Exploring Antioxidant, Antimicrobial and Anti-Inflammatory Effects of Juglans regia and Pfaffia paniculata Extracts: Implications for Intestinal Dysbiosis and Colorectal Cancer Risk Associated with Oral Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Equipment
2.3. Soluble Solids Content in Ethanol
2.4. Determination of Total Phenol Content
2.5. Determination of the Total Flavonoid Content Expressed as Quercetin in Juglans regia and Pfaffia paniculata Extracts
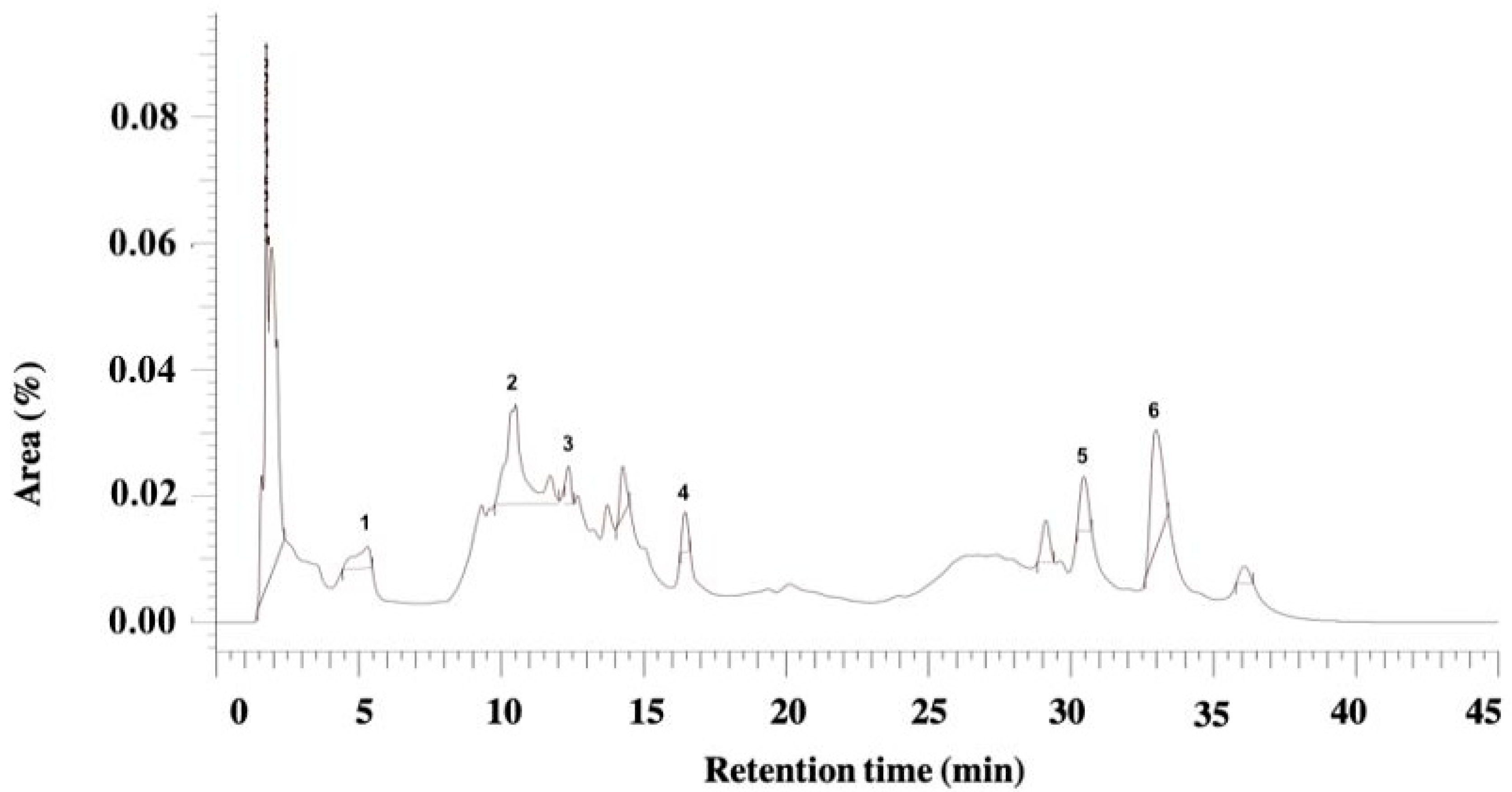
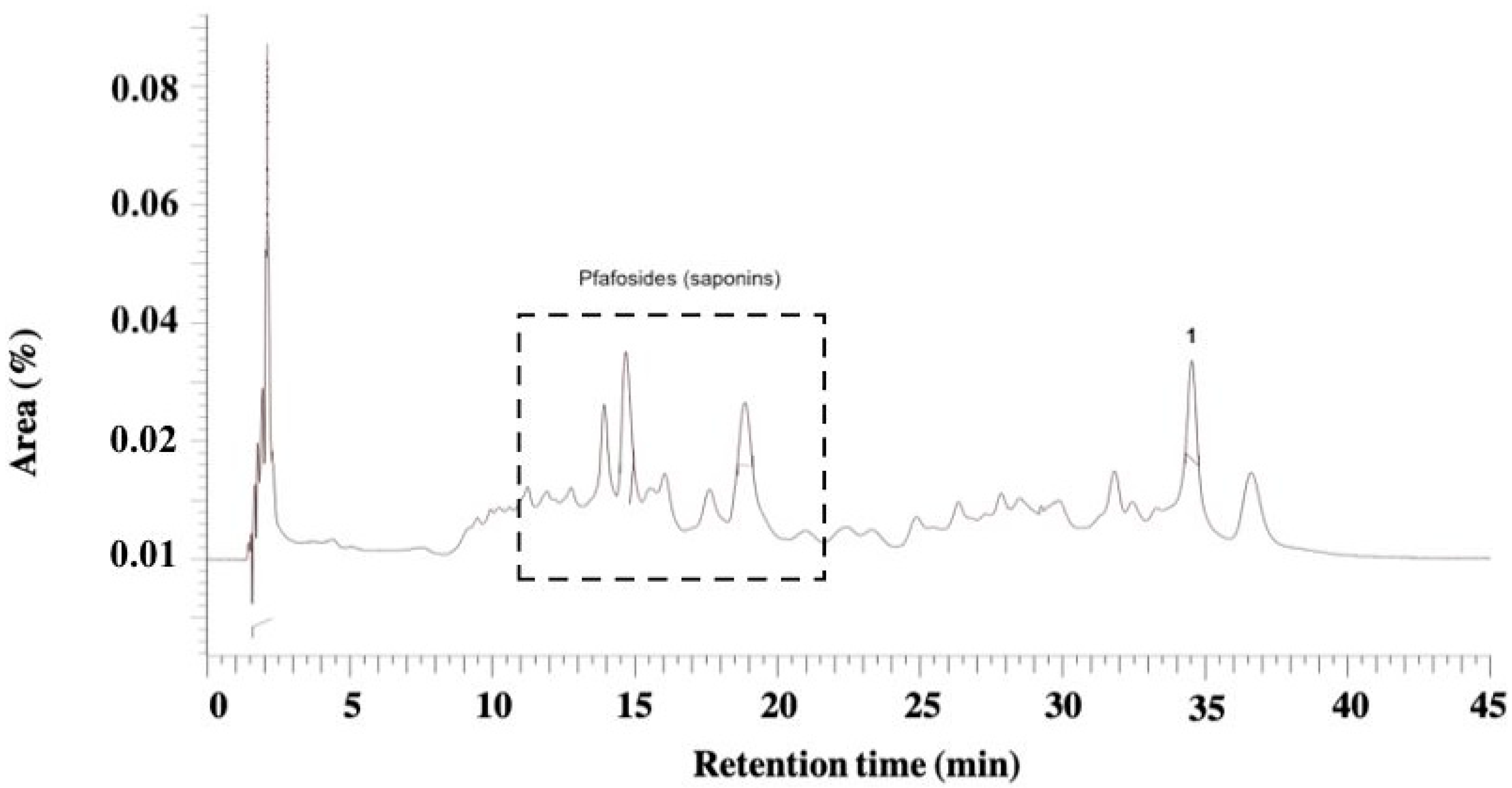
2.6. Phytochemical Analysis of Juglans regia and Pfaffia paniculata Extracts by High-Performance Liquid Chromatography with Diode-Array Detection
2.7. Evaluation of Antioxidant Activity of Juglans regia and Pfaffia paniculata Extracts
2.8. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Juglans regia and Pfaffia paniculata Extracts by CLSI M11-A7
2.9. Antibiofilm Action of Juglans regia and Pfaffia paniculata Extracts
2.10. Metabolic Activity Assessment of Juglans regia and Pfaffia paniculata Extracts on Human Keratinocytes (HaCaT)
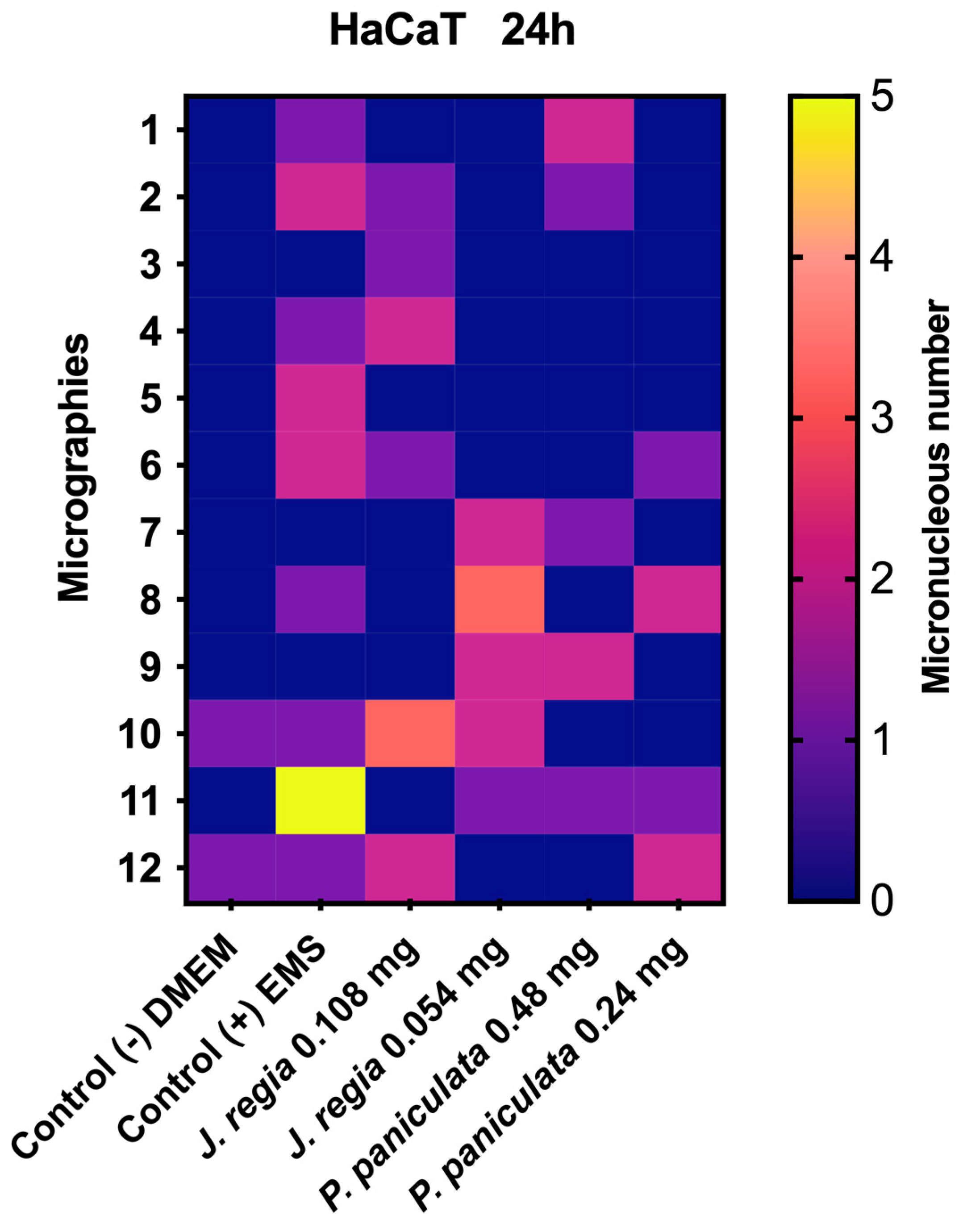
2.11. Micronucleus Test of J. regia and P. paniculata Extracts on Human Keratinocytes (HaCaT)
2.12. Statistical Analysis
3. Results
3.1. Physicochemical and HPLC-DAD Analysis of J. regia and P. paniculata Extracts
3.2. Antioxidant Activity of J. regia and P. paniculata Extracts
3.3. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of J. regia and P. paniculata Extracts
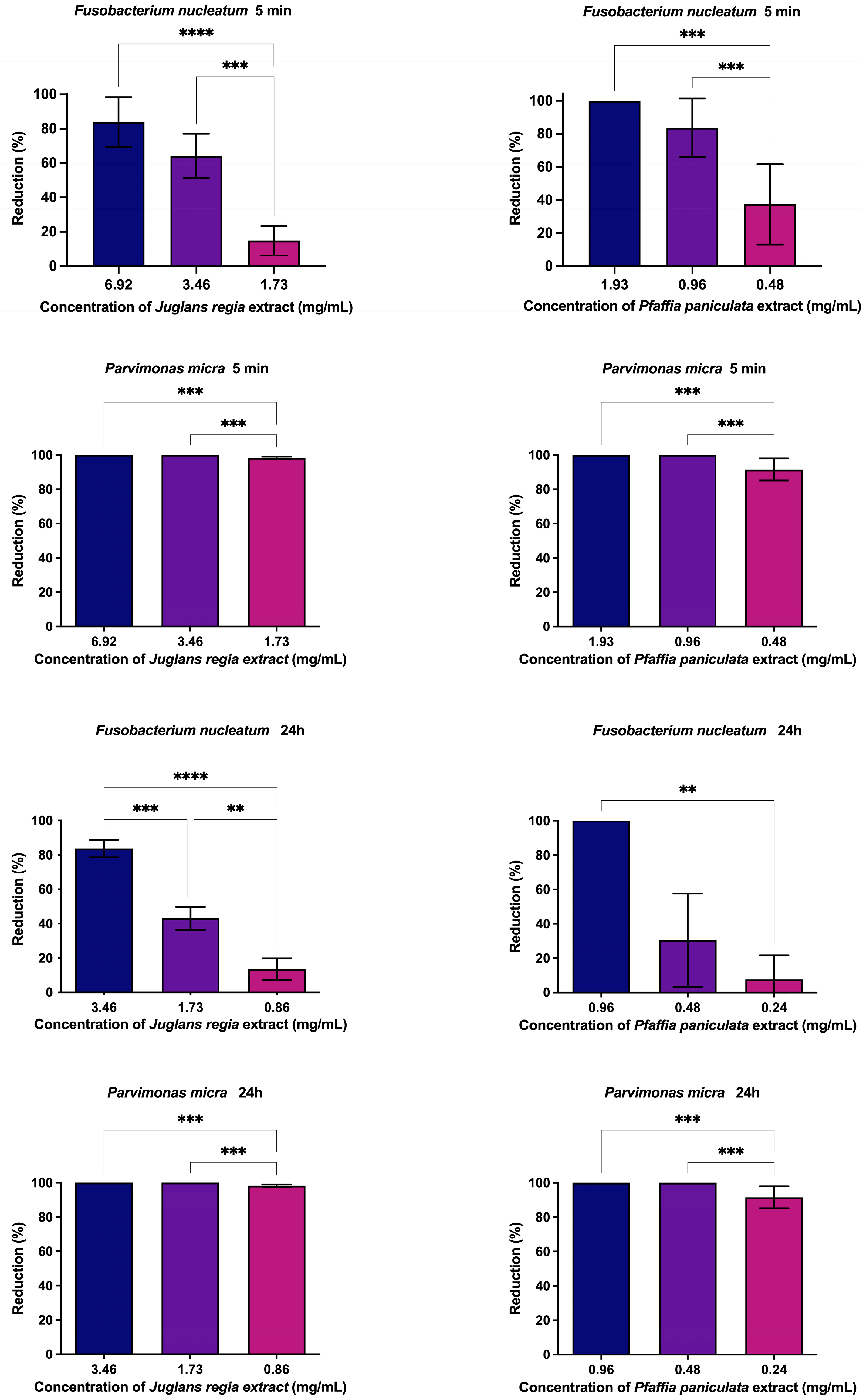
3.4. Evaluation of Antibiofilm Action of J. regia and P. paniculata Extracts
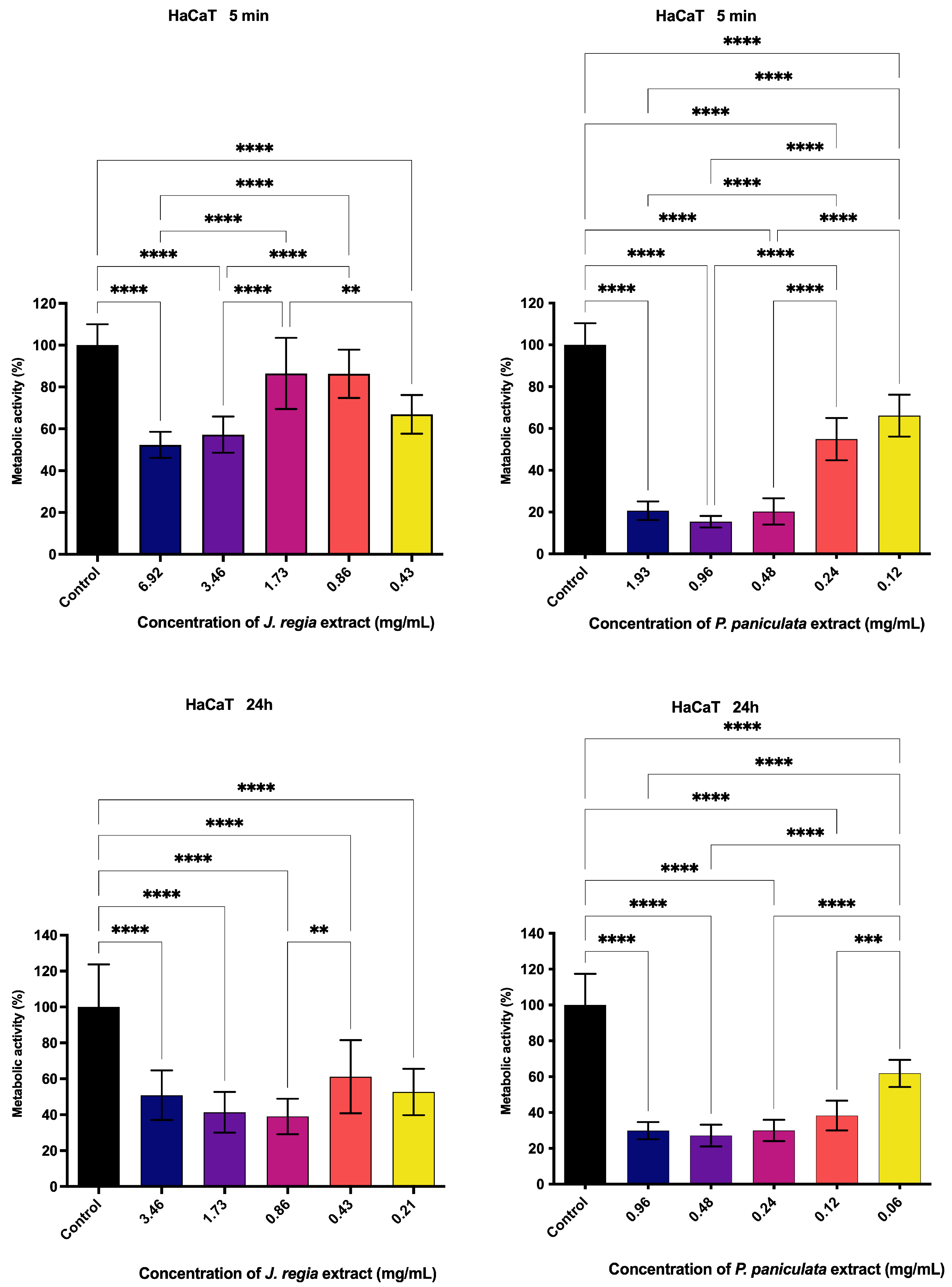
3.5. Metabolic Activity Assessment of J. regia and P. paniculata Extracts by MTT Assay on Human Keratinocytes (HaCat)
3.6. Genotoxicity of J. regia and P. paniculata Extracts Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stokowa-Sołtys, K.; Kierpiec, K.; Szczerba, K.; Wieczorek, R. Can bacteria F. nucleatum be actively involved in colon cancer progression via a radical mediated mechanism? J. Inorg. Biochem. 2023, 246, 112307. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef] [PubMed]
- Petrochilos, D.; Shojaie, A.; Gennari, J.; Abernethy, N. Using random walks to identify cancer-associated modules in expression data. BioData Min. 2013, 6, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef]
- Li, R.; Shen, J.; Xu, Y. Fusobacterium nucleatum and Colorectal Cancer. Infect. Drug Resist. 2022, 15, 1115–1120. [Google Scholar] [CrossRef]
- Kodio, A.; Menu, E.; Ranque, S. Eukaryotic and Prokaryotic Microbiota Interactions. Microorganisms 2020, 8, 2018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.; Zhang, X.; Zhou, Y.; Fu, K.; Lau, H.C.-H.; Chun, T.W.-Y.; Cheung, A.H.-K.; Coker, O.O.; Wei, H.; Wu, W.K.-K.; et al. Parvimonas micra promotes colorectal tumorigenesis and is associated with prognosis of colorectal cancer patients. Oncogene 2022, 41, 4200–4210. [Google Scholar] [CrossRef]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- Bergsten, E.; Mestivier, D.; Donnadieu, F.; Pedron, T.; Barau, C.; Meda, L.T.; Mettouchi, A.; Lemichez, E.; Gorgette, O.; Chamaillard, M.; et al. Parvimonas micra, an oral pathobiont associated with colorectal cancer, epigenetically reprograms human colonocytes. Gut Microbes 2023, 15, 2265138. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, Y.; Otsuka, M.; Shibata, C.; Seimiya, T.; Yamamoto, K.; Ishibashi, R.; Kishikawa, T.; Tanaka, E.; Isagawa, T.; Takeda, N.; et al. Gut Bacteria-derived Membrane Vesicles Induce Colonic Dysplasia by Inducing DNA Damage in Colon Epithelial Cells. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 745–767. [Google Scholar] [CrossRef]
- Lam, G.A.; Albarrak, H.; McColl, C.J.; Pizarro, A.; Sanaka, H.; Gomez-Nguyen, A.; Cominelli, F.; Paes Batista da Silva, A. The Oral-Gut Axis: Periodontal Diseases and Gastrointestinal Disorders. Inflamm. Bowel Dis. 2023, 29, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgård, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate from the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Ferrocino, I.; Del Lupo, V.; Colonna, E.; Thumbigere-Math, V.; Caviglia, G.P.; Franciosa, I.; Mariani, G.M.; Romandini, M.; Ribaldone, D.G.; et al. Effect of Periodontitis and Periodontal Therapy on Oral and Gut Microbiota. J. Dent. Res. 2024, 103, 359–368. [Google Scholar] [CrossRef]
- Löwenmark, T.; Löfgren-Burström, A.; Zingmark, C.; Ljuslinder, I.; Dahlberg, M.; Edin, S.; Palmqvist, R. Tumour colonisation of Parvimonas micra is associated with decreased survival in colorectal cancer patients. Cancers 2022, 14, 5937. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Gu, J. Clinical implications of the associations between intestinal microbiome and colorectal cancer progression. Cancer Manag. Res. 2020, 12, 4117–4128. [Google Scholar] [CrossRef]
- Conde-Pérez, K.; Aja-Macaya, P.; Buetas, E.; Trigo-Tasende, N.; Nasser-Ali, M.; Rumbo-Feal, S.; Nión, P.; Arribas, E.M.; Estévez, L.S.; Otero-Alén, B.; et al. The multispecies microbial cluster of Fusobacterium, Parvimonas, Bacteroides and Faecalibacterium as a precision biomarker for colorectal cancer diagnosis. Mol. Oncol. 2024, 18, 1093–1122. [Google Scholar] [CrossRef]
- Xu, J.; Yang, M.; Wang, D.; Zhang, S.; Yan, S.; Zhu, Y.; Chen, W. Alteration of the abundance of Parvimonas micra in the gut along the adenoma-carcinoma sequence. Oncol. Lett. 2020, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Valdivieso, M.; Bussalleu, A.; Sexton, R.; Thompson, K.C.; Osorio, S.; Novoa Reyes, I.; Crowley, J.J.; Baker, L.H.; Xi, C. Antibiotic resistance among Helicobacter pylori clinical isolates in Lima, Peru. Infect. Drug Resist. 2017, 10, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sandu-Bălan Tăbăcariu, A.; Ifrim, I.L.; Patriciu, O.I.; Ștefănescu, I.A.; Fînaru, A.L. Walnut By-Products and Elderberry Extracts-Sustainable Alternatives for Human and Plant Health. Molecules 2024, 29, 498. [Google Scholar] [CrossRef]
- Mateș, L.; Rusu, M.E.; Popa, D.S. Phytochemicals and Biological Activities of Walnut Septum: A Systematic Review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef]
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2021, 35, 2076–2081. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic Antimicrobial Activity of Camellia sinensis and Juglans regia against Multidrug-Resistant Bacteria. PLoS ONE 2015, 10, e0118431. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Zhang, S.; Yan, W.; Si, C.; Lee, M.H.; Li, Z. Inhibitory Effects of the Extracts of Juglans sigillata Green Husks on the Proliferation, Migration and Survival of KYSE150 and EC9706 Human Esophageal Cancer Cell Lines. Nutr. Cancer 2019, 71, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Oniyangi, O.; Cohall, D.H. Phytomedicines (medicines derived from plants) for sickle cell disease. Cochrane Database Syst. Rev. 2020, 9, CD004448. [Google Scholar] [CrossRef]
- Costa, C.A.R.A.; Quaglio, A.E.V.; Di Stasi, L.C. Pfaffia paniculata (Brazilian ginseng) extract modulates Mapk and mucin pathways in intestinal inflammation. J. Ethnopharmacol. 2018, 213, 21–25. [Google Scholar] [CrossRef]
- Costa, C.A.; Tanimoto, A.; Quaglio, A.E.; Almeida, L.D., Jr.; Severi, J.A.; Di Stasi, L.C. Anti-inflammatory effects of Brazilian ginseng (Pfaffia paniculata) on TNBS-induced intestinal inflammation: Experimental evidence. Int. Immunopharmacol. 2015, 28, 459–469. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.C.; Cogliati, B.; Latorre, A.O.; Akisue, G.; Nagamine, M.K.; Haraguchi, M.; Hansen, D.; Sanches, D.S.; Dagli, M.L.Z. Pfaffosidic Fraction from Hebanthe paniculata Induces Cell Cycle Arrest and Caspase-3-Induced Apoptosis in HepG2 Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 835796. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, M.K.; da Silva, T.C.; Matsuzaki, P.; Pinello, K.C.; Cogliati, B.; Pizzo, C.R.; Akisue, G.; Haraguchi, M.; Górniak, S.L.; Sinhorini, I.L.; et al. Cytotoxic effects of butanolic extract from Pfaffia paniculata (Brazilian ginseng) on cultured human breast cancer cell line MCF-7. Exp. Toxicol. Pathol. 2009, 61, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Badaoui, B.; Mourabit, Y.; Salhi, N.; Alshahrani, M.M.; Al Awadh, A.A.; Bouyahya, A.; Goh, K.W.; et al. Comparative investigation of chemical constituents of kernels, leaves, husk, and bark of Juglans regia L., using HPLC-DAD-ESI-MS/MS analysis and evaluation of their antioxidant, antidiabetic, and anti-inflammatory activities. Molecules 2022, 27, 8989. [Google Scholar] [CrossRef]
- Li, J.; Jadhav, A.N.; Khan, I.A. Triterpenoids from Brazilian ginseng, Pfaffia paniculata. Planta Medica 2010, 76, 635–639. [Google Scholar] [CrossRef]
- Rodrigues, M.V.N.; Souza, K.P.; Rehder, V.L.G.; Vilela, G.F.; Montanari Junior, I.; Figueira, G.M.; Rath, S. Development of na analytical method for the quantification of pfaffic acid in Brazilian ginseng (Hebanthe eriantha). J. Pharm. Biomed. Anal. 2013, 77, 76–82. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diemer, E.; Chadni, M.; Grimi, N.; Ioannou, I. Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts. Foods 2022, 11, 3214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makori, S.I.; Mu, T.H.; Sun, H.N. Physicochemical properties, antioxidant activities, and binding behavior of 3,5-di-O-caffeoylquinic acid with beta-lactoglobulin colloidal particles. Food Chem. 2021, 347, 129084. [Google Scholar] [CrossRef] [PubMed]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Mo-darre-si-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further re-search. Biomed Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Khan, R.; Naseem, I. Antiglycation and antioxidant potential of coumaric acid isomers: A comparative in-vitro study. J. Biomol. Struct. Dyn. 2023, 42, 12090–12104. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Mendoza, L.; Cotoras, M. Alteration of oxidative phosphorylation as a possible mechanism of the antifungal action of p-coumaric acid against Botrytis cinerea. J. Appl. Microbiol. 2017, 123, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.P.; Castillo, Y.P.; Monteiro, M.L.; Menezes, R.R.P.P.B.; Almeida, R.N.; Martins, A.M.C.; Sousa, D.P. Trypanocidal Mechanism of Action and in silico Studies of p-Coumaric Acid Derivatives. Int. J. Mol. Sci. 2019, 20, 5916. [Google Scholar] [CrossRef]
- Costa, P.; Boeing, T.; Somensi, L.B.; Cury, B.J.; Espíndola, V.L.; França, T.C.S.; de Almeida, M.O.; Arruda, C.; Bastos, J.K.; da Silva, L.M.; et al. Hydroalcoholic extract from Baccharis dracunculifolia recovers the gastric ulcerated tissue, and p-coumaric acid is a pivotal bioactive compound to this action. BioFactors 2019, 45, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Ojha, D.; Patil, K.N. p-Coumaric acid inhibits the Listeria monocytogenes RecA protein functions and SOS response: An antimi-crobial target. Biochem. Biophys. Res. Commun. 2019, 517, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Mozar, A.; Charlot, K.; Sandor, B.; Rabaï, M.; Lemonne, N.; Billaud, M.; Hardy-Dessources, M.-D.; Beltan, E.; Pandey, R.C.; Connes, P.; et al. Pfaffia paniculata extract improves red blood cell de-formability in sickle cell patients. Clin. Hemorheol. Microcirc. 2015, 62, 327–333. [Google Scholar] [CrossRef]
- Ochensberger, S.; Alperth, F.; Mitić, B.; Kunert, O.; Mayer, S.; Mourão, M.F.; Turek, I.; Luca, S.V.; Skalicka-Woźniak, K.; Maleš, Ž.; et al. Phenolic compounds of Iris adriatica and their antimyco-bacterial effects. Acta Pharm. 2019, 69, 673–681. [Google Scholar] [CrossRef]
- Lima, A.M.A.; Teixeira, T.R.; Silva, B.F.S.; Raoni, P.; Silva, I.E.P.; Santos, E.G.; Fernandes, M.C.; Gonçalves, V.; Bressan, G.; Mendes, T.; et al. Síntese e avaliação das atividades fotoprotetora, citotóxica e antiviral contra o zika vírus de derivados triazólicos da benzofenona. Quim. Nova 2019, 42, 473–484. [Google Scholar] [CrossRef]
- Bai, M.; Gao, C.H.; Liu, K.; Zhao, L.Y.; Tang, Z.Z.; Liu, Y.H. Two new benzophenones isolated from a mangrove-derived fungus Penicillium sp. J. Antibiot. 2021, 74, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.B.; Chen, W.J.; Shan, T.Z.; Sun, B.Y.; Yan, P.C.; Jiang, W. Antibacterial Diphenyl Ether, Benzophenone and Xanthone Derivatives from Aspergillus flavipes. Chem. Biodivers. 2020, 17, e1900640. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.P.; Osbourn, A.E. Fungal Resistance to Plant Antibiotics as a Mechanism of Pathogenesis. Microbiol. Mol. Biol. Rev. 1999, 63, 708–724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakraborty, A.K.; Saha, S.; Poria, K.; Samanta, T.; Gautam, S.; Mukhopadhyay, J. A saponin-polybromophenol antibiotic (CU1) from Cassia fistula Bark Against Multi-Drug Resistant Bacteria Targeting RNA polymerase. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, X.; Yang, X.; Xue, P.; Zhang, Z.; Ren, G. Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria. BMC Complement. Altern. Med. 2019, 19, 46. [Google Scholar] [CrossRef]
- Silva, L.N.; Trentin Dda, S.; Zimmer, K.R.; Treter, J.; Brandelli, C.L.; Frasson, A.P.; Tasca, T.; da Silva, A.G.; da Silva, M.V.; Macedo, A.J. Anti-infective effects of Brazilian Caatinga plants against pathogenic bacterial biofilm formation. Pharm. Biol. 2015, 53, 464–468. [Google Scholar] [CrossRef][Green Version]
- Coêlho, M.L.; Ferreira, J.H.; de Siqueira Júnior, J.P.; Kaatz, G.W.; Barreto, H.M.; de Carvalho Melo Cavalcante, A.A. Inhibition of the NorA multi-drug transporter by oxygenated monoterpenes. Microb. Pathog. 2016, 99, 173–177. [Google Scholar] [CrossRef]
- Bezerra, L.F.G.; Silva, A.P.S.D.; Cunha, R.X.D.; Oliveira, J.R.S.; Barros, M.D.; Silva, V.M.M.A.D.; Lima, V.L.M. Antioxidant, anti-inflammatory and analgesic activity of Mimosa acutistipula (Mart.) Benth. J. Ethnopharmacol. 2023, 303, 115964. [Google Scholar] [CrossRef]
- Żurek, N.; Pawłowska, A.; Pycia, K.; Grabek-Lejko, D.; Kapusta, I.T. Phenolic Profile and Antioxidant, Antibacterial, and Antiproliferative Activity of Juglans regia L. Male Flowers. Molecules 2022, 27, 2762. [Google Scholar] [CrossRef]
- Eberlin, S.; Del Carmen Velazquez Pereda, M.; de Campos Dieamant, G.; Nogueira, C.; Werka, R.M.; de Souza Queiroz, M.L. Effects of a Brazilian herbal compound as a cosmetic eyecare for periorbital hyperchromia (“dark circles”). J. Cosmet. Dermatol. 2009, 8, 127–135. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Wang, T.; Liu, H.; Shi, J.; Zhang, X. Evaluation of the Oxidative Stress Status in Zebrafish (Danio rerio) Liver Induced by Three Typical Organic UV Filters (BP-4, PABA and PBSA). Int. J. Environ. Res. Public Health 2020, 17, 651. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.E.; Aabed, K.; Benabdelkamel, H.; Shami, A.; Alotaibi, M.O.; Alanazi, M.; Alfadda, A.A.; Rahman, I. Proteomic Pro-fil-ing Reveals Cytotoxic Mechanisms of Action and Adaptive Mechanisms of Resistance in Porphyromonas gingivalis: Treat-ment with Juglans regia and Melaleuca alternifolia. ACS Omega 2023, 8, 12980–12991. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.D.; Topgaard, D.; Sparr, E. Cyclic and Linear Monoterpenes in Phospholipid Membranes: Phase Behavior, Bilayer Structure, and Molecular Dynamics. Langmuir 2015, 31, 11067–11077. [Google Scholar] [CrossRef]
- de Groot, C.; Müller-Goymann, C.C. Saponin Interactions with Model Membrane Systems—Langmuir Monolayer Studies, Hemolysis and Formation of ISCOMs. Planta Medica 2016, 82, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, A.; Kuboniwa, M.; Shimma, S.; Alghamdi, S.A.; Mayumi, S.; Lamont, R.J.; Fukusaki, E.; Amano, A. Fusobacterium nucleatum Metabolically Integrates Commensals and Pathogens in Oral Biofilms. mSystems 2022, 7, e0017022. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Ashraf, M.S.; Alomrani, S.O.; Alreshidi, M.; Tepe, B.; Sachidanandan, M.; Danciu, C.; Patel, M. Sapo-nin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections. Antibiotics 2023, 12, 1415. [Google Scholar] [CrossRef]
- Rahamouz-Haghighi, S.; Sharafi, A. Antiproliferative assay of suma or Brazilian ginseng (Hebanthe eriantha) methanolic extract on HCT116 and 4T1 cancer cell lines, in vitro toxicity on Artemia salinalarvae, and antibacterial activity. Nat. Prod. Res. 2023, 38, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, K.A.; Cho, H.Y.; Kim, D.; Kim, W.K.; Yong, D.; Lee, H.; Yoon, S.S.; Han, D.H.; Han, Y.D.; et al. Association between Fusobacterium nucleatum and patient prognosis in metastatic colon cancer. Sci. Rep. 2021, 11, 20263. [Google Scholar] [CrossRef]
- Veiga, R.S.; De Mendonça, S.; Mendes, P.; Paulino, N.; Mimica, M.; Netto, A.L.; Lira, I.; López, B.-C.; Negrão, V.; Marcucci, M. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef]
| Extract | Soluble Solids (g/100 mL) | Total Phenols (mg/100 mL) | Total Flavonoids (mg/100 mL) |
|---|---|---|---|
| Juglans regia | 6.92 ± 0.27 | 11.53 ± 0.56 | 0.04 ± 0.06 |
| Pfaffia paniculata | 1.93 ± 0.08 | 7.10 ± 0.95 | 1.65 ± 0.14 |
| Extract | EC50 for F. nucleatum | EC50 for P. micra |
|---|---|---|
| Juglans regia | 37.26 μg/mL | 37.26 μg/mL |
| Pfaffia paniculata | 1367.57 μg/mL | 1367.57 μg/mL |
| Extract/Bacteria | F. nucleatum | P. micra | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Juglans regia | nd | 1.73 mg/mL | nd | 1.73 mg/mL |
| Pfaffia paniculata | nd | 0.49 mg/mL | nd | 0.49 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, D.G.; Ramos, L.d.P.; Attik, N.; Silva, N.V.D.H.F.; Camargo, P.C.; de Araujo, G.F.; Lopes, N.F.d.S.; Marcucci, M.C.; Pacheco-Soares, C.; Godoi, B.H.; et al. Exploring Antioxidant, Antimicrobial and Anti-Inflammatory Effects of Juglans regia and Pfaffia paniculata Extracts: Implications for Intestinal Dysbiosis and Colorectal Cancer Risk Associated with Oral Pathogens. Pharmaceutics 2025, 17, 693. https://doi.org/10.3390/pharmaceutics17060693
Miranda DG, Ramos LdP, Attik N, Silva NVDHF, Camargo PC, de Araujo GF, Lopes NFdS, Marcucci MC, Pacheco-Soares C, Godoi BH, et al. Exploring Antioxidant, Antimicrobial and Anti-Inflammatory Effects of Juglans regia and Pfaffia paniculata Extracts: Implications for Intestinal Dysbiosis and Colorectal Cancer Risk Associated with Oral Pathogens. Pharmaceutics. 2025; 17(6):693. https://doi.org/10.3390/pharmaceutics17060693
Chicago/Turabian StyleMiranda, Diego Garcia, Lucas de Paula Ramos, Nina Attik, Nicole Van Der Heijde Fernandes Silva, Pyetra Claro Camargo, Gabriela Ferraz de Araujo, Nicole Fernanda dos Santos Lopes, Maria Cristina Marcucci, Cristina Pacheco-Soares, Bruno Henrique Godoi, and et al. 2025. "Exploring Antioxidant, Antimicrobial and Anti-Inflammatory Effects of Juglans regia and Pfaffia paniculata Extracts: Implications for Intestinal Dysbiosis and Colorectal Cancer Risk Associated with Oral Pathogens" Pharmaceutics 17, no. 6: 693. https://doi.org/10.3390/pharmaceutics17060693
APA StyleMiranda, D. G., Ramos, L. d. P., Attik, N., Silva, N. V. D. H. F., Camargo, P. C., de Araujo, G. F., Lopes, N. F. d. S., Marcucci, M. C., Pacheco-Soares, C., Godoi, B. H., Caires, G. A., Vigerelli, H., & Carrouel, F. (2025). Exploring Antioxidant, Antimicrobial and Anti-Inflammatory Effects of Juglans regia and Pfaffia paniculata Extracts: Implications for Intestinal Dysbiosis and Colorectal Cancer Risk Associated with Oral Pathogens. Pharmaceutics, 17(6), 693. https://doi.org/10.3390/pharmaceutics17060693











