Nano-Liposomal Carrier as Promising Dermal Delivery Platform for Fumaria officinalis L. Bioactives
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Extract Preparation
2.3. Extract-Loaded Liposome Preparation
2.4. Ultraviolet (UV) Irradiation of Liposomes
2.5. Encapsulation Efficiency
2.6. Monitoring of the Plain and Extract-Loaded Liposome Storage Stability
2.7. An Examination of the Rheological Characteristics of the Plain and Extract-Loaded Liposomes
2.8. Anti-ABTS and Anti-DPPH Radical Potential
2.9. Thiobarbituric Acid-Reactive Substances Assay
2.10. In Vitro Polyphenol Diffusion in Simulated Skin Conditions
2.11. Statistical Data Processing
3. Results and Discussion
3.1. Encapsulation Efficiency of Fumaria officinalis Polyphenols in Developed Liposomes
3.2. Physical Properties of Developed Liposomes
3.3. Rheological Characteristics of Liposomes
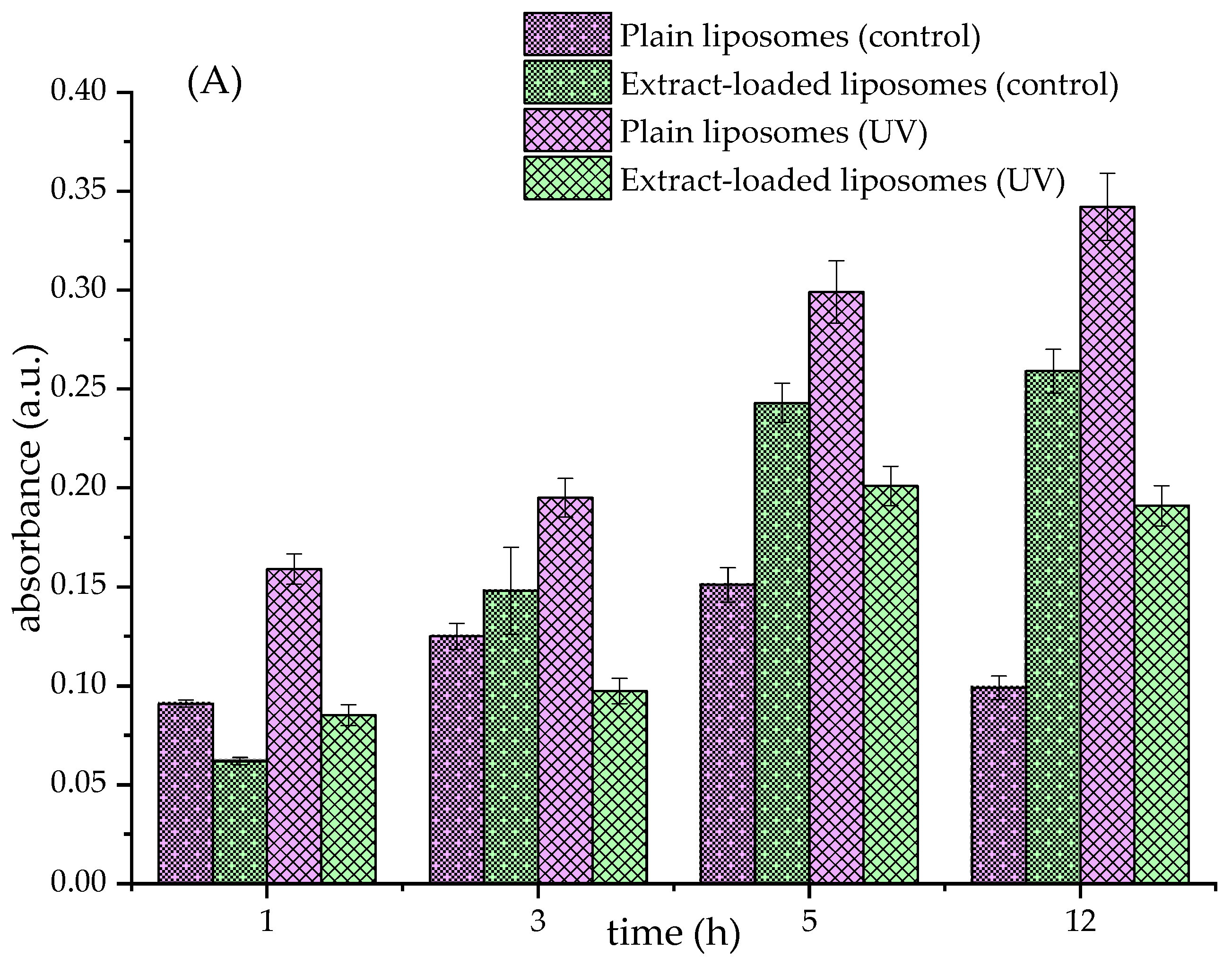
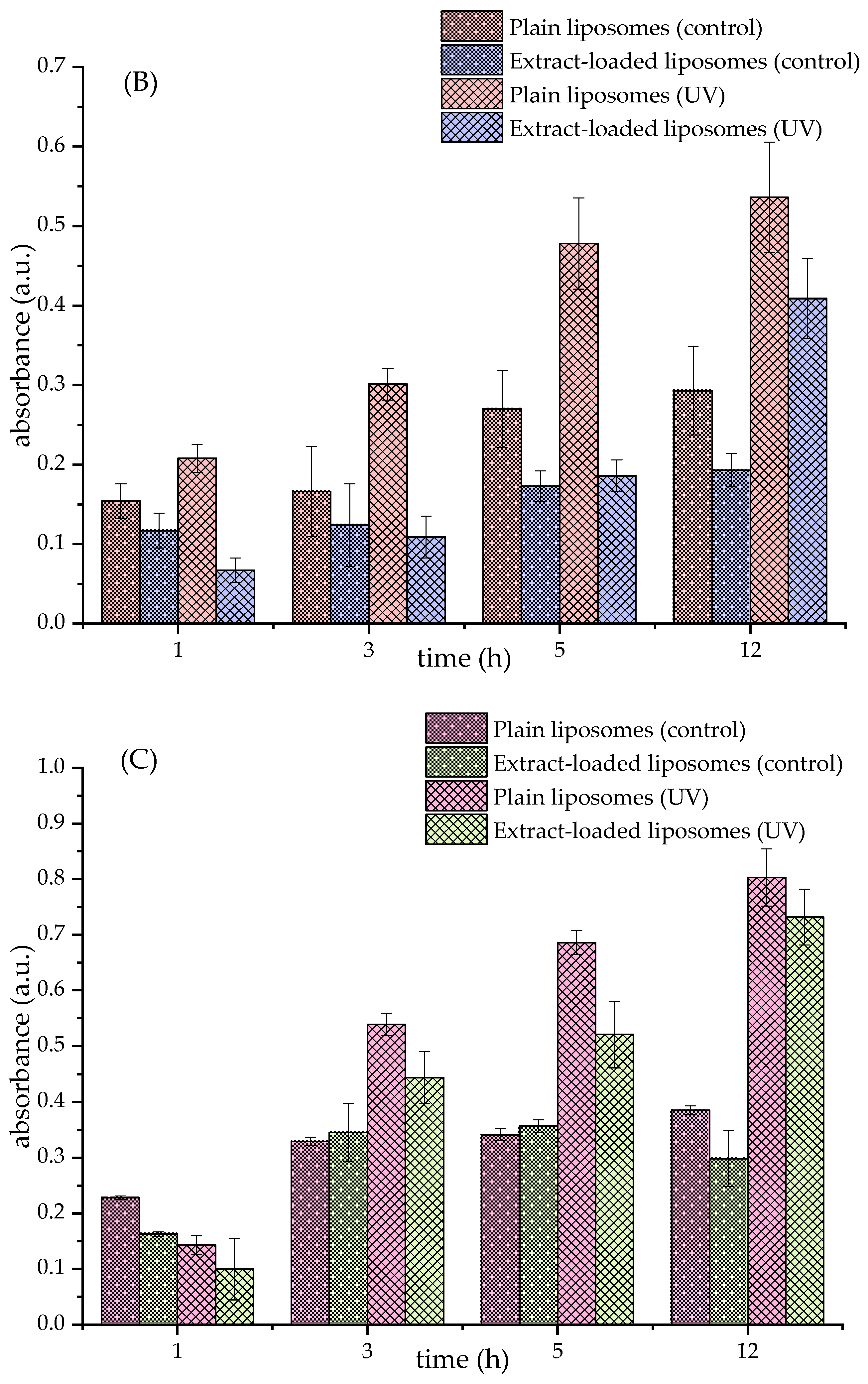
3.4. Lipid Peroxidation in Formulated Liposomes
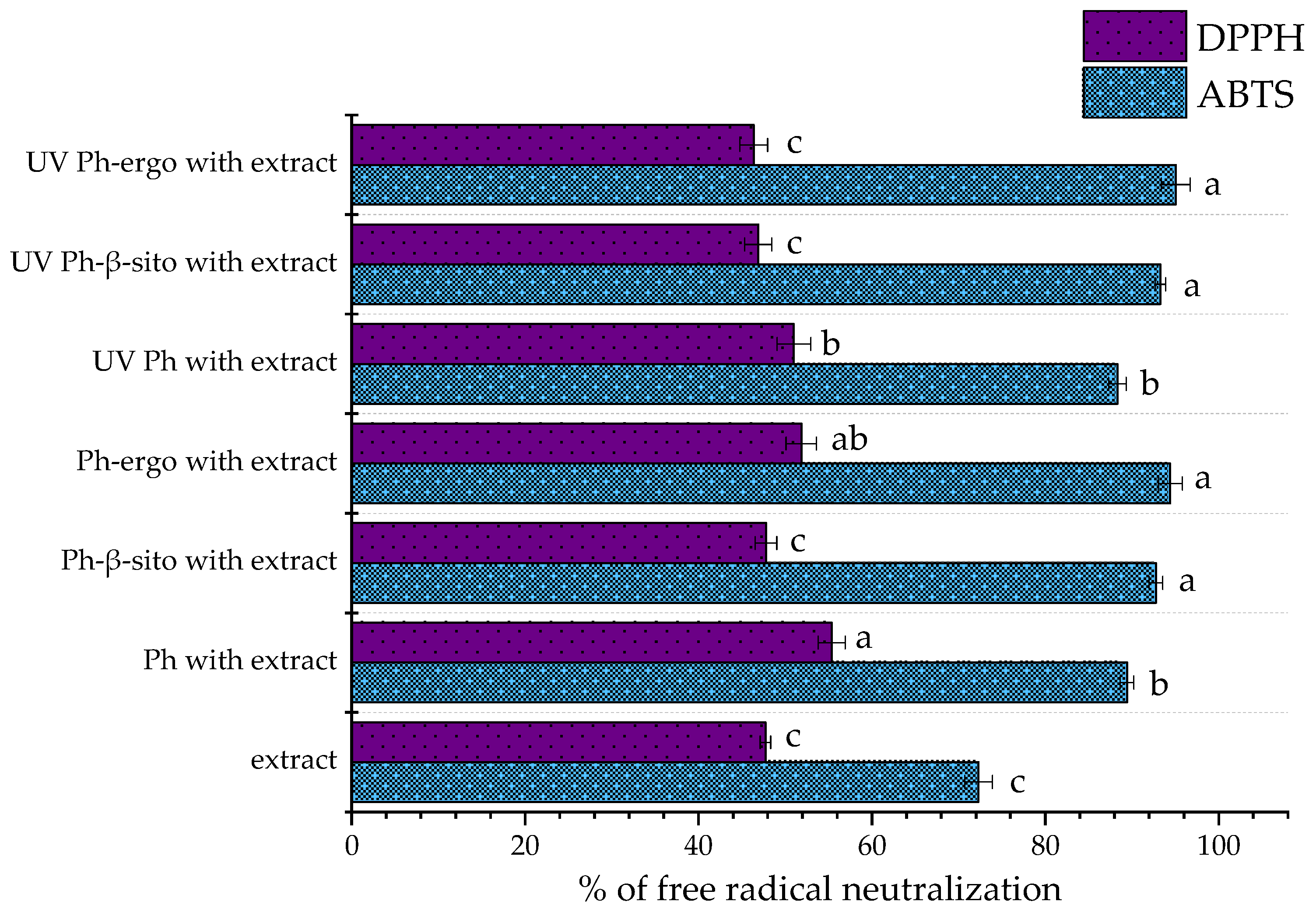
3.5. Antioxidant Potential of Fumitory Extract-Loaded Liposomes
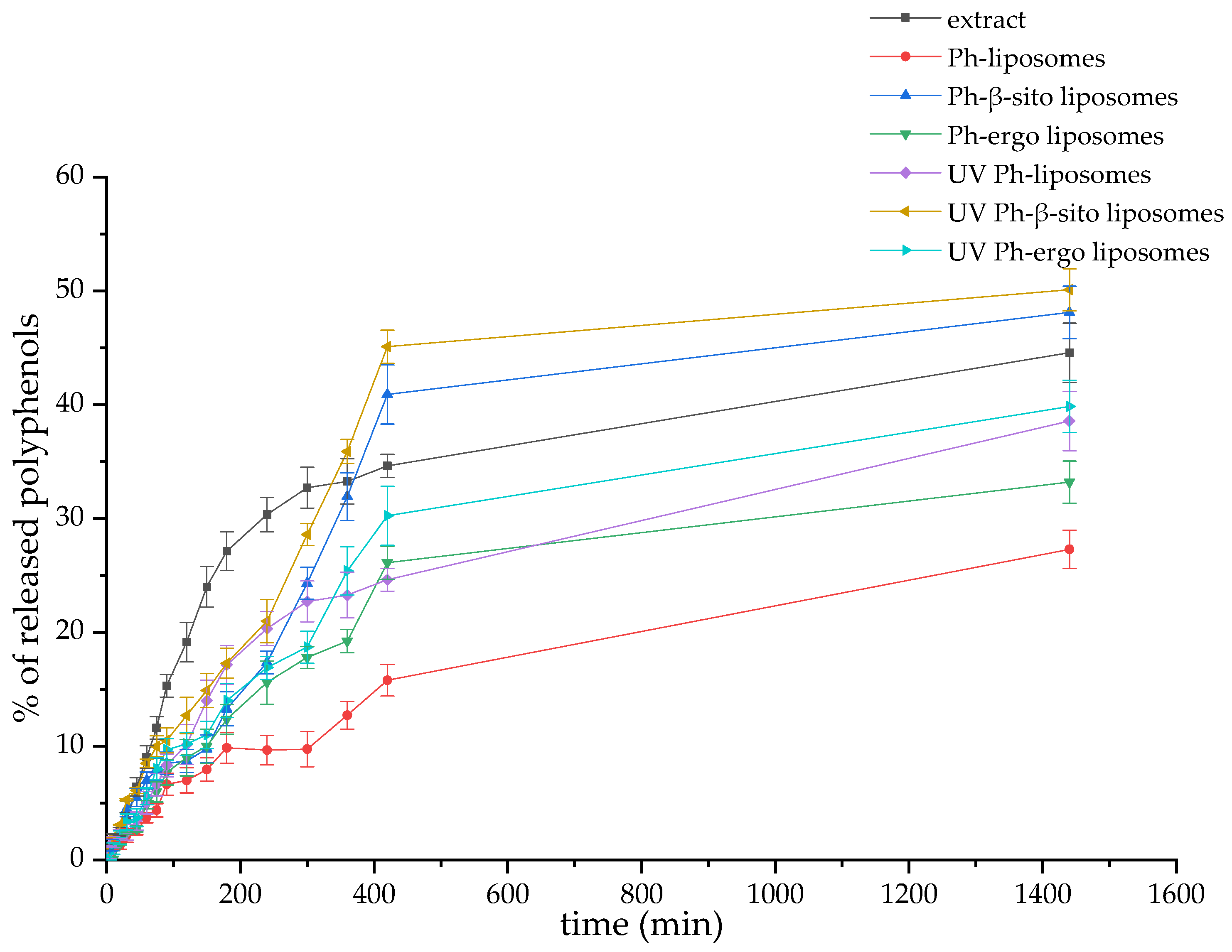
3.6. Polyphenol Release Kinetics from Fumitory Extract and Extract-Loaded Liposomes in Simulated Skin Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, P.C.; Sharma, N.; Rao, C.V. A Review on Ethnobotany, Phytochemistry and Pharmacology of Fumaria indica (Fumitory). Asian Pac. J. Trop. Biomed. 2012, 2, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R. Fumaria officinalis L. active compounds and biological activities: A review. Int. J. Herb. Med. 2023, 11, 144–151. [Google Scholar] [CrossRef]
- Adham, A.N.; Sharef, A.Y.; Ahmad, H.O.; Abdulla, S.S. Evaluation of the Antioxidant and Anti-ulcer Activities of the Ethanolic Extract of Fumaria officinalis. S. Afr. J. Bot. 2022, 151, 816–825. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Nedović, V.; Kalušević, A.; Manojlović, V.; Lević, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef]
- Paramshetti, S.; Angolkar, M.; Talath, S.; Osmani, R.A.M.; Spandana, A.; Al Fatease, A.; Hani, U.; Ramesh, K.V.R.N.S.; Singh, E. Unravelling the in vivo dynamics of liposomes: Insights into biodistribution and cellular membrane interactions. Life Sci. 2024, 346, 122616. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.; Volić, M.; Pećinar, I.; Živković, J.; Šavikin, K.P. Rosehip Extract-Loaded Liposomes for Potential Skin Application: Physicochemical Properties of Non- and UV-Irradiated Liposomes. Plants 2023, 12, 3063. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative Effects of Cholesterol and β-Sitosterol on the Liposome Membrane Characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.K. Development of skin-permeable flexible liposome using ergosterol esters containing unsaturated fatty acids. Chem. Phys. Lipids 2023, 250, 105270. [Google Scholar] [CrossRef]
- Tierney, K.J.; Block, D.E.; Longo, M.L. Elasticity and phase behavior of DPPC membrane modulated by cholesterol, Erg, and ethanol. Biophys. J. 2005, 89, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Chen, J.; Zheng, A.; Tian, S. Effect of sterols on liposomes: Membrane characteristics and physicochemical changes during storage. LWT 2022, 164, 113558. [Google Scholar] [CrossRef]
- Yoda, T. Charged Lipids Influence Phase Separation in Cell-Sized Liposomes Containing Cholesterol or Ergosterol. Membranes 2022, 12, 1121. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; José Alves, M.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Phenolic acids, cinnamic acid, and ergosterol as cosmeceutical ingredients: Stabilization by microencapsulation to ensure sustained bioactivity. Microchem. J. 2019, 147, 469–477. [Google Scholar] [CrossRef]
- European Commission. Available online: https://ec.europa.eu/growth/tools-databases/cosing/details/82630 (accessed on 12 May 2025).
- Lee, D.U.; Park, H.W.; Lee, S.C. Comparing the stability of retinol in liposomes with cholesterol, β-sitosterol, and stigmasterol. Food Sci. Biotechnol. 2021, 30, 389–394. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; He, X.; Wei, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, Y.; Yuan, F. Effect of β-sitosterol on the curcumin-loaded liposomes: Vesicle characteristics, physicochemical stability, in vitro release and bioavailability. Food Chem. 2019, 293, 92–102. [Google Scholar] [CrossRef]
- Abeesh, P.; Guruvayoorappan, C. Preparation and characterization of beta sitosterol encapsulated nanoliposomal formulation for improved delivery to cancer cells and evaluation of its anti-tumor activities against Daltons Lymphoma Ascites tumor models. J. Drug Deliv. Sci. Technol. 2022, 70, 102832. [Google Scholar] [CrossRef]
- Haiyuan, Y.U.; Shen, X.; Liu, D.; Hong, M.; Lu, Y. The protective effects of β-sitosterol and vermicularin from Thamnolia vermicularis (Sw.) Ach. against skin aging in vitro. An. Acad. Bras. Ciênc. 2019, 91, e20181088. [Google Scholar] [CrossRef]
- Han, N.-R.; Kim, H.-M.; Jeong, H.-J. The β-sitosterol attenuates atopic dermatitis-like skin lesions through down-regulation of TSLP. Exp. Biol. Med. 2014, 239, 454–464. [Google Scholar] [CrossRef]
- Ahmoda, R.A.; Pirković, A.; Milutinović, V.; Milošević, M.; Marinković, A.; Jovanović, A.A. Fumaria officinalis Dust as a Source of Bioactives for Potential Dermal Application: Optimization of Extraction Procedures, Phytochemical Profiling, and Effects Related to Skin Health Benefits. Plants 2025, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Luaces, P.; Pascual, M.; Pérez, A.G.; Sanz, C. An Easy-to-Use Procedure for the Measurement of Total Phenolic Compounds in Olive Fruit. Antioxidants 2021, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Balanč, B.; Ota, A.; Djordjević, V.; Šentjurc, M.; Nedović, V.; Bugarski, B.; Poklar Ulrih, N. Resveratrol-loaded liposomes: Interaction of resveratrol with phospholipids. Eur. J. Lipid Sci. Technol. 2015, 117, 1615–1626. [Google Scholar] [CrossRef]
- Abd, E.; Gomes, J.; Sales, C.C.; Yousef, S.; Forouz, F.; Telaprolu, K.C.; Roberts, M.S.; Grice, J.E.; Lopes, P.S.; Leite-Silva, V.R.; et al. Deformable liposomes as enhancer of caffeine penetration through human skin in a Franz diffusion cell test. Int. J. Cosmet. Sci. 2021, 43, 1–10. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Scharinger, A.; Teipel, J.; Buchweitz, M. Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments. Molecules 2021, 26, 4656. [Google Scholar] [CrossRef]
- Tripathy, S.; Srivastav, P.P. Encapsulation of Centella asiatica leaf extract in liposome: Study on structural stability, degradation kinetics and fate of bioactive compounds during storage. Food Chem. Adv. 2023, 2, 100202. [Google Scholar] [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of Physicochemical and Antioxidant Properties of Yogurt Enriched by Olive Leaf Phenolics within Nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef]
- Miere, F.; Vicas, S.I.; Timar, A.V.; Ganea, M.; Zdrinca, M.; Cavalu, S.; Fritea, L.; Vicas, L.; Muresan, M.; Pallag, A.; et al. Preparation and Characterization of Two Different Liposomal Formulations with Bioactive Natural Extract for Multiple Applications. Processes 2021, 9, 432. [Google Scholar] [CrossRef]
- Jahanfar, S.; Ghavami, M.; Khosravi-Darani, K.; Jahad, M. Liposomal Green Tea Extract: Optimization and Physicochemical Characterization. J. Appl. Biotechnol. Rep. 2021, 8, 5–12. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, K.-E.; Kim, J.-J.; Lim, S.-H. The Effect of Cholesterol in the Liposome Bilayer on the Stabilization of Incorporated Retinol. J. Liposome Res. 2005, 15, 157–166. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.; Chen, L. Preparation of liposomes using supercritical carbon dioxide technology: Effects of phospholipids and sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Dave, N.; Liu, J. Protection and Promotion of UV Radiation-Induced Liposome Leakage via DNA-Directed Assembly with Gold Nanoparticles. Adv. Mater. 2011, 23, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.; Lovell, J.F. Mechanisms of light-induced liposome permeabilization. Bioeng. Transl. Med. 2016, 1, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ruozi, B.; Tosi, G.; Forni, F.; Fresta, M.; Vandelli, M.A. Atomic force microscopy and photon correlation spectroscopy: Two techniques for rapid characterization of liposomes. Eur. J. Pharm. Sci. 2005, 25, 81–89. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Edwards, K.A. Liposomes in Analytical Methodologies, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Physical and chemical stability of anthocyanin-rich black carrot extract-loaded liposomes during storage. Food Res. Int. 2018, 108, 491–497. [Google Scholar] [CrossRef]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2014, 38, 28–39. [Google Scholar] [CrossRef]
- Ramli, N.A.; Ali, N.; Hamzah, S.; Yatim, N.I. Physicochemical characteristics of liposome encapsulation of stingless bees’ propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Duman, G.; Corapcıoğlu, G.; Baranauskas, A.; Taralp, A.; Ivanauskas, L.; Bernatoniene, J. Liposomal Incorporation to Improve Dissolution and Stability of Rosmarinic Acid and Carvacrol Extracted from Oregano (O. onites L.). BioMed Res. Int. 2018, 2018, 6147315. [Google Scholar] [CrossRef]
- Šturm, L.; Poklar Ulrih, N. Basic Methods for Preparation of Liposomes and Studying Their Interactions with Different Compounds, with the Emphasis on Polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chu, S.; Hagerman, A.E.; Lorigan, G.A. Probing the Interaction of Polyphenols with Lipid Bilayers by Solid-State NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 6783–6789. [Google Scholar] [CrossRef] [PubMed]
- Uekusa, Y.; Kamihira, M.; Nakayama, T. Dynamic behavior of tea catechins interacting with lipid membranes as determined by NMR spectroscopy. J. Agric. Food Chem. 2007, 55, 9986–9992. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K.; Kumazawa, S.; Naito, A.; Nakayama, T. Solid-state NMR analysis of the orientation and dynamics of epigallocatechin gallate, a green tea polyphenol, incorporated into lipid bilayers. Magn. Reson. Chem. 2008, 46, 174–177. [Google Scholar] [CrossRef]
- Zhao, J.; Mao, S. Tuning the membrane fluidity of liposomes for desirable in vivo fate with enhanced drug delivery. In Advances in Biomembranes and Lipid Self-Assembly; Iglič, A., Rappolt, M., García-Sáez, A.J., Eds.; Academic Press: San Diego, CA, USA, 2021; Volume 34, pp. 67–106. [Google Scholar] [CrossRef]
- Phan, H.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Bosompemaa, G.; Young-Ho, K. Preparation and Characterization of Liposomes Containing Green Tea and Roselle Extracts to be Used in Cosmetics. J. Int. Dev. Coop. 2019, 14, 131–160. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of Rosemary Extract by Liposomes Formulated by Mozafari Method: Physicochemical Characterization and Optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Prevete, G.; Carvalho, L.G.; Razola-Diaz, M.; Verardo, V.; Mancini, G.; Fiore, A.; Mazzonna, M. Ultrasound assisted extraction and liposome encapsulation of olive leaves and orange peels: How to transform biomass waste into valuable resources with antimicrobial activity. Ultrason. Sonochem. 2024, 102, 106765. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Lasch, J.; Weissig, V.; Brandl, M. Preparation of liposomes. In Liposomes: A Practical Approach, 2nd ed.; Torchilin, V., Weissig, V., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 3–29. [Google Scholar]
- Toopkanloo, S.P.; Tan, T.B.; Abas, F.; Azam, M.; Nehdi, I.A.; Tan, C.P. Improving Vesicular Integrity and Antioxidant Activity of Novel Mixed Soy Lecithin-Based Liposomes Containing Squalene and Their Stability against UV Light. Molecules 2020, 25, 5873. [Google Scholar] [CrossRef]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.-M.; Peter Tieleman, D.; Monticelli, L. Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Calvagno, M.G.; Celia, C.; Paolino, D.; Cosco, D.; Iannone, M.; Castelli, F.; Doldo, P.; Frest, M. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr. Drug Deliv. 2007, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Campardelli, R.; Aliakbarian, B.; Perego, P.; Reverchon, E. Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. J. Supercrit. Fluids 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Shashidhar, G.M.; Manohar, B. Nanocharacterization of liposomes for the encapsulation of water soluble compounds from Cordyceps sinensis CS1197 by a supercritical gas anti-solvent technique. RSC Adv. 2018, 8, 34634–34649. [Google Scholar] [CrossRef]
- Narenji, M.; Talaee, M.R.; Moghimi, H.R. Investigating the Effects of Size, Charge, Viscosity and Bilayer Flexibility on Liposomal Delivery under Convective Flow. Int. J. Pharm. 2016, 513, 88–96. [Google Scholar] [CrossRef]
- Dag, D.; Oztop, M.H. Formation and characterization of green tea extract loaded liposomes. J. Food Sci. 2017, 82, 463–470. [Google Scholar] [CrossRef]
- Lopez-Pinto, J.M.; Gonzalez-Rodriguez, M.L.; Rabasco, A.M. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005, 298, 1–12. [Google Scholar] [CrossRef]
- Ricci, M.; Olivia, R.; Del Vecchio, P.; Paolantoni, M.; Moressi, A.; Sassi, P. DMSO-induced perturbation of thermotropic properties of cholesterol-containing DPPC liposomes. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 3024–3031. [Google Scholar] [CrossRef]
- Hu, S.; Niu, M.; Hu, F.; Lu, Y.; Qi, J.; Yin, Z.; Wu, W. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int. J. Pharm. 2013, 441, 693–700. [Google Scholar] [CrossRef]
- Malekar, S.A.; Sarode, A.L.; Bach, A.C.; Worthen, D.R. The Localization of Phenolic Compounds in Liposomal Bilayers and Their Effects on Surface Characteristics and Colloidal Stability. AAPS PharmSciTech 2016, 17, 1468–1476. [Google Scholar] [CrossRef]
- Arias-Alpizar, G.; Kong, L.; Vlieg, R.C.; Rabe, A.; Papadopoulou, P.; Meijer, M.S.; Bonnet, S.; Vogel, S.; van Noort, J.; Kros, A.; et al. Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo. Nat. Commun. 2020, 11, 3638. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.; Petrović, P.M.; Volić, M.; Micić, D.; Živković, J.; Šavikin, K.P. Design and Characterization of Liposomal-Based Carriers for the Encapsulation of Rosa canina Fruit Extract: In Vitro Gastrointestinal Release Behavior. Plants 2024, 13, 2608. [Google Scholar] [CrossRef] [PubMed]
- Karaz, S.; Senses, E. Liposomes Under Shear: Structure, Dynamics, and Drug Delivery Applications. Adv. Nanobiomed Res. 2023, 3, 2200101. [Google Scholar] [CrossRef]
- Demirbay, B.; Akaoğlu, C.; Ulusaraç, İ.; Acar, F.G. Thermal and UV radiation effects on dynamic viscosity of gelatin-based riboflavin solutions. J. Mol. Liq. 2017, 225, 147–150. [Google Scholar] [CrossRef]
- Mathew Thevarkattil, A.; Yousaf, S.; Houacine, C.; Khan, W.; Bnyan, R.; Elhissi, A.; Khan, I. Anticancer Drug Delivery: Investigating the Impacts of Viscosity on Lipid-Based Formulations for Pulmonary Targeting. Int. J. Pharm. 2024, 664, 124591. [Google Scholar] [CrossRef]
- Azarbayjani, A.F.; Jouyban, A.; Chan, S.Y. Impact of surface tension in pharmaceutical sciences. Int. J. Pharm. Pharm. Sci. 2009, 12, 218–228. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.Y.; Gan, L.J.; Zhang, H.; Shin, J.A.; Lee, K.T.; Hong, S.T. Effects of flavonoid glycosides obtained from a Ginkgo biloba extract fraction on the physical and oxidative stabilities of oil-in-water emulsions prepared from a stripped structured lipid with a low omega-6 to omega-3 ratio. Food Chem. 2015, 174, 124–131. [Google Scholar] [CrossRef]
- Luo, Z.; Murray, B.S.; Yusoff, A.; Morgan, M.R.; Povey, M.J.; Day, A.J. Particle-stabilizing effects of flavonoids at the oil−water interface. J. Agric. Food. Chem. 2011, 59, 2636–2645. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mechanism of the Decrease in Surface Tension by Bulk Nanobubbles (Ultrafine Bubbles). Langmuir 2023, 39, 16574–16583. [Google Scholar] [CrossRef]
- Minelli, C.; Sikora, A.; Garcia-Diez, R.; Sparnacci, K.; Gollwitzer, C.; Krumrey, M.; Shard, A.G. Measuring the Size and Density of Nanoparticles by Centrifugal Sedimentation and Flotation. Anal. Methods 2018, 10, 1725–1732. [Google Scholar] [CrossRef]
- Koehler, J.K.; Gedda, L.; Wurster, L.; Schnur, J.; Edwards, K.; Heerklotz, H.; Massing, U. Tailoring the Lamellarity of Liposomes Prepared by Dual Centrifugation. Pharmaceutics 2023, 15, 706. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.-S.; Jung, H.-S. Precise Control of Liposome Size Using Characteristic Time Depends on Solvent Type and Membrane Properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Ebisawa, A.; Tsuchiya, K.; Sakai, K.; Sakai, H. Effect of Lipid Composition on the Characteristics of Liposomes Prepared Using the Polyol Dilution Method. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134609. [Google Scholar] [CrossRef]
- Hlaváč, P.; Božiková, M.; Ardonová, V.; Petrović, A.; Kotoulek, P. Influence of the Selected Factors on the Liquid Food Density. J. Process. Energy Agric. 2018, 22, 53–57. [Google Scholar] [CrossRef]
- Cesquini, M.; Torsoni, M.; Stoppa, G.; Ogo, S. t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed. Pharmacother. 2003, 57, 124–129. [Google Scholar] [CrossRef]
- Noudoost, B.; Noori, N.; Abedini, A.G.; Gandomi, H.; Akhondzadeh Basti, A.; Ashkan, J.; Ghadami, F. Encapsulation of Green Tea Extract in Nanoliposomes and Evaluation of Its Antibacterial, Antioxidant and Prebiotic Properties. J. Med. Plants 2015, 14, 66–78. [Google Scholar]
- Zokti, J.A.; Badlishah, A.; Baharin, S.; Abas, F.; Baharin, B.S. Microencapsulation of Green Tea Extracts and Its Effects on the Physico-chemical and Functional Properties of Mango Drinks. Int. J. Basic Appl. Sci. 2016, 16, 16–32. [Google Scholar]
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Reevaluation of antimicrobial and antioxidant activity of Thymus spp. extracts before and after encapsulation in liposomes. J. Food Prot. 2006, 69, 2998–3005. [Google Scholar] [CrossRef]
- Spigno, G.; Donsì, F.; Amendola, D.; Sessa, M.; Ferrari, G.; De Faveri, D.M. Nanoencapsulation systems to improve solubility and antioxidant efficiency of a grape marc extract into hazelnut paste. J. Food Eng. 2013, 114, 207–214. [Google Scholar] [CrossRef]
- Cortie, C.H.; Else, P.L. An Antioxidant-like Action for Non-Peroxidisable Phospholipids Using Ferrous Iron as a Peroxidation Initiator. Biochim. Biophys. Acta—Biomembr. 2015, 1848, 1303–1307. [Google Scholar] [CrossRef]
- Mora, M.; Tourne-Peteilh, C.; Charveron, M.; Fabre, B.; Milon, A.; Muller, I. Optimisation of Plant Sterols Incorporation in Human Keratinocyte Plasma Membrane and Modulation of Membrane Fluidity. Chem. Phys. Lipids 1999, 101, 255–265. [Google Scholar] [CrossRef]
- Gracià, R.S.; Bezlyepkina, N.; Knorr, R.L.; Lipowsky, R.; Dimova, R. Effect of Cholesterol on the Rigidity of Saturated and Unsaturated Membranes: Fluctuation and Electrodeformation Analysis of Giant Vesicles. Soft Matter 2010, 6, 1472. [Google Scholar] [CrossRef]
- Najafinobar, N.; Mellander, L.J.; Kurczy, M.E.; Dunevall, J.; Angerer, T.B.; Fletcher, J.S.; Cans, A.-S. Cholesterol Alters the Dynamics of Release in Protein Independent Cell Models for Exocytosis. Sci. Rep. 2016, 6, 33702. [Google Scholar] [CrossRef] [PubMed]
- Spratt, T.; Bondurant, B.; O’Brien, D.F. Rapid Release of Liposomal Contents upon Photoinitiated Destabilization with UV Exposure. Biochim. Biophys. Acta—Biomembr. 2003, 1611, 35–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; An, X. Preparation, Microstructure and Function of Liposome with Light Responsive Switch. Colloids Surf. B Biointerfaces 2019, 178, 238–244. [Google Scholar] [CrossRef]
- Rubio-Camacho, M.; Martínez-Tomé, M.J.; Cuestas-Ayllón, C.; de la Fuente, J.M.; Esquembre, R.; Mateo, C.R. Tailoring the Plasmonic Properties of Gold-Liposome Nanohybrids as a Potential Powerful Tool for Light-Mediated Therapies. Colloid Interface Sci. Commun. 2023, 52, 100690. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the Extraction Process of Polyphenols from Thymus serpyllum L. Herb Using Maceration, Heat- and Ultrasound-Assisted Techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Murariu, A.; Oprea, O.; Bojin, D.; Meghea, A. Antioxidant Activity of Solid Lipid Nanoparticles Loaded with Umbelliferone. Soft Mater. 2012, 11, 75–84. [Google Scholar] [CrossRef]
| Day | Liposomes | Encapsulation Efficiency (%) | |
|---|---|---|---|
| 1st | Non-treated | Ph | 72.2 ± 1.3 a* |
| Ph-β-sitosterol | 66.7 ± 1.1 b | ||
| Ph-ergosterol | 62.9 ± 1.2 c | ||
| UV-irradiated | Ph | 71.9 ± 0.5 a | |
| Ph-β-sitosterol | 68.7 ± 1.5 b | ||
| Ph-ergosterol | 64.1 ± 1.0 c | ||
| 30th | Non-treated | Ph | 73.0 ± 2.1 a |
| Ph-β-sitosterol | 65.5 ± 1.0 b | ||
| Ph-ergosterol | 61.4 ± 0.9 c | ||
| UV-irradiated | Ph | 70.8 ± 1.9 a | |
| Ph-β-sitosterol | 66.4 ± 2.0 b | ||
| Ph-ergosterol | 63.0 ± 1.7 bc | ||
| Day | Liposomes | Diameter (nm) | PdI | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| 1st | Non-treated | Plain Ph | 420.6 ± 4.3 g* | 0.259 ± 0.013 c | −17.6 ± 0.3 b |
| Plain Ph-β-sitosterol | 683.1 ± 12.9 a | 0.205 ± 0.026 d | −20.6 ± 0.6 a | ||
| Plain Ph-ergosterol | 596.0 ± 15.6 cd | 0.232 ± 0.017 cd | −20.9 ± 0.5 a | ||
| Ph with extract | 270.6 ± 5.5 k | 0.302 ± 0.026 b | −5.99 ± 0.62 d | ||
| Ph-β-sitosterol with extract | 311.7 ± 13.5 i | 0.289 ± 0.020 bc | −6.11 ± 1.03 d | ||
| Ph-ergosterol with extract | 345.8 ± 9.5 h | 0.295 ± 0.048 bc | −5.74 ± 0.89 d | ||
| UV-irradated | Plain Ph | 442.8 ± 5.5 f | 0.253 ± 0.030 cd | −17.3 ± 0.4 b | |
| Plain Ph-β-sitosterol | 667.2 ± 13.0 a | 0.206 ± 0.020 d | −20.9 ± 0.8 a | ||
| Plain Ph-ergosterol | 586.7 ± 16.0 cd | 0.256 ± 0.021 cd | −19.1 ± 1.7 ab | ||
| Ph with extract | 291.9 ± 2.6 j | 0.398 ± 0.019 a | −5.31 ± 0.29 d | ||
| Ph-β-sitosterol with extract | 315.1 ± 7.1 i | 0.319 ± 0.025 b | −5.69 ± 0.99 d | ||
| Ph-ergosterol with extract | 354.8 ± 10.2 h | 0.373 ± 0.018 a | −6.03 ± 0.54 d | ||
| 30th | Non-treated | Plain Ph | 558.0 ± 11.2 e | 0.273 ± 0.049 bc | −17.1 ± 0.2 b |
| Plain Ph-β-sitosterol | 663.3 ± 9.5 a | 0.223 ± 0.052 cd | −18.0 ± 0.4 b | ||
| Plain Ph-ergosterol | 616.2 ± 23.3 bc | 0.242 ± 0.059 cd | −16.1 ± 1.2 bc | ||
| Ph with extract | 279.8 ± 4.9 k | 0.375 ± 0.050 ab | −5.65 ± 0.56 d | ||
| Ph-β-sitosterol with extract | 326.4 ± 14.3 hi | 0.287 ± 0.019 bc | −5.48 ± 1.12 d | ||
| Ph-ergosterol with extract | 350.1 ± 11.1 h | 0.351 ± 0.025 ab | −6.14 ± 1.04 d | ||
| UV-irradated | Plain Ph | 581.6 ± 3.4 d | 0.269 ± 0.038 bc | −16.9 ± 0.9 bc | |
| Plain Ph-β-sitosterol | 658.7 ± 31.9 ab | 0.201 ± 0.086 d | −16.3 ± 0.4 c | ||
| Plain Ph-ergosterol | 622.9 ± 25.4 bc | 0.295 ± 0.023 bc | −15.4 ± 0.7 c | ||
| Ph with extract | 294.3 ± 4.2 j | 0.389 ± 0.008 a | −5.48 ± 0.06 d | ||
| Ph-β-sitosterol with extract | 328.8 ± 17.5 hi | 0.272 ± 0.047 bc | −5.91 ± 0.84 d | ||
| Ph-ergosterol with extract | 346.9 ± 10.1 h | 0.385 ± 0.021 a | −5.63 ± 1.09 d | ||
| Day | Liposomes | Viscosity (mPa × s) | Surface tension (mN/m) | Density (g/cm3) | |
|---|---|---|---|---|---|
| 1st | Non-treated | Plain Ph | 2.58 ± 0.11 c* | 23.8 ± 1.0 a | 0.998 ± 0.002 a |
| Plain Ph-β-sitosterol | 2.43 ± 0.29 c | 22.6 ± 1.3 ab | 0.999 ± 0.000 a | ||
| Plain Ph-ergosterol | 2.57 ± 0.17 c | 20.3 ± 1.4 bc | 1.001 ± 0.002 a | ||
| Ph with extract | 6.09 ± 0.33 a | 17.6 ± 0.5 d | 1.000 ± 0.001 a | ||
| Ph-β-sitosterol with extract | 6.78 ± 0.45 a | 16.6 ± 0.9 d | 1.002 ± 0.005 a | ||
| Ph-ergosterol with extract | 6.49 ± 0.22 a | 18.7 ± 1.3 cd | 0.998 ± 0.003 a | ||
| UV-irradiated | Plain Ph | 3.04 ± 0.51 c | 24.5 ± 0.5 a | 1.001 ± 0.001 a | |
| Plain Ph-β-sitosterol | 3.10 ± 0.38 c | 21.7 ± 0.9 ab | 1.000 ± 0.001 a | ||
| Plain Ph-ergosterol | 2.92 ± 0.27 c | 20.9 ± 0.7 b | 1.003 ± 0.005 a | ||
| Ph with extract | 6.31 ± 0.50 a | 18.2 ± 0.8 cd | 0.997 ± 0.004 a | ||
| Ph-β-sitosterol with extract | 6.92 ± 0.43 a | 17.0 ± 1.1 d | 1.000 ± 0.001 a | ||
| Ph-ergosterol with extract | 6.81 ± 0.38 a | 17.9 ± 0.5 d | 0.999 ± 0.004 a | ||
| 30th | Non-treated | Plain Ph | 1.87 ± 0.22 d | 17.5 ± 1.1 d | 1.004 ± 0.002 a |
| Plain Ph-β-sitosterol | 1.69 ± 0.15 d | 18.7 ± 1.3 cd | 1.005 ± 0.004 a | ||
| Plain Ph-ergosterol | 1.57 ± 0.29 d | 17.8 ± 1.0 d | 1.002 ± 0.005 a | ||
| Ph with extract | 5.17 ± 0.40 b | 14.2 ± 0.9 f | 0.999 ± 0.003 a | ||
| Ph-β-sitosterol with extract | 4.98 ± 0.83 b | 13.9 ± 1.0 f | 1.000 ± 0.001 a | ||
| Ph-ergosterol with extract | 5.06 ± 0.64 b | 15.0 ± 0.9 ef | 0.999 ± 0.004 a | ||
| UV-irradiated | Plain Ph | 2.00 ± 0.12 d | 18.6 ± 1.2 cd | 1.000 ± 0.001 a | |
| Plain Ph-β-sitosterol | 1.76 ± 0.31 d | 18.0 ± 0.7 cd | 1.000 ± 0.003 a | ||
| Plain Ph-ergosterol | 1.97 ± 0.13 d | 16.9 ± 1.2 de | 0.997 ± 0.005 a | ||
| Ph with extract | 5.60 ± 0.15 b | 15.1 ± 1.0 ef | 1.002 ± 0.004 a | ||
| Ph-β-sitosterol with extract | 5.19 ± 0.19 b | 14.9 ± 0.9 ef | 0.999 ± 0.004 a | ||
| Ph-ergosterol with extract | 4.84 ± 0.54 b | 14.3 ± 0.5 f | 1.002 ± 0.004 a | ||
| Samples | D (m2/s) | R (s/m) | |
|---|---|---|---|
| Fumitory extract | 5.09 × 10−9 | 8.01 × 105 | |
| Non-treated | Ph-liposomes | 3.48 × 10−9 | 1.17 × 106 |
| Ph-β-sitosterol liposomes | 4.02 × 10−9 | 9.48 × 105 | |
| Ph-ergosterol liposomes | 4.30 × 10−9 | 1.43 × 106 | |
| UV-irradiated | Ph-liposomes | 5.42 × 10−9 | 7.51 × 105 |
| Ph-β-sitosterol liposomes | 1.10 × 10−8 | 4.23 × 105 | |
| Ph-ergosterol liposomes | 9.64 × 10−9 | 3.69 × 105 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmoda, R.A.; Milošević, M.; Marinković, A.; Jovanović, A.A. Nano-Liposomal Carrier as Promising Dermal Delivery Platform for Fumaria officinalis L. Bioactives. Pharmaceutics 2025, 17, 782. https://doi.org/10.3390/pharmaceutics17060782
Ahmoda RA, Milošević M, Marinković A, Jovanović AA. Nano-Liposomal Carrier as Promising Dermal Delivery Platform for Fumaria officinalis L. Bioactives. Pharmaceutics. 2025; 17(6):782. https://doi.org/10.3390/pharmaceutics17060782
Chicago/Turabian StyleAhmoda, Rabiea Ashowen, Milena Milošević, Aleksandar Marinković, and Aleksandra A. Jovanović. 2025. "Nano-Liposomal Carrier as Promising Dermal Delivery Platform for Fumaria officinalis L. Bioactives" Pharmaceutics 17, no. 6: 782. https://doi.org/10.3390/pharmaceutics17060782
APA StyleAhmoda, R. A., Milošević, M., Marinković, A., & Jovanović, A. A. (2025). Nano-Liposomal Carrier as Promising Dermal Delivery Platform for Fumaria officinalis L. Bioactives. Pharmaceutics, 17(6), 782. https://doi.org/10.3390/pharmaceutics17060782










