Quality by Design and In Silico Approach in SNEDDS Development: A Comprehensive Formulation Framework
Abstract
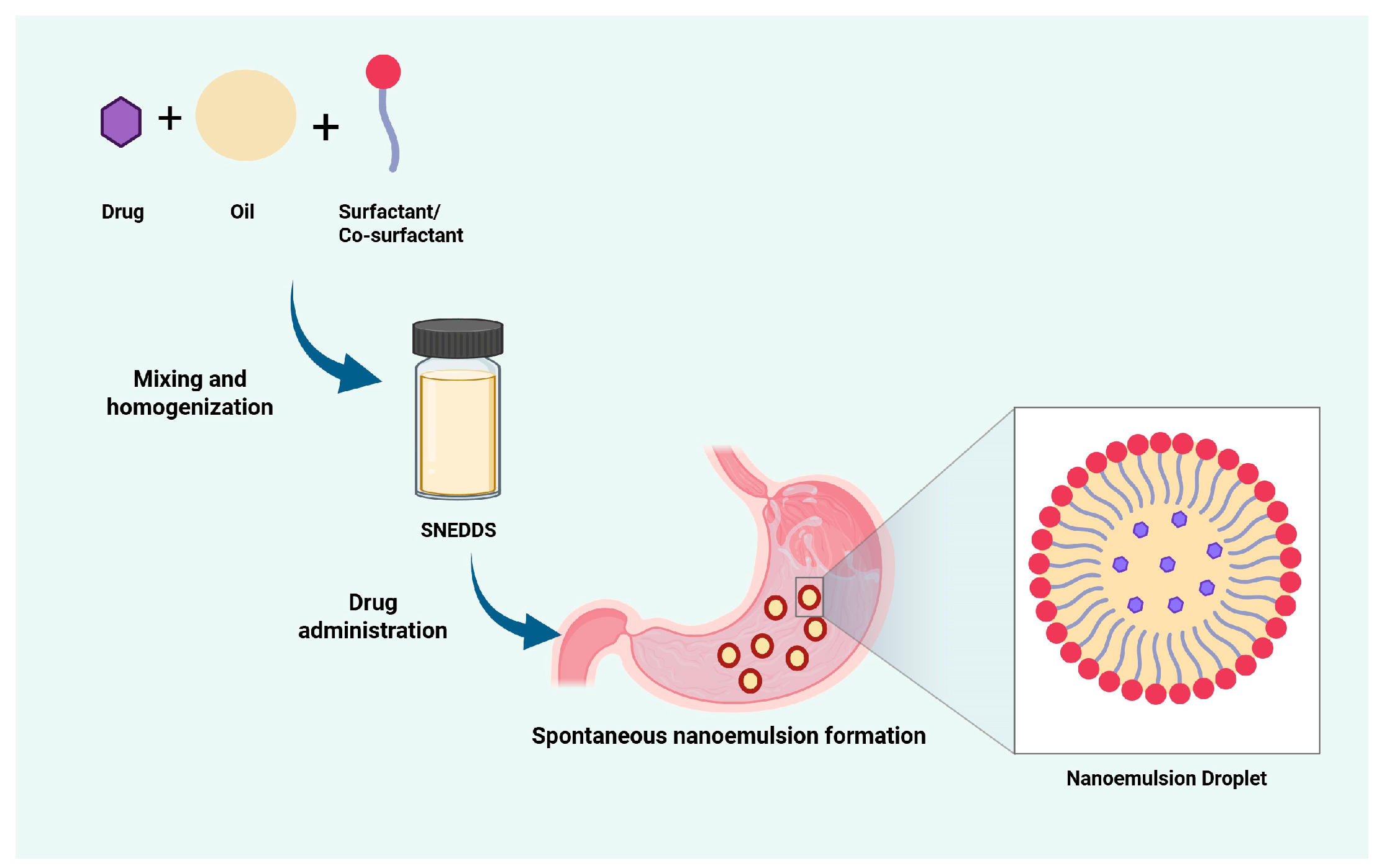
1. Introduction
2. Basic Principles of SNEDDS Formulation
2.1. Solubility Testing
2.2. Emulsification Efficiency
2.3. Construction Diagram Pseudo-Ternary
2.4. Optimization of SNEDDS Formulation
3. The Principle of QbD in Product Development
4. Application of QbD in SNEDDS Development
4.1. Define of QTPP in SNEDDS Development
4.2. Identify CQAs in SNEDDS Development
4.3. Risk Assessment in SNEDDS Development
5. Application of DoE in SNEDDS Development
5.1. Mixture Design
5.2. Response Surface Methodology
5.3. Factorial Design
6. Optimization Stages in SNEDDS Development Based on DoE
6.1. Determination of Factors and Responses
6.2. Selecting Experimental Design
6.3. Establishment of Experimental Points
6.4. Preparation and Characterization of SNEDDS
6.5. Data Analysis and Polynomial Modelling
6.6. Analysis of the Relationship Between Factors and Responses
6.7. Desirability Function Analysis and Validation
7. In Vitro, Ex Vivo, and In Vivo Performances of Optimized SNEDDS
7.1. The Influence of SNEDDS on the Drug Release Profile
7.2. The Influence of SNEDDS on Drug Permeation
7.3. The Influence of SNEDDS on Pharmacokinetic Profiles
7.4. The Influence of SNEDDS on Therapeutic Efficacy
8. In Silico Approach in SNEDDS Development
| Active Ingredient [Ref] | Components | Computational Method Used | Results Obtained |
|---|---|---|---|
| Meloxicam [19] | Labrafil M 1944 CS (oil), Cremophor RH40 (surfactant), and Transcutol HP (co-surfactant) |
|
|
| Palm Kernel Oil Wax Esters (PKOEs) [144] | PKOEs (oil) and Tween 80 (surfactant) |
|
|
| Black Cumin Oil (BCO [145] | Tween 20, Tween 80, Span 20, Span 80, and Lecithin in equal proportions with 10 BCO molecules |
|
|
| Benzalkonium chloride [147] | Cyclohexane (oil), Benzalkonium chloride (surfactant), and Ethanol (cosurfactant) |
|
|
| Curcumin [146] | Soybean oil-tween 80 in two conditions: without curcumin (OL: TW80:H2O = 1.67:15:83.33) and with curcumin (OL:TW80:H2O: CUR = 0.17:1.66:15:83.19:0.17) |
|
|
9. Challenges and Limitations
10. Conclusions
11. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| API | Active Pharmaceutical Ingredient |
| AUC | Area Under the Curve |
| BBD | Box–Behnken Design |
| CCD | Central Composite Design |
| CMA | Critical Material Attributes |
| Cmax | Maximum Concentration |
| CPP | Critical Process Parameters |
| Co-S | Co-Surfactant |
| DL | Drug Loading |
| DoE | Design of Experiment |
| DR | Drug Release |
| ET | Emulsification Time |
| FMEA | Failure Mode and Effects Analysis |
| GS | Globule Size |
| ICH | International Council for Harmonisation |
| MD | Molecular Dynamic |
| ML | Machine Learning |
| PBPK | Physiologically Based Pharmacokinetic |
| PDI | Polydispersity Index |
| QbD | Quality by Design |
| QTPP | Quality Target Product Profile |
| RAM | Risk Assessment Matrix |
| REM | Risk Estimation Matrix |
| RSM | Response Surface Methodology |
| SLD | Simplex Lattice Design |
| Smix | Surfactant and Co-Surfactant Mixture |
| SNEDDS | Self-Nanoemulsifying Drug Delivery System |
| ZP | Zeta Potential |
References
- Tucker, G.; DeSilva, B.; Dressman, J.; Ito, M.; Kumamoto, T.; Mager, D.; Mahler, H.-C.; der Zee, A.H.M.-V.; Pauletti, G.M.; Sasaki, H.; et al. Current Challenges and Potential Opportunities for the Pharmaceutical Sciences to Make Global Impact: An FIP Perspective. J. Pharm. Sci. 2016, 105, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Aryal, S.; Regmi, Y.; Rajarajan, S. Cocrystallization: An Approach to Improve Bioavailability by Altering Physicochemical Properties of Poorly Soluble API’s. Int. J. Pharm. Pharm. Res. 2021, 20, 381–397. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Chandel, A.K.S. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar] [CrossRef]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Morakul, B. Self-nanoemulsifying drug delivery systems (SNEDDS): An advancement technology for oral drug delivery. Pharm. Sci. Asia 2020, 47, 205–220. [Google Scholar] [CrossRef]
- Cherniakov, I.; Domb, A.J.; Hoffman, A. Self-nano-emulsifying drug delivery systems: An update of the biopharmaceutical aspects. Expert Opin. Drug Deliv. 2015, 12, 1121–1133. [Google Scholar] [CrossRef]
- Sailor, G.U. Self-Nanoemulsifying Drug Delivery Systems (SNEDDS): An Innovative Approach to Improve Oral Bioavailability. In Nanocarriers: Drug Delivery System: An Evidence Based Approach; Springer: Singapore, 2021; pp. 255–280. [Google Scholar] [CrossRef]
- Jvus, C.; Kothuri, N.; Singh, S.; Verma, S.; Shafi, H.; Reddy, D.V.S.; Kedar, A.; Rana, R.; Mishra, K.; Sharma, D.; et al. A Quality by Design Approach for Developing SNEDDS Loaded with Vemurafenib for Enhanced Oral Bioavailability. AAPS PharmSciTech 2024, 25, 14. [Google Scholar] [CrossRef]
- Aru, P.B.; Gulhane, M.S.; Katekar, V.A.; Deshmukh, S.P. Deshmukh. Quality by Design (QbD) in pharmaceutical development: A comprehensive review. GSC Biol. Pharm. Sci. 2024, 26, 328–340. [Google Scholar] [CrossRef]
- Ali, J.; Pramod, K.; Tahir, M.A.; Charoo, N.A.; Ansari, S.H. Pharmaceutical product development: A quality by design approach. Int. J. Pharm. Investig. 2016, 6, 129–138. [Google Scholar] [CrossRef]
- ter Horst, J.P.; Turimella, S.L.; Metsers, F.; Zwiers, A. Implementation of Quality by Design (QbD) Principles in Regulatory Dossiers of Medicinal Products in the European Union (EU) Between 2014 and 2019. Ther. Innov. Regul. Sci. 2021, 55, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, A.S.; Fernandes, G.J.; Naha, A.; Rathnanand, M.; Kumar, L. Design of Experiments in Pharmaceutical Development. Pharm. Chem. J. 2019, 53, 730–735. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline Q10 on Pharmaceutical Quality System; European Medicines Agency: Amsterdam, The Netherlands, 2015; p. 44.
- International Council for Harmonisation. ICH Guideline Q9 on Quality Risk Management; International Conference on Harmonization: Geneva, Switzerland, 2015; p. 44. [Google Scholar]
- International Council for Harmonisation. ICH Guideline Q8 (R2) on Pharmaceutical Development; European Medicines Agency: Amsterdam, The Netherlands, 2017.
- Hsieh, C.-M.; Yang, T.-L.; Putri, A.D.; Chen, C.-T. Application of Design of Experiments in the Development of Self-Microemulsifying Drug Delivery Systems. Pharmaceuticals 2023, 16, 283. [Google Scholar] [CrossRef]
- Lamidi, S.; Olalere, R.; Yekinni, A.; Adesina, K. Design of Experiments (DOE): Applications and Benefits in Quality Control and Assurance. In Quality Control and Quality Assurance—Techniques and Applications; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Gao, H.; Jia, H.; Dong, J.; Yang, X.; Li, H.; Ouyang, D. Integrated in silico formulation design of self-emulsifying drug delivery systems. Acta Pharm. Sin. B 2021, 11, 3585–3594. [Google Scholar] [CrossRef]
- Akiladevi, D.; Prakash, H.; Biju, G.; Madumitha, N. Nano-novel approach: Self nano emulsifying drug delivery system (SNEDDS)—Review article. Res. J. Pharm. Technol. 2020, 13, 983–990. [Google Scholar] [CrossRef]
- Buya, A.B.; Ucakar, B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDSs) for senicapoc. Int. J. Pharm. 2020, 580, 119180. [Google Scholar] [CrossRef]
- Kumar, M.; Jain, C.P.; Shukla, A.K.; Verma, G.; Yadav, V.K. Terminology and Mechanisms of Self-Emulsifying Systems for Biomedical Applications: A Comprehensive Review. Colloid J. 2023, 85, 917–929. [Google Scholar] [CrossRef]
- Park, H.; Ha, E.-S.; Kim, M.-S. Current status of supersaturable self-emulsifying drug delivery systems. Pharmaceutics 2020, 12, 365. [Google Scholar] [CrossRef]
- Suyal, J.; Kumar, B.; Jakhmola, V. Novel Approach Self Nanoemulsifying Drug Delivery System: A Review. Adv. Pharmacol. Pharm. 2023, 11, 131–139. [Google Scholar] [CrossRef]
- Parveen, N.; Sheikh, A.; Abourehab, M.A.; Karwasra, R.; Singh, S.; Kesharwani, P. Self-nanoemulsifying drug delivery system for pancreatic cancer. Eur. Polym. J. 2023, 190, 111993. [Google Scholar] [CrossRef]
- Kazi, M.; Khan, M.F.; A Nasr, F.; Ahmed, M.Z.; Alqahtani, A.S.; Ali, M.M.; Aldughaim, M.S. Development of Curcumin and Piperine-Loaded Bio-Active Self-Nanoemulsifying Drugs and Investigation of Their Bioactivity in Zebrafish Embryos and Human Hematological Cancer Cell Lines. Int. J. Nanomed. 2023, 18, 1793–1808. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing oral bioavailability of apigenin using a bioactive self-nanoemulsifying drug delivery system (Bio-SNEDDS): In vitro, in vivo and stability evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alanazi, Y.; Kumar, A.; Shahba, A.A.-W.; Ahamad, S.R.; Alghamdi, K.M. Oral Bioactive Self-Nanoemulsifying Drug Delivery Systems of Remdesivir and Baricitinib: A Paradigmatic Case of Drug Repositioning for Cancer Management. Molecules 2023, 28, 2237. [Google Scholar] [CrossRef]
- Hamdy, A.; El-Badry, M.; Fathy, M.; El-Sayed, A.M. Impact of oil type on the development and oral bioavailability of self-nanoemulsifying drug delivery systems containing simvastatin. Sci. Rep. 2024, 14, 22584. [Google Scholar] [CrossRef]
- Izgelov, D.; Shmoeli, E.; Domb, A.J.; Hoffman, A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int. J. Pharm. 2020, 580, 119201. [Google Scholar] [CrossRef]
- Oliveira, L.T.; Castanheira, R.G.; Vilela, J.M.C.; Andrade, M.S.; de Oliveira, M.A.; Mosqueira, V.C.F. Impact of non-ionic surfactants on release kinetics, toxicity and colloidal characteristics of benznidazole self-emulsifying delivery system evidenced by flow field-flow fractionation. J. Chromatogr. A 2024, 1740, 465565. [Google Scholar] [CrossRef]
- Rathod, S.; Desai, H.; Patil, R.; Sarolia, J. Non-ionic Surfactants as a P-Glycoprotein(P-gp) Efflux Inhibitor for Optimal Drug Delivery—A Concise Outlook. AAPS PharmSciTech 2022, 23, 55. [Google Scholar] [CrossRef]
- Saikumar, D.; Prasanna, J.L. A Literature Review on Self Nanoemulsifying Drug Delivery System (SNEDDS). Int. J. Pharm. Sci. Rev. Res. 2021, 70, 85–94. [Google Scholar] [CrossRef]
- Parmar, N.; Singla, N.; Amin, S.; Kohli, K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf B Biointerfaces 2011, 86, 327–338. [Google Scholar] [CrossRef]
- Makkad, S.; Sheikh, M.; Shende, S.; Jirvankar, P. Pharmaceutical Excipients: Functions, Selection Criteria, and Emerging Trends. Int. J. Pharm. Investig. 2025, 15, 361–376. [Google Scholar] [CrossRef]
- Chatterjee, P.; Alvi, M.M. Excipients and active pharmaceutical ingredients. In AAPS Advances in the Pharmaceutical Sciences Series; Springer: New York, NY, USA, 2014; Volume 11. [Google Scholar] [CrossRef]
- Bravo-Alfaro, D.A.; Ochoa-Rodríguez, L.R.; Villaseñor-Ortega, F.; Luna-Barcenas, G.; García, H.S. Self-nanoemulsifying drug delivery system (SNEDDS) improves the oral bioavailability of betulinic acid. J. Mol. Liq. 2022, 364, 119946. [Google Scholar] [CrossRef]
- Buddhadev, S.S.; Garala, K.C.; Saisivam, S.; Rahamathulla, M.; Ahmed, M.M.; Farhana, S.A.; Pasha, I. Quality by design aided self-nano emulsifying drug delivery systems development for the oral delivery of Benidipine: Improvement of biopharmaceutical performance. Drug Deliv. 2023, 31, 2288801. [Google Scholar] [CrossRef] [PubMed]
- Gardouh, A.R.; Nasef, A.M.; Mostafa, Y.; Gad, S. Design and evaluation of combined atorvastatin and ezetimibe optimized self- nano emulsifying drug delivery system. Drug Deliv. Sci. Technol. 2020, 60, 102093. [Google Scholar] [CrossRef]
- Verma, R.; Kaushik, D. Design and optimization of candesartan loaded self-nanoemulsifying drug delivery system for improving its dissolution rate and pharmacodynamic potential. Drug Deliv. 2020, 27, 756–771. [Google Scholar] [CrossRef]
- Saifullah, S.; Kanwal, T.; Ullah, S.; Kawish, M.; Habib, S.M.; Ali, I.; Munir, A.; Imran, M.; Shah, M.R. Design and development of lipid modified chitosan containing muco-adhesive self-emulsifying drug delivery systems for cefixime oral delivery. Chem. Phys. Lipids 2021, 235, 105052. [Google Scholar] [CrossRef]
- Mohite, P.; Sule, S.; Pawar, A.; Alharbi, H.M.; Maitra, S.; Subramaniyan, V.; Kumarasamy, V.; Uti, D.E.; Ogbu, C.O.; Oodo, S.I.; et al. Development and characterization of a self-nano emulsifying drug delivery system (SNEDDS) for Ornidazole to improve solubility and oral bioavailability of BCS class II drugs. Sci. Rep. 2024, 14, 27724. [Google Scholar] [CrossRef]
- Patel, J.; Kevin, G.; Patel, A.; Raval, M.; Sheth, N. Design and development of a self-nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int. J. Pharm. Investig. 2011, 1, 112–118. [Google Scholar] [CrossRef]
- Jhawat, V.; Gulia, M.; Sharma, A.K. Pseudoternary phase diagrams used in emulsion preparation. In Chemoinformatics and Bioinformatics in the Pharmaceutical Sciences; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Abushal, A.S.; Aleanizy, F.S.; Alqahtani, F.Y.; Shakeel, F.; Iqbal, M.; Haq, N.; Alsarra, I.A. Self-Nanoemulsifying Drug Delivery System (SNEDDS) of Apremilast: In Vitro Evaluation and Pharmacokinetics Studies. Molecules 2022, 27, 3085. [Google Scholar] [CrossRef]
- Rathore, C.; Hemrajani, C.; Sharma, A.K.; Gupta, P.K.; Jha, N.K.; Aljabali, A.A.A.; Gupta, G.; Singh, S.K.; Yang, J.-C.; Dwivedi, R.P.; et al. Self-nanoemulsifying drug delivery system (SNEDDS) mediated improved oral bioavailability of thymoquinone: Optimization, characterization, pharmacokinetic, and hepatotoxicity studies. Drug Deliv. Transl. Res. 2022, 13, 292–307. [Google Scholar] [CrossRef]
- Usta, D.Y.; Timur, B.; Teksin, Z.S. Formulation development, optimization by Box-Behnken design, characterization, in vitro, ex-vivo, and in vivo evaluation of bosentan-loaded self-nanoemulsifying drug delivery system: A novel alternative dosage form for pulmonary arterial hypertension treatment. Eur. J. Pharm. Sci. 2022, 174, 106159. [Google Scholar] [CrossRef]
- Verch, T.; Campa, C.; Chéry, C.C.; Frenkel, R.; Graul, T.; Jaya, N.; Nakhle, B.; Springall, J.; Starkey, J.; Wypych, J.; et al. Analytical Quality by Design, Life Cycle Management, and Method Control. AAPS J. 2022, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Blue, L.E.; Guan, X.; Joubert, M.K.; Kuhns, S.T.; Moore, S.; Semin, D.J.; Wikström, M.; Wypych, J.; Goudar, C.T. State-of-the-art and emerging trends in analytical approaches to pharmaceutical-product commercialization. Curr. Opin. Biotechnol. 2022, 78, 102800. [Google Scholar] [CrossRef] [PubMed]
- Amancio, L.; Dorneles, C.F.; Dalip, D.H. Recency and quality-based ranking question in CQAs: A Stack Overflow case study. Inf. Process. Manag. 2021, 58, 102552. [Google Scholar] [CrossRef]
- Yenduri, G.; Costa, A.P.; Xu, X.; Burgess, D.J. Impact of critical process parameters and critical material attributes on the critical quality attributes of liposomal formulations prepared using continuous processing. Int. J. Pharm. 2022, 619, 121700. [Google Scholar] [CrossRef] [PubMed]
- Mohseni-Motlagh, S.F.; Dolatabadi, R.; Baniassadi, M.; Baghani, M. Application of the Quality by Design Concept (QbD) in the Development of Hydrogel-Based Drug Delivery Systems. Polymers 2023, 15, 4407. [Google Scholar] [CrossRef]
- Kumar, V.P.; Vishal Gupta, N. A review on quality by design approach (QBD) for pharmaceuticals. Int. J. Drug Dev. Res. 2015, 7, 52–60. [Google Scholar]
- Madondo, N.I.; Chetty, M. Anaerobic co-digestion of sewage sludge and bio-based glycerol: Optimisation of process variables using one-factor-at-a-time (OFAT) and Box-Behnken Design (BBD) techniques. South Afr. J. Chem. Eng. 2022, 40, 87–99. [Google Scholar] [CrossRef]
- Gurba-Bryśkiewicz, L.; Maruszak, W.; Smuga, D.A.; Dubiel, K.; Wieczorek, M. Quality by Design (QbD) and Design of Experiments (DOE) as a Strategy for Tuning Lipid Nanoparticle Formulations for RNA Delivery. Biomedicines 2023, 11, 2750. [Google Scholar] [CrossRef]
- Kuruba, G.; Reddy, K.A.N.; Poli, S.; Ramnarayanan, C. Quality by Design Based Development of Self Nano Emulsifying Drug Delivery System of Ritonavir. J. Young Pharm. 2020, 12, 215–220. [Google Scholar] [CrossRef]
- Jahan, R.N.; Khan, Z.; Akhtar, S.; Ansari, M.D.; Solanki, P.; Ahmad, F.J.; Aqil, M.; Sultana, Y. Development of Bedaquiline-Loaded SNEDDS Using Quality by Design (QbD) Approach to Improve Biopharmaceutical Attributes for the Management of Multidrug-Resistant Tuberculosis (MDR-TB). Antibiotics 2023, 12, 1510. [Google Scholar] [CrossRef]
- Panigrahi, K.C.; Patra, C.N.; Rao, M.E.B. Quality by Design Enabled Development of Oral Self-Nanoemulsifying Drug Delivery System of a Novel Calcimimetic Cinacalcet HCl Using a Porous Carrier: In Vitro and In Vivo Characterisation. AAPS PharmSciTech 2019, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Shrivastava, N.; Kumar, S.; Singh, A.K.; Ali, J.; Baboota, S. Designing and development of omega-3 fatty acid based self-nanoemulsifying drug delivery system (SNEDDS) of docetaxel with enhanced biopharmaceutical attributes for management of breast cancer. J. Drug Deliv. Sci. Technol. 2022, 68, 103117. [Google Scholar] [CrossRef]
- Arun, J.K.; Vodeti, R.; Shrivastava, B.; Bakshi, V. Integrated quality by design approach for developing nanolipidic drug delivery systems of olmesartan medoxomil with enhanced antihypertensive action. Adv. Pharm. Bull. 2020, 10, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Gausuzzaman, S.A.L.; Saha, M.; Dip, S.J.; Alam, S.; Kumar, A.; Das, H.; Sharker, S.M.; Rashid, A.; Kazi, M.; Reza, H.M. A QbD Approach to Design and to Optimize the Self-Emulsifying Resveratrol–Phospholipid Complex to Enhance Drug Bioavailability through Lymphatic Transport. Polymers 2022, 14, 3220. [Google Scholar] [CrossRef]
- Shrivastava, N.; Parikh, A.; Dewangan, R.P.; Biswas, L.; Verma, A.K.; Mittal, S.; Ali, J.; Garg, S.; Baboota, S. Solid Self-Nano Emulsifying Nanoplatform Loaded with Tamoxifen and Resveratrol for Treatment of Breast Cancer. Pharmaceutics 2022, 14, 1486. [Google Scholar] [CrossRef]
- Jagtap, K.; Chaudhari, B.; Redasani, V. Quality by Design (QbD) concept Review in Pharmaceuticals. Asian J. Res. Chem. 2022, 15, 303–307. [Google Scholar] [CrossRef]
- Khan, A.; Naquvi, K.J.; Haider, F.; Khan, M.A. Quality by design- newer technique for pharmaceutical product development. Intell. Pharm. 2024, 2, 122–129. [Google Scholar] [CrossRef]
- Sitre, D.; Kamble, R. Articulation of Quality By Design Elements for Product Development and its Unique Applications. J. Drug Deliv. Ther. 2020, 10, 253–261. [Google Scholar] [CrossRef]
- Namjoshi, S.; Dabbaghi, M.; Roberts, M.S.; Grice, J.E.; Mohammed, Y. Quality by design: Development of the quality target product profile (QTPP) for semisolid topical products. Pharmaceutics 2020, 12, 287. [Google Scholar] [CrossRef]
- Saliy, O.; Los, O.; Palchevska, T.; Nebylytsia, K. Implementation of the Quality by Design approach for developing the composition and the manufacturing technology of an injectable drug for intra-articular introduction. News Pharm. 2021, 1, 28–37. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, J.-K.; Jee, J.-P.; Jang, D.-J.; Park, Y.-J.; Kim, J.-E. Quality by Design (QbD) application for the pharmaceutical development process. J. Pharm. Investig. 2022, 52, 649–682. [Google Scholar] [CrossRef]
- Grangeia, H.B.; Silva, C.; Simões, S.P.; Reis, M.S. Quality by design in pharmaceutical manufacturing: A systematic review of current status, challenges and future perspectives. Eur. J. Pharm. Biopharm. 2020, 147, 19–37. [Google Scholar] [CrossRef]
- Kumah, A.; Nwogu, C.N.; Issah, A.-R.; Obot, E.; Kanamitie, D.T.; Sifa, J.S.; Aidoo, L.A. Cause-and-Effect (Fishbone) Diagram: A Tool for Generating and Organizing Quality Improvement Ideas. Glob. J. Qual. Saf. Healthc. 2024, 7, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Zahir-Jouzdani, F.; Shahbaz, S.; Andarzbakhsh, K.; Dinarvand, S.; Nasab, M.H.F.; Amoli, F.A.; Asgharian, R.; Atyabi, F. Triamcinolone-loaded self nano-emulsifying drug delivery systems for ocular use: An alternative to invasive ocular surgeries and injections. Int J Pharm. 2024, 653, 123840. [Google Scholar] [CrossRef]
- Zingale, E.; Bonaccorso, A.; D’amico, A.G.; Lombardo, R.; D’agata, V.; Rautio, J.; Pignatello, R. Formulating Resveratrol and Melatonin Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Ocular Administration Using Design of Experiments. Pharmaceutics 2024, 16, 125. [Google Scholar] [CrossRef]
- Farooqui, H.; Upadhyay, S.; Upadhyay, P. Transdermal Patches Approach Towards Self-Nano-Emulsifying Drug Delivery System (SNEDDS) Using Essential Oil as Penetration Enhancer. Micro Nanosyst. 2022, 14, 314–340. [Google Scholar] [CrossRef]
- Leichner, C.; Baus, R.A.; Jelkmann, M.; Plautz, M.; Barthelmes, J.; Dünnhaupt, S.; Bernkop-Schnürch, A. In vitro evaluation of a self-emulsifying drug delivery system (SEDDS) for nasal administration of dimenhydrinate. Drug Deliv. Transl. Res. 2019, 9, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.S. Design of experiments: A design to improve pharmaceutical research. J. Adv. Pharm. Technol. Res. 2023, 14, 1. [Google Scholar] [CrossRef]
- Kasemiire, A.; Avohou, H.T.; De Bleye, C.; Sacre, P.-Y.; Dumont, E.; Hubert, P.; Ziemons, E. Design of experiments and design space approaches in the pharmaceutical bioprocess optimization. Eur. J. Pharm. Biopharm. 2021, 166, 144–154. [Google Scholar] [CrossRef]
- Jankovic, A.; Chaudhary, G.; Goia, F. Designing the design of experiments (DOE)—An investigation on the influence of different factorial designs on the characterization of complex systems. Energy Build. 2021, 250, 111298. [Google Scholar] [CrossRef]
- Visetvichaporn, V.; Kim, K.-H.; Jung, K.; Cho, Y.-S.; Kim, D.-D. Formulation of self-microemulsifying drug delivery system (SMEDDS) by D-optimal mixture design to enhance the oral bioavailability of a new cathepsin K inhibitor (HL235). Int. J. Pharm. 2020, 573, 118772. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, D.; Gao, X. Optimization of mixture proportions by statistical experimental design using response surface method—A review. J. Build. Eng. 2021, 36, 102101. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Felimban, R.I.; Tayeb, H.H.; Rizg, W.Y.; Alnadwi, F.H.; Alotaibi, H.A.; Alhakamy, N.A.; Abd-Allah, F.I.; Mohamed, G.A.; Zidan, A.S.; et al. Development of multi-compartment 3d-printed tablets loaded with self-nanoemulsified formulations of various drugs: A new strategy for personalized medicine. Pharmaceutics 2021, 13, 1733. [Google Scholar] [CrossRef]
- Al-Amodi, Y.A.; Hosny, K.M.; Alharbi, W.S.; Safo, M.K.; El-Say, K.M. Investigating the potential of transmucosal delivery of febuxostat from oral lyophilized tablets loaded with a self-nanoemulsifying delivery system. Pharmaceutics 2020, 12, 534. [Google Scholar] [CrossRef]
- Fayez, S.M.; Elnahas, O.S.; Fayez, A.M.; El-Mancy, S.S. Coconut oil based self-nano emulsifying delivery systems mitigate ulcerogenic NSAIDs side effect and enhance drug dissolution: Formula optimization, in-vitro, and in-vivo assessments. Int. J. Pharm. 2023, 634, 122666. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Bakhaidar, R.B.; Zaki, R.M.; Abualsunun, W.A.; Alkhalidi, H.M.; Bahmdan, R.H.; Shadab; Hassan, A.H. Formulation and optimization of neomycin Sulfate–Thioctic acid loaded in a eucalyptus oil self-nanoemulsion to enhance the beneficial activity of the substances and limit the side effects associated with the treatment of hepatic coma. J. Drug Deliv. Sci. Technol. 2021, 61, 102108. [Google Scholar] [CrossRef]
- El-Say, K.M.; Alamri, S.H.; Alsulimani, H.H.; Alharbi, W.S.; Omar, A.M.; Safo, M.K.; Ahmed, T.A. Incorporating valsartan in sesame oil enriched self-nanoemulsifying system-loaded liquisolid tablets to improve its bioavailability. Int. J. Pharm. 2023, 639, 122966. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Alrobaian, M.; Kazmi, I.; Afzal, O.; Altamimi, A.S.A.; Al-Abbasi, F.A.; Almalki, W.H.; Baothman, A.A.; Choudhry, H.; Rahman, M.; et al. Cationic self-nanoemulsifying formulations of tamoxifen with improved biopharmaceutical attributes and anticancer activity: Systematic development and evaluation. J. Mol. Liq. 2020, 320, 114534. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Fahmy, U.A.; Aldawsari, H.M.; Ahmed, O.A.A.; Alhakamy, N.A.; Okbazghi, S.Z.; El-Moselhy, M.A.; Alghaith, A.F.; Anter, A.; Matouk, A.I.; et al. Optimized Self-Nanoemulsifying Delivery System Based on Plant-Derived Oil Augments Alpha-Lipoic Acid Protective Effects Against Experimentally Induced Gastric Lesions. Dose-Response 2021, 19, 15593258211001259. [Google Scholar] [CrossRef]
- Gao, S.; Chen, J.; Peng, W.; Yang, Y.; Hua, L.; Guo, Y.; Wang, Y.; Zhang, X. The preparation and relative bioavailability of an artemisin in self-emulsifying drug delivery system. Drug Deliv. 2023, 30, 2168794. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Al-Maghrabi, P.M.; Alavi, O.; Lind, T.; Sassene, P.J.; Kirkensgaard, J.J.; Mota-Santiago, P.; Rades, T.; Müllertz, A. Design, evaluation, and in vitro–in vivo correlation of self-nanoemulsifying drug delivery systems to improve the oral absorption of exenatide. J. Control. Release 2025, 379, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Alotaibi, H.A.; Almehmady, A.M.; Safo, M.K.; El-Say, K.M. Influences of Glimepiride Self-Nanoemulsifying Drug Delivery System Loaded Liquisolid Tablets on the Hypoglycemic Activity and Pancreatic Histopathological Changes in Streptozotocin-Induced Hyperglycemic Rats. Nanomaterials 2022, 12, 3966. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Famta, P.; Vambhurkar, G.; Srinivasarao, D.A.; Kumar, K.C.; Bagasariya, D.; Begum, N.; Sharma, A.; Jain, N.; Shahrukh, S.; et al. Quality by design accredited self-nanoemulsifying delivery of ibrutinib for extenuating the fast-fed variability, ameliorating the anticancer activity and oral bioavailability in prostate cancer. J. Drug Deliv. Sci. Technol. 2023, 89, 105052. [Google Scholar] [CrossRef]
- Verma, R.; Kaushik, A.; Almeer, R.; Rahman, H.; Abdel-Daim, M.M.; Kaushik, D. Improved pharmacodynamic potential of rosuvastatin by self-nanoemulsifying drug delivery system: An in vitro and in vivo evaluation. Int. J. Nanomed. 2021, 16, 905–924. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alhakamy, N.A.; Almodhwahi, M.A.; Kurakula, M.; Almehmady, A.M.; Elgebaly, S.S. Self-nanoemulsifying system loaded with sildenafil citrate and incorporated within oral lyophilized flash tablets: Preparation, optimization, and in vivo evaluation. Pharmaceutics 2020, 12, 1124. [Google Scholar] [CrossRef]
- Rasoanirina, B.N.V.; Lassoued, M.A.; Kamoun, A.; Bahloul, B.; Miladi, K.; Sfar, S. Voriconazole-loaded self-nanoemulsifying drug delivery system (SNEDDS) to improve transcorneal permeability. Pharm. Dev. Technol. 2020, 25, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Buya, A.B.; Terrasi, R.; Mbinze, J.K.; Muccioli, G.G.; Beloqui, A.; Memvanga, P.B.; Préat, V. Quality-by-design-based development of a voxelotor self-nanoemulsifying drug-delivery system with improved biopharmaceutical attributes. Pharmaceutics 2021, 13, 1388. [Google Scholar] [CrossRef]
- Ali, S.A.; Alhakamy, N.A.; Hosny, K.M.; Alfayez, E.; Bukhary, D.M.; Safhi, A.Y.; Badr, M.Y.; Mushtaq, R.Y.; Alharbi, M.; Huwaimel, B.; et al. Rapid oral transmucosal delivery of zaleplon–lavender oil utilizing self-nanoemulsifying lyophilized tablets technology: Development, optimization and pharmacokinetic evaluation. Drug Deliv. 2022, 29, 2773–2783. [Google Scholar] [CrossRef]
- Radwan, M.F.; El-Moselhy, M.A.; Alarif, W.M.; Orif, M.; Alruwaili, N.K.; Alhakamy, N.A. Optimization of Thymoquinone-Loaded Self-Nanoemulsion for Management of Indomethacin-Induced Ulcer. Dose-Response 2021, 19, 15593258211013655. [Google Scholar] [CrossRef]
- Beg, S. Mixture Designs and Their Applications in Pharmaceutical Product Development. In Design of Experiments for Pharmaceutical Product Development Volume I: Basics and Fundamental Principles; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Galvan, D.; Effting, L.; Cremasco, H.; Conte-Junior, C.A. Recent applications of mixture designs in beverages, foods, and pharmaceutical health: A systematic review and meta-analysis. Foods 2021, 10, 1941. [Google Scholar] [CrossRef]
- Abo-Neima, S.E.; Elsehly, E.M.; Al-Otibi, F.O.; El-Metwally, M.M.; Helmy, Y.A.; Eldadamony, N.M.; Saber, W.I.; El-Morsi, A.A. Simplex-lattice design and decision tree optimization of endophytic Trichoderma-multi-walled carbon nanotube composite for enhanced methylene blue removal. Heliyon 2024, 10, e39949. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.A.N.; Kunchithapatham, J.; Dang, R.; Ramnarayanan, C. Design and development of darunavir loaded self micro emulsifying drug delivery system using extreme vertices mixture design in a quality by design framework. Indian J. Pharm. Educ. Res. 2020, 54, 337–348. [Google Scholar] [CrossRef]
- Adem, A.A.; Belete, A.; Lai, K.K.; Hage, C.; Neubert, R.H.; Gebre-Mariam, T. Nanoemulgel formulation for topical delivery of plant glucosylceramide: Characterization and optimization. J. Drug Deliv. Sci. Technol. 2023, 79, 104056. [Google Scholar] [CrossRef]
- Zafar, A.; Yasir, M.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alshehri, S.; Ghoneim, M.M.; Alquraini, A.; Rawaf, A.; Ansari, M.J.; et al. Formulation of Self-Nanoemulsifying Drug Delivery System of Cephalexin: Physiochemical Characterization and Antibacterial Evaluation. Polymers 2022, 14, 1055. [Google Scholar] [CrossRef]
- Corrie, L.; Gulati, M.; Awasthi, A.; Vishwas, S.; Kaur, J.; Khursheed, R.; Kumar, R.; Kumar, A.; Imran, M.; Chellappan, D.; et al. Polysaccharide, fecal microbiota, and curcumin-based novel oral colon-targeted solid self-nanoemulsifying delivery system: Formulation, characterization, and in-vitro anticancer evaluation. Mater. Today Chem. 2022, 26, 101165. [Google Scholar] [CrossRef]
- Mao, J.; Liu, X.; Zhang, L.; Chen, Y.; Zhou, S.; Liu, Y.; Ye, J.; Xu, X.; Zhang, Q. Self-Nanoemulsifying Drug Delivery System of Morin: A New Approach for Combating Acute Alcohol Intoxication. Int. J. Nanomed. 2024, 19, 10569–10588. [Google Scholar] [CrossRef]
- Echeverry, S.M.; Rey, D.; Valderrama, I.H.; de Araujo, B.V.; Aragón, D.M. Development of a self-emulsifying drug delivery system (SEDDS) to improve the hypoglycemic activity of Passiflora ligularis leaves extract. J. Drug Deliv. Sci. Technol. 2021, 64, 102604. [Google Scholar] [CrossRef]
- Zafar, A.; Imam, S.S.; Alruwaili, N.K.; Alsaidan, O.A.; Elkomy, M.H.; Ghoneim, M.M.; Alshehri, S.; Ali, A.M.A.; Alharbi, K.S.; Yasir, M.; et al. Development of piperine-loaded solid self-nanoemulsifying drug delivery system: Optimization, in-vitro, ex-vivo, and in-vivo evaluation. Nanomaterials 2021, 11, 2920. [Google Scholar] [CrossRef]
- Kamble, P.R.; Shaikh, K.S. Optimization and Evaluation of Self-nanoemulsifying Drug Delivery System for Enhanced Bioavailability of Plumbagin. Planta Medica 2022, 88, 79–90. [Google Scholar] [CrossRef]
- Uttreja, P.; Youssef, A.A.A.; Karnik, I.; Sanil, K.; Narala, N.; Wang, H.; Elkanayati, R.M.; Vemula, S.K.; Repka, M.A. Formulation Development of Solid Self-Nanoemulsifying Drug Delivery Systems of Quetiapine Fumarate via Hot-Melt Extrusion Technology: Optimization Using Central Composite Design. Pharmaceutics 2024, 16, 324. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, P.; Jin, Y.; Tang, M.; Shen, M.; Xu, S.; Huang, S.; Chen, Y. Using Self-Nanoemulsifying System to Improve Oral Bioavailability of a Pediatric Antiepileptic Agent Stiripentol: Formulation and Pharmacokinetics Studies. AAPS PharmSciTech 2020, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Rajana, N.; Chary, P.S.; Pooja, Y.S.; Bhavana, V.; Singh, H.; Guru, S.K.; Singh, S.B.; Mehra, N.K. Quality by design approach-based fabrication and evaluation of self-nanoemulsifying drug delivery system for improved delivery of venetoclax. Drug Deliv. Transl. Res. 2023, 14, 1277–1300. [Google Scholar] [CrossRef]
- Breig, S.J.M.; Luti, K.J.K. Response surface methodology: A review on its applications and challenges in microbial cultures. Mater. Today Proc. 2021, 42, 2277–2284. [Google Scholar] [CrossRef]
- Kumar, R.; Reji, M. Response surface methodology (RSM): An overview to analyze multivariate data. Indian J. Microbiol. Res. 2022, 9, 241–248. [Google Scholar] [CrossRef]
- Szpisják-Gulyás, N.; Al-Tayawi, A.N.; Horváth, Z.H.; László, Z.; Kertész, S.; Hodúr, C. Methods for experimental design, central composite design and the Box–Behnken design, to optimise operational parameters: A review. Acta Aliment. 2023, 52, 521–537. [Google Scholar] [CrossRef]
- Li, Z.; Shu, X. Roller design and optimization based on RSM with categoric factors in power spinning of Ni-based superalloy. Int. J. Adv. Manuf. Technol. 2022, 120, 447–469. [Google Scholar] [CrossRef]
- Yadav, P.; Rastogi, V.; Verma, A. Application of Box–Behnken design and desirability function in the development and optimization of self-nanoemulsifying drug delivery system for enhanced dissolution of ezetimibe. Futur. J. Pharm. Sci. 2020, 6, 7. [Google Scholar] [CrossRef]
- Galatage, S.T.; Trivedi, R.; Bhagwat, D.A. Oral self-emulsifying nanoemulsion systems for enhancing dissolution, bioavailability and anticancer effects of camptothecin. J. Drug Deliv. Sci. Technol. 2022, 78, 103929. [Google Scholar] [CrossRef]
- Tekeli, M.C.; Aktas, Y.; Celebi, N. Oral self-nanoemulsifying formulation of GLP-1 agonist peptide exendin-4: Development, characterization and permeability assesment on Caco-2 cell monolayer. Amino Acids. 2021, 53, 73–88. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Alyousef, A.M.; Altamimi, M.; Alsulays, B.; Shakeel, F. Flufenamic acid-loaded self-nanoemulsifying drug delivery system for oral delivery: From formulation statistical optimization to preclinical anti-inflammatory assessment. J. Oleo Sci. 2020, 69, 1257–1271. [Google Scholar] [CrossRef]
- Galatage, S.T.; Manjappa, A.S.; Bhagwat, D.A.; Trivedi, R.; Salawi, A.; Sabei, F.Y.; Alsalhi, A. Oral self-nanoemulsifying drug delivery systems for enhancing bioavailability and anticancer potential of fosfestrol: In vitro and in vivo characterization. Eur. J. Pharm. Biopharm. 2023, 193, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhan, M.; Wang, Z.; Wang, Z.; Li, X.-M.; Miao, M. Development of an orally bioavailable isoliquiritigenin self-nanoemulsifying drug delivery system to effectively treat ovalbumin-induced asthma. Int. J. Nanomed. 2020, 15, 8945–8961. [Google Scholar] [CrossRef]
- Galatage, S.T.; Manjappa, A.S.; Salawi, A.; Desai, J.L.; Kumbar, V.M.; Ghagane, S.; Hebalkar, A.S.; Dhobale, S.V. Palbociclib-letrozole loaded solid self-nano emulsifying drug delivery system for oral treatment of breast cancer: In-vitro and In-vivo characterization. J. Drug Deliv. Sci. Technol. 2025, 104, 106469. [Google Scholar] [CrossRef]
- More, S.M.; Rashid, A.; Kharwade, R.S.; Taha, M.; Alhamhoom, Y.; Elhassan, G.O.; Gangane, P.; Asar, T.O.; Pise, A.; Kaleem, M.; et al. Development of Solid Self-Nanoemulsifying Drug Delivery System of Rhein to Improve Biopharmaceutical Performance: Physiochemical Characterization, and Pharmacokinetic Evaluation. Int. J. Nanomed. 2025, 20, 267–291. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Singh, B.; Shah, J.; Jacob, S.; Aldhubiab, B.; Sreeharsha, N.; Morsy, M.A.; Venugopala, K.N.; Attimarad, M.; Shinu, P. Formulation and Evaluation of Self-Nanoemulsifying Drug Delivery System Derived Tablet Containing Sertraline. Pharmaceutics 2022, 14, 336. [Google Scholar] [CrossRef]
- El-Halim, S.M.A.; A Mamdouh, M.; Eid, S.M.; Ibrahim, B.M.; Labib, D.A.A.; Soliman, S.M. The potential synergistic activity of zolmitriptan combined in new self-nanoemulsifying drug delivery systems: Atr-ftir real-time fast dissolution monitoring and pharmacodynamic assessment. Int. J. Nanomed. 2021, 16, 6395–6412. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, R.; Azer, M.S.; Makky, A.; Zaghloul, A.; El-Nabarawi, M.; Nada, A. Development, characterization, and in-vivo pharmacokinetic study of lamotrigine solid self-nanoemulsifying drug delivery system. Drug Des. Dev. Ther. 2020, 14, 4343–4362. [Google Scholar] [CrossRef]
- Beg, S.; Sandhu, P.S.; Batra, R.S.; Khurana, R.K.; Singh, B. QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv. 2014, 22, 765–784. [Google Scholar] [CrossRef] [PubMed]
- Beg, S. (Ed.) Design of Experiments for Pharmaceutical Product Development; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Sopyan, I.; Gozali, D.; Sriwidodo; Guntina, R.K. Design-Expert Software (Doe): An Application Tool For Optimization In Pharmaceutical Preparations Formulation. Int. J. Appl. Pharm. 2022, 14, 55–63. [Google Scholar] [CrossRef]
- Kraber, S. Improving Your DOE—Analysis with Response Transformations. J. Plast. Film Sheeting 2022, 38, 15–20. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Kim, J.; Jin, F.; Jun, M.; Cheong, M.; Yammarino, F.J. Polynomial regression analysis and response surface methodology in leadership research. Leadersh. Q. 2022, 33, 101592. [Google Scholar] [CrossRef]
- Earle, R.; Gadela, V. Optimization and characterization of self-nanoemulsifying drug delivery system of iloperidone using box-behnken design and desirability function. Ann. Pharm. Fr. 2022, 81, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Baloch, J.; Sohail, M.F.; Sarwar, H.S.; Kiani, M.H.; Khan, G.M.; Jahan, S.; Rafay, M.; Chaudhry, M.T.; Yasinzai, M.; Shahnaz, G. Self-nanoemulsifying drug delivery system (Snedds) for improved oral bioavailability of chlorpromazine: In vitro and in vivo evaluation. Medicina 2019, 55, 210. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for poorly water-soluble talinolol: Preparation, in vitroand in vivoAssessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef]
- Neelam, U.K.; Daveedu, B.; Ambabhai, V.N.; Siripragada, M.R.; Kumar, S.R.; Balasubramanian, S. Physicochemical aspects and comparative analysis of Voxelotor and its salt and cocrystal. J. Mol. Struct. 2023, 1271, 134024. [Google Scholar] [CrossRef]
- Pathan, L.S.; Modi, C.D.; Gohel, M.C.; Udhwani, N.H.; Thakkar, V.T.; Rana, H.B. Introducing novel hybridization technique for solubility enhancement of Bosentan formulation. Food Hydrocoll. Health 2022, 2, 100055. [Google Scholar] [CrossRef]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 Cell Line Standardization with Pharmaceutical Requirements and In Vitro Model Suitability for Permeability Assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical formulations with p-glycoprotein inhibitory effect as promising approaches for enhancing oral drug absorption and bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.; Qi, J.; Wu, W. An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm. Sin. B 2021, 11, 2449–2468. [Google Scholar] [CrossRef]
- Kumari, L.; Choudhari, Y.; Patel, P.; Das Gupta, G.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Das Kurmi, B. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef]
- Khan, A.D.; Tabish, M.; Kaushik, R.; Saxena, V.; Kesharwani, P.; Gupta, S.; Alam, M.N.; Sharma, V. Hydrotropy: Recent advancements in enhancement of drug solubility and formulation development. Int. J. Drug Deliv. Technol. 2021, 11, 1092–1102. [Google Scholar]
- Schonlau, M.; Zou, R.Y. The random forest algorithm for statistical learning. Stata J. Promot. Commun. Stat. Stata 2020, 20, 3–29. [Google Scholar] [CrossRef]
- Aulifa, D.L.; Al Shofwan, A.A.; Megantara, S.; Fakih, T.M.; Budiman, A. Elucidation of Molecular Interactions Between Drug–Polymer in Amorphous Solid Dispersion by a Computational Approach Using Molecular Dynamics Simulations. Adv. Appl. Bioinform. Chem. 2024, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.; Bruininks, B.M.H.; Singh, P.; dos Santos, L.; Pizzol, C.D.; Dieamant, G.D.C.; Kruger, O.; Martin, A.A.; Marrink, S.J.; Souza, P.C.T.; et al. Complex nanoemulsion for vitamin delivery: Droplet organization and interaction with skin membranes. Nanoscale 2022, 14, 506–514. [Google Scholar] [CrossRef]
- Karjiban, R.A.; Huan, Q.-Y.; Rahman, M.B.A.; Basri, M.; Tejo, B.A. Self-assembly of Palm Kernel Oil Wax Esters in Aqueous Media: A Molecular Dynamics Study. Int. J. Chem. 2015, 7, 133. [Google Scholar] [CrossRef]
- Hidayat, A.F.; Fakih, T.M. Self-Assembly of Black Cumin Oil-Based Nanoemulsion on Various Surfactants: A Molecular Dynamics Study. Makara J. Sci. 2021, 25, 8–264. [Google Scholar] [CrossRef]
- Moghaddasi, F.; Housaindokht, M.R.; Darroudi, M.; Bozorgmehr, M.R.; Sadeghi, A. Soybean oil-based nanoemulsion systems in absence and presence of curcumin: Molecular dynamics simulation approach. J. Mol. Liq. 2018, 264, 242–252. [Google Scholar] [CrossRef]
- Pirhadi, S.; Amani, A. Molecular dynamics simulation of self-assembly in a nanoemulsion system. Chem. Pap. 2020, 74, 2443–2448. [Google Scholar] [CrossRef]
- Kazi, M.; Almarri, F.; Shahba, A.A.-W.; Ahmad, A.; Albraiki, S.; Alanazi, F.K. Nutraceutically-enhanced oral delivery of vitamin D3 via Bio-SNEDDS: Demonstrating in vivo superiority over pediatric formulations. Biochem. Biophys. Res. Commun. 2024, 709, 149852. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, N.; Zhao, X.; Wang, Z.; Liu, Z.; Cui, Y. Application of physiologically based pharmacokinetic modeling of novel drugs approved by the U.S. food and drug administration. Eur. J. Pharm. Sci. Eur. J. Pharm. Sci. 2024, 200, 106838. [Google Scholar] [CrossRef]
- Demeester, C.; Robins, D.; Edwina, A.E.; Tournoy, J.; Augustijns, P.; Ince, I.; Lehmann, A.; Vertzoni, M.; Schlender, J.F. Physiologically based pharmacokinetic (PBPK) modelling of oral drug absorption in older adults—An AGePOP review. Eur. J. Pharm. Sci. 2023, 188, 106496. [Google Scholar] [CrossRef] [PubMed]
- Abrahim-Vieira, B.A.; DE Souza, A.M.; Barros, R.C.; Carmo, F.A.D.; DE Abreu, L.C.; Moreira, R.S.; Honório, T.S.; Rodrigues, C.R.; DE Sousa, V.P.; Cabral, L.M. In silico studies of novel sildenafil self-emulsifying drug delivery system absorption improvement for pulmonary arterial hypertension. An. Da Acad. Bras. De Cienc. 2020, 92, e20191445. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.A.; Hathout, R.M. Computer-Assisted Drug Formulation Design: Novel Approach in Drug Delivery. Mol. Pharm. 2015, 12, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, H.; Leuenberger, M.N. Impact of the digital revolution on the future of pharmaceutical formulation science. Eur. J. Pharm. Sci. 2016, 87, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wu, Z.; Xu, H.; Ouyang, D. FormulationAI: A novel web-based platform for drug formulation design driven by artificial intelligence. Brief. Bioinform. 2024, 25, bbad419. [Google Scholar] [CrossRef]


| API [Ref] | QTPP Elements | Risk Assesment Method | CQAs | CPPs | CMAs |
|---|---|---|---|---|---|
| Ritonavir [56] | Dosage type, dosage strength, route of administration, packaging, pharmacokinetic parameter, stability | Ishikawa fish-bone diagram follows with REM | Globule size, emulsification time, PDI, and % transmittance | - | Conc. of oil, surf, and co-S |
| Bedaquiline [57] | Dosage form, dosage type, dosage strength, route of administration, stability, container closure system, alternative method for administration | Ishikawa fish-bone diagram | Globule size, PDI, % transmittance | Sonication time | Conc. of oil and Smix |
| Benidipine [38] | Dosage form, dosage type, dosage strength, route of administration, pharmacokinetics, packaging, container closure system, different methods of administration, stability | Ishikawa fish-bone diagram, | Emulsification time, globule size, % drug release, % transmittance | - | Conc. of oil, surf, and co-S |
| Cinacalcet HCl [58] | Dosage form, dosage type, drug absorption, dispersity | Ishikawa fish-bone diagram followed by FMEA | % drug release, emulsification time, globule size, PDI | - | Conc. of oil, surf, and co-S |
| Docetaxel [59] | Drug delivery system, dosage type, route of administration, and drug release | Ishikawa fish-bone diagram | Globule size, PDI, % transmittance, emulsification time | Sonication time | Conc. of oil and Smix |
| Olmesartan medoxomil [60] | Dosage form, dosage type, dosage strength, route of administration, pharmacokinetics, packaging, stability | Ishikawa fish-bone diagram followed by RAM | Globule size, emulsification time, % drug release, mean dissolution time, % dissolution efficiency | - | Conc. of oil, surf, and co-S |
| Resveratrol [61] | Clinical target, route of Administration, dosage form design, stability, container closure system | RAM | Emulsification time, globule size, PDI, % drug release | - | Conc. of oil, surf, and co-S |
| Tamoxifen and Resveratrol [62] | Dosage form, dosage type, route of administration, stability | Ishikawa fish-bone diagram | Globule size, PDI, % transmittance | - | Conc. of oil and Smix |
| QTPP Element 1 | Target | Justification |
|---|---|---|
| Clinical Target | Improved bioavailability of poorly water-soluble drugs | SNEDDS enhances drug solubility and absorption by forming nanoemulsions in the gastrointestinal tract. |
| Dosage Form | SNEDDS/Lipid-based drug delivery system | SNEDDS is a lipid-based drug delivery system that can enhance the bioavailability of poorly water-soluble compounds with good stability. |
| Dosage Type | Immediate release | A quicker onset of action results in improved therapeutic effects. |
| Dosage Strength | Defined on drug solubility and therapeutic dose | The strength must ensure an optimal dose that achieves the desired pharmacokinetic profile. |
| Route of Administration | Oral | SNEDDS is designed for oral delivery to enhance drug absorption in the gastrointestinal tract. |
| Packaging/Container Closure System | Soft or hard capsules/airtight glass bottles | Protects the formulation from environmental factors and prevents drug–lipid interactions |
| Pharmacokinetic Parameters | Increased Cmax and AUC compared to conventional formulations | SNEDDS improves drug dissolution, leading to enhanced systemic exposure and faster onset of action. |
| Stability | Compliance with ICH guidelines | Ensures that the formulation remains effective and does not degrade under storage conditions |
| CQAs 1 | Target | Justification | Method |
|---|---|---|---|
| Globule Size | <200 nm | Enhances drug absorption and bioavailability | Dynamic Light Scattering of the dilution system |
| Emulsification Time | <1 min | Ensures rapid self-nanoemulsification in the GI tract | Visual observation with gentle agitation in aqueous media |
| % Transmittance | >90% | Indicates a clear and stable nanoemulsion | UV-Vis spectrophotometry of the dilution system |
| Drug Release | ~100 %(the limit varies for each API) | Ensures efficient and rapid drug release | Dissolution testing in appropriate media |
| Zeta Potential | ±30 mV or higher | It prevents globule aggregation and enhances stability. | Electrophoretic Light Scattering of the dilution system |
| Polydispersity Index | <0.5 | Indicates uniform globule size distribution | Dynamic Light Scattering of the dilution system |
| API [Ref] | Experimental Design | Correlation Factors and Responses | Optimal Formulation | Product Performances |
|---|---|---|---|---|
| Glimepiride + Rosuvastatin [80] | DoE: extreme vertices MD Runs: 13 Factors: % oil, surf, Co-S Responses: globule size | GS ↑: Oil and surf ↑, Co-S ↓ | Oil: Curcuma longa oil (15%) Surf: Tween 80 (10%) Co-S: PEG 400 (75%) Characteristics: GS of 94.43 ± 3.55 nm and PDI of 0.544 |
|
| Febuxostat [81] | DoE: extreme vertices MD Runs: 14 Factors: % oil, surf, Co-S Responses: globule size, Stability Index | GS ↑: Oil and Co-S ↑, Surf ↓ Stability index ↑: Oil and Surf ↓, Co-S ↑ | Oil: Corn oil (10%) Surf: Labrasol (40%) Co-S: Transcutol HP (50%) Characteristics: GS of 175.7 nm |
|
| Diclofenac potassium and coconut oil [82] | DoE: simplex lattice design Runs: 13 Factors: % of oil, surf, co-S Responses: globule size, PDI | GS ↑: Oil and Co-S ↑, Surf ↓ PDI ↑: Oil ↑ | Oil: Coconut oil (10%) Surf: Tween 80 (70%) Co-S: Ethanol (20%) Characteristics: GS of 160 ± 7.5 nm, PDI of 0.380 ± 0.06, ZP: −38.2 ± 1.9 mV |
|
| Neomycin Sulfate–Thioctic acid [83] | DoE: simplex lattice design Runs: 11 Factors: % oil, surf, co-S Responses: globule size | GS ↑: Oil ↑, Surf and Co-S ↓ | Oil: Eucalyptus oil (13%) Surf: Tween 80 (46%) Co-S: Propyleneglycol (39%) Characteristics: GS of 150 nm |
|
| Valsartan [84] | DoE: simplex lattice design Runs: 16 Factors: % oil, surf, co-S Responses: globule size; % drug load | GS ↑: Oil ↑, Surf and Co-S ↓ DL↑: Oil ↑ | Oil: Sesame oil (24.9%) Surf: Tween 80 (33.3%) Co-S: PEG 400 (41.8%) Characteristics: GS of 174.6 nm, PDI of 0.184, and a ZP of 31.2 mV |
|
| Tamoxifen [85] | DoE: mixture design Runs: 16 runs Factors: % oil, surf, co-S Responses: globule size, PDI, zeta potential, % drug release | NA | Oil: Corn oil (34%) Surf: Labrasol (48%) Co-S: Transcutol (18%) Characteristics: GS of 138 nm. PDI of 0.31, and ZP of +35.45 mV |
|
| Alpha-lipoic acid [86] | DoE: D-optimal mixture Runs: 16 Factors: % oil, surf, co-S Responses: globule size | GS ↑: Oil ↑ | Oil: Pumpkin oil (10%) Surf: Tween 80 Co-S: PEG 200 Characteristics: GS of 97.12 nm |
|
| Artemisin [87] | DoE: optimal mixture design Runs: 16 Factors: % oil, surf, co-S Responses: % drug loading, solution, emulsification time | DL↑: Co-S ↓ Solution ↑: Oil ↑ ET ↑: Oil ↓, Surf and Co-S ↑ | Oil: Labrafil M 1944 (50%) Surf: Cremophor EL (20%) Co-S: Transcutol P (30%) Characteristics: ET of 231 s, GS of 128.0 nm, ZP of −4.29 mV |
|
| Atorvastatin and ezetimibe [39] | DoE: D-optimal mixture design Runs: 16 Factors: % oil, surf, co-S Responses: globule size, zeta potential, % drug release, PDI | NA | Oil: Capryol 90 (10%) Surfactant: Tween 80/Koliphor RH 40 (42.71%) Co-S: Transcutol HP (47.29%) Characteristics: GS of 101.3 ± 0.47 nm; ZP of 23 mv, PDI of 0.241, dispersibility: grade A |
|
| Candesartan [40] | DoE: D-optimal mixture design Runs: 14 Factors: % oil, surf, co-S Responses: globule size, % drug release, self-emulsification time | GS ↑: Oil ↑, surf and Co-S ↓ %DR ↑: Oil ↓, surf and Co-S ↑ ET ↑: Oil ↑, surf and Co-S ↓ | Oil: Capmul PG-8 (5%) Surfactant: Kolliphor EL (32%) Co-S: Transcutol P (63%) Characteristics: GS of 13.91 nm, ET of 16s, ZP of 0.32 mV |
|
| Cefixime [41] | DoE: D-optimal design Runs: 20 Factors: % oil, surf, co-S Responses: globule size | GS ↑: Oil ↑, surf ↓ | Oil: Cinnamon oil (40%) Surfactant: Tween 80 (40%) Co-S: PEG 200 (20%) Characteristics: GS of 130.73 ± 19.39 nm, PDI of 0.26 ± 0.01 nm, ZP of −9.50 ± 1.76 mV |
|
| Exenatide [88] | DoE: D-optimal design Runs: 15 Factors: % MCT, MGDG, surf Responses: globule size | GS ↑: MCT ↑, MGDG and surf ↓ | Oil: Captex 300/Capmul MCM (70%) Surfactant: Kolliphor RH 40 (30%) Characteristics: 26 ± 4 nm (Kolliphor rich), 231 ± 8 (MCT Rich) |
|
| Glimepiride [89] | DoE: mixture design Runs: 17 runs Factors: % oil, surf, co-S Responses: globule size, solubility | GS ↑: Oil ↑, surf ↓ solubility ↑: surf↑ | Oil: Black seed oil (15%) Surfactant: Tween 80 (40%) Co-S: PEG 400 (45%) Characteristics: GS of 34.64 nm and solubility of 36.67% |
|
| Ibrutinib [90] | DoE: I-optimal mixture Runs: 16 Factors: % oil, surf, co-S Responses: globule size, PDI, drug loading | GS ↑: Oil ↑, surf and Co-S ↓ PDI ↑: Oil ↑, surf and Co-S ↓ DL ↑: Oil ↑, surf and Co-S | Oil: Eugenol (5%) Surfactant: Tween 80 (80%) Co-S: PEG 200 (15%) Characteristics: GS of 60.85 nm, PDI of 0.195, ZP −11.9 mV, ET: 7 s |
|
| Resveratrol [61] | DoE: mixer design Runs: 13 Factors: % oil, surf, co-S Responses: globule size, PDI, emulsification time, % drug release | GS ↑: Oil ↑, surf and Co-S ↓ PDI ↑: Surf ↑, Co-S ↓ ET↑: Oil, surf, co-S ↑ %DR: co-S ↑ | Oil: Labrafil® M 1944 (23%) Surfactant: Kolliphor RH 40 (41%) Co-S: Transcutol HP (36%) Characteristics: GS of 22.07 nm, PDI of 0.148 nm, ET of 21.67 s |
|
| Rosuvastatin [91] | DoE: D-optimal mixture design Runs: 14 Factors: % oil, surf, and co-S Responses: globule size, % drug release, emulsification time | GS ↑: Oil and Co-S ↑, Surf ↓ ET↑: Oil ↑, surf ↓ %DR↑: Oil ↓, surf ↑ | Oil: Capmul MCM EP (14%) Surfactant: Tween 20 (50%) Co-S: Transcutol P (36%) Characteristics: GS of 14.69 nm, PDI < 0.5, ZP of −4.09 mV |
|
| Sildenafil Citrate [92] | DoE: mixture design Runs: 16 Factors: % of oil, surf, and co-S Responses: globule size | GS ↑: Cons of Oil ↑, Surf ↓ | Oil: Clove oil/oleic acid (10%) Surfactant: Tween 20 (60%) Co-S, Propylene glycol (30%) Characteristics: GS of 103.5 nm |
|
| Voxelotor [94] | DoE: D-optimal mixture design Runs: 16 Factors: % of oil, surf, and co-S Responses: globule size, PDI, emulsification time | GS ↑: Oil ↑, Surf and Co-S ↓ PDI ↑: Oil ↑, Surf ↓ | Oil: Capryol PGMC (40%) Surfactant: Cremophor-E L (43%) Co-S: Labrafil M 1944 (17%) Characteristics: GS of 34.9 nm, PDI of 0.2, ZP of −8.4 mV, and ET of 32.4 s |
|
| Zaleplon [95] | DoE: mixture design Runs: 18 Factors: % of oil, surf, and co-S Responses: globule size, % drug load | GS ↑: Oil ↑, Surf and Co-S ↓ DL ↑: Oil ↑, Surf ↓ | Oil: Lavender oil (13%) Surfactant: Sorbeth-20 (49%) Co-S: HCO-60 (38%) Characteristics: GS of 87 nm, DL: 185 mg/mL |
|
| Thymoquinone [96] | DoE: mixture design Runs: 22 Factors: % of oil, surf, and co-S Responses: globule size | GS ↑: Oil ↑ | Oil: Almond oil Surfactant: Tween 80 Co-S: PEG 200 Characteristics: GS 64.8 nm |
|
| API [Ref] | Experimental Design | Correlation Factors and Responses | Optimal Formulation | Product Performances |
|---|---|---|---|---|
| Bedaquiline [57] | DoE: Box–Benkhen design Runs: 14 Factors: % oil, Smix, sonication time Responses: globule size, PDI, % transmittance | GS ↑: Oil ↑, Surf and Co-S ↓ PDI ↑: Oil ↑, Surf and Co-S ↓ | Oil: Caprylic acid (20%) Smix: Propylene glycol/Transcutol-P (40%) Sonication time: 30 S Characteristics: GS of 98.88 ± 2.10 nm, PDI of 0.3 ± 0.09, ZP of 21.16 ± 3.4 mV, ET of 15 ± 3 s |
|
| Cinacalcet hydrochloride [58] | DoE: Box–Behnken design Runs: 15 Factors: mg of oil, surf, and co-S Responses: drug release, emulsification time, globule size, PDI | %DR ↑: Suf ↑ ET ↑: Surf ↓ GS ↑: Co-S ↑ PDI ↑: Surf and Co-S ↓ | Oil: Capmul MCM (50 mg) Surfactant: Tween 20 (150 mg) Co-S: Transcutol P (55 mg) Characteristics: GS of 89.5 nm, PDI of 0.211, ET of 23.3 s |
|
| Bosentan [47] | DoE: Box–Behnken design Runs: 15 Factors: % of oil, Surf, and co-S Responses: globule size, PDI | GS ↑: Oil ↑, Surf ↓ PDI ↑: Oil ↑, Surf ↓ | Oil: Maisine 35-1 (10%) Surfactant: Kolliphor RH 40 (81%) Co-S: Labrasol (9%) Characteristics: GS of 17.11 nm, PDI of 0.180, ET < 1 min |
|
| Cephalexin [102] | DoE: Box–Behnken design Runs: 17 Factors: % of oil, surf, and co-S Responses: globule size, % transmittance, emulsification time | GS ↑: Oil ↑, Surf and Co-S ↓ ET↑: Oil ↑, surf co-S↓ %T ↑: Oil ↓, Surf and Co-S ↑ | Oil: Lauroglycol 90 (14%) Surfactant: Poloxamer 188 (59%) Co-S: Transcutol-HP (32%) Characteristics: GS 87.25 3.16 nm, PDI of 0.25, ZP of 24.37 mV, ET of 52 1.7 s |
|
| Curcumin [103] | DoE: Box–Behnken design Runs: 17 Factors: µL of oil, surf, and co-S Responses: PDI, zeta potential, % drug loading, globule size Responses: | GS ↑: Oil ↑, Smix ↓ PDI ↑: Oil ↑, Smix ↓ | Oil: Labrafil M 1994 CS (100 µL) Surfactant: Tween 80 (450 µL) Co-S: Transcutol P (450 µL) Characteristics: PDI of 0.14; ZP of −22.3 mV; DL of 95.9% and GS of 76.10 nm) |
|
| Ezetimibe [115] | DoE: Box Behnken design Runs: 15 Factors: % of oil, surf, and co-S Responses: globule size; %Transmittance, Emulsification time, % drug release | GS ↑: Oil ↑, Surf and Co-S ↓ ET↑: Oil ↑, surf co-S↓ | Oil: Peceol (10%) Surfactant: Tween 80 (60%) Co-S: transcutol P (27%) Characteristics: GS of 24.4 ± 2.07 nm, ET of 55 s |
|
| Morin [104] | DoE: Box–Behnken design Runs: 17 Factors: Smix (ratio), % oil, Dosage Responses: Globule size, PDI | GS ↑: oil and dosage ↑, Smix ratio ↓ %DR ↑: Oil ↓, Smix ↑ | Oil: GTCC (21%) Surfactant/Co-S: Cremophor RH 60/PEG 400 (1:5) Characteristics: GS of 32.8 nm, PDI of 0.089 |
|
| Passiflora ligularis leaves extract [105] | DoE: Box–Behnken design Runs: 27 runs Factors: oil, surf, co-S, polymer Responses: globule size, PDI, Z-potential | GS↑: Oil, co-S, polymer ↑, Surf ↓ ZP ↑: Surf ↑ | Oil: Castor oil (31) Surfactant: Cremophor EL (120) Co-S: Propylene glycol (80) Polymer: 30 Characteristics: GS of 22.371 ± 0.387, PDI 0.27 ± 0.03, and ZP −10.92 ± 0.42 mV |
|
| Piperine [106] | DoE: Box–Behnken design Runs: 17 Factors: % of oil, surf, and co-S Responses: globule size, % transmittance, emulsification time | GS ↑: Oil ↑, Surf and Co-S ↓ ET ↑: Oil ↑, Surf and Co-S %T ↑: Oil ↓, Surf and Co-S ↑ | Oil: Glyceryl monolinoleat (25%) Surfactant: Poloxamer 188 (46%) Co-S: Transcutol HP (25%) Characteristics: GS of 70.34 ± 3.27 nm, ET of 53 ± 2 s |
|
| Vemurafenib [9] | DoE: Box–Behnken design Runs: 15 Factors: % oil, surf, co-S Responses: globule size, PDI, %transmittance | GS ↑: Oil, Surf, co-S ↓ PDI ↑: Oil and Surf ↑, co-S ↓ | Oil: Capryol 90 (16%) Surfactant: Tween 80 (65%) Co-S: Transcutol HP (25%) Characteristics: GS of 43.27 ± 5 nm., PDI of 0.276 ± 0.005, ZP of −8.2 mV |
|
| Benidipine [38] | DoE: Central Composite Design Runs: 15 Factors: % oil, surf, co-S Responses: emulsification time, globule size, % drug release, % transmittance | NA | Oil: Labrafil M 2125 CS (30%) Surfactant: Kolliphor EL (38%) Co-S: Transcutol P (40%) Characteristic: GS of 156.20 ± 2.40 nm, PDI of 0.25, ZP of −17.36 ± 0.18 mV, ET of 65.21 ± 1.95 s |
|
| Docetaxel [59] | DoE: Central composite rotatable design Runs: 17 Factors: vol oil, vol Smix, sonication time (s) Responses: globule size, PDI, %transmittance, emulsification time | S ↑: Oil ↑, Smix ↓, ST ↓ PDI ↑: Oil ↑, Smix ↓, ST ↓ %ET ↑: Oil ↑, Smix ↓, ST ↓ %T ↑: Oil ↓, Smix ↑ | Oil: Fish oil (0.145 mL) Smix: Tween 80/PEG 400 (0.92 mL) Sonication time: 15 s Characteristic: GS of 121.5 nm, PDI of 0.338, and ET of 22 s |
|
| Plumbagin [107] | DoE: Central Composite Design Runs: 14 Factors: %Oil, Smix ratio Responses: globule size, emulsification time, %drug release, equilibrium solubility | GS ↑: Oil ↑, Smix ↓ %ET ↑: Oil ↑, Smix ↓ %DR ↑: Oil ↓, Smix ↑ | Oil: Capmul MCM Smix (Tween 20/PPG) ratio: 1.35:1 Characteristics: GS of 58.500 ± 1.170 nm, PDI of 0.228 ± 0.012, ET of 17.660 ± 1.520 s, ZP of −28.20 ± 1.20 mV |
|
| Quetiapine Fumarate [108] | DoE: Central Composite Design Runs: 22 Factors: %Oil, Smix ratio, Surf type Responses: globule size, emulsification time | GS ↑: Oil ↑, Smix ↓ %ET ↑: Oil ↑, Smix ↓ | Oil: Capmul MCM (10%) Smix (Gelucire 48/16/PPG) ratio: 4:1 Characteristics: GS of 92.27 nm, ET of 3.38 min, PDI of 0.32 ± 0.02, ZP of −17.1 ± 3.4 mV |
|
| Stiripentol [109] | DoE: Central Composite Design Runs: 13 Factors: % Oil, Smix ratio Responses: globule size, zeta potential, % drug release | GS ↑: oil ↑, Ratio Smix ↓ ZP ↑: oil ↓, Ratio Smix ↑ %DR ↑: oil ↑, Ratio Smix ↓ | Oil: ethyl oleate (40%) Surfactant: Cremophor RH 40 (43%) Co-S: 1,2-propanediol (17%) Characteristics: GS of 45.52 ± 1.99 nm, ZP of −21.67 ± 0.24 mV, and PDI of 0.076 ± 0.011 |
|
| Tamoxifen and Resveratrol [62] | DoE: Central composite rotatable design Runs: 13 Factors: vol of oil and Smix Responsed: globule size; PDI, %transmittance | GS ↑: Cons of Oil ↑, Smix ↓ PDI ↑: Smix ↓ %T ↑: Oil ↓, Smix ↑, ST ↑ | Oil: Capmul MCM (0.6 mL) Smix: Tween 80/Transcutol-HP (1.86 mL) Characteristics: GS of 104.5 nm and PDI of 0.211 |
|
| Venetoclax [110] | DoE: Central Composite Design Runs: 32 Factors: mg of oil, surf, co-surf, rate of stirring, stirring time Responses: globule size, PDI, emulsification time, % transmittance | GS ↑: Oil ↑ PDI ↑: Oil ↑, Surf ↓ ET: Oil ↑, Smix ↓ %T ↑: Surf ↑ | Oil: Cinnamon oil (100 mg) Surfactant: Cremophor RH40 (300 mg) Co-S: Transcutol P (250 mg) Rate of stirring: 150 rpm Stirring time: 10 min Characteristics: GS of 71.32 ± 2.85 nm, PDI of 0.113 ± 0.01, ET of 16.4 ± 0.81 s |
|
| Olmesartan Medoxomil [60] | DoE: D-optimal response surface design Runs: 16 Factors: mg of oil, surf, and co-surf Responses: globule size, emulsification time, % drug release | GS ↑: Oil and Co-S ↑, Surf ↓ %DR ↑: Cons of Oil Surf ↓ | Oil: Capmul MCM (393 mg) Surfactant: Tween 80 (423 mg) Co-S: Transcutol HP (184 mg) Characteristics: GS of 64.2 nm and ZP of −25.4 mV |
|
| API [Ref] | Experimental Design | Correlation Factors and Responses | Optimal Formulation | Product Performances |
|---|---|---|---|---|
| Camptothecin [116] | Full Factorial Design Runs: 13 Factors: % of oil, Smix Responsed: % transmittance, emulsification time, % drug release | %T ↑: Oil ↓, Smix ↑: ET ↑: Oil ↑, Smix ↓ %DR ↑: Oil ↓, Smix ↑, | Oil: Omega oil (12.5%) Smix: Cremophor RH40/Labrafil M2125 (40%) Characteristics: GS of 47 nm, PDI of 0.176, ZP of 35.2 mV, and ET of 11 s |
|
| Exendin-4 [117] | factorial D-optimal design Runs: 13 Factors: gram of oil, Smix Responses: globule size, PDI, Z-potential | DS ↑: Oil ↑, Surf ↓ PDI ↑: Oil ↑, Surf ↓ ZP ↑: Oil ↑, Surf ↓ | Oil: Ethyl oleate (15.42) Surfactant: Cremophor EL (42.5) Co-S: Labrasol/ethanol (21/5) Characteristics: GS of 20.66 ± 0.40 nm, PDI of 0.1 ± 0.01 and ZP of −5.99 ± 0.43 mV |
|
| Flufenamic Acid [118] | DoE: 32 Full Factorial Design Runs: 9 Factors: mg of oil, Surf Responses: globule size, Z-potential, PDI | GS ↑: Oil ↑, Surf ↓ | Oil: Miglyol 812 (150 mg) Smix: Labrasol/Cremophor EL (300 mg) Characteristics: GS of 61.12 nm, ZP of −25.53 mV and PDI of 0.432 |
|
| Fosfestrol [119] | Full Factorial Design Runs: 13 Factors: % of oil and Smix Responses: % transmittance, emulsification time, % drug release | %T ↑: Oil ↓, Smix ↑: ET ↑: Oil ↑, Smix ↓ %DR ↑: Oil ↓, Smix ↑ | Oil: Soyabean oil (10%) Smix: Labrasol ALF/Labrafil-M212 (39.48%) Characteristics: GS of 52 nm, PDI of 0.158, ET 24 s |
|
| Isoliquiritigenin [120] | DoE: 22 Full Factorial Design Runs: 9 Factors: % oil, Smix ratio Responses: globule size, % drug loading | %GL ↑: Oil ↑, Ratio Smix ↑ | Oil: Ethyl oleate Surfactant: Tween 80 Co-S: PEG 400 Characteristics: GS of 20.63 ± 1.95 nm, PDI of 0.11 ± 0.03, and ZP of −12.64 ± 2.12 mV |
|
| Lamotrigine [125] | DoE: D-optimal factorial design Runs: 19 Factors: % oil, co-S type, Smix ratio Responses: globule size, % drug release | NA | Oil: Rose oil (30%) Surfactant: Cremophor EL (47%) Co-S: PEG 400 (23%) Characteristics: GS of 15.013 ± 0.158 nm, PDI of 0.245 ± 0.018, ZP of −7.97 mV |
|
| Palbociclib-letrozole [121] | Full factorial Design Runs: 9 Factors: %oil, Smix Responses: % transmittance, emulsification time, % drug release | %T: Oil ↓, Smix ↑: ET ↑: Oil ↑, Smix ↓ %DR ↑: Oil ↓, Smix ↑ | Oil: Maisine (10%) Smix: Cremophor-RH40/Labrasol (35%) Characteristics: GS of 71 ± 3 nm, PDI of 0.28 ± 0.09, ET of 80 ± 1.13 s, ZP: 31 ± 2 mV |
|
| Rhein [122] | Full Factorial Design Runs: 10 Factors: % oil, Smix Responses: globule size, % transmittance, emulsification time | GS ↑: Oil ↑, Smix ↓ %T ↑: Oil ↓,Smix ↑: ET ↑: Oil ↑, Smix ↓ | Oil: Eucalyptus oil (50%) Smix: Tween 80/PEG 400 (50%) Characteristics: GS of 129.3 ± 1.57 nm, ZP of −24.6 mV ± 0.34, EE of 98.86 ± 0.23%. |
|
| Sertraline [123] | Full Factorial Design Runs: 8 Factors: mg of oil, surf, Co-S Responses: dissolution efficiency, globule size, and emulsification time | DE ↑: oil and co-S ↓, Surf ↑ GS ↑: surf ↑ ET ↑: oil ↑, Smix ↓ | Oil: Glycerol triacetate (100 mg) Surfactant: Tween 80 (133 mg) Co-S: PEG 200 (66 mg) Characteristics: GS of 76.03 nm, ET of 29 s, PDI of 0.422, and ZP of 25.5 mV |
|
| Zolmitriptan [124] | Full Factorial Design Runs: 9 Factors: % oil and Smix Responses: globule size, zetapotential, % drug release | GS ↑: Oil ↑, Surf ↓ ns of Oil ↑, Surf ↓ | Oil: Lavender oil (30%) Smix: P35HC/Transcutol HP (20%) Characteristics: GS of 19.59 ± 0.17 nm, ZP −23.5 ± 1.17 mV, and ET of 121 ± 2.51 s |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priani, S.E.; Fakih, T.M.; Wilar, G.; Chaerunisaa, A.Y.; Sopyan, I. Quality by Design and In Silico Approach in SNEDDS Development: A Comprehensive Formulation Framework. Pharmaceutics 2025, 17, 701. https://doi.org/10.3390/pharmaceutics17060701
Priani SE, Fakih TM, Wilar G, Chaerunisaa AY, Sopyan I. Quality by Design and In Silico Approach in SNEDDS Development: A Comprehensive Formulation Framework. Pharmaceutics. 2025; 17(6):701. https://doi.org/10.3390/pharmaceutics17060701
Chicago/Turabian StylePriani, Sani Ega, Taufik Muhammad Fakih, Gofarana Wilar, Anis Yohana Chaerunisaa, and Iyan Sopyan. 2025. "Quality by Design and In Silico Approach in SNEDDS Development: A Comprehensive Formulation Framework" Pharmaceutics 17, no. 6: 701. https://doi.org/10.3390/pharmaceutics17060701
APA StylePriani, S. E., Fakih, T. M., Wilar, G., Chaerunisaa, A. Y., & Sopyan, I. (2025). Quality by Design and In Silico Approach in SNEDDS Development: A Comprehensive Formulation Framework. Pharmaceutics, 17(6), 701. https://doi.org/10.3390/pharmaceutics17060701







