Combining Advanced Therapies with Alternative Treatments: A New Approach to Managing Antimicrobial Resistance?
Abstract
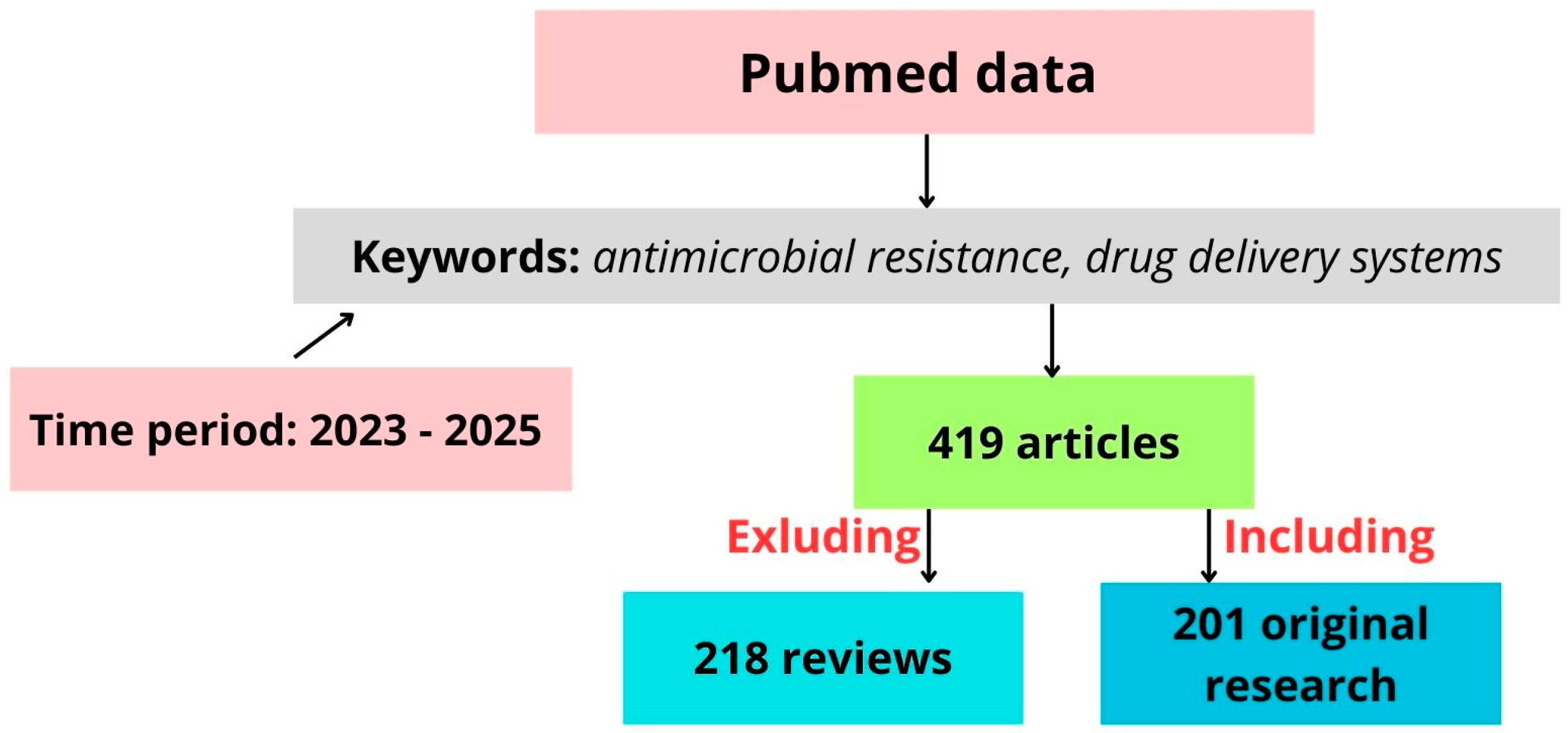
1. Introduction
2. Trends in Antimicrobial Drug Formulations
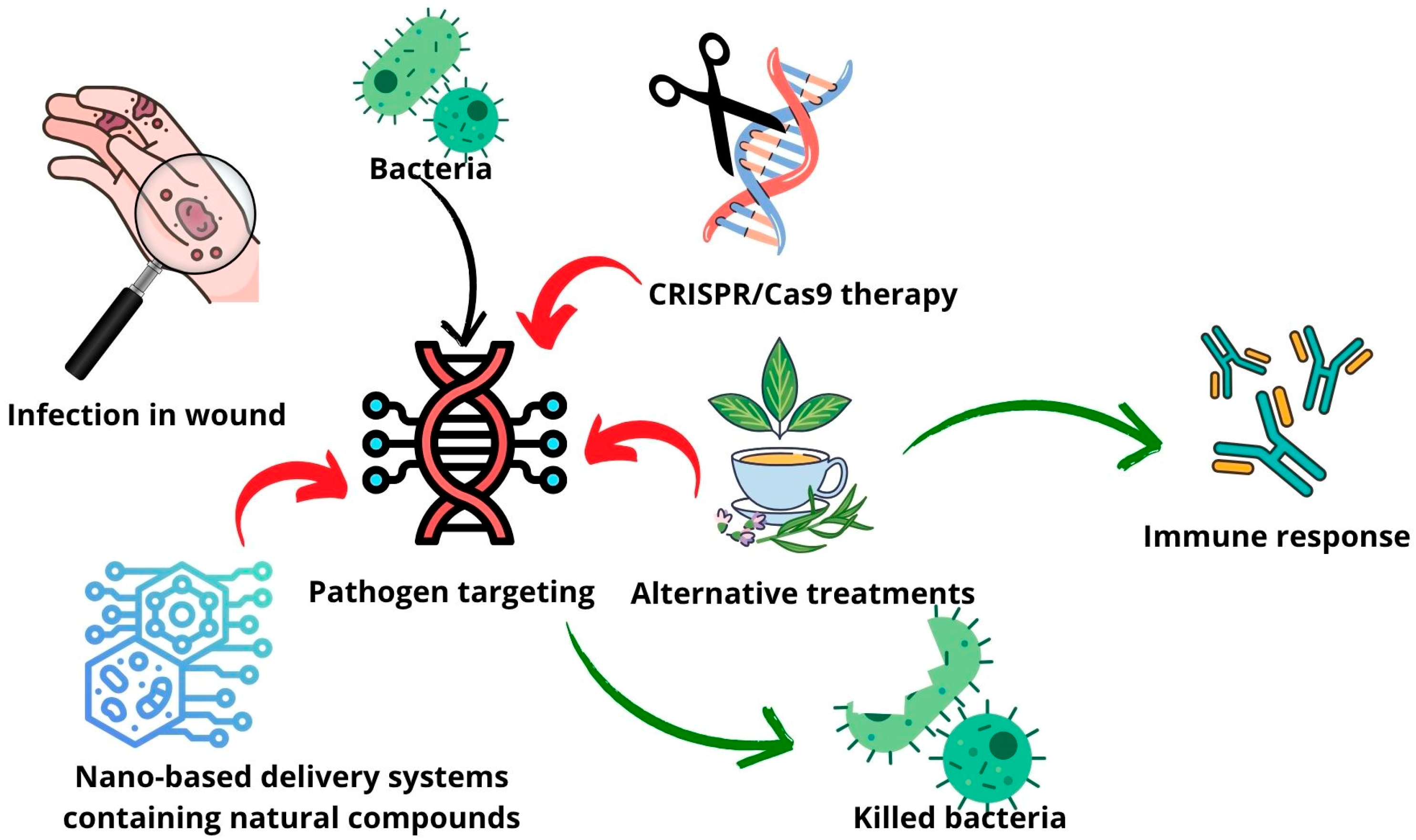
3. Advanced Therapy
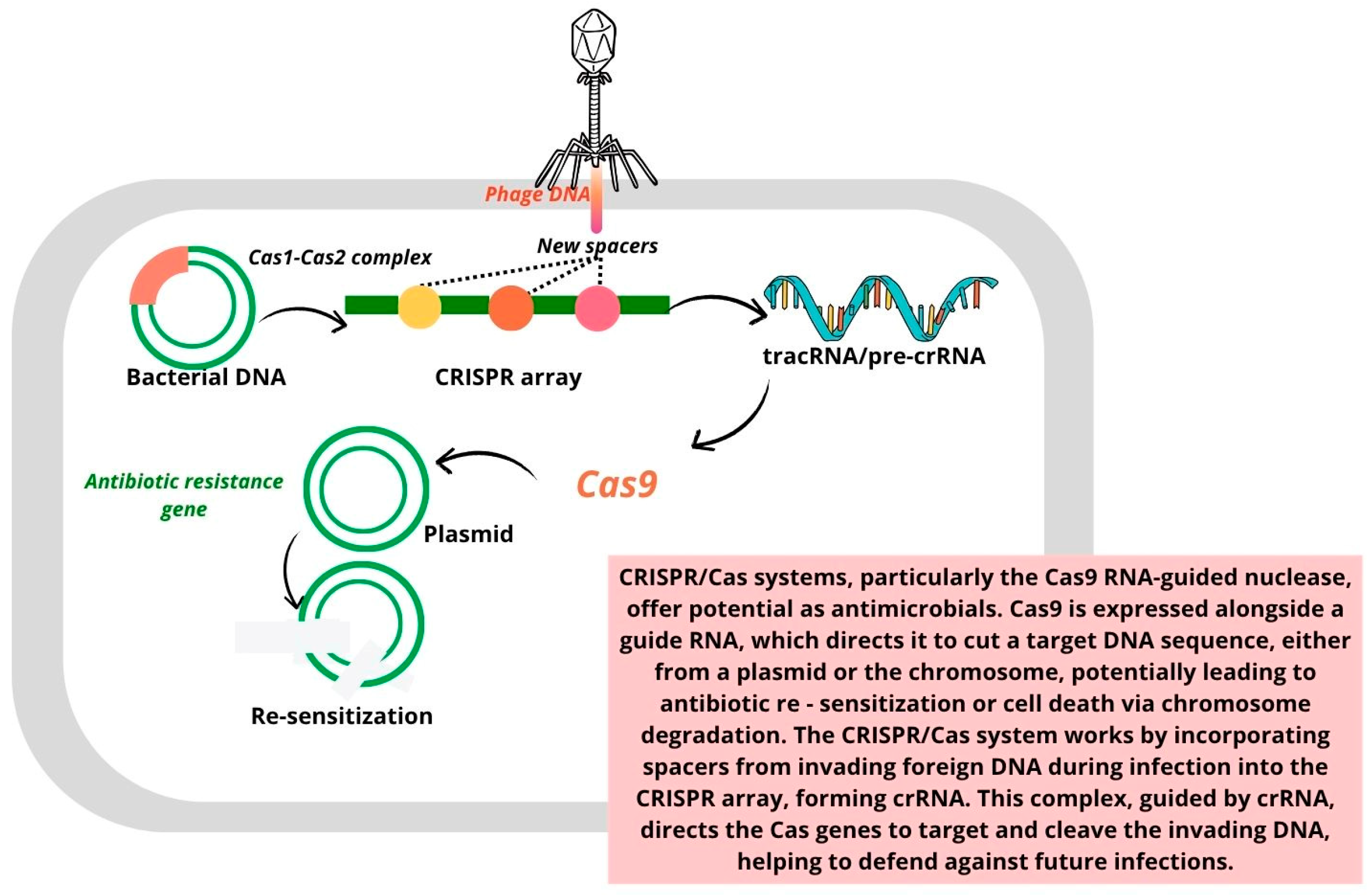
- I.
- Adaptation stage: During this phase, spacer sequences are acquired from invading genetic material, such as viruses or plasmids.
- II.
- Expression stage: In this stage, the CRISPR array is transcribed to generate crRNA (CRISPR RNA), which, along with the Cas protein, forms the essential components for targeting invading DNA.
- III.
- Interference stage: The mature crRNA binds to the Cas protein, forming a nucleic acid–protein complex. This complex can then recognize and bind to complementary sequences in the target nucleic acid, triggering endonuclease activity that cleaves and degrades the foreign genetic material.
4. Drug Delivery Systems
| Drug Delivery System | Activity | Application |
|---|---|---|
| OnG6 MOFs serve as mesoporous metal–organic frameworks capable of loading multiple antibiotics, including isoniazid and ciprofloxacin [111]. | MOFs loaded with ciprofloxacin exhibited strong antimicrobial activity against S. aureus and E. coli. | These MOFs are designed for treating bacterial infections, particularly tuberculosis, by releasing active drugs like ASA in vivo and enhancing the delivery efficiency. |
| A smart, bacteria-responsive carrier system (CMC-EFT@ZIF-8) was developed using ZIF-8 and carboxymethyl cellulose to deliver the antibiotic ceftiofur [112]. | The system demonstrated enhanced drug release under acidic and cellulase-rich conditions, resulting in the 99% elimination of P. aeruginosa in vitro. | This delivery platform showed strong therapeutic potential in a mouse skin wound model, offering a promising approach for treating resistant bacterial infections. |
| A thyme-oil-based nanoemulsion (NE) was developed to enhance the stability and bioavailability and control the release of the volatile and easily oxidized essential oil compound thymol [113]. | The NE showed potent antibacterial effects against B. subtilis, E. coli, P. aeruginosa, and S. aureus, and antitumor activity by inducing apoptosis and cell cycle arrest in HepG2 liver cancer cells. | This nanoemulsion system offers a dual-function therapeutic platform for bacterial infection control and cancer treatment, making it a promising alternative to conventional therapies. |
| Lavender oil was incorporated into niosomes to enhance delivery, reduce volatility, and improve cellular compatibility in biomedical applications [114]. | The niosomes maintained a high cell viability across the tested concentrations in adipose-derived stem cells and myometrial cells, indicating a low cytotoxicity and biocompatibility. | This system showed strong potential for regenerative medicine and pharmaceutical therapies, offering a safe and natural alternative for future biomedical formulations. |
| Solid lipid microparticles (SLM) were developed using hardfat and palm oil carriers via spray-chilling to encapsulate cinnamon bark oleoresin (CO), protecting its bioactive components from degradation [115]. | The SLMs exhibited strong antimicrobial activity, maintaining effective inhibition against Candida pseudointermedia and Penicillium paneum, with an enhanced performance over a 28-day period. | These lipid-based microparticles offer a stable and controlled-release system for natural antimicrobials, making them suitable for applications in food preservation, pharmaceuticals, and possibly topical therapeutics. |
| A bioabsorbable, controlled-release nanoemulgel of quercetin was developed using cinnamon oil, tween 80, Carbitol®, and poloxamer 407 as the base, with the aim of enhancing its solubility and bioavailability for periodontitis treatment [116]. | The nanoemulgel showed a significant drug release of 92.4% quercetin within 6 h, far surpassing the release from a pure quercetin-loaded gel (<3% release), indicating efficient drug delivery. | This nanoemulgel system holds potential as an effective therapeutic delivery platform for treating periodontitis, improving the clinical outcomes by enhancing quercetin’s antimicrobial and anti-inflammatory effects. |
| A Pickering emulsion was developed using zein–tannic acid complexes to co-load tannic acid and cinnamon essential oil, enhancing the interfacial stability and controlled release [117]. | The optimized formulation exhibited strong antimicrobial activity against the spoilage organisms Pseudomonad paralactis MN10 and Lactobacillus sakei VMR17. | This system presents a novel approach for food preservation, enabling the effective delivery of multiple natural antimicrobials through a stable, bio-based emulsion interface. |
| Propolis-based chitosan varnishes were formulated in 5%, 10%, and 15% concentrations to ensure controlled release and strong adhesion to tooth surfaces [118]. | The system demonstrated strong antimicrobial activity—comparable or superior to chlorhexidine—against cariogenic biofilm-forming bacteria, with the sustained release of active compounds for over one week. | These formulations show promise for preventive dental care, specifically in managing and preventing dental caries, and warrant further clinical investigation. |
| A targeted NP carrier system (PBCA-NP) functionalized with polysorbate 80 to adsorb apolipoprotein E was developed to deliver propolis across the blood–brain barrier [119]. | The propolis-loaded PBCA-NPs exhibited significant antifungal activity against Cryptococcus neoformans in vitro and reduced fungal virulence and burden in both Galleria mellonella and mouse models. | This system demonstrates strong potential for treating cerebral cryptococcosis, overcoming bioavailability issues and targeting the central nervous system through a blood–brain-barrier-crossing mechanism. |
| TTO was formulated into dry powder inhalers using β-cyclodextrin inclusion complexes (TTO-β-CD) to enable effective pulmonary delivery [120]. | TTO-β-CD powders showed superior antipneumonic activity compared to TTO alone, and performed comparably to fluconazole and penicillin in rat models of fungal and bacterial pneumonia, respectively, through antimicrobial and anti-inflammatory mechanisms. | The TTO-β-CD dry powder inhalers offer a promising inhalable therapy for managing fungal and bacterial pneumonia, with their advantages including s high lung deposition, stability, and self-administration suitability. |
| Hydrogels incorporating TTO-loaded nanocapsules and nanoemulsions were formulated using Carbopol Ultrez, offering stable and skin-compatible topical delivery [121]. | These formulations showed significant anti-inflammatory (antiedematogenic) effects following UVB exposure, and enhanced wound healing, with nanocapsule-based hydrogels outperforming other treatments in reducing the wound area. | This study supports the topical use of nanostructured tea tree oil hydrogels for managing skin inflammation and cutaneous wound repair, demonstrating their potential in dermatological therapy. |
| Air-filled lysozyme microbubbles functionalized with gold NPs and alkaline phosphatase [122]. | Enhanced antimicrobial activity against M. lysodeikticus and effective biosensing of paraoxon in aqueous solutions. | The use of air-filled lysozyme microbubbles functionalized with gold NPs and alkaline phosphatase includes antimicrobial therapy and biosensing, specifically for detecting paraoxon in aqueous solutions. |
| Injectable paste composed of mannitol, chitosan, and polyethylene glycol designed for localized antibiotic delivery [123]. | Mannitol reactivated dormant S. aureus persister cells, enhancing the antibiotic susceptibility, and when combined with vancomycin or amikacin in a paste formulation, it extended drug release by up to 7 days and reduced biofilm viability by up to 95.5%, with mannitol alone also contributing to biofilm disruption. | This represents a promising adjunctive treatment for musculoskeletal infections, especially where biofilm-forming bacteria like S. aureus are involved. |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| ATMPs | advanced therapy medicinal products |
| EV | extracellular vesicle |
| MOL | molnupiravir |
| MSC | mesenchymal stem cells |
| MV | methyl vanillate |
| NPs | nanoparticles |
| SCC | somatic cell therapy |
| SLM | solid lipid microparticles |
| TTO | tea tree oil |
| ZnO | zinc oxide |
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Gimza, B.D.; Cassat, J.E. Mechanisms of Antibiotic Failure During Staphylococcus aureus Osteomyelitis. Front. Immunol. 2021, 12, 638085. [Google Scholar] [CrossRef] [PubMed]
- Ronga, L.; Abbasciano, A.; Calia, C.; Mosca, A.; Battista, M.; Sparapano, E.; De Carlo, C.; Miragliotta, G.; Del Prete, R. Trends in the Antibiotic Resistance of S. Aureus Clinical Isolates: A 4 Years Retrospective Study in a Teaching Hospital in South Italy. Infez. Med. 2019, 27, 266–273. [Google Scholar]
- Shemetov, O.; Faustova, M.; Perepelova, T.; Balia, H.; Pavlish, I.; Loban’, G. Forecasting the Development of Antimicrobial Resistance of S. aureus. Front. Oral. Health 2025, 5, 1514070. [Google Scholar] [CrossRef] [PubMed]
- Araya, S.; Gebreyohannes, Z.; Tadlo, G.; Gessew, G.T.; Negesso, A.E. Epidemiology and Multidrug Resistance of Pseudomonas aeruginosa and Acinetobacter baumanni Isolated from Clinical Samples in Ethiopia. Infect. Drug Resist. 2023, 16, 2765–2773. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef] [PubMed]
- Ray, H.; Weis, C.; Nwaeze, C.; Zhou, V.; Basu, P.; Mitra, A. Development and Control of Biofilms in Diabetic Foot Infections: A Narrative Review. Acta Microbiol. Hell. 2025, 70, 9. [Google Scholar] [CrossRef]
- Idrees, M.; Khan, I.; Ullah, A.; Shah, S.M.M.; Ullah, H.; Khan, M.A.; Almeer, R.; Shah, Z.A.; Nadeem, T. Bacterial Spectrum from Diabetic Foot Ulcers: A Study of Antibiotic Resistance Patterns and Phylogenetic Diversity. J. King Saud. Univ. Sci. 2024, 36, 103320. [Google Scholar] [CrossRef]
- Guo, H.; Song, Q.; Mei, S.; Xue, Z.; Li, J.; Ning, T. Distribution of Multidrug-Resistant Bacterial Infections in Diabetic Foot Ulcers and Risk Factors for Drug Resistance: A Retrospective Analysis. PeerJ 2023, 11, e16162. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Ding, L.; Hu, F. China’s New National Action Plan to Combat Antimicrobial Resistance (2022–2025). J. Antimicrob. Chemother. 2023, 78, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Committee for Advanced Therapies (CAT); CAT Scientific Secretariat; Schneider, C.K.; Salmikangas, P.; Jilma, B.; Flamion, B.; Todorova, L.R.; Paphitou, A.; Haunerova, I.; Maimets, T.; et al. Challenges with Advanced Therapy Medicinal Products and How to Meet Them. Nat. Rev. Drug Discov. 2010, 9, 195–201. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. CRISPR-Cas-Based Antimicrobials: Design, Challenges, and Bacterial Mechanisms of Resistance. ACS Infect. Dis. 2023, 9, 1283–1302. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Balderas-Cisneros, F.d.J.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Morones-Ramírez, J.R. Synthetic Biology Tools for Engineering Microbial Cells to Fight Superbugs. Front. Bioeng. Biotechnol. 2022, 10, 869206. [Google Scholar] [CrossRef] [PubMed]
- Angeles Flores, G.; Cusumano, G.; Venanzoni, R.; Angelini, P. Advancements in Antibacterial Therapy: Feature Papers. Microorganisms 2025, 13, 557. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a Novel Antibacterial Agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential Oils and Its Antibacterial, Antifungal and Anti-Oxidant Activity Applications: A Review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Marzaman, A.N.F.; Roska, T.P.; Sartini, S.; Utami, R.N.; Sulistiawati, S.; Enggi, C.K.; Manggau, M.A.; Rahman, L.; Shastri, V.P.; Permana, A.D. Recent Advances in Pharmaceutical Approaches of Antimicrobial Agents for Selective Delivery in Various Administration Routes. Antibiotics 2023, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Current Trends and Definitions in High-Performance Antimicrobial Strategies. Curr. Opin. Biomed. Eng. 2022, 23, 100407. [Google Scholar] [CrossRef]
- Maxson, T.; Mitchell, D.A. Targeted Treatment for Bacterial Infections: Prospects for Pathogen-Specific Antibiotics Coupled with Rapid Diagnostics. Tetrahedron 2016, 72, 3609–3624. [Google Scholar] [CrossRef]
- Lamba, S.; Heruka De Zoysa, G.; Wang, K.; Lu, J.; Swift, S.; Sarojni, V. Homo and Hetero-Branched Lipopeptide Dendrimers: Synthesis and Antimicrobial Activity. Bioorganic Chem. 2024, 150, 107567. [Google Scholar] [CrossRef]
- Berking, B.B.; Poulladofonou, G.; Karagrigoriou, D.; Wilson, A.D.; Neumann, K. Zwitterionic Polymeric Sulfur Ylides with Minimal Charge Separation Open a New Generation of Antifouling and Bactericidal Materials. Angew. Chem. Int. Ed. 2023, 62, e202308971. [Google Scholar] [CrossRef]
- Geng, Z.; Cao, Z.; Liu, J. Recent Advances in Targeted Antibacterial Therapy Basing on Nanomaterials. Exploration 2023, 3, 20210117. [Google Scholar] [CrossRef]
- Mohammadi, M.; Rahmani, S.; Ebrahimi, Z.; Nowroozi, G.; Mahmoudi, F.; Shahlaei, M.; Moradi, S. In Situ Forming Hydrogel Reinforced with Antibiotic-Loaded Mesoporous Silica Nanoparticles for the Treatment of Bacterial Keratitis. AAPS PharmSciTech 2024, 25, 254. [Google Scholar] [CrossRef]
- Jadhav, K.; Jhilta, A.; Singh, R.; Ray, E.; Kumar, V.; Yadav, A.B.; Singh, A.K.; Verma, R.K. Effective Cerebral Tuberculosis Treatment via Nose-to-Brain Transport of Anti-TB Drugs Using Mucoadhesive Nano-Aggregates. Nanoscale 2024, 16, 16485–16499. [Google Scholar] [CrossRef]
- Madrid Sani, A.T.; Ramos-Rocha, K.L.V.; Sarcinelli, M.A.; Chaves, M.H.d.C.; Rocha, H.V.A.; Léo, P.; Cerize, N.N.P.; Zanin, M.H.A.; Feitosa, V.A.; Rangel-Yagui, C.d.O. Development of a Dry Powder Formulation for Pulmonary Delivery of Azithromycin-Loaded Nanoparticles. J. Pharm. Pharm. Sci. 2024, 27, 13635. [Google Scholar] [CrossRef]
- Coksu, I.; Bozkurt, Y.; Akmayan, I.; Demirci, H.; Ozbek, T.; Acar, S. Ketoconazole-Loading Strategy to Improve Antifungal Activity and Overcome Cytotoxicity on Human Renal Proximal Tubular Epithelial Cells. Nanotechnology 2023, 35, 115702. [Google Scholar] [CrossRef]
- Alneghery, L.M.; Al-Zharani, M.; Nasr, F.A.; Eldin, Z.E.; Al Hujran, T.A.; Tawfeek, H.M.; Fayed, M.H.; Elbeltagi, S. Fabrication and Optimization of Naringin-Loaded MOF-5 Encapsulated by Liponiosomes as Smart Drug Delivery, Cytotoxicity, and Apoptotic on Breast Cancer Cells. Drug Dev. Ind. Pharm. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Enoch, K.; Somasundaram, A.A. Unravelling the Rheological and Multifunctionality of Justicia Adhatoda-Impregnated Carboxymethyl Cellulose Hydrogels for Drug Delivery Systems. Int. J. Biol. Macromol. 2025, 306, 141419. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Farley, A.J.M.; Lankapalli, A.; Zhang, Y.; Premchand-Branker, S.; Cook, K.; Baran, A.; Gray-Hammerton, C.; Orbegozo Rubio, C.; Suna, E.; et al. The Triple Combination of Meropenem, Avibactam, and a Metallo-β-Lactamase Inhibitor Optimizes Antibacterial Coverage Against Different β-Lactamase Producers. Engineering 2024, 38, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Haddaji, N.; Bahloul, B.; Bahia, W.; Bechambi, O.; Mahdhi, A. Development of Nanotechnology-Based Drug Delivery Systems for Controlling Clinical Multidrug-Resistant Staphylococcus aureus and Escherichia coli Associated with Aerobic Vaginitis. Pharmaceutics 2023, 15, 2133. [Google Scholar] [CrossRef]
- Aliakbari, E.; Nural, Y.; Zamiri, R.E.; Yabalak, E.; Mahdavi, M.; Yousefi, V. Design and Synthesis of Silver Nanoparticle Anchored Poly(Ionic Liquid)s Mesoporous for Controlled Anticancer Drug Delivery with Antimicrobial Effect. Int. J. Environ. Health Res. 2024, 34, 90–102. [Google Scholar] [CrossRef]
- Vatankhah, M.; Dadashzadeh, S.; Mahboubi, A.; Haeri, A.; Jandaghi Alaee, K.; Mostafavi Naeini, S.B.; Abbasian, Z. Preparation of Multivesicular Liposomes for the Loco-Regional Delivery of Vancomycin Hydrochloride Using Active Loading Method: Drug Release and Antimicrobial Properties. J. Liposome Res. 2024, 34, 77–87. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Nada, A.I.; Farrag, M.A.; Alatawi, K.; Alalawy, A.I.; Al-Qahtani, S.D.; El-Mehasseb, I.M. Spectroscopic Study to Verify the Anti-Hepatitis C Virus (HCV) Treatment through a Delivery System of the Sofosbuvir Drug on Chitosan and Pycnogenol Nanoparticles Surface. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 302, 123063. [Google Scholar] [CrossRef]
- Abid, F.; Savaliya, B.; Parikh, A.; Kim, S.; Amirmostofian, M.; Cesari, L.; Song, Y.; Page, S.W.; Trott, D.J.; Garg, S. Nanotechnology and Narasin: A Powerful Combination against Acne. Nanoscale 2023, 15, 13728–13739. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, T.S.; Bezerra de Lima, L.E.; Alves-Pereira, E.L.; Alves-Silva, M.F.; Dourado, D.; Fernandes-Pedrosa, M.d.F.; Figueiredo, R.C.B.Q.d.; da Silva-Junior, A.A. Cationic and Anionic PLGA-Cholesterol Hybrid Nanoparticles as Promising Platforms to Enhance the Trypanocidal Efficacy of Benznidazole and Drug Delivery in Trypanosoma cruzi-Infected Cells. Biomed. Pharmacother. 2025, 183, 117782. [Google Scholar] [CrossRef] [PubMed]
- Patamia, V.; Zagni, C.; Fiorenza, R.; Fuochi, V.; Dattilo, S.; Riccobene, P.M.; Furneri, P.M.; Floresta, G.; Rescifina, A. Total Bio-Based Material for Drug Delivery and Iron Chelation to Fight Cancer through Antimicrobial Activity. Nanomaterials 2023, 13, 2036. [Google Scholar] [CrossRef]
- AMR-X Collaborators. System-Wide Approaches to Antimicrobial Therapy and Antimicrobial Resistance in the UK: The AMR-X Framework. Lancet Microbe 2024, 5, e500–e507. [Google Scholar] [CrossRef]
- Makhlouf, Z.; Ali, A.A.; Al-Sayah, M.H. Liposomes-Based Drug Delivery Systems of Anti-Biofilm Agents to Combat Bacterial Biofilm Formation. Antibiotics 2023, 12, 875. [Google Scholar] [CrossRef]
- Yao, J.; Zou, P.; Cui, Y.; Quan, L.; Gao, C.; Li, Z.; Gong, W.; Yang, M. Recent Advances in Strategies to Combat Bacterial Drug Resistance: Antimicrobial Materials and Drug Delivery Systems. Pharmaceutics 2023, 15, 1188. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; Vishwakarma, N.; Lehr, C.-M.; Prestidge, C.A.; Thomas, N.; Roberts, R.J.; Thorn, C.R.; Melero, A. Antibiotic Resistance and Tolerance: What Can Drug Delivery Do against This Global Threat? Drug Deliv. Transl. Res. 2024, 14, 1725–1734. [Google Scholar] [CrossRef]
- Mulukutla, A.; Shreshtha, R.; Kumar Deb, V.; Chatterjee, P.; Jain, U.; Chauhan, N. Recent Advances in Antimicrobial Peptide-Based Therapy. Bioorganic Chem. 2024, 145, 107151. [Google Scholar] [CrossRef]
- Advanced Therapy Medicinal Products: Overview. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/human-regulatory-overview/advanced-therapy-medicinal-products-overview (accessed on 5 May 2025).
- Regulation—1394/2007. Available online: https://eur-lex.europa.eu/eli/reg/2007/1394/oj/eng (accessed on 5 May 2025).
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The Approved Gene Therapy Drugs Worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef]
- Cuende, N.; Izeta, A. 2—European Regulatory Framework for the Development of Cell-Based Medicines. In Guide to Cell Therapy GxP; Vives, J., Carmona, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 15–47. ISBN 978-0-12-803115-5. [Google Scholar]
- Joyce, K.; Buljovcic, Z.; Rosic, G.; Kaszkin-Bettag, M.; Pandit, A. Issues with Tissues: Trends in Tissue-Engineered Products in Clinical Trials in the European Union. Tissue Eng. Part. B Rev. 2023, 29, 78–88. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Liang, W. The Application of the CRISPR-Cas System in Antibiotic Resistance. Infect. Drug Resist. 2022, 15, 4155–4168. [Google Scholar] [CrossRef]
- Al-Fadhli, A.H.; Jamal, W.Y. Recent advances in gene-editing approaches for tackling antibiotic resistance threats: A review. Front. Cell Infect. Microbiol. 2024, 14, 1410115. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Naha, S.; Rao, A.; Basu, S. Chapter Five—CRISPR-Cas System, Antibiotic Resistance and Virulence in Bacteria: Through a Common Lens. In Progress in Molecular Biology and Translational Science; Advances in CRISPR/Cas and Related, Technologies; Ghosh, D., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 178, pp. 123–174. [Google Scholar]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas Systems for the Detection and Control of Antibiotic-Resistant Infections. J. Nanobiotechnology 2021, 19, 401. [Google Scholar] [CrossRef]
- Javed, M.U.; Hayat, M.T.; Mukhtar, H.; Imre, K. CRISPR-Cas9 System: A Prospective Pathway toward Combatting Antibiotic Resistance. Antibiotics 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Callaway, T.R.; Rollin, E.; Ryman, V.E. Association of Pre-Treatment Somatic Cell Counts with Bacteriological Cure Following Diagnosis of Intramammary Infection. Res. Vet. Sci. 2022, 152, 537–545. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Impastato, R.; Coy, J.; Strumpf, A.; Dow, S. Antibacterial Activity of Human Mesenchymal Stem Cells Mediated Directly by Constitutively Secreted Factors and Indirectly by Activation of Innate Immune Effector Cells. Stem Cells Transl. Med. 2020, 9, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Cano-Vicent, A.; Sabater i Serra, R.; El-Tanani, M.; Aljabali, A.; Tambuwala, M.M.; Mishra, Y.K. Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications. Mater. Today Bio. 2022, 16, 100412. [Google Scholar] [CrossRef]
- Coppola, B.; Menotti, F.; Longo, F.; Banche, G.; Mandras, N.; Palmero, P.; Allizond, V. New Generation of Osteoinductive and Antimicrobial Polycaprolactone-Based Scaffolds in Bone Tissue Engineering: A Review. Polymers 2024, 16, 1668. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Huang, S.; Tang, X.; Yao, G.; Sun, L. Mesenchymal Stem Cells Enhance Pulmonary Antimicrobial Immunity and Prevent Following Bacterial Infection. Stem Cells Int. 2020, 2020, 3169469. [Google Scholar] [CrossRef]
- Castro Ramos, A.; Widjaja Lomanto, M.Y.; Vuong, C.-K.; Ohneda, O.; Fukushige, M. Antibacterial Effects of Human Mesenchymal Stem Cells and Their Derivatives: A Systematic Review. Front. Microbiol. 2024, 15, 1430650. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, T.; Saleem, A.; Farooq, N.; Dar, L.A.; Nazir, J.; Saleem, S.; Ismail, S.; Gugjoo, M.B.; Shiekh, A.P.; Ahmad, S.M. Extracellular Vesicles Derived from Mesenchymal Stem Cells—A Novel Therapeutic Tool in Infectious Diseases. Inflamm. Regen. 2023, 43, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-Z.; Yang, R.-L.; Wei, H.-X.; Yang, K.; Yang, Y.-B.; Wang, N.-X.; Zhang, Q.; Chen, F.; Zhang, T. Advances in the Research of Immunomodulatory Mechanism of Mesenchymal Stromal/Stem Cells on Periodontal Tissue Regeneration. Front. Immunol. 2025, 15, 1449411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, H.; Liu, R.; Cui, R. Advances in Immunomodulatory Mechanisms of Mesenchymal Stem Cells-Derived Exosome on Immune Cells in Scar Formation. Int. J. Nanomed. 2023, 18, 3643–3662. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Soleimanian, S.; Azarpira, N.; Asvar, Z.; Pakbaz, S. Stem Cell-Derived Exosome as Potential Therapeutics for Microbial Diseases. Front. Microbiol. 2022, 12, 786111. [Google Scholar] [CrossRef]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface Charge-Switching Polymeric Nanoparticles for Bacterial Cell Wall-Targeted Delivery of Antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Huang, T.-H.; Yang, S.-C.; Chen, C.-C.; Fang, J.-Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M. New Zein Protein Composites with High Performance in Phosphate Removal, Intrinsic Antibacterial, and Drug Delivery Capabilities. ACS Appl. Mater. Interfaces 2024, 16, 37468–37485. [Google Scholar] [CrossRef]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Mohammed, S.N.F.; Syed Abd Halim, S.A.; Ghafar, N.A.; Abdul Jalil, N.A. Therapeutic Potential of Honey and Propolis on Ocular Disease. Pharmaceuticals 2022, 15, 1419. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Jiang, H.; Zhang, Y.; Cao, J.; Jiang, W. Advances in Propolis and Propolis Functionalized Coatings and Films for Fruits and Vegetables Preservation. Food Chem. 2023, 414, 135662. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of Honey and Propolis for Wound Care. Biotechnol. Bioeng. 2023, 120, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Puteo, C.; Lombardi, R.; Garcea, G.; Lupia, C.; Spano, A.; Liguori, G.; Palma, E.; Britti, D.; Castagna, F. Antimicrobial Properties of Hive Products and Their Potential Applications in Human and Veterinary Medicine. Antibiotics 2025, 14, 172. [Google Scholar] [CrossRef]
- Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Available online: https://www.mdpi.com/1420-3049/26/18/5701 (accessed on 5 May 2025).
- Javed, S.; Mangla, B.; Ahsan, W. From Propolis to Nanopropolis: An Exemplary Journey and a Paradigm Shift of a Resinous Substance Produced by Bees. Phytother. Res. 2022, 36, 2016–2041. [Google Scholar] [CrossRef]
- Barboza, A.d.S.; Ribeiro de Andrade, J.S.; Ferreira, M.L.; Peña, C.L.D.; da Costa, J.S.; Fajardo, A.R.; Lund, R.G. Propolis Controlled Delivery Systems for Oral Therapeutics in Dental Medicine: A Systematic Review. Dent. J. 2023, 11, 162. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Cardoso, M.L.C.; Lucchesi, M.B.; Gremião, M.P.D. Gelatin Microparticles Containing Propolis Obtained by Spray-Drying Technique: Preparation and Characterization. Int. J. Pharm. 2003, 264, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sangboonruang, S.; Semakul, N.; Sookkree, S.; Kantapan, J.; Ngo-Giang-Huong, N.; Khamduang, W.; Kongyai, N.; Tragoolpua, K. Activity of Propolis Nanoparticles against HSV-2: Promising Approach to Inhibiting Infection and Replication. Molecules 2022, 27, 2560. [Google Scholar] [CrossRef]
- Rassu, G.; Cossu, M.; Langasco, R.; Carta, A.; Cavalli, R.; Giunchedi, P.; Gavini, E. Propolis as Lipid Bioactive Nano-Carrier for Topical Nasal Drug Delivery. Colloids Surf. B Biointerfaces 2015, 136, 908–917. [Google Scholar] [CrossRef]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.-V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of Essential Oils as Efficient Tools against Antimicrobial Resistance: A Review of the Type and Physical-Chemical Properties of the Delivery Systems and Applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles-Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Carreño, H.; Stashenko, E.E.; Escobar, P. Essential Oils Distilled from Colombian Aromatic Plants and Their Constituents as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2023, 28, 2872. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Lai, Y.; Zou, J.; Yang, P.; Wu, Z.; He, W. Deep Eutectic Solvents for Essential-Oil Delivery and Bacterial-Infected Wound Healing. Langmuir 2024, 40, 23766–23779. [Google Scholar] [CrossRef]
- Muthalagu, S.; Natarajan, S. Deciphering the Antimicrobial and Antibiofilm Efficiency of Thyme Essential Oil Encapsulated Zeolitic Imidazole Framework-8 Against Foodborne Pathogens. Curr. Microbiol. 2024, 82, 49. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Jesil, A.M.; Lewis, S.A.; Raja, S.; Paul, A.; Ghosal, K.; Mahmood, S.; Ansari, M.D. Design and Evaluation of Microemulsion-Based Drug Delivery Systems for Biofilm-Based Infection in Burns. AAPS PharmSciTech 2024, 25, 203. [Google Scholar] [CrossRef]
- Development of Stability, Antioxidant, and Antimicrobial Properties of Biopolymeric Chitosan Modified Starch Nanocapsules Containing Essential Oil—Maryam Hasani, Seyed Mahdi Ojagh, Mohammad Amir Hasani, Shirin Hasani. 2024. Available online: https://journals.sagepub.com/doi/10.1177/10820132231168449 (accessed on 5 May 2025).
- Kaspute, G.; Arunagiri, B.D.; Alexander, R.; Ramanavicius, A.; Samukaite-Bubniene, U. Development of Essential Oil Delivery Systems by ‘Click Chemistry’ Methods: Possible Ways to Manage Duchenne Muscular Dystrophy. Materials 2023, 16, 6537. [Google Scholar] [CrossRef]
- Kaspute, G.; Ramanavicius, A.; Prentice, U. Molecular Imprinting Technology for Advanced Delivery of Essential Oils. Polymers 2024, 16, 2441. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Abd El-Aziz, S.; Nabil-Adam, A.; Tamer, T.M. Formulation of Novel Bioactive Gelatin Inspired by Cinnamaldehyde for Combating Multi-Drug Resistant Bacteria: Characterization, Molecular Docking, Pharmacokinetic Analyses, and in Vitro Assessments. Int. J. Pharm. 2024, 652, 123827. [Google Scholar] [CrossRef]
- Nichitoi, M.M.; Josceanu, A.M.; Isopescu, R.D.; Isopencu, G.O.; Geana, E.-I.; Ciucure, C.T.; Lavric, V. Polyphenolics Profile Effects upon the Antioxidant and Antimicrobial Activity of Propolis Extracts. Sci. Rep. 2021, 11, 20113. [Google Scholar] [CrossRef]
- Luo, L.; Huang, W.; Zhang, J.; yu, Y.; Sun, T. Metal-Based Nanoparticles as Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2024, 7, 2529–2545. [Google Scholar] [CrossRef]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Stepanov, N.; Aslanli, A.; Lyagin, I.; Senko, O.; Maslova, O. Combination of Enzymes with Materials to Give Them Antimicrobial Features: Modern Trends and Perspectives. J. Funct. Biomater. 2023, 14, 64. [Google Scholar] [CrossRef]
- Weldick, P.J.; Wang, A.; Halbus, A.F.; Paunov, V.N. Emerging Nanotechnologies for Targeting Antimicrobial Resistance. Nanoscale 2022, 14, 4018–4041. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Xiao, Z.; Luo, Y. Sustainable Nanotechnology for Food Preservation: Synthesis, Mechanisms, and Applications of Zinc Oxide Nanoparticles. J. Agric. Food Res. 2025, 19, 101743. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.-H.; Shim, K.-D.; Kim, D.-J.; Jhee, K.-H.; Lee, H.-W.; Heo, C.-H.; Kim, H.-M.; Jang, E.-S. Antibacterial Mechanism of ZnO Nanoparticles under Dark Conditions. J. Ind. Eng. Chem. 2017, 45, 430–439. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Li, M.; Jiang, X.; Yu, H.; Sun, D. An Efficient Metal–Organic Framework-Based Drug Delivery Platform for Synergistic Antibacterial Activity and Osteogenesis. J. Colloid. Interface Sci. 2023, 640, 521–539. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Mostafa, Y.S.; Ramadan, M.S.; El-Mehasseb, I.M. Enhancement Efficiency Delivery of Antiviral Molnupiravir-Drug via the Loading with Self-Assembly Nanoparticles of Pycnogenol and Cellulose Which Are Decorated by Zinc Oxide Nanoparticles for COVID-19 Therapy. Bioorg Chem. 2024, 143, 107028. [Google Scholar] [CrossRef]
- Salama, S.A.; Essam, D.; Tagyan, A.I.; Farghali, A.A.; Khalil, E.M.; Abdelaleim, Y.F.; Hozzein, W.N.; Mubarak, M.; Nasr, F.A.; Eweis, A.A.; et al. Novel composite of nano zinc oxide and nano propolis as antibiotic for antibiotic-resistant bacteria: A promising approach. Sci. Rep. 2024, 14, 20894. [Google Scholar] [CrossRef]
- Ghaffari, P.; Zeighami, H.; Najdalizade, M.; Eftekhar, L. In Vitro Antibacterial Effect of a Nano-Zinc Oxide Eugenol Sealer Alone and in Combination with Chitosan, Propolis, and Nanosilver on Enterococcus faecalis. Dent. Res. J. 2024, 21, 56. [Google Scholar] [CrossRef]
- Zayed, H.S.; Saleh, S.; Omar, A.E.; Saleh, A.K.; Salama, A.; Tolba, E. Development of Collagen-Chitosan Dressing Gel Functionalized with Propolis-Zinc Oxide Nanoarchitectonics to Accelerate Wound Healing. Int. J. Biol. Macromol. 2024, 261, 129665. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Liu, Y.; Shao, S.; Zheng, X.; Tang, K. Synergistic Effect of Nano Zinc Oxide and Tea Tree Essential Oil on the Properties of Soluble Soybean Polysaccharide Films. Int. J. Biol. Macromol. 2023, 239, 124361. [Google Scholar] [CrossRef]
- AbouAitah, K.; Geioushy, R.A.; Nour, S.A.; Emam, M.T.H.; Zakaria, M.A.; Fouad, O.A.; Shaker, Y.M.; Kim, B.S. A Combined Phyto- and Photodynamic Delivery Nanoplatform Enhances Antimicrobial Therapy: Design, Preparation, In Vitro Evaluation, and Molecular Docking. ACS Appl. Bio Mater. 2024, 7, 6873–6889. [Google Scholar] [CrossRef]
- Ahmed, A.; Kelly, A.; Leonard, D.; Saleem, W.; Bezrukov, A.; Efthymiou, C.G.; Zaworotko, M.J.; Tiana, D.; Boyd, A.; Papatriantafyllopoulou, C. Synthesis and Characterisation of Antimicrobial Metal-Organic Frameworks as Multi-Drug Carriers. Dalton Trans. 2024, 53, 11867–11875. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhang, M.; Wang, S.; Li, L.; Zuo, R.; Qu, S. Bacteria-Responsive Drug Delivery System Utilizing Carboxymethyl Cellulose-Functionalized Metal-Organic Framework for Enhanced Antibacterial Efficacy. ACS Biomater. Sci. Eng. 2025, 11, 2216–2225. [Google Scholar] [CrossRef]
- Nasra, S.; Meghani, N.; Kumar, A. Nanoemulsion-Based System as a Novel and Promising Approach for Enhancing the Antimicrobial and Antitumoral Activity of Thymus Vulgaris (L.) Oil in Human Hepatocellular Carcinoma Cells. Appl. Biochem. Biotechnol. 2024, 196, 949–970. [Google Scholar] [CrossRef] [PubMed]
- Vilela, J.d.M.V.; Moghassemi, S.; Dadashzadeh, A.; Dolmans, M.-M.; Azevedo, R.B.; Amorim, C.A. Safety of Lavender Oil-Loaded Niosomes for In Vitro Culture and Biomedical Applications. Nanomaterials 2022, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Procopio, F.R.; Oriani, V.B.; Paulino, B.N.; do Prado-Silva, L.; Pastore, G.M.; Sant’Ana, A.S.; Hubinger, M.D. Solid Lipid Microparticles Loaded with Cinnamon Oleoresin: Characterization, Stability and Antimicrobial Activity. Food Res. Int. 2018, 113, 351–361. [Google Scholar] [CrossRef]
- Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations. Molecules 2018, 23, 1363. [Google Scholar] [CrossRef]
- Fan, S.; Yang, Q.; Wang, D.; Zhu, C.; Wen, X.; Li, X.; Richel, A.; Fauconnier, M.-L.; Yang, W.; Hou, C.; et al. Zein and Tannic Acid Hybrid Particles Improving Physical Stability, Controlled Release Properties, and Antimicrobial Activity of Cinnamon Essential Oil Loaded Pickering Emulsions. Food Chem. 2024, 446, 138512. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; De Luca, M.P.; Ribeiro, T.G.; Castilho, R.O.; Moreira, A.N.; Santos, V.R.; Faraco, A.A.G. Propolis--Based Chitosan Varnish: Drug Delivery, Controlled Release and Antimicrobial Activity against Oral Pathogen Bacteria. BMC Complement. Altern. Med. 2014, 14, 478. [Google Scholar] [CrossRef]
- Thammasit, P.; Tharinjaroen, C.S.; Tragoolpua, Y.; Rickerts, V.; Georgieva, R.; Bäumler, H.; Tragoolpua, K. Targeted Propolis-Loaded Poly (Butyl) Cyanoacrylate Nanoparticles: An Alternative Drug Delivery Tool for the Treatment of Cryptococcal Meningitis. Front. Pharmacol. 2021, 12, 723727. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Zhang, T.; Liu, B.; Du, L.; Jin, Y. Pulmonary Delivery of Tea Tree Oil-β-Cyclodextrin Inclusion Complexes for the Treatment of Fungal and Bacterial Pneumonia. J. Pharm. Pharmacol. 2017, 69, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; De Lima, J.A.; Da Silva, C.R.; Benvegnú, D.; Ferreira, J.; Burger, M.E.; Beck, R.C.R.; Rolim, C.M.B.; Rocha, M.I.U.M.; Da Veiga, M.L.; et al. Hydrogels Containing Nanocapsules and Nanoemulsions of Tea Tree Oil Provide Antiedematogenic Effect and Improved Skin Wound Healing. J. Nanosci. Nanotechnol. 2015, 15, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Micheli, L.; Kaliappan, S.; Teo, B.M.; Zhou, M.; Palleschi, G.; Ashokkumar, M. Antimicrobial and Biosensing Ultrasound-Responsive Lysozyme-Shelled Microbubbles. ACS Appl. Mater. Interfaces 2013, 5, 464–471. [Google Scholar] [CrossRef]
- Pace, L.R.; Harrison, Z.L.; Brown, M.N.; Haggard, W.O.; Jennings, J.A. Characterization and Antibiofilm Activity of Mannitol-Chitosan-Blended Paste for Local Antibiotic Delivery System. Mar. Drugs 2019, 17, 517. [Google Scholar] [CrossRef]

| Example | Details | Reference |
|---|---|---|
| Biosurfactant-based nanoemulsions | Broad-spectrum antibacterial activity and significant antibiofilm effects against E. coli and S. aureus. | [37] |
| Silver-poly(ionic liquid) nanocomposite | Antibacterial activity against E. coli and S. aureus. | [38] |
| Vancomycin hydrochloride-loaded multivesicular liposomes | An encapsulation efficiency of over 90%, with the drug released over a sustained period—up to 19 days—compared to 6–8 h for free vancomycin hydrochloride. Effective antibacterial activity against osteomyelitis-causing pathogens. | [39] |
| Hybrid nanocomposite (Cs@Pyc.SOF) of sofosbuvir, pycnogenol, and chitosan NPs | An 83% drug-loading efficiency and a controlled release of up to 94% over 48 h. | [40] |
| A self-nanomicellizing solid dispersion system using Soluplus® | Encapsulated narasin achieved a 100-fold increase in solubility, demonstrated superior skin penetration compared to free narasin, and exhibited strong antibacterial activity. | [41] |
| Naringin-loaded Zn–organic framework 5 (NG-MOF-5) coated with liponiosomes (LNs) | The NG-MOF-5@LNs exhibited monodispersed spherical particles with excellent antimicrobial activity, an IC50 of 21 µg/mL against MCF-7 breast cancer cells, and a significant apoptosis effect of 68.2%, as indicated by the MTT assay and inhibition zone results. | [34] |
| Cationic and anionic PLGA–cholesterol hybrid NPs for the intracellular delivery of benznidazole | Demonstrated enhanced trypanocidal activity against intracellular amastigotes and a superior performance in anionic NPs, attributed to effective internalization and endo-/lysosomal residence. | [42] |
| Carboxymethylcellulose-based hydrogels delivering Justicia adhatoda | Improved mechanical properties, antimicrobial activity, reduced biofilm formation, and antioxidant effects. | [35] |
| Halloysite nanotubes combined with the iron-chelating capabilities of kojic acid | Strong antibacterial activity against all tested pathogens and successfully loaded resveratrol and curcumin as potential drug carriers, offering dual functionality as both an antimicrobial agent and a drug delivery system. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspute, G.; Zebrauskas, A.; Streckyte, A.; Ivaskiene, T.; Prentice, U. Combining Advanced Therapies with Alternative Treatments: A New Approach to Managing Antimicrobial Resistance? Pharmaceutics 2025, 17, 648. https://doi.org/10.3390/pharmaceutics17050648
Kaspute G, Zebrauskas A, Streckyte A, Ivaskiene T, Prentice U. Combining Advanced Therapies with Alternative Treatments: A New Approach to Managing Antimicrobial Resistance? Pharmaceutics. 2025; 17(5):648. https://doi.org/10.3390/pharmaceutics17050648
Chicago/Turabian StyleKaspute, Greta, Arunas Zebrauskas, Akvile Streckyte, Tatjana Ivaskiene, and Urte Prentice. 2025. "Combining Advanced Therapies with Alternative Treatments: A New Approach to Managing Antimicrobial Resistance?" Pharmaceutics 17, no. 5: 648. https://doi.org/10.3390/pharmaceutics17050648
APA StyleKaspute, G., Zebrauskas, A., Streckyte, A., Ivaskiene, T., & Prentice, U. (2025). Combining Advanced Therapies with Alternative Treatments: A New Approach to Managing Antimicrobial Resistance? Pharmaceutics, 17(5), 648. https://doi.org/10.3390/pharmaceutics17050648







