] [CrossRef]
- Taghizadeh, S.M.; Moghimi-Ardakani, A.; Mohamadnia, F. A Statistical Experimental Design Approach to Evaluate the Influence of Various Penetration Enhancers on Transdermal Drug Delivery of Buprenorphine. J. Adv. Res. 2015, 6, 155–162. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, F.; Zhang, Q.; Liu, J.; Quan, D.; Song, W. Molecular Perspective of Efficiency and Safety Problems of Chemical Enhancers: Bottlenecks and Recent Advances. Drug Deliv. Transl. Res. 2022, 12, 1376–1394. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Li, R.; Pang, L.; Zhu, S.; Ma, J.; Du, L.; Jin, Y. Electroporation-Enhanced Transdermal Drug Delivery: Effects of logP, pKa, Solubility and Penetration Time. Eur. J. Pharm. Sci. 2020, 151, 105410. [Google Scholar] [CrossRef]
- Tsoneva, I.; Semkova, S.; Bakalova, R.; Zhelev, Z.; Nuss, P.; Staneva, G.; Nikolova, B. Electroporation, Electrochemotherapy and Electro-Assisted Drug Delivery in Cancer: A State-of-the-Art Review. Biophys. Chem. 2022, 286, 106819. [Google Scholar] [CrossRef]
- Wong, T.W. Electrical, Magnetic, Photomechanical and Cavitational Waves to Overcome Skin Barrier for Transdermal Drug Delivery. J. Control. Release 2014, 193, 257–269. [Google Scholar] [CrossRef]
- Narasimha Murthy, S.; Sen, A.; Zhao, Y.-L.; Hui, S.W. Temperature Influences the Postelectroporation Permeability State of the Skin. J. Pharm. Sci. 2004, 93, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Aqil, M.; Talegaonkar, S.; Azeem, A.; Sultana, Y.; Ali, A. Enhanced Transdermal Drug Delivery Techniques: An Extensive Review of Patents. Recent Pat. Drug Deliv. Formul. 2009, 3, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-Based Drug Delivery Systems: Microfabrication, Drug Delivery, and Safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, C.; Awosika, O.; Rengifo-Pardo, M.; Ehrlich, A. Review of Applications of Microneedling in Dermatology. Clin. Cosmet. Investig. Dermatol. 2017, 10, 289–298. [Google Scholar] [CrossRef]
- Ilyas, M.; Zhang, N.; Sharma, A. Residual Squamous Cell Carcinoma After Shave Biopsy in Solid Organ Transplant Recipients. Dermatol. Surg. 2018, 44, 370–374. [Google Scholar] [CrossRef]
- Keen, M.; Hassan, I. Vitamin E in Dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for Drug and Vaccine Delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Cengiz, E.; Tamborlane, W.V. A Tale of Two Compartments: Interstitial Versus Blood Glucose Monitoring. Diabetes Technol. Ther. 2009, 11, S-11–S-16. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of Skin Resealing after Insertion of Microneedles in Human Subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumar, K.B.; Hegde, G.M.; Ramachandra, S.G.; Nayak, M.M.; Dinesh, N.S.; Rajanna, K. Development of Cup Shaped Microneedle Array for Transdermal Drug Delivery. Biointerphases 2015, 10, 021008. [Google Scholar] [CrossRef]
- Olatunji, O.; Igwe, C.C.; Ahmed, A.S.; Alhassan, D.O.A.; Asieba, G.O.; Diganta, B.D. Microneedles from Fish Scale Biopolymer. J. Appl. Polym. Sci. 2014, 131, 40377. [Google Scholar] [CrossRef]
- Kaur, M.; Ita, K.B.; Popova, I.E.; Parikh, S.J.; Bair, D.A. Microneedle-Assisted Delivery of Verapamil Hydrochloride and Amlodipine Besylate. Eur. J. Pharm. Biopharm. 2014, 86, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Ita, K.B.; Parikh, S.J.; Popova, I.E.; Bair, D.A. Transdermal Delivery of Captopril and Metoprolol Tartrate with Microneedles. Drug Deliv. Lett. 2014, 4, 236–243. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, G.; Dong, P.; Gong, Z.; Li, G.; Zhang, K.; Wu, C. Investigation on Fabrication Process of Dissolving Microneedle Arrays to Improve Effective Needle Drug Distribution. Eur. J. Pharm. Sci. 2015, 66, 148–156. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Koutsonanos, D.G.; Del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving Polymer Microneedle Patches for Influenza Vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and Biodegradable Microneedle Technologies for Transdermal Sustained Delivery of Drug and Vaccine. Drug Des. Dev. Ther. 2013, 2013, 945–952. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, Z.; Zhao, Y.; Zhang, Z.; Li, L.; Ding, D.; Guo, J.; Zhang, J.; Liu, H.; et al. A Transdermal Drug Delivery System Based on Dissolving Microneedles for Boron Neutron Capture Therapy of Melanoma. Biomater. Sci. 2023, 11, 7568–7578. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Gao, W.; Zhang, L.; Wei, F.; Liu, H.; Wang, S.; Li, Y.; Zhao, W.; Ma, T.; et al. Combination and Efficiency: Preparation of Dissolving Microneedles Array Loaded with Two Active Ingredients and Its Anti-Pigmentation Effects on Guinea Pigs. Eur. J. Pharm. Sci. 2021, 160, 105749. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Alkilani, A.Z.; McCrudden, M.T.C.; O’Neill, S.; O’Mahony, C.; Armstrong, K.; McLoone, N.; Kole, P.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays Exhibit Antimicrobial Properties: Potential for Enhanced Patient Safety. Int. J. Pharm. 2013, 451, 76–91. [Google Scholar] [CrossRef]
- Tekko, I.A.; Chen, G.; Domínguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larrañeta, E.; McElnay, J.C.; McCarthy, H.O.; Rooney, M.; et al. Development and Characterisation of Novel Poly (Vinyl Alcohol)/Poly (Vinyl Pyrrolidone)-Based Hydrogel-Forming Microneedle Arrays for Enhanced and Sustained Transdermal Delivery of Methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Prausnitz, M.R. Separable Arrowhead Microneedles. J. Control. Release 2011, 149, 242–249. [Google Scholar] [CrossRef]
- Khan, H.; Mehta, P.; Msallam, H.; Armitage, D.; Ahmad, Z. Smart Microneedle Coatings for Controlled Delivery and Biomedical Analysis. J. Drug Target. 2014, 22, 790–795. [Google Scholar] [CrossRef]
- Haj-Ahmad, R.; Khan, H.; Arshad, M.; Rasekh, M.; Hussain, A.; Walsh, S.; Li, X.; Chang, M.-W.; Ahmad, Z. Microneedle Coating Techniques for Transdermal Drug Delivery. Pharmaceutics 2015, 7, 486–502. [Google Scholar] [CrossRef]
- DeMuth, P.C.; Min, Y.; Huang, B.; Kramer, J.A.; Miller, A.D.; Barouch, D.H.; Hammond, P.T.; Irvine, D.J. Polymer Multilayer Tattooing for Enhanced DNA Vaccination. Nat. Mater. 2013, 12, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; Van Den Berg, D.J.; Dijksman, J.A.; Van Hecke, M.; Verpoorten, H.; Van Den Berg, A.; Luttge, R.; Bouwstra, J.A. Improved Piercing of Microneedle Arrays in Dermatomed Human Skin by an Impact Insertion Method. J. Control. Release 2008, 128, 80–88. [Google Scholar] [CrossRef]
- Yuzhakov, V.V. The AdminPenTM Microneedle Device for Painless & Convenient Drug Delivery. Drug Deliv. Technol. 2010, 10, 32–36. [Google Scholar]
- Lyon, B.J.; Aria, A.I.; Gharib, M. Fabrication of Carbon Nanotube—Polyimide Composite Hollow Microneedles for Transdermal Drug Delivery. Biomed. Microdevices 2014, 16, 879–886. [Google Scholar] [CrossRef]
- Cheung, K.; Das, D.B. Microneedles for Drug Delivery: Trends and Progress. Drug Deliv. 2016, 23, 2338–2354. [Google Scholar] [CrossRef] [PubMed]
- Van Der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle Technologies for (Trans)Dermal Drug and Vaccine Delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Kher, G.; Trehan, S.; Misra, A. Antisense Oligonucleotides and RNA Interference. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 325–386. ISBN 978-0-12-384964-9. [Google Scholar]
- Torchilin, V. Antibody-Modified Liposomes for Cancer Chemotherapy. Expert Opin. Drug Deliv. 2008, 5, 1003–1025. [Google Scholar] [CrossRef]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Increasing Bioavailability of Silymarin Using a Buccal Liposomal Delivery System: Preparation and Experimental Design Investigation. Int. J. Pharm. 2006, 308, 140–148. [Google Scholar] [CrossRef]
- Zhang, Z.; Mei, L.; Feng, S.-S. Paclitaxel Drug Delivery Systems. Expert Opin. Drug Deliv. 2013, 10, 325–340. [Google Scholar] [CrossRef]
- Yi, H.; Lu, W.; Liu, F.; Zhang, G.; Xie, F.; Liu, W.; Wang, L.; Zhou, W.; Cheng, Z. ROS-Responsive Liposomes with NIR Light-Triggered Doxorubicin Release for Combinatorial Therapy of Breast Cancer. J. Nanobiotechnol. 2021, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Elsamaligy, M.; Afifi, N.; Mahmoud, E. Evaluation of Hybrid Liposomes-Encapsulated Silymarin Regarding Physical Stability and in Vivo Performance. Int. J. Pharm. 2006, 319, 121–129. [Google Scholar] [CrossRef]
- Mehnert, W. Solid Lipid Nanoparticles Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Luo, H.; Lu, L.; Liu, N.; Li, Q.; Yang, X.; Zhang, Z. Curcumin Loaded Sub-30 Nm Targeting Therapeutic Lipid Nanoparticles for Synergistically Blocking Nasopharyngeal Cancer Growth and Metastasis. J. Nanobiotechnol. 2021, 19, 224. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated Polymer Nanoparticles: Preparation, Properties, Functionalization and Biological Applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.-N.; Ma, W.; Yang, Y.; Chen, G.; Fu, H.; Cui, C.; Yu, Z.; Wang, X. Multifunctional AIE Iridium (III) Photosensitizer Nanoparticles for Two-Photon-Activated Imaging and Mitochondria Targeting Photodynamic Therapy. J. Nanobiotechnol. 2021, 19, 254. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric Nano- and Microparticulate Drug Delivery Systems for Treatment of Biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and Polymer Nanoparticles for Drug Delivery to Bacterial Biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Tacrolimus-Loaded Chitosan Nanoparticles for Enhanced Skin Deposition and Management of Plaque Psoriasis. Carbohydr. Polym. 2021, 268, 118238. [Google Scholar] [CrossRef]
- Chaudhary, H.; Kohli, K.; Kumar, V. A Novel Nano-Carrier Transdermal Gel against Inflammation. Int. J. Pharm. 2014, 465, 175–186. [Google Scholar] [CrossRef]
- Hensel, J.K.; Carpenter, A.P.; Ciszewski, R.K.; Schabes, B.K.; Kittredge, C.T.; Moore, F.G.; Richmond, G.L. Molecular Characterization of Water and Surfactant AOT at Nanoemulsion Surfaces. Proc. Natl. Acad. Sci. USA 2017, 114, 13351–13356. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Chen, Y.; Li, X.; Chen, H.; Liu, J.; Yang, J.; Cao, Y.; Li, M.; Ma, J.; et al. Hydroxyl-Based Acid-Hypersensitive Acetal Ester Bond: Design, Synthesis and the Application Potential in Nanodrugs. Eur. J. Med. Chem. 2025, 283, 117153. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Zuo, Y.; Wang, M.; Chen, H.; Wang, C.; Cao, Y.; Zeng, Y.; Chen, Y.; Zhang, T.; et al. Dual Targeting of FR+CD44 Overexpressing Tumors by Self-Assembled Nanoparticles Quantitatively Conjugating Folic Acid-Hyaluronic Acid to the GSH-Sensitively Modified Podophyllotoxin. Chem. Eng. J. 2025, 505, 159276. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Soliva-Fortuny, R.; Rojas-Graü, M.A.; McClements, D.J.; Martín-Belloso, O. Edible Nanoemulsions as Carriers of Active Ingredients: A Review. Annu. Rev. Food Sci. Technol. 2017, 8, 439–466. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Anton, N.; Akram, S.; Er-Rafik, M.; Anton, H.; Klymchenko, A.; Yu, W.; Vandamme, T.F.; Serra, C.A. A New Method for the Formulation of Double Nanoemulsions. Soft Matter 2017, 13, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, C.; Navarini, A.A. The Continuing Evolution of Targeted Therapy for Inflammatory Skin Disease. Semin. Immunopathol. 2016, 38, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of Atopic Dermatitis: Clinical Implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Dubertret, L. Retinoids, Methotrexate and Cyclosporine. In Current Problems in Dermatology; Yawalkar, N., Ed.; KARGER: Basel, Switzerland, 2009; Volume 38, pp. 79–94. ISBN 978-3-8055-9151-5. [Google Scholar]
- West, D.; Roberts, A.M.; Stroebel, B.; Abuabara, K. The Epidemiology of Inflammatory Skin Disease in Older Adults. JAAD Int. 2025, 18, 151–153. [Google Scholar] [CrossRef]
- Polaskey, M.T.; Chang, C.H.; Daftary, K.; Fakhraie, S.; Miller, C.H.; Chovatiya, R. The Global Prevalence of Seborrheic Dermatitis: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2024, 160, 846–855. [Google Scholar] [CrossRef]
- Cipriani, F.; Dondi, A.; Ricci, G. Recent Advances in Epidemiology and Prevention of Atopic Eczema. Pediatr. Allergy Immunol. 2014, 25, 630–638. [Google Scholar] [CrossRef]
- Gao, J.; Shen, X.; Ko, R.; Huang, C.; Shen, C. Cognitive Process of Psoriasis and Its Comorbidities: From Epidemiology to Genetics. Front. Genet. 2021, 12, 735124. [Google Scholar] [CrossRef]
- Hoegsberg, T.; Iversen, L.; Lange, M.M.; Bissonette, R.; Carvalho, A.V.E.; Van De Kerkhof, P.C.; Kirby, B.; Kleyn, C.E.; Van Der Walt, J.M.; Wu, J.J.; et al. Topical Treatment of Psoriasis: Questionnaire Results on Topical Therapy as Long-Term Continuous Treatment and Use on Specific Body Sites. J. Dermatol. Treat. 2021, 32, 916–921. [Google Scholar] [CrossRef]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.H.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient Enhancement of the Skin Barrier from Birth Offers Effective Atopic Dermatitis Prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef]
- Chalmers, J.R.; Haines, R.H.; Bradshaw, L.E.; Montgomery, A.A.; Thomas, K.S.; Brown, S.J.; Ridd, M.J.; Lawton, S.; Simpson, E.L.; Cork, M.J.; et al. Daily Emollient during Infancy for Prevention of Eczema: The BEEP Randomised Controlled Trial. Lancet 2020, 395, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of Care for the Management of Atopic Dermatitis. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A pH/ROS Dual-Responsive and Targeting Nanotherapy for Vascular Inflammatory Diseases. Biomaterials 2020, 230, 119605. [Google Scholar] [CrossRef] [PubMed]
- Titus, S.; Hodge, J. Diagnosis and Treatment of Acne. Am. Fam. Physician 2012, 86, 734–740. [Google Scholar] [PubMed]
- Sidbury, R.; Paller, A.S. The Diagnosis and Management of Acne. Pediatr. Ann. 2000, 29, 17–24. [Google Scholar] [CrossRef]
- Krautheim, A.; Gollnick, H. Transdermal Penetration of Topical Drugs Used in the Treatment of Acne. Clin. Pharmacokinet. 2003, 42, 1287–1304. [Google Scholar] [CrossRef]
- Manouchehri, A.; Abbaszadeh, S.; Ahmadi, M.; Nejad, F.K.; Bahmani, M.; Dastyar, N. Polycystic Ovaries and Herbal Remedies: A Systematic Review. JBRA Assist. Reprod. 2022, 27, 85–91. [Google Scholar] [CrossRef]
- Magin, P.J.; Adams, J.; Pond, C.D.; Smith, W. Topical and Oral CAM in Acne: A Review of the Empirical Evidence and a Consideration of Its Context. Complement. Ther. Med. 2006, 14, 62–76. [Google Scholar] [CrossRef]
- Fisk, W.A.; Lev-Tov, H.A.; Sivamani, R.K. Botanical and Phytochemical Therapy of Acne: A Systematic Review. Phytother. Res. 2014, 28, 1137–1152. [Google Scholar] [CrossRef]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.M.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of Treatment Goals for Moderate to Severe Psoriasis: A European Consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef]
- West, J.; Ogston, S.; Berg, J.; Palmer, C.; Fleming, C.; Kumar, V.; Foerster, J. HLA-Cw6-Positive Patients with Psoriasis Show Improved Response to Methotrexate Treatment. Clin. Exp. Dermatol. 2017, 42, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.C.Y.; Griffiths, C.E.M.; Berth-Jones, J.; Papp, K.A.; Vanaclocha, F.; Dauden, E.; Beard, A.; Puvanarajan, L.; Paul, C. Intermittent Short Courses of Cyclosporine Microemulsion for the Long-Term Management of Psoriasis: A 2-Year Cohort Study. J. Am. Acad. Dermatol. 2001, 44, 643–651. [Google Scholar] [CrossRef]
- Schafer, P.; Parton, A.; Gandhi, A.; Capone, L.; Adams, M.; Wu, L.; Bartlett, J.; Loveland, M.; Gilhar, A.; Cheung, Y.; et al. Apremilast, a cAMP Phosphodiesterase-4 Inhibitor, Demonstrates Anti-inflammatory Activity in Vitro and in a Model of Psoriasis. Br. J. Pharmacol. 2010, 159, 842–855. [Google Scholar] [CrossRef]
- Oehrl, S.; Prakash, H.; Ebling, A.; Trenkler, N.; Wölbing, P.; Kunze, A.; Döbel, T.; Schmitz, M.; Enk, A.; Schäkel, K. The Phosphodiesterase 4 Inhibitor Apremilast Inhibits Th1 but Promotes Th17 Responses Induced by 6-Sulfo LacNAc (Slan) Dendritic Cells. J. Dermatol. Sci. 2017, 87, 110–115. [Google Scholar] [CrossRef]
- Gesser, B.; Johansen, C.; Rasmussen, M.K.; Funding, A.T.; Otkjaer, K.; Kjellerup, R.B.; Kragballe, K.; Iversen, L. Dimethylfumarate Specifically Inhibits the Mitogen and Stress-Activated Kinases 1 and 2 (MSK1/2): Possible Role for Its Anti-Psoriatic Effect. J. Investig. Dermatol. 2007, 127, 2129–2137. [Google Scholar] [CrossRef]
- Harper, R.A. Specificity in the Synergism between Retinoic Acid and EGF on the Growth of Adult Human Skin Fibroblasts. Exp. Cell Res. 1988, 178, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Brand, N.; Petkovich, M.; Krust, A.; Chambon, P.; De Thé, H.; Marchio, A.; Tiollais, P.; Dejean, A. Identification of a Second Human Retinoic Acid Receptor. Nature 1988, 332, 850–853. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.D.; McLean, W.H.I.; Leung, D.Y.M. Filaggrin Mutations Associated with Skin and Allergic Diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef]
- Lowe, A.J.; Leung, D.Y.M.; Tang, M.L.K.; Su, J.C.; Allen, K.J. The Skin as a Target for Prevention of the Atopic March. Ann. Allergy. Asthma Immunol. 2018, 120, 145–151. [Google Scholar] [CrossRef]
- Obaidat, R.; Shameh, A.A.; Aljarrah, M.; Hamed, R. Preparation and Evaluation of Polyvinylpyrrolidone Electrospun Nanofiber Patches of Pioglitazone for the Treatment of Atopic Dermatitis. AAPS PharmSciTech 2022, 23, 51. [Google Scholar] [CrossRef]
- Jeong, J.H.; Back, S.K.; An, J.H.; Lee, N.; Kim, D.; Na, C.S.; Jeong, Y.; Han, S.Y. Topical Film Prepared with Rhus Verniciflua Extract-Loaded Pullulan Hydrogel for Atopic Dermatitis Treatment. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-Functional Transdermal Drug Delivery System with Controllable Drug Loading Based on Thermosensitive Poloxamer Hydrogel for Atopic Dermatitis Treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, B.Z.; Zhang, X.P.; Zheng, H.; Li, Z.; Zhang, C.Y.; Guo, X.D. Conductive Microneedle Patch with Electricity-Triggered Drug Release Performance for Atopic Dermatitis Treatment. ACS Appl. Mater. Interfaces 2022, 14, 31645–31654. [Google Scholar] [CrossRef]
- Choi, J.-H.; Song, Y.-S.; Lee, H.-J.; Kim, G.-C.; Hong, J.-W. The Topical Application of Low-Temperature Argon Plasma Enhances the Anti-Inflammatory Effect of Jaun-Ointment on DNCB-Induced NC/Nga Mice. BMC Complement. Altern. Med. 2017, 17, 340. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of Triptolide-Nanoemulsion Gels for Percutaneous Administration: Physicochemical, Transport, Pharmacokinetic and Pharmacodynamic Characteristics. J. Nanobiotechnol. 2017, 15, 88. [Google Scholar] [CrossRef]
- Latif, M.S.; Al-Harbi, F.F.; Nawaz, A.; Rashid, S.A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N. Formulation and Evaluation of Hydrophilic Polymer Based Methotrexate Patches: In Vitro and in Vivo Characterization. Polymers 2022, 14, 1310. [Google Scholar] [CrossRef]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.H.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Wu, C.; Tong, X.; Shi, Y.; Chen, S. Microneedle Patch Delivery of Methotrexate-Loaded Albumin Nanoparticles to Immune Cells Achieves a Potent Antipsoriatic Effect. Int. J. Nanomed. 2022, 17, 3841–3851. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Yin, Y.; Li, J.; Wang, M.; Liu, Y.; Ren, X. Advancements in the Transdermal Drug Delivery Systems Utilizing Microemulsion-Based Gels. Curr. Pharm. Des. 2024, 30, 2753–2764. [Google Scholar] [CrossRef]
- Dahmash, E.Z.; Attiany, L.M.; Ali, D.; Assaf, S.M.; Alkrad, J.; Alyami, H. Development and Characterization of Transdermal Patches Using Novel Thymoquinone-L-Arginine-Based Polyamide Nanocapsules for Potential Use in the Management of Psoriasis. AAPS PharmSciTech 2024, 25, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, X.; Song, Y.; Lan, L.; Wang, J.; Xu, X.; Du, Y. Nano Transdermal System Combining Mitochondria-Targeting Cerium Oxide Nanoparticles with All-Trans Retinoic Acid for Psoriasis. Asian J. Pharm. Sci. 2023, 18, 100846. [Google Scholar] [CrossRef]
- Zhan, Z.-Y.; Zhang, Z.-H.; Sun, R.-H.; Wu, Y.-L.; Nan, J.-X.; Lian, L.-H. A Therapeutic Strategy of Parthenolide in Improving Imiquimod-Induced Psoriasis-like Skin Inflammation Targeting IL-36/NETs through Skin Transdermal Therapeutic System. Int. Immunopharmacol. 2024, 131, 111824. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Chang, T.; Jiang, K.; Wang, J.; Cui, X.; Cheng, M.; Yan, F.; Song, B.; Wang, Y. ROS-Sensitive Calcipotriol Nano-Micelles Prepared by Methoxypolyethylene Glycol (mPEG)–Modified Polymer for the Treatment of Psoriasis. Drug Deliv. 2022, 29, 1903–1913. [Google Scholar] [CrossRef]
- L’homme, L.; Dombrowicz, D. Astrotactin 1–Derived Peptide: A New Skin-Penetrating Peptide against Inflammatory Skin Diseases. J. Allergy Clin. Immunol. 2018, 141, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.J.; Kim, J.Y.; Jeong, D.H.; Lee, M.-S.; Kim, G.M.; Bae, J.M.; Lee, J.H. Additional Use of Hyaluronic Acid-Based Dissolving Microneedle Patches to Treat Psoriatic Plaques: A Randomized Controlled Trial. Ann. Dermatol. 2025, 37, 105. [Google Scholar] [CrossRef]
- Wu, C.; Yang, X.; Yang, K.; Yu, Q.; Huang, C.; Li, F.; Zhang, L.; Zhu, D. Compensatory Effect-Based Oxidative Stress Management Microneedle for Psoriasis Treatment. Bioact. Mater. 2025, 46, 229–241. [Google Scholar] [CrossRef]
- Vats, A.; Sharma, P. Formulation and Evaluation of Topical Anti Acne Formulation of Coriander Oil. Int. J. Pharm. Pharm. Sci. Res. 2012, 2, 61–66. [Google Scholar]
- Baghel, S.; Gidwani, B.; Kaur, C.D. Novel Drug Delivery Systems of Herbal Constituents Used in Acne. Asian J. Res. Pharm. Sci. 2017, 7, 57–67. [Google Scholar] [CrossRef]
- Nangare, S.; Bhatane, D.; Mali, R.; Shitole, M. Development of a Novel Freeze-Dried Mulberry Leaf Extract-Based Transfersome Gel. Turk. J. Pharm. Sci. 2021, 18, 44–55. [Google Scholar] [CrossRef]
- Wang, Q.; Gan, Z.; Wang, X.; Li, X.; Zhao, L.; Li, D.; Xu, Z.; Mu, C.; Ge, L.; Li, D. Dissolving Hyaluronic Acid-Based Microneedles to Transdermally Deliver Eugenol Combined with Photothermal Therapy for Acne Vulgaris Treatment. ACS Appl. Mater. Interfaces 2024, 16, 21595–21609. [Google Scholar] [CrossRef] [PubMed]
- Mahant, S.; Kumar, S.; Nanda, S.; Rao, R. Microsponges for Dermatological Applications: Perspectives and Challenges. Asian J. Pharm. Sci. 2020, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Alalaiwe, A.; Lin, C.-F.; Hsiao, C.-Y.; Chen, E.-L.; Lin, C.-Y.; Lien, W.-C.; Fang, J.-Y. Development of Flavanone and Its Derivatives as Topical Agents against Psoriasis: The Prediction of Therapeutic Efficiency through Skin Permeation Evaluation and Cell-Based Assay. Int. J. Pharm. 2020, 581, 119256. [Google Scholar] [CrossRef] [PubMed]
- Raison-Peyron, N.; Guillot, B. Allergic Contact Dermatitis Caused by Rotigotine in a Transdermal Therapeutic System. Contact Dermat. 2016, 75, 121–122. [Google Scholar] [CrossRef]
- Pérez-Calderón, R.; Gonzalo-Garijo, M.A.; Rodríguez-Nevado, I. Generalized Allergic Contact Dermatitis from Nitroglycerin in a Transdermal Therapeutic System. Contact Dermat. 2002, 46, 303. [Google Scholar] [CrossRef]
- Thakur, S.; Riyaz, B.; Patil, A.; Kaur, A.; Kapoor, B.; Mishra, V. Novel Drug Delivery Systems for NSAIDs in Management of Rheumatoid Arthritis: An Overview. Biomed. Pharmacother. 2018, 106, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S. Bioavailability and Bioequivalence of Topical Glucocorticoids. Eur. J. Pharm. Biopharm. 2008, 68, 453–466. [Google Scholar] [CrossRef]
- Cheng, T.; Tai, Z.; Shen, M.; Li, Y.; Yu, J.; Wang, J.; Zhu, Q.; Chen, Z. Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System. Pharmaceutics 2023, 15, 2165. [Google Scholar] [CrossRef]

| Study | Transdermal Technique | Type of ISD | Drug | Outcome | Reference |
|---|---|---|---|---|---|
| Rana Obaidat et al., 2022 | Nanofiber (NF) microfiber system | Different skin inflammatory conditions | Pioglitazone | The flux of PGZ was enhanced by five times using NFs compared to the casted film. PGZ is highly retained in the skin layers, which could be beneficial for achieving local delivery of PGZ into the skin for the management of skin conditions, with minimal amount reaching the blood circulation. A change in the diameter and re-crystallization of PGZ occurred upon storage. | [145] |
| Ji Heun Jeong et al., 2019 | A pullulan hydrogel incorporating Rhus verniciflua extract | Atopic dermatitis | Rhus verniciflflua extract | RVE@PH exerts therapeutic effects through dual functions: the hydrogel film-mediated physical and the RVE-mediated pharmaceutical actions. | [146] |
| Wenyi Wang et al., 2016 | P407/CMCS composite thermosensitive hydrogel | Atopic dermatitis | P407/CMCS hydrogel | FE-SEM images showed that CMCs can endow the hydrogel formulation of P407/CMCs with excellent porous structures, which was found to facilitate the diffusional release of CM across the skin. The sol-gel transition temperatures of P407/CMCs composite hydrogel can be tailored simply by controlling the addition of CMCs without causing the alteration of the sol-gel transition property. The rheological property, however, showed significant change, with a remarked increase in the storage modulus and complex viscosity. | [147] |
| Yuan Yang et al., 2022 | Conductive transdermal drug delivery system | Atopic dermatitis-related skin inflammation; enables on-demand drug loading and release via electrical stimulation | Various drug types integrated with biosensors in smart drug delivery systems | Achieve on-demand release drug by ES and regulate the drug release rate by applying different potentials; c-TDDS can be used as a versatile device with a high drug-loading capacity and electrotriggered drug release profile to effectively deliver one or more anionic drugs, cationic drugs, and neutral drugs for the treatment of many diseases, such as arthritis, mental illness, and analgesia. | [148] |
| Jeong Hae Choi et al., 2017 | Low-temperature atmospheric-pressure plasma-Jaun ointment | Atopic dermatitis | Jaun ointment (JO) | Enhance the drug penetration; regulate the activity of NFκB. | [149] |
| Meng Yang et al., 2017 | Triptolide nano-lotion gel | Dermatitis | Triptolide | Replenish keratin; alter the stratum corneum structure by disrupting it; modify lipid arrangements; moisturize keratin; enhance skin drug absorption; and mitigate adverse digestive, urinary, and reproductive system reactions associated with oral triptolide administration. The TPL-nanoemulsion gels provided higher percutaneous amounts than other carriers did. | [150] |
| Muhammad Shahid Latif et al., 2022 | Transdermal patches made of CMC-Na and HPMC | Psoriasis-induced skin inflammation | Methotrexate | CMC-Na at a high concentration mainly affected proteins (ceramides and proteins) of the skin, resulting in higher penetration and retention of methotrexate in the skin. Reduced serum concentrations; led to better patient compliance; and reduced systemic toxicities. | [151] |
| Muhammad Shahid Latif et al., 2021 | Methotrexate-containing patch using HPMC and ethyl cellulose | Psoriasis | Methotrexate | Among all formulated patches (F1–F9), the F5 formulation exhibited the best in vitro drug release pattern and ex vivo drug permeation ability, having the highest deposition of methotrexate compared to other formulated patches. | [152] |
| Huaiji Wang et al., 2021 | Hyaluronic acid (HA)-based microneedle patch (HM/MN patch) loaded with methotrexate nanoparticles | Psoriasis | Methotrexate nanoparticles | Reduce skin thickening in psoriasis, lower IL-6 and TNF-α levels in the body, mitigate inflammation more effectively than traditional methods, and target the immune system with fewer side effects. | [153] |
| Yongjian Song et al., 2024 | Thermoresponsive hydrogel derived from ionic liquid microemulsion (IL microemulsion) | Psoriasis | Methotrexate | Enhance skin affinity, strengthen formulation adhesion, improve bioavailability, target drug delivery, and extend release characteristics. | [154] |
| Eman Zmaily Dahmash et al., 2024 | Thymoquinone polyamide-based arginine (TQ-Arg-PA) nanocapsules | Psoriasis | Thymoquinone | The TQ-Arg-PA nanocapsules were incorporated into transdermal patches containing EVA, Eudragit E100, glycerin, Span 60, and aloe vera as a penetration enhancer. The patches containing aloe vera provided good penetration enhancement, with an increase in thymoquinone fux (Jss) of 42.64%. | [155] |
| Wang, W., et al., 2023 | Nano-transdermal delivery system combining 9-ol and TCeO2 nanoparticles | Psoriasis | TCeO2-TRA-FNL | Integrate TRA to prevent excessive keratinocyte growth and TCeO2 to target mitochondria and reduce reactive oxygen species (ROS) levels and inflammation. | [156] |
| Zi-Ying Zhan et al., 2024 | Parthenolide with transdermal administration | Psoriasis | Parthenolide | Par down-regulated the expression of IL-36 and improved psoriasis-like skin inflammation induced by imiquimod. | [157] |
| Yulin Hua et al., 2022 | Methoxypol-yethylene glycol thioether thiol-calcipotriol (mPEG-SS-CPT, PSC) nanomicelle TDDS en-capsulates calcipotriol | Psoriasis | Calcipotriol | ROS sensitivity, good biocompatibility, safe route of administration, and short treatment cycle. | [158] |
| Hyun Jeong Ju et al., 2025 | Hyaluronic acid-based dissolving microneedle patches | Psoriasis | Hyaluronic acid | Sufficient skin penetration strength and enhanced drug delivery in the in vitro study. | [160] |
| Chaoxiong Wu et al., 2025 | Compensatory effect-based oxidative stress management microneedle | Psoriasis | DNA nanostructures | Manage ROS levels, inhibit pyroptosis and abnormal immune activation, modulate ROS levels, and enhance the therapeutic impact of IL-17A siRNA. | [161] |
| Laurent L’homme et al., 2017 | Atromentin-1-derived peptide protein tyrosine phosphatase (AP-PTPP) conjugate | Psoriatic dermatitis; contact dermatitis animal | The astrotactin 1-derived peptide (AP) to EGFP and dTomato | TC-PTP dephosphorylates a wide range of proteins, such as various JAKs and STATs8. Conjugation of a truncated TC-PTP, containing only the protein tyrosine phosphatase domain, with AP (AP-rPTP) led to a functional cell-penetrating phosphatase. AP-rPTP decreased the phosphorylation of STATs induced by cytokines in keratinocytes in vitro. Skin thickening and inflammation in conditions like contact dermatitis and psoriatic dermatitis were alleviated. | [159] |
| Sopan Nangare et al., 2021 | Transfer gel formulation of mulberry leaf extract | Acne | Novel freeze-dried mulberry leaf extract | The optimized batch MF5 provided 86.23% entrapment efficiency of quercetin in the vesicles and 95.79% drug release. The MG1 formulation provided superior antioxidant activity, drug content, and entrapment efficiency, ex vivo drug release, spreadability, homogeneity, and stability to MG2. quercetin in the extract and gel formulation was confirmed by using high-performance thin-layer chromatography. Skin permeability and antioxidant activity were improved. | [164] |
| Qi Wang et al., 2024 | High-molecular-weight hyaluronic acid (HA)-based mi-croneedle system enriched with eugenol | Acne | Eugenol | A transdermal delivery function of cavity-loaded eugenol achieved by rapid dissolution after insertion into the skin tissue. Used for favorable photothermal properties, blood compatibility, cytocompatibility, in vivo biocompatibility, the promotion of cell proliferation, and migration of fibroblasts. Delivers eugenol, exhibits photothermal properties, has antibacterial activity against acne-causing bacteria, promotes sebaceous gland atrophy, reduces inflammation, and aids skin healing. | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Deng, T.; Cheng, H.; Lu, J.; Wu, J. Advances in Transdermal Drug Delivery Systems and Clinical Applications in Inflammatory Skin Diseases. Pharmaceutics 2025, 17, 746. https://doi.org/10.3390/pharmaceutics17060746
Liu S, Deng T, Cheng H, Lu J, Wu J. Advances in Transdermal Drug Delivery Systems and Clinical Applications in Inflammatory Skin Diseases. Pharmaceutics. 2025; 17(6):746. https://doi.org/10.3390/pharmaceutics17060746
Chicago/Turabian StyleLiu, Sizhuo, Tinghan Deng, Hongbin Cheng, Jun Lu, and Jingping Wu. 2025. "Advances in Transdermal Drug Delivery Systems and Clinical Applications in Inflammatory Skin Diseases" Pharmaceutics 17, no. 6: 746. https://doi.org/10.3390/pharmaceutics17060746
APA StyleLiu, S., Deng, T., Cheng, H., Lu, J., & Wu, J. (2025). Advances in Transdermal Drug Delivery Systems and Clinical Applications in Inflammatory Skin Diseases. Pharmaceutics, 17(6), 746. https://doi.org/10.3390/pharmaceutics17060746









