Improving Pulmonary Delivery of Budesonide Suspensions Nebulized with Constant-Output Vibrating Mesh Nebulizers by Using Valved Holding Chamber
Abstract
1. Introduction
2. Materials and Methods
2.1. Nabulizers and BUD Products
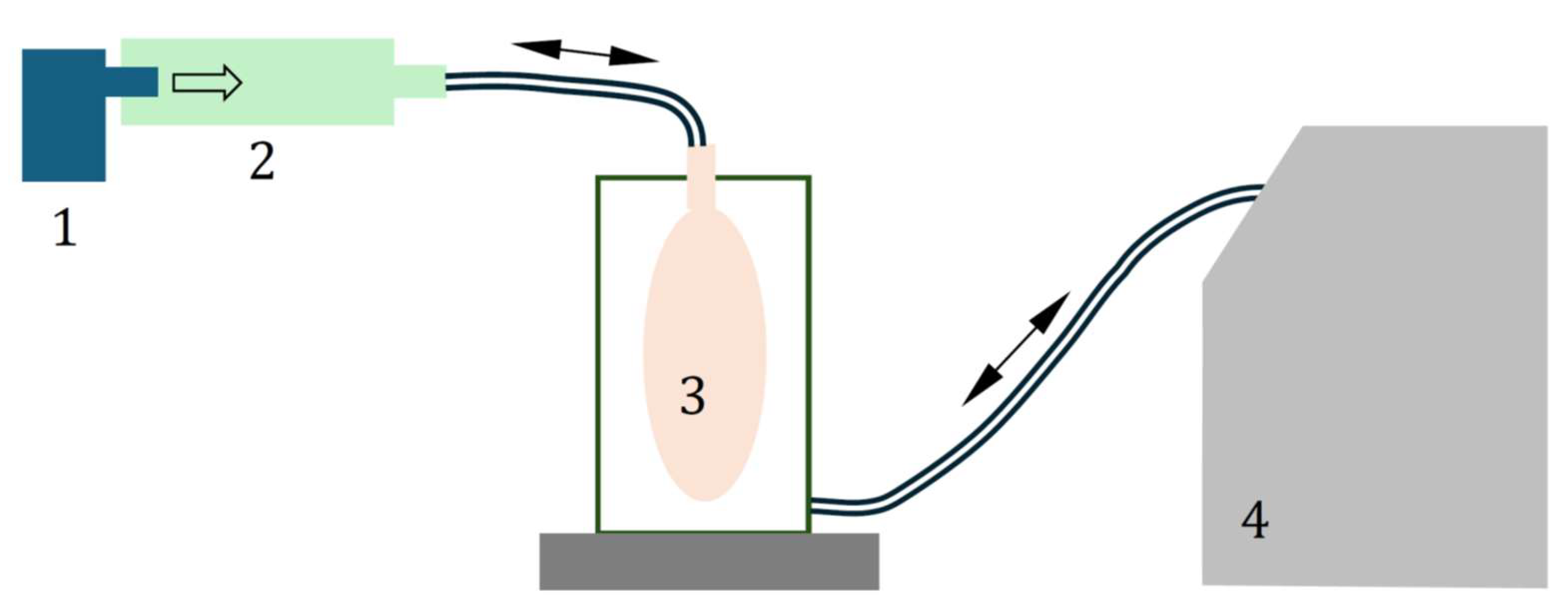
2.2. Mass Output of VMNs Operated Without and with VHC
2.3. Aerosol Droplet Size Distribution for VMNs Operated Without and with VHC
2.4. The Calculation of the Relative Increase in the Total and Pulmonary Drug Availability
3. Results
3.1. The Aerosol Mass Output of the VMNs Without and with the VHC
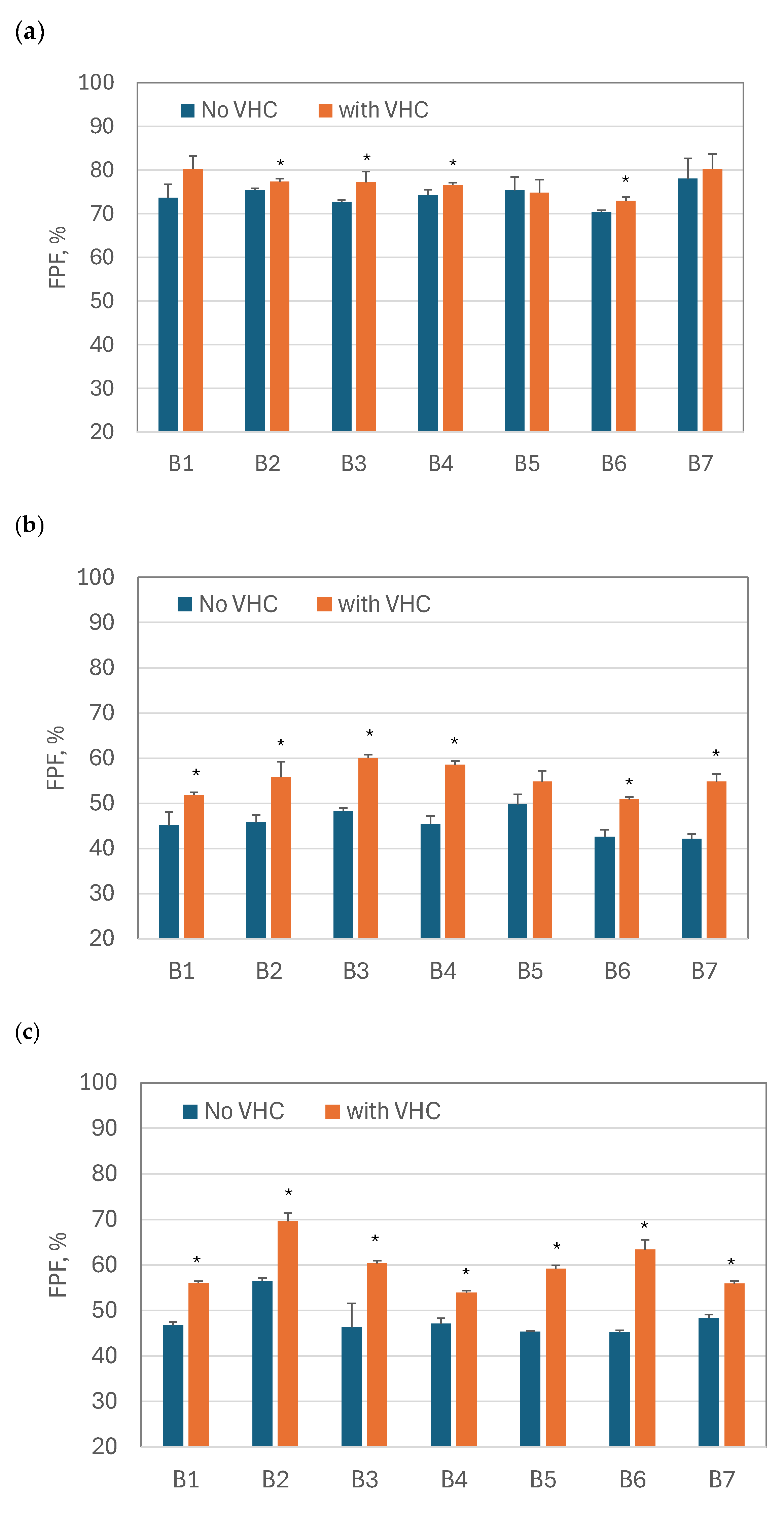
3.2. Fine Particle Fraction and MMAD of Nebulized BUD Products
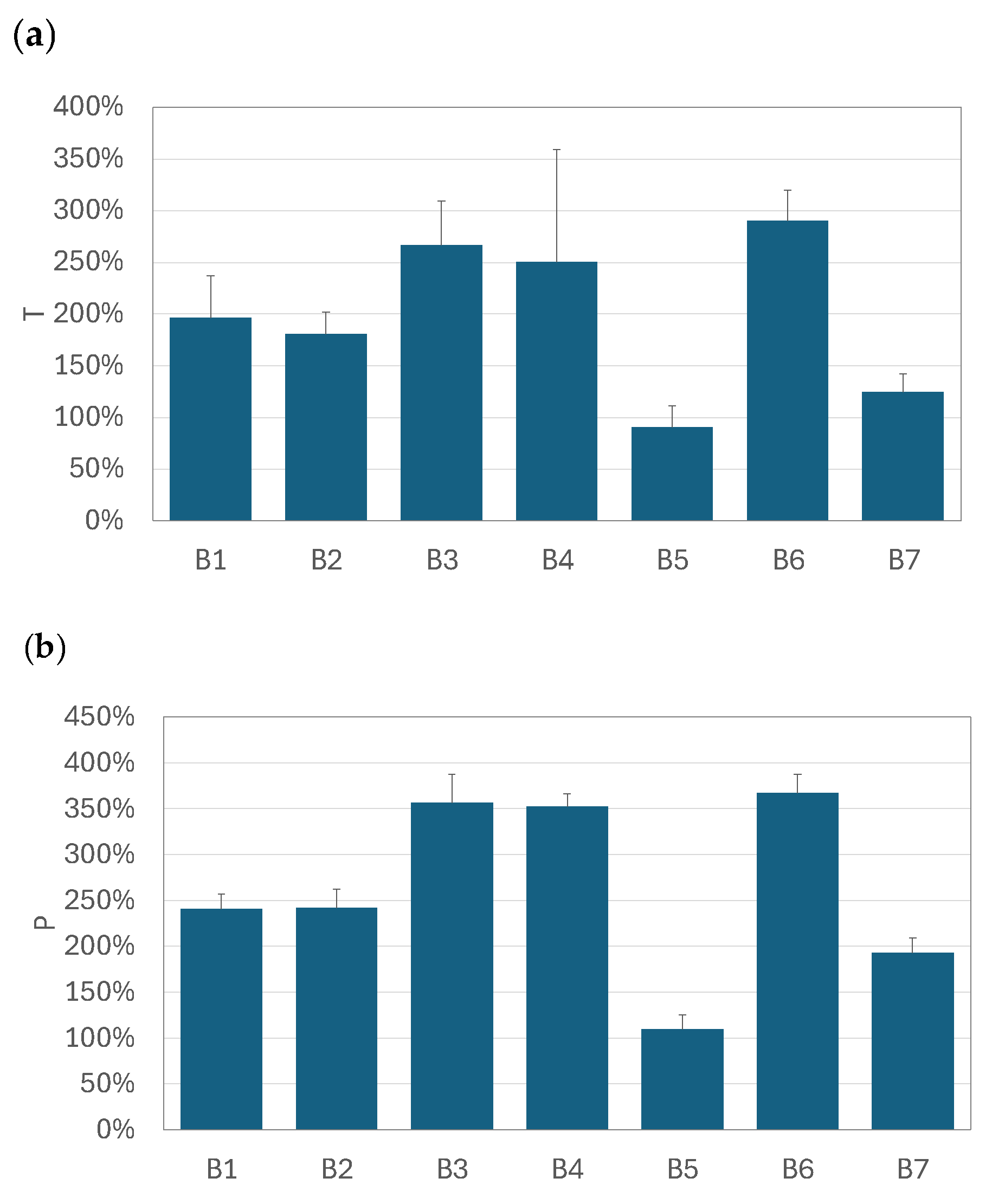
3.3. Relative Increase in Total and Pulmonary Drug Availability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| BUD | Budesonide |
| COPD | Chronic obstructive pulmonary disease |
| DPI | Dry powder inhaler |
| DSD | Droplet size distribution |
| Dv50 | Median diameter of volumetric DSD, μm |
| FM | Fast Mesh (nebulizer) |
| FPF | Fine particle fraction, % |
| FPM | Fine particle mass, mg |
| MDI | Metered dose inhaler |
| MMAD | Mass median aerodynamic diameter, μm |
| OP | One Pro (nebulizer) |
| P | Relative increase in pulmonary drug availability, % |
| PDA | Pulmonary drug availability, mg/min |
| SM | Silent Mesh (nebulizer) |
| T | Relative increase in total drug availability, % |
| TDA | Total drug availability, mg/min |
| VHC | Valved holding chamber |
| VMN | Vibrating mesh nebulizer |
References
- O’Connell, E.J. Review of the unique properties of budesonide. Clin. Ther. 2003, 25 (Suppl. C), C42–C60. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Lipworth, B.; Brattsand, R. Benefit: Risk profile of budesonide in obstructive airways disease. Drugs 2019, 79, 1757–1775. [Google Scholar] [CrossRef]
- Barnes, P.J.; Pedersen, S.; Busse, W.W. Efficacy and safety of inhaled corticosteroids: New developments. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 2, S1–S53. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R. Regional deposition: Targeting. J. Aerosol Med. Pulm. Drug Deliv. 2021, 34, 1–10. [Google Scholar] [CrossRef]
- Hvizdos, K.M.; Jarvis, B. Budesonide inhalation suspension: A review of its use in infants, children and adults with inflammatory respiratory disorders. Drugs 2000, 60, 1141–1178. [Google Scholar] [CrossRef]
- Szefler, S.J.; Eigen, H. Budesonide inhalation suspension: A nebulized corticosteroid for persistent asthma. J. Allergy Clin. Immunol. 2002, 109, 729–742. [Google Scholar] [CrossRef]
- Food and Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020929s052lbl.pdf (accessed on 20 March 2025).
- Dobrowolska, K.; Emeryk, A.; Janeczek, K.; Krzosa, R.; Pirożyński, M.; Sosnowski, T.R. Influence of physicochemical properties of budesonide micro-suspensions on their expected lung delivery using a vibrating mesh nebulizer. Pharmaceutics 2023, 15, 752. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2015, 12, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.N.; Hatley, R.H.; Denyer, J.; Hollen, D.V. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther. Deliv. 2018, 9, 121–136. [Google Scholar] [CrossRef]
- Fink, J.B.; Simmons, R. Nebulization of steroid suspension: An in vitro evaluation of the Aeroneb® Go and the Pari LC Plus™ nebulizers. Chest 2004, 126, S816–S817. [Google Scholar] [CrossRef]
- Zhou, J.F.; Zhang, Y.B.; Zhang, Z.W. Comparison of clinical outcomes following delivery of budesonide by both vibrating mesh nebulizer and jet nebulizer in premature infants with bronchopulmonary dysplasia. Front. Pediatr. 2023, 11, 1258846. [Google Scholar] [CrossRef] [PubMed]
- Rottier, B.L.; van Erp, C.J.; Sluyter, T.S.; Heijerman, H.G.; Frijlink, H.W.; de Boer, A.H. Changes in performance of the Pari eFlow® rapid and Pari LC Plus™ during 6 months use by CF patients. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 263–269. [Google Scholar] [CrossRef]
- Dugernier, J.; Hesse, M.; Vanbever, R.; Depoortere, V.; Roeseler, J.; Michotte, J.B.; Laterre, P.F.; Jamar, F.; Reychler, G. SPECT-CT comparison of lung deposition using a system combining a vibrating-mesh nebulizer with a valved holding chamber and a conventional jet nebulizer: A randomized Cross-over Study. Pharm. Res. 2017, 34, 290–300. [Google Scholar] [CrossRef]
- McGrath, J.A.; O’Sullivan, A.; Bennett, G.; O’Toole, C.; Joyce, M.; Byrne, M.A.; MacLoughlin, R. Investigation of the quantity of exhaled aerosols released into the environment during nebulisation. Pharmaceutics 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R.; Vilkotsky, A.I.; Emeryk, A. Vibrating mesh nebulizers with a valved inhalation chamber for increased drug delivery to the lower airways. In Respiratory Drug Delivery 2022; Dalby, R.N., Peart, J., Suman, J.D., Young, P.M., Traini, D., Watts, A., Eds.; RDD Online: Richmond, VA, USA, 2002; Volume 1, pp. 551–554. [Google Scholar]
- Sosnowski, T.R. Towards more precise targeting of inhaled aerosols to different areas of the respiratory system. Pharmaceutics 2024, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, R.M.; Elberry, A.A.; Abdelwahab, N.S.; Rabea, H.; Salem, M.N.; Abdelrahim, M.E. Effect of a nebulizer holding chamber on aerosol delivery. Respir. Care 2018, 63, 1125–1131. [Google Scholar] [CrossRef]
- Aerogen Ultra. Available online: https://www.aerogen.com/products/aerogen-ultra (accessed on 20 March 2025).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2024. Available online: www.ginasthma.org (accessed on 14 February 2025).
- European Directorate for the Quality of Medicines and HealthCare (EDQM). Chapter 2.9.18. Preparations for inhalation: Aerodynamic assessment of fine particles. In European Pharmacopeia, 10th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM): Strasbourg, France, 2021. [Google Scholar]
- Mitchell, J.; Copley, M.; Sizer, Y.; Russell, T.; Solomon, D. Adapting the Abbreviated Impactor Measurement (AIM) concept to make appropriate inhaler aerosol measurements to compare with clinical data: A scoping study with the “Alberta” Idealized Throat (AIT) inlet. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 188–197. [Google Scholar] [CrossRef]
- Sosnowski, T.R. Powder particles and technologies for medicine delivery to the respiratory system: Challenges and opportunities. KONA Powder Part. J. 2018, 35, 122–138. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Janeczek, K.; Grzywna, K.; Emeryk, A. Mass and volume balances of nebulization processes for the determination of the expected dose of liquid medicines delivered by inhalation. Chem. Process Eng. 2021, 42, 253–261. [Google Scholar]
- O’Riordan, T.G. Formulations and nebulizer performance. Respir. Care 2002, 47, 1305–1312. [Google Scholar]
- Montefusco-Pereira, C.V. Steps toward nebulization in-use studies to understand the stability of new biological entities. Drug Discov. Today 2023, 28, 103461. [Google Scholar] [CrossRef] [PubMed]
- Hye, T.; Moinuddin, S.M.; Sarkar, T.; Nguyen, T.; Saha, D.; Ahsan, F. An evolving perspective on novel modified release drug delivery systems for inhalational therapy. Expert Opin. Drug Deliv. 2023, 20, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ari, A. Jet, ultrasonic, and mesh nebulizers: An evaluation of nebulizers for better clinical outcomes. Eurasian J. Pulmonol. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- Fink, J.B.; Stapleton, K.W. Nebulizers. J. Aerosol Med. Pulm. Drug Deliv. 2024, 37, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Waldrep, J.C.; Dhand, R. Advanced nebulizer designs employing vibrating mesh/aperture plate technologies for aerosol generation. Curr. Drug Deliv. 2008, 5, 114–119. [Google Scholar] [CrossRef]
- Elhissi, A.M.; Faizi, M.; Naji, W.F.; Gill, H.S.; Taylor, K.M. Physical stability and aerosol properties of liposomes delivered using an air-jet nebulizer and a novel micropump device with large mesh apertures. Int. J. Pharm. 2007, 334, 62–70. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Oesterheld, N. Electrolyte type and nozzle composition affect the process of vibrating-membrane nebulization. Eur. J. Pharm. Biopharm. 2017, 119, 11–16. [Google Scholar] [CrossRef]
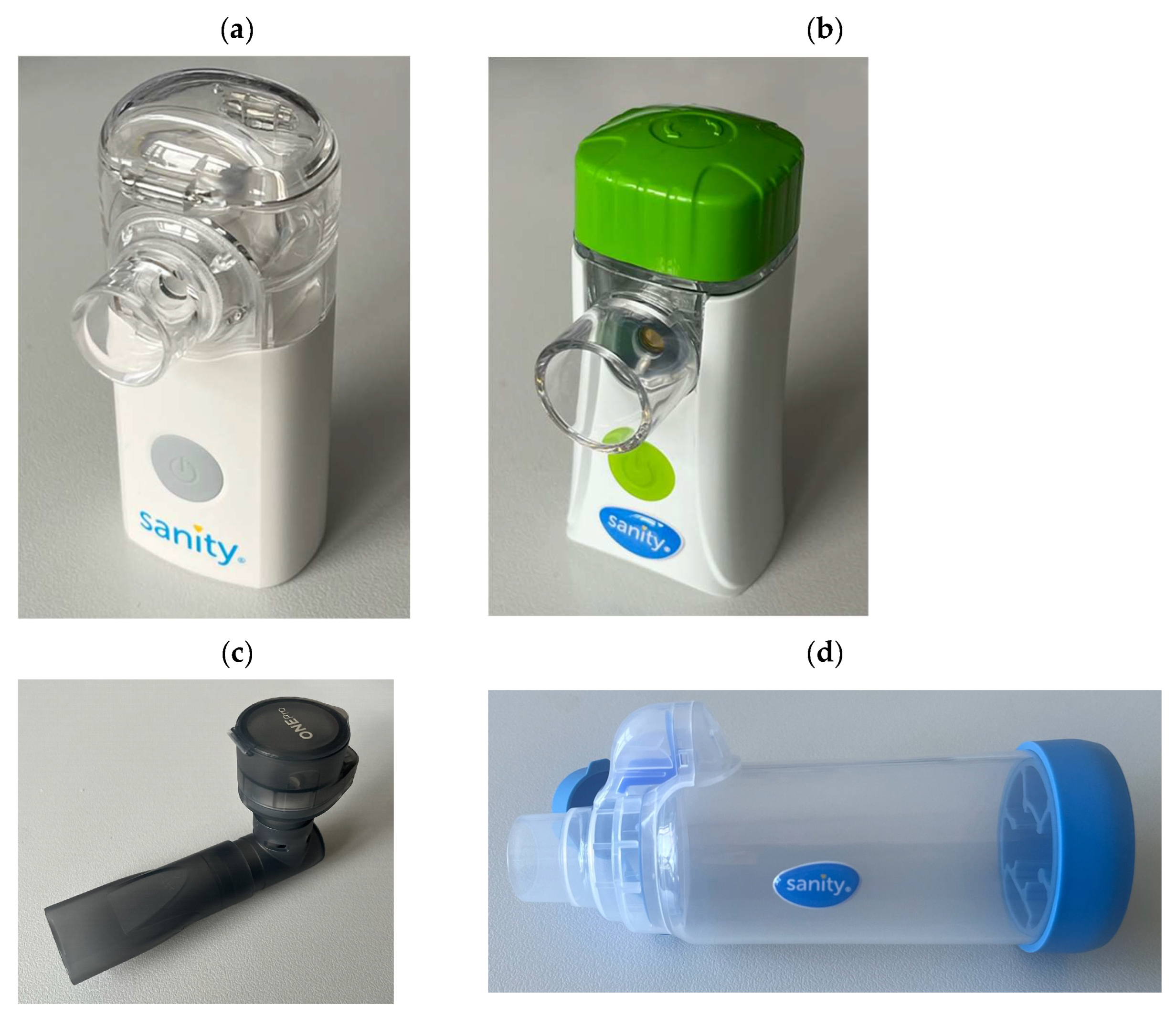


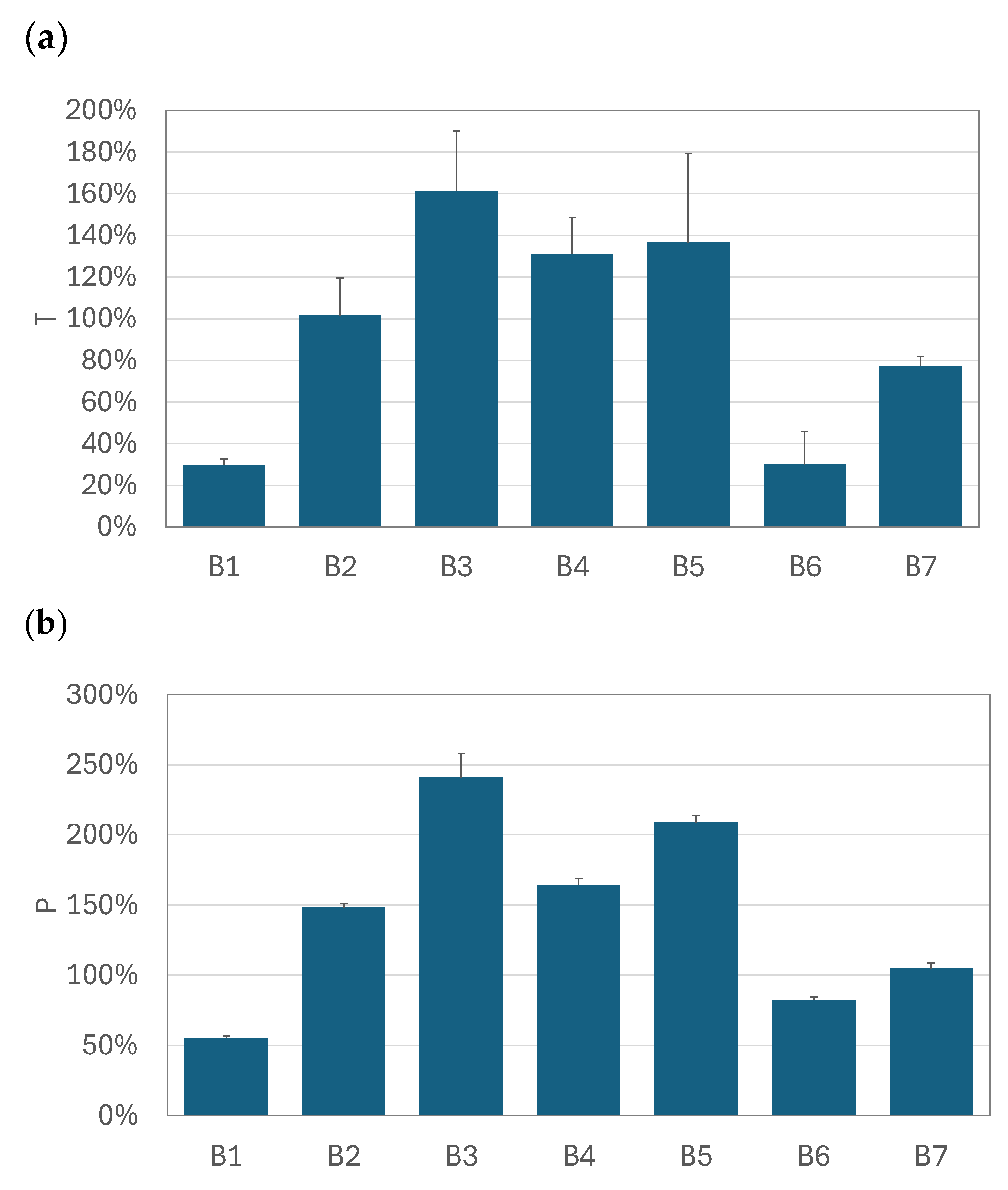

| BUD Product | Manufacturer (Manufacturing Authorization Holder) |
|---|---|
| BDS N, 0.25 mg/mL | Aurovitas Pharma Polska, Warsaw, Poland |
| Benodil, 0.25 mg/mL | Polpharma SA, Starogard Gdański, Poland |
| Budixon NEB, 0.25 mg/mL | Adamed Pharma, Pieńków, Poland |
| Nebbud, 0.25 mg/mL | Teva Pharmaceuticals Polska, Warsaw, Poland |
| Ondemet, 0.25 mg/mL | Zentiva Polska, Warsaw, Poland |
| Pulmicort, 0.25 mg/mL | AstraZeneca Pharma Poland, Warsaw, Poland |
| Resbud, 0.5 mg/2 mL | Lek-AM Sp. z o.o., Zakroczym, Poland |
| BUD | No VHC | SD | With VHC | SD |
|---|---|---|---|---|
| B1 | 0.217 | 0.043 | 0.201 | 0.034 |
| B2 | 0.202 | 0.025 | 0.214 | 0.030 |
| B3 | 0.187 | 0.030 | 0.227 | 0.013 |
| B4 | 0.188 | 0.010 | 0.173 | 0.001 |
| B5 | 0.224 | 0.026 | 0.227 | 0.028 |
| B6 | 0.230 | 0.044 | 0.219 | 0.028 |
| B7 | 0.219 | 0.011 | 0.196 | 0.003 |
| BUD | No VHC | SD | With VHC | SD |
|---|---|---|---|---|
| B1 | 0.191 | 0.017 | 0.361 | 0.032 |
| B2 | 0.179 | 0.019 | 0.368 | 0.021 |
| B3 | 0.187 | 0.030 | 0.359 | 0.009 |
| B4 | 0.216 | 0.014 | 0.406 | 0.011 |
| B5 | 0.243 | 0.057 | 0.383 | 0.006 |
| B6 | 0.212 | 0.021 | 0.416 | 0.057 |
| B7 | 0.214 | 0.031 | 0.368 | 0.056 |
| BUD | No VHC | SD | With VHC | SD |
|---|---|---|---|---|
| B1 | 0.269 | 0.010 | 0.209 | 0.020 |
| B2 | 0.279 | 0.003 | 0.260 | 0.034 |
| B3 | 0.263 | 0.007 | 0.324 | 0.039 |
| B4 | 0.246 | 0.008 | 0.265 | 0.051 |
| B5 | 0.271 | 0.010 | 0.291 | 0.021 |
| B6 | 0.289 | 0.006 | 0.167 | 0.034 |
| B7 | 0.279 | 0.015 | 0.258 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnowski, T.R.; Kazimierczak, I.; Sawczuk, A.; Janeczek, K.; Emeryk, A. Improving Pulmonary Delivery of Budesonide Suspensions Nebulized with Constant-Output Vibrating Mesh Nebulizers by Using Valved Holding Chamber. Pharmaceutics 2025, 17, 696. https://doi.org/10.3390/pharmaceutics17060696
Sosnowski TR, Kazimierczak I, Sawczuk A, Janeczek K, Emeryk A. Improving Pulmonary Delivery of Budesonide Suspensions Nebulized with Constant-Output Vibrating Mesh Nebulizers by Using Valved Holding Chamber. Pharmaceutics. 2025; 17(6):696. https://doi.org/10.3390/pharmaceutics17060696
Chicago/Turabian StyleSosnowski, Tomasz R., Izabela Kazimierczak, Aleksandra Sawczuk, Kamil Janeczek, and Andrzej Emeryk. 2025. "Improving Pulmonary Delivery of Budesonide Suspensions Nebulized with Constant-Output Vibrating Mesh Nebulizers by Using Valved Holding Chamber" Pharmaceutics 17, no. 6: 696. https://doi.org/10.3390/pharmaceutics17060696
APA StyleSosnowski, T. R., Kazimierczak, I., Sawczuk, A., Janeczek, K., & Emeryk, A. (2025). Improving Pulmonary Delivery of Budesonide Suspensions Nebulized with Constant-Output Vibrating Mesh Nebulizers by Using Valved Holding Chamber. Pharmaceutics, 17(6), 696. https://doi.org/10.3390/pharmaceutics17060696







