Evaluation of the Effectiveness of Chitosan-Modified Bone Regeneration Materials: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Eligibility Criteria
- Research articles in English;
- Published in the period January 2016—January 2025;
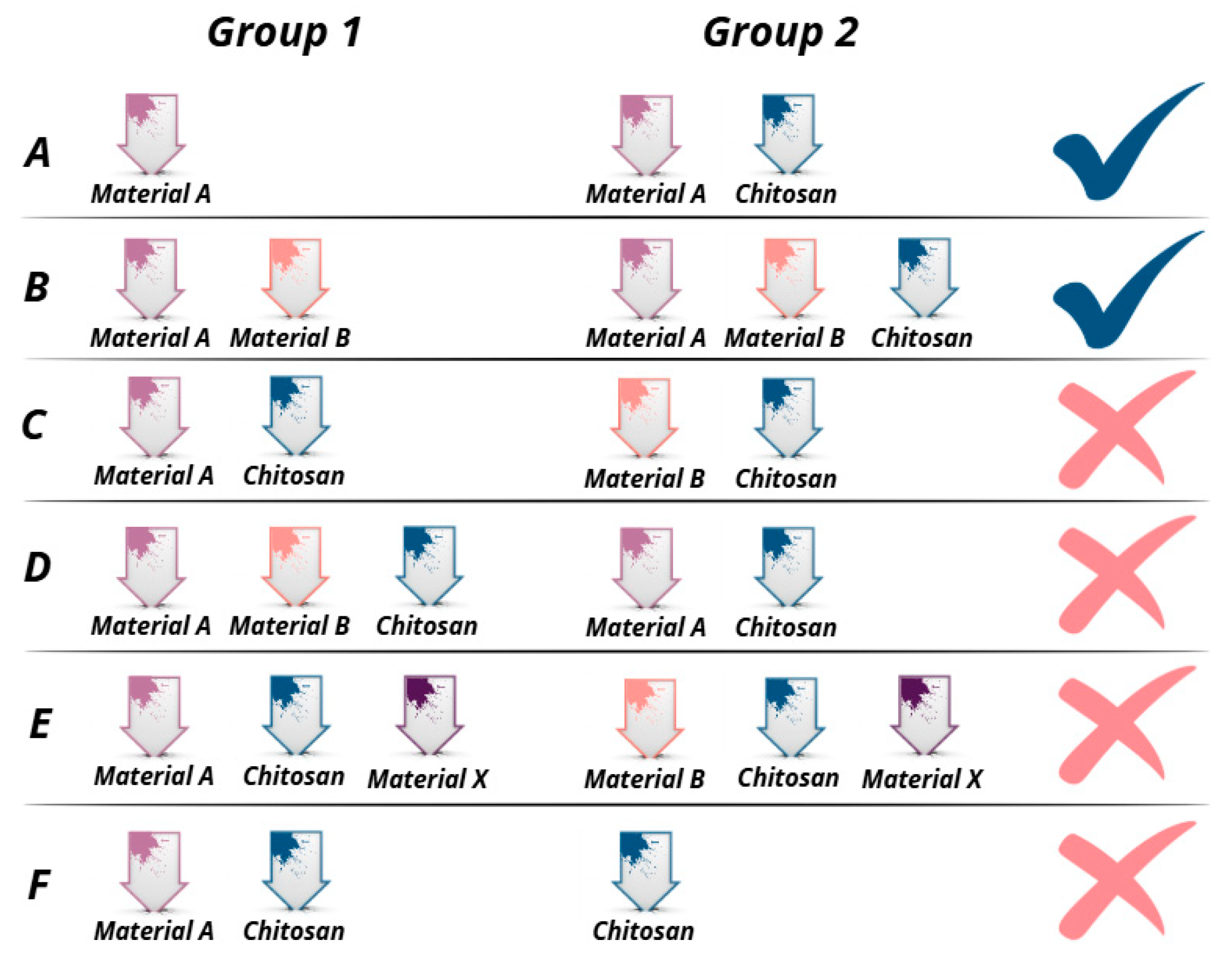
- Studies including research on groups of bone grafts described in Figure 3A,B.
- Books, book chapters, reviews, case reports, case series, and abstracts;
- Articles published in 2014 and earlier;
- Articles written in non-English languages;
- Studies that do not evaluate the effectiveness of chitosan-modified bone regeneration materials;
- Studies investigating a variety of bone regeneration materials, but in which chitosan was included in all groups investigated (Figure 3C,D);
- Studies investigating the effectiveness of various bone grafts after the addition of chitosan in combination with another material (Figure 3E);
- Studies that examined chitosan-modified bone grafts, but the control group represented self-administered chitosan (Figure 3F).
2.3. Information Sources
2.4. Search Strategy
- Web of science—TS=(chitosan) AND (TS=(bone) AND (TS=(graft) OR TS=(substitute))) AND TS=(bone regeneration) AND TS=(biological properties)
- Scopus—(ALL (chitosan) AND ALL (“bone graft” OR “bone substitute”) AND ALL (“bone regeneration”) AND ALL (“biological properties”)) AND PUBYEAR > 2014 AND PUBYEAR < 2026 AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”))
- PubMed—(chitosan) AND (bone AND (graft OR substitute)) AND (bone regeneration) AND (biological properties)
2.5. Study Selection and Data Collection Process
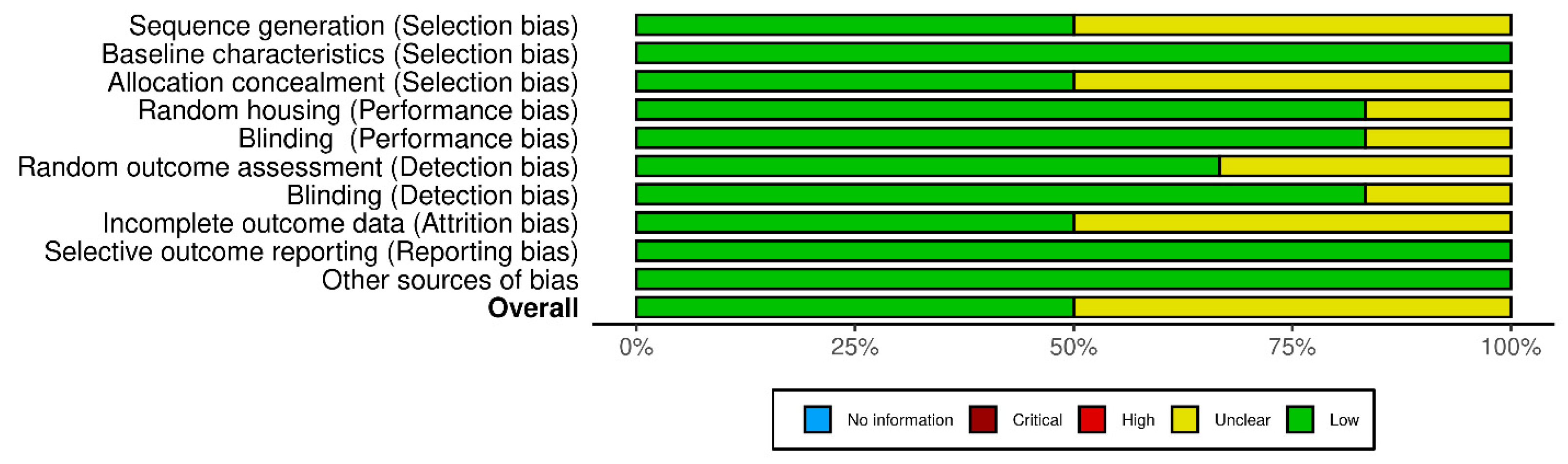
2.6. Risk of Bias Assessment
3. Results
3.1. In Vitro Studies
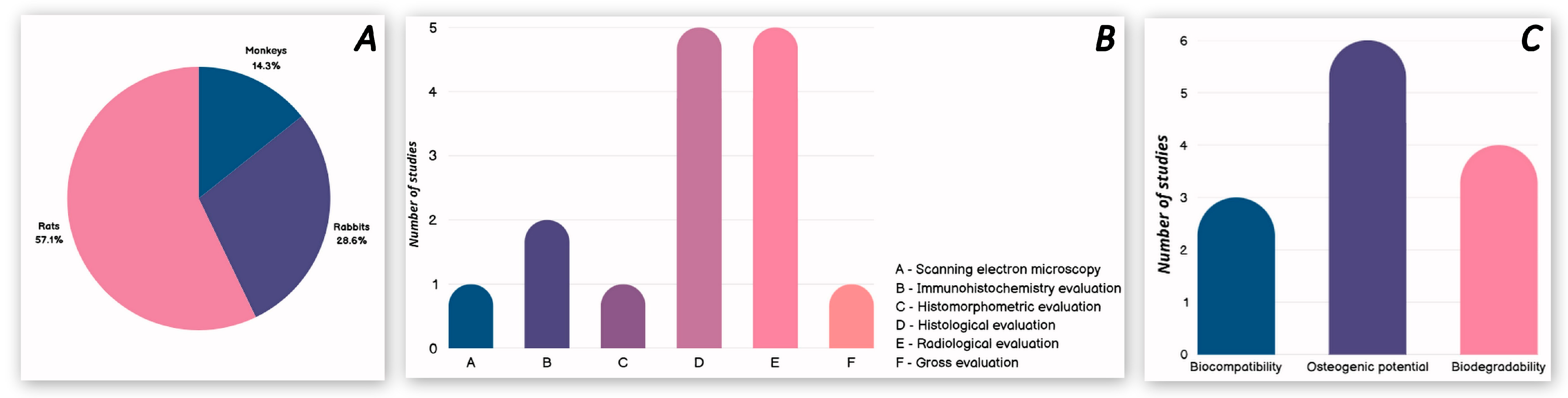
3.2. In Vivo Studies with Animals
3.3. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| SEM | Scanning electron microscopy |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| ELISA | Enzyme-linked immunosorbent assay |
| FTIR | Fourier transform infrared spectroscopy |
| IFC | Imaging flow cytometry |
| TE | Tissue engineering |
| CS | Chitosan |
| HA | Hydroxyapatite |
| BMP-2 | Bone morphogenetic protein 2 |
| Il-1 | Interleukin-1 |
| BCP | Biphasic calcium phosphate |
References
- Yotsova, R.; Peev, S. Biological Properties and Medical Applications of Carbonate Apatite: A Systematic Review. Pharmaceutics 2024, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Kim, S.-K.; Murugan, S.S.; Dalavi, P.A.; Gupta, S.; Anil, S.; Seong, G.H.; Venkatesan, J. Biomimetic chitosan with biocomposite nanomaterials for bone tissue repair and regeneration. Beilstein J. Nanotechnol. 2022, 13, 1051–1067. [Google Scholar] [CrossRef]
- Pourhajrezaei, S.; Abbas, Z.; Khalili, M.A.; Madineh, H.; Jooya, H.; Babaeizad, A.; Gross, J.D.; Samadi, A. Bioactive polymers: A comprehensive review on bone grafting biomaterials. Int. J. Biol. Macromol. 2024, 278, 134615. [Google Scholar] [CrossRef]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef]
- Ansari, M.A.A.; Dash, M.; Camci-Unal, G.; Jain, P.K.; Nukavarapu, S.; Ramakrishna, S.; Falcone, N.; Dokmeci, M.R.; Najafabadi, A.H.; Khademhosseini, A.; et al. Engineered stimuli-responsive smart grafts for bone regeneration. Curr. Opin. Biomed. Eng. 2023, 28, 100493. [Google Scholar] [CrossRef]
- Hao, S.; Wang, M.; Yin, Z.; Jing, Y.; Bai, L.; Su, J. Microenvironment-targeted strategy steers advanced bone regeneration. Mater. Today Bio 2023, 22, 100741. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Yang, Y.; Li, P. The Microenvironment That Regulates Vascular Wall Stem/Progenitor Cells in Vascular Injury and Repair. Biomed. Res. Int. 2022, 2022, 9377965. [Google Scholar] [CrossRef]
- Jadoun, S.; Riaz, U.; Budhiraja, V. Biodegradable conducting polymeric materials for biomedical applications: A review. Med. Devices Sens. 2021, 4, e10141. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.P.; Augustus, A.R.; Chandramohan, S.; Bhat, S.A.; Priya, V.V.; Rajagopalan, R. A review on biomaterials-based scaffold: An emerging tool for bone tissue engineering. Mater. Today Commun. 2023, 34, 105124. [Google Scholar] [CrossRef]
- Yunus Basha, R.; Sampath Kumar, T.S.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2024, 265, 130870. [Google Scholar] [CrossRef]
- Vaidya, G.; Pramanik, S.; Kadi, A.; Rayshan, A.R.; Abualsoud, B.M.; Ansari, M.J.; Masood, R.; Michaelson, J. Injecting hope: Chitosan hydrogels as bone regeneration innovators. J. Biomater. Sci. Polym. Ed. 2024, 35, 756–797. [Google Scholar] [CrossRef]
- Alkaron, W.; Almansoori, A.; Balázsi, C.; Balázsi, K. A Critical Review of Natural and Synthetic Polymer-Based Biological Apatite Composites for Bone Tissue Engineering. J. Compos. Sci. 2024, 8, 523. [Google Scholar] [CrossRef]
- Sugiyanti, D.; Darmadji, P.; Anggrahini, S.; Anwar, C.; Santoso, U. Preparation and characterization of chitosan from Indonesian Tambak Lorok shrimp shell waste and crab shell waste. Pak. J. Nutr. 2018, 17, 446–453. [Google Scholar] [CrossRef]
- Kapadnis, G.; Dey, A.; Dandekar, P.; Jain, R. Effect of degree of deacetylation on solubility of low-molecular-weight chitosan produced via enzymatic breakdown of chitosan. Polym. Int. 2019, 68, 1054–1063. [Google Scholar] [CrossRef]
- Gholap, A.D.; Rojekar, S.; Kapare, H.S.; Vishwakarma, N.; Raikwar, S.; Garkal, A.; Mehta, T.A.; Jadhav, H.; Prajapati, M.K.; Annapure, U. Chitosan scaffolds: Expanding horizons in biomedical applications. Carbohydr. Polym. 2024, 323, 121394. [Google Scholar] [CrossRef]
- Menazea, A.; El-Newehy, M.H.; Thamer, B.M.; El-Naggar, M.E. Synthesis, characterization and antibacterial activity of poly (vinyl alcohol)/chitosan film embedded with the as-prepared vanadium oxide nanoparticles via laser ablation. J. Mol. Struct. 2020, 1225, 129163. [Google Scholar] [CrossRef]
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D printing to innovate biopolymer materials for demanding applications: A review. Mater. Today Chem. 2021, 20, 100459. [Google Scholar] [CrossRef]
- Dong, J.; Ding, H.; Wang, Q.; Wang, L. A 3D-Printed Scaffold for Repairing Bone Defects. Polymers 2024, 16, 706. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2024, 131, 1038–1042. [Google Scholar] [CrossRef]
- Guo, W.; Peng, Z.; Ning, D.; Wu, Y.; Mao, Y.; Wang, E.; Zhang, M.; Zhang, Y.; Zhang, W.; You, H.; et al. Chitosan microporous foam filled 3D printed polylactic acid-pearl macroporous scaffold: Dual-scale porous structure, biological and mechanical properties. Int. J. Biol. Macromol. 2025, 303, 140508. [Google Scholar] [CrossRef]
- Huang, J.; Ratnayake, J.; Ramesh, N.; Dias, G.J. Development and Characterization of a Biocomposite Material from Chitosan and New Zealand-Sourced Bovine-Derived Hydroxyapatite for Bone Regeneration. ACS Omega 2020, 5, 16537–16546. [Google Scholar] [CrossRef]
- Galotta, A.; Rubenis, K.; Locs, J.; Sglavo, V.M. Dissolution-precipitation synthesis and cold sintering of mussel shells-derived hydroxyapatite and hydroxyap-atite/chitosan composites for bone tissue engineering. Open Ceram. 2023, 15, 100418. [Google Scholar] [CrossRef]
- Koski, C.; Vu, A.A.; Bose, S. Effects of chitosan-loaded hydroxyapatite on osteoblasts and osteosarcoma for chemopreventative applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111041. [Google Scholar] [CrossRef]
- Gong, C.; Fang, S.; Xia, K.; Chen, J.; Guo, L.; Guo, W. Enhancing the mechanical properties and cytocompatibility of magnesium potassium phosphate cement by incorporating oxygen-carboxymethyl chitosan. Regen. Biomater. 2020, 8, rbaa048. [Google Scholar] [CrossRef]
- Liao, Y.; Li, H.; Shu, R.; Chen, H.; Zhao, L.; Song, Z.; Zhou, W. Mesoporous Hydroxyapatite/Chitosan Loaded With Recombinant-Human Amelogenin Could Enhance Antibacterial Effect and Promote Periodontal Regeneration. Front. Cell Infect. Microbiol. 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Sun, D.; Li, M.; Zhou, J.; Yang, R.; Zhang, J.; Chai, W.; Wang, Y. Modification of hydroxyapatite (HA) powder by carboxymethyl chitosan (CMCS) for 3D printing bioceramic bone scaffolds. Ceram. Int. 2023, 49, 538–547. [Google Scholar] [CrossRef]
- Yang, L.; He, Y.; Huang, J.; Li, W.; Xie, W.; Huang, J. Osteogenic differentiation and biocompatibility of rabbit bone marrow mesenchymal stem cells promoted by hydroxyapatite-chitosan composites. J. Biotech. Res. 2022, 13, 46–54. [Google Scholar]
- Najafabadi, F.M.; Karbasi, S.; Benisi, S.Z.; Shojaei, S. Physical, mechanical, and biological performance of chitosan-based nanocomposite coating deposited on the polycapro-lactone-based 3D printed scaffold: Potential application in bone tissue engineering. Int. J. Biol. Macromol. 2024, 283, 137877. [Google Scholar] [CrossRef]
- Lv, B.H.; Tan, W.; Zhu, C.C.; Shang, X.; Zhang, L. Properties of a Stable and Sustained-Release Formulation of Recombinant Human Parathyroid Hormone (rhPTH) with Chitosan and Silk Fibroin Microparticles. Med. Sci. Monit. 2018, 24, 7532–7540. [Google Scholar] [CrossRef]
- Sampath, V.; Krishnasamy, V. Synthesis and characterization of hydroxyapatite self-assembled nanocomposites on graphene oxide sheets from seashell waste: A green process for regenerative medicine. J. Mech. Behav. Biomed. Mater. 2024, 151, 106383. [Google Scholar] [CrossRef]
- Yildizbakan, L.; Iqbal, N.; Giannoudis, P.V.; Jha, A. Synthesis of Chitosan and Ferric-Ion (Fe3+)-Doped Brushite Mineral Cancellous Bone Scaffolds. Biomimetics 2024, 9, 308. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Tan, W.S.; Cai, H. The chitosan/carboxymethyl cellulose/montmorillonite scaffolds incorporated with epigallocatechin-3-gallate-loaded chitosan microspheres for promoting osteogenesis of human umbilical cord-derived mesenchymal stem cell. Bioresour. Bioprocess 2022, 9, 36. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Noroozi, P.N.; Fathi, M.; Omidi, Y. The design of polycaprolactone-polyurethane/chitosan composite for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127895. [Google Scholar] [CrossRef]
- Skubis-Sikora, A.; Hudecki, A.; Sikora, B.; Wieczorek, P.; Hermyt, M.; Hreczka, M.; Likus, W.; Markowski, J.; Siemianowicz, K.; Kolano-Burian, A.; et al. Toxicological Assessment of Biodegradable Poli-ε-Caprolactone Polymer Composite Materials Containing Hydroxyapatite, Bioglass, and Chitosan as Potential Biomaterials for Bone Regeneration Scaffolds. Biomedicines 2024, 12, 1949. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Gong, M.; Xing, F.; Wu, S.; Xiang, Z. Urine-derived stem cells loaded onto a chitosan-optimized biphasic calcium-phosphate scaffold for repairing large segmental bone defects in rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2014–2029. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, R.; Shi, W.; Lei, S.; Zhang, X.; Jiang, N.; Lin, Y.; Li, Z.; Nie, M. Zinc and chitosan-enhanced β-tricalcium phosphate from calcined fetal bovine bone for mandible reconstruction. Front. Bioeng. Biotechnol. 2024, 12, 1355493. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Moshiri, A. Comparative study on the role of gelatin, chitosan and their combination as tissue engineered scaffolds on healing and regeneration of critical sized bone defects: An in vivo study. J. Mater. Sci. Mater. Med. 2016, 27, 155. [Google Scholar] [CrossRef]
- Gani, A.; Yulianty, R.; Supiaty, S.; Rusdy, M.; Asri, G.D.; Satya, D.E.; Feblina, A.R.; Achmad, H. Effectiveness of Combination of Chitosan Gel and Hydroxyapatite from Crabs Shells (Portunus pelagicus) Waste as Bonegraft on Periodontal Network Regeneration through IL-1 and BMP-2 Analysis. Int. J. Biomater. 2022, 2022, 1817236. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Xu, J.; Wang, J.; Li, C.; Wang, L. Tissue engineering using 3D printed nano-bioactive glass loaded with NELL1 gene for repairing alveolar bone defects. Regen. Biomater. 2023, 5, 213–220, Erratum in Regen Biomater. 2023, 10, rbad065. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Soundarya, S.P.; Sanjay, V.; Menon, A.H.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Ait Said, H.; Mabroum, H.; Lahcini, M.; Oudadesse, H.; Barroug, A.; Ben Youcef, H.; Noukrati, H. Manufacturing methods, properties, and potential applications in bone tissue regeneration of hydroxyapatite-chitosan biocomposites: A review. Int. J. Biol. Macromol. 2023, 243, 125150. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wei, Q.; Chang, P.; Hu, K.; Okoro, O.V.; Shavandi, A.; Nie, L. Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals 2021, 11, 353. [Google Scholar] [CrossRef]
- Rosenberg, N.; Rosenberg, O. Safety and efficacy of in vitro generated bone-like material for in vivo bone regeneration—A feasibility study. Heliyon 2020, 6, e03191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Xiao, K.; Li, Z.; Ma, Q.; Li, W.; Shen, S.; Weng, X. Self-healing and injectable hybrid hydrogel for bone regeneration of femoral head necrosis and defect. Biochem. Biophys. Res. Commun. 2019, 508, 25–30. [Google Scholar] [CrossRef]
- Sigusch, B.; Kranz, S.; von Hohenberg, A.C.; Wehle, S.; Guellmar, A.; Steen, D.; Berg, A.; Rabe, U.; Heyder, M.; Reise, M. Histological and Histomorphometric Evaluation of Implanted Photodynamic Active Biomaterials for Periodontal Bone Regeneration in an Animal Study. Int. J. Mol. Sci. 2023, 24, 6200. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, B.; Feng, L.; Xu, X.; Wang, C.; Lee, Y.; Wang, M.; Lu, X.; Qin, L.; Lin, S.; et al. Augmenting osteoporotic bone regeneration through a hydrogel-based rejuvenating microenvironment. Bioact. Mater. 2024, 41, 440–454. [Google Scholar] [CrossRef]
- Shah, F.A.; Ruscsák, K.; Palmquist, A. 50 years of scanning electron microscopy of bone—A comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy. Bone Res. 2019, 7, 15. [Google Scholar] [CrossRef]
- de Lacerda Schickert, S.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Jansen, J.A. Pre-Clinical Evaluation of Biological Bone Substitute Materials for Application in Highly Loaded Skeletal Sites. Biomolecules 2020, 10, 883. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Huang, X.; Zhu, X.; Zhi, W.; Wang, J.; Sun, D.; Chen, X.; Zhu, X.; Zhang, X. Accelerated osteogenesis of bone graft by optimizing the bone microenvironment formed by electrical signals dependent on driving micro vibration stimulation. Mater. Today Bio 2023, 23, 100891. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Kotze, M.J.; Bütow, K.W.; Olorunju, S.A.; Kotze, H.F. A radiological evaluation of alveolar bone regeneration between the left and right mandibles and maxillae of the Chacma baboon. J. S. Afr. Vet. Assoc. 2016, 87, e1–e6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sargolzaie, N.; Kadkhodazadeh, M.; Ebadian, A.R.; Shafieian, R.; Pourkaveh, S.; Naghibi, N.; Ramandie, M.F. Histological Evaluation of Bone Regeneration Using Hydroxyapatite Based Bone Substitute Derived from Antler: An Animal Study. J. Long Term Eff. Med. Implant. 2022, 32, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Gordo, P.M.; Costa, B.F.O.; Alves, P. Formulation and Characterization of Chitosan-Based Mixed-Matrix Scaffold for Tissue Engineering. Macromol 2024, 4, 253–268. [Google Scholar] [CrossRef]
- Jagdale, S.; Agarwal, B.; Dixit, A.; Gaware, S. Chitosan as excellent bio-macromolecule with myriad of anti-activities in biomedical applications—A review. Int. J. Biol. Macromol. 2024, 257, 128697. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhu, L.; Liu, T.; Huang, L. Chitosan-based biomaterials for bone tissue engineering. Int. J. Biol. Macromol. 2025, 304, 140923. [Google Scholar] [CrossRef]
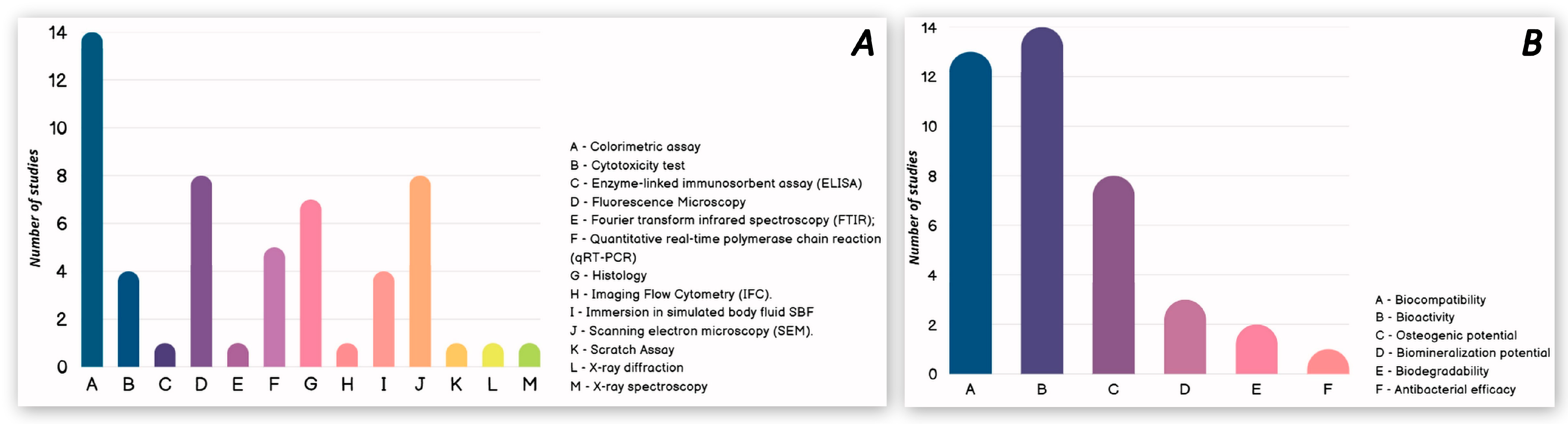

| Refs. | Authors | Studied Materials | Concentrations and Form of Chitosan | Study Methods | Biological Properties |
|---|---|---|---|---|---|
| [26] | Guo et al. (2025) | PLA-P 1 0.25% CS 2/PLA-P 1% CS/PLA-P 3% CS/PLA-P | CS microporous foams produced with 0.25 wt%, 1 wt%, and 3 wt% | Colorimetric assay; Fluorescence Microscopy; Histology; Immersion in simulated body fluid SBF. | Biocompatibility |
| Bioactivity | |||||
| Osteogenic potential | |||||
| Biomineralization potential | |||||
| [27] | Huang et al. (2020) | BHA 3 8% CS-BHA | CS solution (8% w/v)—by dissolving 4 g of CS in 50 mL of 1% acetic acid solution | Colorimetric assay; Fluorescence Microscopy. | Biocompatibility |
| Bioactivity | |||||
| [28] | Gallota et al. (2023) | HA 4 CS/HA | CS powder (10 wt% with respect to HA) | Immersion in simulated body fluid SBF | Biomineralization potential |
| [29] | Koski et al. (2020) | HA CS/HA | CS solution—by dissolving CS in 0.1 M acetic acid and pipetted on top of the HA disks at initial concentrations of 10 μg | Colorimetric assay | Biocompatibility |
| Bioactivity | |||||
| [30] | Gong et al. (2020) | K-struvite 1% O-CMC 5 + K-struvite 6 2.5% O-CMC + K-struvite 5% O-CMC + K-struvite | O-CMC powders (0, 1, 2.5 and 5 wt.% with respect to K-struvite powder phase) | Colorimetric assay; Fluorescence Microscopy; Quantitative real-time polymerase chain reaction (qRT-PCR); Scanning electron microscopy (SEM). | Bioactivity |
| Osteogenic potential | |||||
| Biodegradability | |||||
| [31] | Liao et al. (2020) | 3 mg/mL mHA 7 2.25 mg/mL mHA/CS 4.5 mg/mL mHA/CS | CS solution—by dissolving 1 g of CS in 50 mL of 2% acetic acid solution. Chitosan was mixed with mHA at a mass ratio of 1:2 | Fluorescence Microscopy | Antibacterial efficacy |
| [32] | Wei et al. (2023) | HA 1% CMCS 8/HA 3% CMCS/HA | CMCS powders (0, 1, and 3 wt.% with respect to HA) | Colorimetric assay; Cytotoxicity test; Fourier transform infrared spectroscopy (FTIR); Immersion in simulated body fluid SBF; Scanning electron microscopy (SEM). | Biocompatibility |
| Bioactivity | |||||
| Biomineralization potential | |||||
| Biodegradability | |||||
| [33] | Yang et al. (2022) | HA CS/HA | Not stated | Colorimetric assay; Histology; Imaging Flow Cytometry (IFC). | Biocompatibility |
| Bioactivity | |||||
| Osteogenic potential | |||||
| [34] | Najafabadi et al. (2024) | PMA 9 PMA/CS 10 | CS solution—by dissolving 2 wt% of the Cs in acetic acid solution | Colorimetric assay; Histology; Immersion in simulated body fluid SBF; Scanning electron microscopy (SEM); X-ray diffraction; X-ray spectroscopy. | Biocompatibility |
| Bioactivity | |||||
| Osteogenic potential | |||||
| [35] | Lv et al. (2018) | SF 11 CS/SF | CS solution (1% w/v)—by dissolving CS in acetic acid solution | Cytotoxicity test; Enzyme-linked immunosorbent assay (ELISA); Scanning Electron Microscopy (SEM). | Bioactivity |
| [36] | Sampath & Krishnasamy (2024) | HA-GO 12 HA-GO-CS 13 Different concentrations (0.2, 0.4, 0.6, 0.8 и 1.0 μg/mL) of GO-HA and GO-HA-CS | CS solution—40 mL of 0.02 mg/mL chitosan was dispersed in 1% acetic acid | Colorimetric assay | Biocompatibility |
| [37] | Yildizbakan et al. (2024) | Fe3+-DCPD 14 Fe3+-DCPD: CS (20:80) Fe3+-DCPD: CS (30:70) Fe3+-DCPD: CS (40:60) Fe3+-DCPD: CS (50:50) | CS solution with 3 wt%—by dissolving high-molecular-weight chitosan flakes in 2% acetic acid | Colorimetric assay | Biocompatibility |
| Bioactivity | |||||
| [38] | Wang et al. (2022) | CS/CMC 15/MMT 16 CS/CMC/MMT-CM 17 | CS solution—by dissolving 400 mg CS in 20 mL 1% glacial acetic acid solution | Colorimetric assay; Fluorescence Microscopy; Histology; Quantitative real-time polymerase chain reaction (qRT-PCR) assay. | Biocompatibility |
| Bioactivity | |||||
| Osteogenic potential | |||||
| [39] | Amiryaghoubi et al. (2022) | PCLUU (3%) 18 PCLUU (3%)/CS (1.5%) | CS solution—CS (3% w/v) was prepared in 2% acetic acid | Colorimetric assay; Fluorescence Microscopy; Histology; Quantitative real-time polymerase chain reaction (qRT-PCR) assay; Scanning electron microscopy (SEM). | Biocompatibility |
| Bioactivity | |||||
| Osteogenic potential | |||||
| [40] | Skubis-Sikora et al. (2024) | PCL 19 PCL/CS 20 | Not stated | Colorimetric assay; Cytotoxicity test; Fluorescence Microscopy; Scanning electron microscopy (SEM); Scratch Assay. | Biocompatibility |
| Bioactivity | |||||
| [41] | Liu et al. (2021) | BCP 21 CS/BCP | CS solution with 2 wt%—80 mg CS was dissolved in 0.1 M acetic acid | Colorimetric assay; Fluorescence Microscopy; Histology; Scanning Electron Microscopy (SEM); Quantitative real-time polymerase chain reaction (qRT-PCR). | Biocompatibility |
| Bioactivity | |||||
| Osteogenic potential | |||||
| [42] | Zhou et al. (2024) | CFBB 22 CFBB/CS CFBB/Zn2+ CFBB/Zn2+/CS | CS solution—10 g/L solution of CS-acetic acid | Colorimetric assay; Cytotoxicity test; Histology; Scanning Electron Microscopy (SEM); Quantitative real-time polymerase chain reaction (qRT-PCR) assay. | Biocompatibility |
| Bioactivity | |||||
| Osteogenic potential | |||||
| Biodegradability |
| Refs. | Authors | Studied Materials | Concentrations and Form of Chitosan |
Model, Sample Size | Studied Period | Application | Study Methods | Biological Properties |
|---|---|---|---|---|---|---|---|---|
| [26] | Guo et al. (2025) | PLA-P 1 1% CS 2/PLA-P | CS microporous foams produced with 1 wt% | rats, X | 8 and 12 weeks | Bone defect regeneration (skull) | Radiological evaluation | Osteogenic potential |
| [41] | Liu et al. (2021) | BCP 3 CS/BCP | CS solution with 2 wt%—80 mg CS was dissolved in 0.1 M acetic acid | rats, 4 | 2 and 4 weeks | Subcutaneous implantation (Degradability and inflammatory response of scaffolds) | Histological evaluation | Biocompatibility Biodegradability |
| [42] | Zhou et al. (2024) | CFBB 4 CFBB/CS CFBB/Zn2+ CFBB/Zn2+/CS | CS solution—10 g/L solution of CS-acetic acid | rabbits, 12 | 6 and 12 weeks | Bone defect regeneration (ulna, unilateral) | Radiological evaluation; Histological evaluation. | Osteogenic potential |
| rabbits, 48 | 4, 8 and 12 weeks | Bone defect regeneration: (mandibula, bilaterally) | Radiological evaluation; Histological evaluation; Immunohistochemistry evaluation. | Osteogenic potential Biodegradability | ||||
| [43] | Oryan et al. (2016) | Gel 5 CS/Gel | CS solution—CS 2% was dissolved in a 1% acetic acid | rats, 10 | 8 weeks | Bone defect regeneration (radius, bilaterally) | Radiological evaluation; Gross evaluation; Histological evaluation; Histomorphometric evaluation; Scanning electron microscopy. | Biocompatibility Osteogenic potential Biodegradability |
| [44] | Gani et al. (2022) | HA 6 CS/HA | Not stated | rats, 18 | 1, 2 and 3 weeks | Bone defect regeneration (femur, bilaterally) | Immunohistochemistry evaluation | Biocompatibility Osteogenic potential |
| [45] | Zhang et al. (2018) | BG 7 + BMSCs BG/CSn 8 + BMSCs 9 | CS solution—200 μL chitosan solution and 20 μL saline | monkeys, 4 | 12 weeks | Bone defect regeneration (extracted first and second premolars in the 4 quadrants) | Radiological evaluation; Histological evaluation. | Osteogenic potential Biodegradability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerova-Vatsova, T.; Peev, S.; Yotsova, R.; Rogova, V.-V. Evaluation of the Effectiveness of Chitosan-Modified Bone Regeneration Materials: A Systematic Review. Pharmaceutics 2025, 17, 665. https://doi.org/10.3390/pharmaceutics17050665
Gerova-Vatsova T, Peev S, Yotsova R, Rogova V-V. Evaluation of the Effectiveness of Chitosan-Modified Bone Regeneration Materials: A Systematic Review. Pharmaceutics. 2025; 17(5):665. https://doi.org/10.3390/pharmaceutics17050665
Chicago/Turabian StyleGerova-Vatsova, Tsvetalina, Stefan Peev, Ralitsa Yotsova, and Varvara-Velika Rogova. 2025. "Evaluation of the Effectiveness of Chitosan-Modified Bone Regeneration Materials: A Systematic Review" Pharmaceutics 17, no. 5: 665. https://doi.org/10.3390/pharmaceutics17050665
APA StyleGerova-Vatsova, T., Peev, S., Yotsova, R., & Rogova, V.-V. (2025). Evaluation of the Effectiveness of Chitosan-Modified Bone Regeneration Materials: A Systematic Review. Pharmaceutics, 17(5), 665. https://doi.org/10.3390/pharmaceutics17050665









