Pharmacokinetics of Different Tacrolimus Formulations in the Early Post-Liver Transplant Period: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
- Population: LT recipients (>18 years old) during the first month post-transplant.
- Intervention: Immunosuppressive treatment with TAC (despite the combination with other immunosuppressant drugs).
- Comparison: With (different formulations of TAC) or without a comparator.
- Outcomes: PK parameters and/or plasma concentrations of TAC.
- Study design: Clinical trials and observational studies.
- All articles that did not meet the inclusion criteria were excluded:
- Studies focused exclusively on the pharmacogenetics of TAC.
- Studies in which TAC was not administered via the systemic route.
- Studies in which the type of TAC formulation used could not be identified.
2.2. Data Source and Search Strategy
2.3. Study Screening and Selection
2.4. Data Extraction
- Author and year of publication.
- Study design.
- Population: number of subjects, sex, age, and ethnicity.
- Main objective of the study.
- Primary variable of interest.
- Immunosuppressive regimen.
- Follow-up period.
- Key PK findings.
- TAC regimen and time to first dose post-LT.
- Time to PK analysis.
- PK target range, as concentration or area under the curve (AUC).
- TAC exposure:
- -
- Concentrations: Minimum concentration (Cmin), which is the concentration immediately prior to dose administration, the steady-state concentration (Css), or the maximum concentration (Cmax).
- -
- AUC: A measure of bioavailability, represents the total amount of the drug that reaches systemic circulation.
- PK parameters: Related to absorption, absorption rate constant (Ka), absolute bioavailability (F), time to maximum plasma concentration (Tmax); related to distribution, apparent volume of distribution (Vd); and related to elimination clearance (Cl) or elimination half-life (t1/2).
2.5. Quality Assessment
3. Results
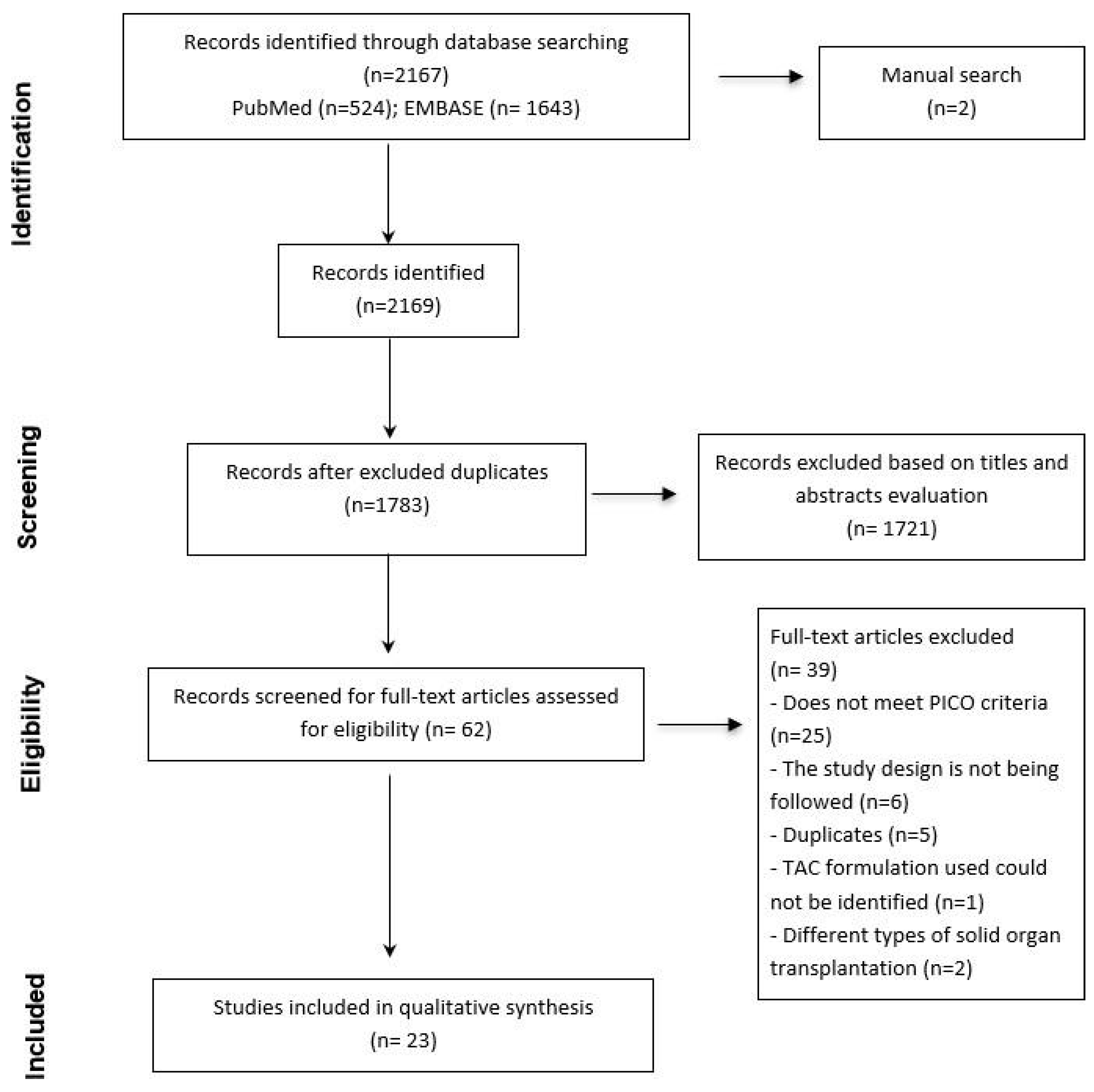
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Characteristics of Tacrolimus Treatment and Pharmacokinetic Analysis
3.4. Pharmacokinetics Outcomes
3.4.1. Intravenous Formulation
3.4.2. Immediate-Release Formulations
3.4.3. Prolonged-Release Formulations
3.4.4. Extended-Release Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Riff, C.; Debord, J.; Monchaud, C.; Marquet, P.; Woillard, J.B. Population pharmacokinetic model and Bayesian estimator for 2 tacrolimus formulations in adult liver transplant patients. Br. J. Clin. Pharmacol. 2019, 85, 1740–1750. [Google Scholar] [CrossRef]
- Undre, N.; Dickinson, J. Relative bioavailability of single doses of prolonged-release tacrolimus administered as a suspension, orally or via a nasogastric tube, compared with intact capsules: A phase 1 study in healthy participants. BMJ Open 2017, 7, e012252. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, R.; Stocker, S.L.; Hennig, S.; Day, R.O.; Carland, J.E. Population Pharmacokinetic Models of Tacrolimus in Adult Transplant Recipients: A Systematic Review. Clin. Pharmacokinet. 2020, 59, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Tett, S.E. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin. Pharmacokinet. 2004, 43, 623–653. [Google Scholar] [CrossRef]
- Wallemacq, P.; Armstrong, V.W.; Brunet, M.; Haufroid, V.; Holt, D.W.; Johnston, A.; Kuypers, D.; Le Meur, Y.; Marquet, P.; Oellerich, M.; et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther. Drug Monit. 2009, 31, 139–152. [Google Scholar] [CrossRef]
- Oteo, I.; Lukas, J.C.; Leal, N.; Suarez, E.; Valdivieso, A.; Gastaca, M.; de Urbina, J.O.; Calvo, R. Tacrolimus pharmacokinetics in the early post-liver transplantation period and clinical applicability via Bayesian prediction. Eur. J. Clin. Pharmacol. 2013, 69, 65–74. [Google Scholar] [CrossRef]
- Yano, I.; Masuda, S.; Egawa, H.; Sugimoto, M.; Fukudo, M.; Yoshida, Y.; Hashi, S.; Yoshizawa, A.; Ogura, Y.; Ogawa, K.; et al. Significance of trough monitoring for tacrolimus blood concentration and calcineurin activity in adult patients undergoing primary living-donor liver transplantation. Eur. J. Clin. Pharmacol. 2012, 68, 259–266. [Google Scholar] [CrossRef][Green Version]
- Undre, N.; Baccarani, U.; Britz, R.; Popescu, I. Pharmacokinetic Profile of Prolonged-Release Tacrolimus When Administered via Nasogastric Tube in De Novo Liver Transplantation: A Sub-Study of the DIAMOND Trial. Ann. Transplant. 2019, 24, 268–272. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Jain, A.B.; Abu-Elmagd, K.; Abdallah, H.; Warty, V.; Fung, J.; Todo, S.; Starzl, T.E.; Venkataramanan, R. Pharmacokinetics of FK506 in liver transplant recipients after continuous intravenous infusion. J. Clin. Pharmacol. 1993, 33, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Cantarovich, M.; Fridell, J.; Barkun, J.; Metrakos, P.; Besner, J.G.; Deschênes, M.; Alpert, E.; Aalamian, Z.; Tchervenkov, J. Optimal time points for the prediction of the area-under-the-curve in liver transplant patients receiving tacrolimus. Transplant. Proc. 1998, 30, 1460–1461. [Google Scholar] [CrossRef]
- Trunečka, P.; Boillot, O.; Seehofer, D.; Pinna, A.D.; Fischer, L.; Ericzon, B.G.; Troisi, R.I.; Baccarani, U.; de Urbina, J.O.; Wall, W.; et al. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am. J. Transplant. 2010, 10, 2313–2323, Erratum in Am. J. Transplant. 2010, 10, 2730. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Miyata, Y.; Kaneko, J.; Tamura, S.; Aoki, T.; Sakamoto, Y.; Hasegawa, K.; Yamashiki, N.; Kokudo, N. Once-daily tacrolimus in living donor liver transplant recipients. Biosci. Trends 2011, 5, 156–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oteo, I.; Lukas, J.C.; Leal, N.; Suarez, E.; Valdivieso, A.; Gastaca, M.; de Urbina, J.O.; Calvo, R. Pathophysiological idiosyncrasies and pharmacokinetic realities may interfere with tacrolimus dose titration in liver transplantation. Eur. J. Clin. Pharmacol. 2011, 67, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Trunečka, P.; Gridelli, B.; Roy, A.; Vitale, A.; Valdivieso, A.; Varo, E.; Seehofer, D.; Lynch, S.; Samuel, D.; et al. Pharmacokinetics for once-daily versus twice-daily tacrolimus formulations in de novo liver transplantation: A randomized, open-label trial. Liver Transpl. 2011, 17, 167–177. [Google Scholar] [CrossRef]
- Mita, A.; Ikegami, T.; Masuda, Y.; Katsuyama, Y.; Ohno, Y.; Urata, K.; Nakazawa, Y.; Kobayashi, A.; Miyagawa, S. Optimal initial dose of orally administered once-daily extended-release tacrolimus following intravenous tacrolimus therapy after liver transplantation. Transplant. Proc. 2014, 46, 794–796. [Google Scholar] [CrossRef]
- Gastaca, M.; Valdivieso, A.; Bustamante, J.; Ruiz, P.; Fernandez, J.R.; Ventoso, A.; Ortiz de Urbina, J. De novo once-daily tacrolimus in liver transplantation: Long-term outcomes of a single center cohort of 150 patients. In Proceedings of the 17th Congress of the European Society for Organ Transplantation, Brussels, Belgium, 29 June–2 July 2015; Volume 28. [Google Scholar] [CrossRef]
- TruneČka, P.; Klempnauer, J.; Bechstein, W.O.; Pirenne, J.; Friman, S.; Zhao, A.; Isoniemi, H.; Rostaing, L.; Settmacher, U.; Mönch, C.; et al. Renal Function in De Novo Liver Transplant Recipients Receiving Different Prolonged-Release Tacrolimus Regimens-The DIAMOND Study. Am. J. Transplant. 2015, 15, 1843–1854. [Google Scholar] [CrossRef]
- Song, G.W.; Lee, S.G.; Hwang, S.; Kim, K.H.; Kim, W.J.; Sin, M.H.; Tak, E.Y. A Pilot Study of the Pharmacokinetics of the Modified-Release Once-Daily Tacrolimus Formulation Administered to Living-Donor Liver Transplant Recipients. Exp. Clin. Transplant. 2016, 14, 412–418. [Google Scholar] [CrossRef][Green Version]
- Mas-Serrano, P.; Ferrer, M.L.B.; Nalda-Molina, R.; Gonzalez, M.D.; Rodriguez-Laiz, G.; Melgar, P.; Soler, M.R.; Carnicer, F.; Lluis, F.; Otaolaurruchi, J.S. Experience of once daily tacrolimus individualised dosing through a bayesian approach in de novo liver transplant recipients. In Proceedings of the 22nd Annual Congress of the European Association of Hospital Pharmacists, EAHP 2017, Cannes, France, 22–24 March 2017; Volume 24. [Google Scholar] [CrossRef]
- Ericzon, B.G.; Varo, E.; Trunečka, P.; Fischer, L.; Colledan, M.; Gridelli, B.; Valdivieso, A.; O’Grady, J.; Dickinson, J.; Undre, N. Pharmacokinetics of prolonged-release tacrolimus versus immediate-release tacrolimus in de novo liver transplantation: A randomized phase III substudy. Clin Transplant. 2017, 31, e12958. [Google Scholar] [CrossRef]
- Shin, M.H.; Song, G.W.; Lee, S.G.; Hwang, S.; Kim, K.H.; Ahn, C.S.; Moon, D.; Ha, T.; Jung, D.; Park, G.; et al. Once-daily, prolonged-release tacrolimus vs twice-daily, immediate-release tacrolimus in de novo living-donor liver transplantation: A Phase 4, randomized, open-label, comparative, single-center study. Clin. Transplant. 2018, 32, e13376. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Yano, I.; Fukatsu, S.; Hashi, S.; Yamamoto, Y.; Sugimoto, M.; Fukudo, M.; Masuda, S.; Nakagawa, S.; Yonezawa, A.; et al. Pharmacokinetics and Pharmacodynamics of Once- Daily Tacrolimus Compared with Twice-Daily Tacrolimus in the Early Stage After Living Donor Liver Transplantation. Ther. Drug Monit. 2018, 40, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, U.; Velkoski, J.; Pravisani, R.; Adani, G.L.; Lorenzin, D.; Cherchi, V.; Falzone, B.; Baraldo, M.; Risaliti, A. MeltDose Technology vs Once-Daily Prolonged Release Tacrolimus in De Novo Liver Transplant Recipients. Transplant. Proc. 2019, 51, 2971–2973. [Google Scholar] [CrossRef]
- Allard, M.; Puszkiel, A.; Conti, F.; Chevillard, L.; Kamar, N.; Noé, G.; White-Koning, M.; Thomas-Schoemann, A.; Simon, T.; Vidal, M.; et al. Pharmacokinetics and Pharmacodynamics of Once-daily Prolonged-release Tacrolimus in Liver Transplant Recipients. Clin. Ther. 2019, 41, 882–896.e3. [Google Scholar] [CrossRef]
- DuBay, D.A.; Teperman, L.; Ueda, K.; Silverman, A.; Chapman, W.; Alsina, A.E.; Tyler, C.; Stevens, D.R. Pharmacokinetics of Once-Daily Extended- Release Tacrolimus Tablets Versus Twice-Daily Capsules in De Novo Liver Transplant. Clin. Pharmacol. Drug Dev. 2019, 8, 995–1008. [Google Scholar] [CrossRef]
- Venkatakrishnan, G.; Abhijith, K.; Kathirvel, M.; Menon, R.; Gopalakrishnan, U.; Balakrishnan, D.; Sudhindran, S. Randomised trial of sustained release tacrolimus Vs twice daily tacrolimus in adult LDLT. In Proceedings of the 2021 Virtual International Congress of ILTS, ELITA and LICAGE, Virtual, 4–8 May 2021; Volume 105. [Google Scholar] [CrossRef]
- Bilbao, I.; Gómez Bravo, M.Á.; Otero, A.; Lladó, L.; Montero, J.L.; González Dieguez, L.; Graus, J.; Miñano, J.A.P. Effectiveness and safety of once-daily tacrolimus formulations in de novo liver transplant recipients: The PRETHI study. Clin. Transplant. 2023, 37, e15105. [Google Scholar] [CrossRef] [PubMed]
- Fukatsu, S.; Yano, I.; Igarashi, T.; Hashida, T.; Takayanagi, K.; Saito, H.; Inui, K. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur. J. Clin. Pharmacol. 2001, 57, 479–484. [Google Scholar] [CrossRef]
- Coilly, A.; Calmus, Y.; Chermak, F.; Dumortier, J.; Duvoux, C.; Guillaud, O.; Houssel-Debry, P.; Neau-Cransac, M.; Stocco, J. Once-daily prolonged release tacrolimus in liver transplantation: Experts’ literature review and recommendations. Liver Transpl. 2015, 21, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Strategy |
|---|---|
| PubMed | ((“liver transplantation*”[MeSH Terms] OR “liver transplantation*”[Title/Abstract] OR “liver transplant*”[Title/Abstract] OR “liver transplant recipient*”[Title/Abstract]) AND (“tacrolimus/pharmacokinetic*”[MeSH Terms] OR “population pharmacokinetic*”[Title/Abstract] OR “Drug Monitoring”[MeSH Terms] OR “Drug Monitoring”[Title/Abstract]) AND (“Tacrolimus”[MeSH Terms] OR “Tacrolimus”[Title/Abstract])) |
| EMBASE | (‘liver transplantation’/exp OR ‘liver transplantation’:ab,ti OR ‘liver graft’/exp OR ‘liver graft’:ti,ab) AND (‘tacrolimus’/exp OR ‘tacrolimus’:ab,ti) AND (‘pharmacokinetics’/exp OR ‘pharmacokinetics’:ab,ti OR ‘population pharmacokinetics’:ab,ti OR ‘drug monitoring’/exp OR ‘drug monitoring’:ab,ti) |
| Author, Year | Study Design | Population (N° Subjects, Sex, Age * and Ethnicity) | Main Objective | Primary Variable | IS Regimen | Follow-Up Period (Post-LT) | Key PK Findings |
|---|---|---|---|---|---|---|---|
| Jain AB., 1993 [11] | Prospective study | N = 9 (8 males) Age: 24–64 | To characterize the iv TAC PK during the immediate PODs and to compare actual and predicted TAC-Css. To develop a popPK model. | Css | TAC + CS | 3 to 6 days, until the last dose of iv TAC | - Two-compartment model without covariables best describes TAC PK profile. - Liver function highly influences TAC-Css. - Time until steady stage using continuous infusions is long (45 h) due to TAC’s extended half-life. |
| Cantaro vich M., 1998 [12] | Prospective study | N = 9 (4 males) Age: 55 ± 9 | To determine a single time point TAC measurement that predicts AUC after LT. | Cmin | TAC + CS + AZA | Until 12 weeks | - Correlation of C2, C3, or C4 and the AUC0–6h suggests that it could be considered for TAC TDM and should be correlated with the AUC0–12h. |
| Truneča P., 2010 [13] | Multicenter, phase III, randomized, double-blind, double-dummy, parallel-group, comparative CT | N = 471 (331 males) IRT: 234, PRT: 237 Age: - IRT: 52.8 ± 9.5 - PRT: 52.7 ± 9.1 Caucasian (458), others (12) | To compare the efficacy and safety of IRT and PRT. | The event rate of local biopsy-proven acute rejection within 24 weeks post-LT | TAC + CS ± MMF (when acute rejection) | 12 months | - Initial dose of PRT should be higher than IRT to achieve similar exposure and to avoid underexposure in early PODs. - TAC levels on day 1 were higher after nasogastric than oral administration. |
| Sugawar a Y., 2011 [14] | Prospective study | N = 12 (5 males) PK profile: 9 subjects Age: 49 (37–61) | To determine the safety and tolerability of OD TAC. | Cmin | TAC + CS | No data | - OD TAC shows high correlation between TAC exposure (AUC0–24 h) and levels (Cmin). - OD regimen improves compliance and maintains immunosuppression. |
| Oteo I., 2011 [15] | Retrospective study | N = 75 | To address the inherent difficulties in empirical TAC TDM by rationally integrating the sources of variability between Cmin and Cmin/dose. | Cmin | TAC + CS ± AZA | Until 15 days | - Bayes prediction based on a population model is the optimum methodology because it does not require reaching SS and provides individual PK parameters with only one value of Cmin after dose. |
| Fischer L., 2011 [16] | Phase II, randomized, open-label, prospective clinical trial | N = 129 (94 males) PRT: 67, IRT: 62 (PK profile: 77) Age: - PRT: 49.4 (24–65) - IRT: 52.4 (27–68) Caucasian (126), Others (3) | To evaluate and compare PK parameters of PRT and IRT following 1st administration and under SS conditions. | AUC0–24h | TAC + CS ± MMF ± AZA | 6 weeks | - AUC0–24h on day 1 for PRT was 50% lower than IRT at equivalent doses. On day 4, AUC0–24h was similar for both formulations (IRT and PRT). - Initial dose of PRT should be higher than IRT to achieve similar exposure. |
| Yano I., 2011 [8] | Prospective study | N = 14 (8 males) Age: 58 ± 6 | To evaluate the relationship between blood TAC-C at each sampling time and drug exposure during the dosing intervals. | Cmin | TAC + CS | 3 weeks | - C2, C4, and C8 TAC levels correlate better with AUC0–12h than trough levels. - Rejection risk in the first 10 days post-LT is linked to average trough TAC levels on PODs 2–4. |
| Oteo I., 2012 [7] | Retrospective and prospective study | N = 75 (development) N = 10 (validation) N = 15 (applicability) | To develop a population PK model for TAC in the first 2 weeks post-LT, and to estimate individual PK to demonstrate its applicability for dose individualization. | Cmin | TAC + CS ± AZA | Until 15 days | - One-compartment model best describes TAC PK profile. - AST, ALB, HCT, and PODs are key predictors of TAC PK variability in early post-LT PK model development. - High variability in TAC t1/2 explains delayed SS in some patients. |
| Mita A., 2014 [17] | Prospective study | N = 10 (4 males) Age: 44.9 ± 16 | To determine the optimal initial dose of orally administered PRT following iv TAC after LT. | AUC0–24h | TAC + CS | No data | - Interpatient variation in absorption may affect PRT more significantly than IRT. - Optimal PRT dose is 8 times the iv TAC dose. |
| Gastaca M., 2015 [18] | Retrospective study | N = 150 Age: 55.5 (20–67) | To describe the long-term efficacy of PRT. | Cmin | TAC ± MMF ± CS ± antibody | 5 years | - Long-term immunosuppression with PRT in LT is effective. |
| Truneča P., 2015 [19] | Multicenter, phase IIIb, randomized, open-label, parallel-group clinical trial (DIAMOND trial) | N = 844 (594 males) Age: 53.7–54.3 ± 10.6–9.1 Black/African (15), Asian (5), other (25) | To determine whether delaying PRT until day 5 or reducing its initial dose improves renal function versus to an immediate post-LT dose. | Renal function estimated by eGFR at week 24 | TAC + MMF ± BAS ± CS | 24 weeks | - Target TAC-C was achieved within 48 h after PRT initiation in all groups. |
| Song GW., 2016 [20] | Phase IV, non-randomized, prospective, open-label pilot study | N = 11 (9 males) (PK profile: 10) Age: 51.6 ± 5.8 Asian | To examine the PK of PRT after the 1st oral dose and under SS conditions (before the 10th oral dose). | AUC0–24h days 6 and 14 | TAC + MMF + CS ± BAS | 12 weeks | - IV TAC in the first days post-LT prevents underexposure due to reduced gastrointestinal function. - AUC0–24h and Cmin correlation was stronger after the 2nd PRT dose than at SS. |
| Más-Serrano P., 2017 [21] | Retrospective study | N = 99 (83 males) Age: 57 | To analyze the efficacy and safety of PRT individualized dosing through a Bayesian approach. | Cmin | TAC + MMF + CS | 4 years | - Target TAC-C was achieved within 48 h after PRT initiation in 75% of patients. |
| Ericzon BG., 2017 [22] | Substudy of a phase III, double-blind, randomized clinical trial | N = 25 (19 males) Age: - PRT: 53.4 - IRT: 55.7 Caucasian | To compare AUC0–24h of TAC between PRT and IRT. | AUC0–24h | TAC | 2 weeks | - AUC0–24h on day 1 for PRT was lower than IRT with the same dose (0.1 mg/kg/day). With a higher dose (0.2 mg/kg/day), PRT exposure was greater than IRT. - Initial dose of PRT should be higher than IRT to achieve similar exposure. - TDM strategies are valid for both formulation (IRT and PRT). |
| Shin MH., 2018 [23] | Phase IV, randomized, open-label, controlled, comparative clinical trial | N = 100 (84 males) (PK profile: 86) Age: - PRT: 53.8 ± 7.6 - IRT: 50.6 ± 7.7 Asian/Oriental | To evaluate the PK of PRT and IRT under early and SS conditions. | AUC0–24h on days 6 and 21 | TAC + CS ± MMF ± BAS | 24 weeks | - PRT and IRT show a similar correlation between AUC0–24h and Cmin. - Initial dose of PRT should be higher than IRT to achieve similar exposure. - IV TAC in the first days post-LT prevents underexposure. |
| Iwasaki M., 2018 [24] | PRT: prospective study; IRT: retrospective study | N = 22 (13 males) PRT: 9, IRT: 13 Age: - PRT: 51 ± 9 - IRT: 58 ± 6 | To investigate the PK of PRT with simultaneous measurements of blood TAC-C in the early stage after LT and compare with previous IRT data. | Cmin | TAC + MMF + CS | 3 weeks | - PRT daily maintenance dose was nearly double that of IRT, though C0 levels were similar, and AUC0–24h for PRT tended to be higher than IRT. - PRT and IRT may require different C0 targets in early post-LT period due to higher systemic exposure with PRT. |
| Baccaran i U., 2019 [25] | Retrospective study | N = 35 (27 males) LCPT: 16, PRT: 19 Age: - LCPT: 59 ± 9 - PRT: 59 ± 8 | To compare LCPT and PRT in terms of therapeutic trough levels and daily dosage after LT. | Cmin | TAC + CS | 30 days | - Target TAC-C was achieved in the 1st month earlier for LCPT than PRT, despite a 25% lower median dose for LCPT. - LCPT dose compared to PRT dose may be reduced to reach target TAC-C. |
| Allard M., 2019 [26] | Multicenter, randomized, prospective clinical trial (CONVERSI ON trial) | N = 90 (PK profile: 24, 20 males) Age: - Group A: 57 (53–60) - Group B: 59 (54–62) | To develop a popPK model for PRT. | Cmin and AUC0–24h | TAC | 180 days | - Two-compartment model best describes TAC PK profile. |
| Undre N., 2019 [9] | Substudy of the DIAMOND trial, phase IIIb, randomized, controlled | N = 11 (PK profile: 10) | To assess the absorption and PK profile of PRT when administered by nasogastric tube immediately post-LT. | Cmin and AUC0–24h on days 1 and 3 | TAC + MMF + CS ± BAS | 3 days | - TAC absorption was not significantly altered when opened PRT capsules were administered via nasogastric tube compared to intact capsules administered orally. |
| DuBay DA., 2019 [27] | Multicenter, phase II, randomized, open-label clinical trial | N = 58 (40 males) (PK profile: 44) Age: 55 (21–72) Hispanic, American, Asian, Black | To analyze the PK of LCPT (AUC0–24h and Cmax) and 24 h trough concentrations (C24) within the first 14 days post-LT, and to compare with IRT, the patients who achieved TAC-C within the first 14 days post-LT. | AUC0–24h, Cmax and C24 | TAC + CS ± MMF | 12 months | - LCPT showed the highest correlation between AUC0–24h and Cmin on day 14 compared to IRT. - LCPT and IRT showed similar efficacy and safety when used de novo in LT. |
| Riff C., 2019 [1] | Multicenter, phase II, randomized, open-label, prospective clinical trial | N = 80 | To develop a pop PK model and BE for PRT and IRT in early and late post-LT periods and to evaluate their performance in predicting TAC AUC and dose requirements. | Cmin, AUC0–12h and AUC0–24h | TAC + CS | 6 weeks ± 7 days | - One-compartment model best describes TAC PK profile. - ‘In stable post-LT, imprecision of TAC AUC and dose estimation was lower than in immediate post-LT for both PRT and IRT. - AUC0–24h and Cmin correlation for IRT was stronger than PRT on day 7, and, on week 6, was stronger for PRT than IRT. |
| Venkata krishnan G., 2021 [28] | Randomized trial | N = 72 | To compare the safety and efficacy of PRT versus IRT. | eGFR at 1, 3, and 6 months following transplant | TAC + BAS + MMF | 6 months | - Cmin of PRT was significantly lower than IRT for an equivalent dose during the first month. |
| Bilbao I., 2023 [29] | Multicenter, prospective, observational study | N = 163 (121 males) LCPT: 87 PRT: 76 Age: 57.3 Caucasian (159) | To compare the effectiveness of LCPT and PRT, | Incidence of treatment failure ** | TAC ± MMF ± CS ± BAS | 48 weeks | - LCPT achieved similar blood TAC-C with a lower total daily dose than PRT. |
| Ref | TAC Regimen and Time to First Dose Post-LT | Time to PK Analysis | PK Target: Concentration (ng/mL) or AUC (ng/mL/h) | TAC Exposure: Concentration (ng/mL) or AUC (ng/mL/h) | PK Parameters | |
|---|---|---|---|---|---|---|
| IV TAC pharmacokinetic studies | ||||||
| [11] | IV TAC 0.15 mg/kg/day 1st dose: 2 to 4 h post-LT | Days 3 to 6 post-LT (Until last dose iv TAC) | No data | Css: 8.15 | No data | Cl: 105.6 ± 105 L/h Vd (Vss): 1252 ± 668 L t1/2: 15.5 ± 11.2 h |
| [14] | IV TAC: 2.5 mg/kg/h 1st dose: Immediately post-LT | Daily, until conversion to PRT | Css: 17–18 | Css: 15.41 ± 0.67 | AUC0–24h: 369.94 ± 16.17 | Cl: 29.7 mL/h/kg |
| [17] | IV TAC: 1.1 ± 0.6 mg/day | Before conversion to PRT | Cmin: 15–20 (1st two weeks post-LT) | Css: 16.6 ± 2.6 | AUC0–24h: 399 ± 53.5 | No data |
| [20] | Day 0 to 4 iv TAC: 0.025 to 0.05 mg/kg/day. 1st dose: Day of transplant | Day 4 | Css: 10–20 | Css: 12.3 ± 3.7 | No data | No data |
| [23] | Day 0 to 4 iv TAC: 0.025–0.05 mg/kg/day 1st dose: after LT | Day 5 (prior to switch) | Css: 10–15 | Css: 11.3 ± 4 | No data | No data |
| IRT pharmacokinetic studies | ||||||
| [12] | IRT: 0.15 mg/kg/day TD 1st dose: Immediately post-LT | Cmin: Daily AUC: week 1 and 4 | Cmin: 10–20 | Cmin: 13 ± 4.7 | AUC0–6h:118.4 ± 37 AUC0–12h:201.9 ± 55.2 | CL: 32.7 ± 16 L/h |
| [13] | IRT: 0.1 mg/kg/day TD 1st dose: within 24 h post-LT | Days 1 and 7 | Cmin: 10–20 | Cmin: Day 1: 11.6 ± 8.5 (via nasogastric) or 9.6 ± 8.8 (oral) Day 7: 9.5 ± 4.5 | No data | No data |
| [14] | IRT: 0.1 mg/kg/day TD 1st dose: Immediately post-LT | Daily, from day 0 until day 15 post-LT or day of release from hospital | Cmin: 10–20 | Cmin: 13.61 (1.5–30) 14.28 (normal AST) 12.45 (elevated AST) * 1. Cmin divided for PODs, AST, ALB and HCT | No data | No data |
| [16] | IRT: 0.10 to 0.15 mg/kg/day TD 1st dose: Within 6–12 h post-LT | Days 1 and 14 | Cmin: 10–20 | Day 1: Cmin: 8.98 ± 5.9 Cmax: 19.75 ± 8.48 Day 14: Cmin: 8.53 ± 2.85 Cmax: 25.07 ± 12.13 | AUC0–24h: Day 1: 263.82 ± 153.36 Day 14: 286.99 ± 88.03 | Tmax (h): Day 1: 2.9 ± 1.8 Day 14: 1.9 ± 1.5 |
| [8] | IRT: 0.05 mg/kg/day TD 1st dose: Within 12 h post-LT | Weeks 1 and 3 | Cmin: 10–15 (PODs 1–7), 8–12 (PODs 8–14), 6–10 (after POD 15) | Week 1: Cmin: 9.8 ± 2.6 Cmax: 16 ± 5.4 Week 3: Cmin: 7.7 ± 2.7 Cmax: 12.5 ± 4.9 | AUC0–12h: Week 1: 140 ± 38 Week 3: 110 ± 40 | Tmax (h): Week 1: 2 (1–8) Week 3: 2 (0–4) Cl/F (L/h/kg): Week 1: 0.15 ± 0.11 Week 3: 0.27 ± 0.22 |
| [7] | IRT: 0.1 mg/kg/day TD 1st dose: In the first hours post-LT | Days 0 to 15 post-LT | Cmin: 8–12 | Cmin (range): 1.5–30 Cmin/Dose: 0.1–6.1 (0–3 PODs) Cmin/Dose: 0.4–21 (4–15 PODs) | No data | Ka: 4.48 h-1 Cl/F: 11.9 ± 12.77 L/h V/F: 153 ± 29.74 L * 2 Final model parameter estimates for two periods t1/2: 7.68–90.74 h |
| [22] | IRT: 0.1 mg/kg/day TD 1st dose: Within 12 h post-LT | Days 1, 3, 7, and 14 (SS conditions) | Cmin: 10–20 | Day 1: Cmin: 9.18 ± 7.26 Cmax: 12.21 ± 8.7 Day 3: Cmin: 10.41 ± 7.61 Cmax: 19.47 ± 11.69 Day 7: Cmin: 7.43 ± 2.63 Cmax: 19.94 ± 12.18 Day 14: Cmin: 8.89 ± 2.24 Cmax: 29.34 ± 22.36 | AUC0–12h: Day 1: 82.87 ± 62.63 Day 3: 161.14 ± 109.77 Day 7: 134.95 ± 60.28 Day 14: 155.54 ± 60.2 AUC0–24h: Day 1: 216.63 ± 132.51 Day 3: 317.9 ± 218.63 Day 7: 249.12 ± 96.4 Day 14: 283.19± 92.21 | Tmax: 1–2 h Day 1: 2 h (1–12) Day 3: 1.5 h (0.5–8) Day 7: 1.5 h (0.5–6) Day 14: 1 h (1–4) |
| [23] | Initial iv TAC and switch to IRT (Day 5) (iv:IRT 1:4) | Days 6 and 21 (SS conditions) | Cmin: 10–15 | Day 6: Cmin: 9.73 ± 2.54 Cmax: 17.4 Day 21: Cmin: 9.08 ± 3.21 Cmax: 18.8 | AUC0–24h: Day 6: 265.8 Day 21: 239.3 | Tmax (h): Day 6: 4.5 Day 21: 2.8 |
| [24] | IRT: 0.03 ± 0.01 mg/kg/day TD 1st dose: In the morning on the day post-LT | Daily until 3 weeks after LT | Cmin: 10–15 (PODs 1–7), 8–12 (PODs 8–14), 6–10 (after POD 15) | Week 3: Cmin: 7.7 ± 2.6 Cmax: 12.5 ± 4.7 | AUC0–24h: Week 3: 220 ± 76 | Week 3: Tmax: 2 h (0–4) Cl/F: 0.267 ± 0.213 L/h/kg |
| [27] | IRT: 0.10–0.15 mg/kg/day TD. 1st dose: Between 1 and 3 days post-LT | Days 1, 7, and 14 | Cmin: 5–20 | Day 1: Cmin: 4.42 ± 2.83 Cmax: 12.7 ± 6.29 Day 7: Cmin: 7.61 ± 4.06 Cmax: 21.1 ± 8.97 Day 14: Cmin: 7.56 ± 2.64 Cmax: 22.95 ± 14.57 | AUC0–24h: Day 1: 135.62 ± 73.92 Day 7: 245.47 ± 102.03 Day 14: 241.22 ± 79.9 | Tmax (h): Day 1: 2.67 h (1–20) Day 7: 1.51 h (0.67–16.5) Day 14: 2 h (1–14) |
| [2] | IRT: 0.05 mg/kg/12 h 1st dose: within 6–12 h post-LT | Day 7 | AUC0–12h: 120–150 | 8.2 (3.8–16.1) | AUC0–12h: 152.8 (60.6–260.2) | Cl/F:39.3 (20.1–75.6) L/h Vd/F: 278 (101–444) L |
| [28] | IRT: 1 mg | Weeks 1, 2, and 3 and month 1 | Cmin: 5 | Cmin: Week 1: 3.8 Week 2: 3.76 Week 3: 4.08 Month 1: 3.72 | No data | No data |
| PRT pharmacokinetic studies | ||||||
| [13] | PRT: 0.2 mg/kg/day 1st dose: within 24 h post-LT | Days 1 and 7 | Cmin: 10–20 | Cmin: Day 1: 9.4 ± 6.8 (via nasogastric) or 6 ± 5.8 (oral) Day 7: 12 ± 5.9 | No data | No data |
| [14] | Initial iv TAC (7–20 days) and switch to PRT | PK profile 7 days after conversion to PRT | No data | Cmin: 14.63 ± 2.68 Cmax: 27.65 ± 3.76 | AUC0–24h: 459.54 ± 76.85 | F: 10.6 ± 4% Cl: 316 mL/h/kg |
| [16] | PRT: 0.10 to 0.15 mg/kg/day 1st dose: Within 6–12 h post-LT | Days 1 and 14 | Cmin: 10–20 | Day 1: Cmin: 4.21 ± 3.31 Cmax: 10.59 ± 6.26 Day 14: Cmin: 8.82 ± 3.18 Cmax: 25.65 ± 11.61 | AUC0–24h: Day 1: 145.97 ± 103.03 Day 14: 324.19 ± 119.07 | Tmax: Day 1: 5 ± 4 h Day 14: 2.8 ± 2.5 h |
| [17] | Initial iv TAC and switch to PRT: 8.3 ± 6.7 mg/day (iv:PRT 1:8) | Days 1, 3, and 5 during conversion TAC iv to PRT | Cmin: 15 | No data | AUC0–24h: Day 1: 374.8 ± 65 Day 3: 369 ± 47.4 Day 5: 412.4 ± 132.1 | F: 13 ± 9% |
| [18] | PRT: 0.15 mg/kg/day (only TAC) or 0.1 mg/kg/day (if TAC + MMF) | 1st week | No data | Cmin: 7.85 | No data | No data |
| [19] | PRT: 0.2 mg/kg/day (Arm1) 1st dose: in the morning or within 18 h post-LT | Daily for 14 days and then on days 21 and 28 | Cmin: 5–15 (until day 42) | Day 1: 3.92 ± 7.42; Days 2–14: 8.48 ± 4.58 to 12.48 ± 9.43 Day 21: 8.43 ± 3.85; Day 28: 8.74 ± 3.6 | No data | No data |
| PRT: 0.15–0.175 mg/kg/day (Arm2) 1st dose: in the morning or within 18 h post-LT | Day 1: 2.38 ± 5.83; Days 2–14: 7.16 ± 3.96 to 10.48 ± 8.1 Day 21: 7.82 ± 3.1; Day 28: 8.43 ± 3.78 | |||||
| PRT: 0.2 mg/kg/day (Arm3) 1st dose: Day 5 post-LT | Day 6: 6.23 ± 6.08; Day 7–14: 9.33 ± 5.77 to 10.54 ± 6.7; Day 21: 9.12 ± 4.18; Day 28: 9.38 ± 4.43 | |||||
| [20] | Initial IV-TAC and switch to PRT (day 5): 0.15–0.3 mg/kg/day | Cmin: week 4; PK profile: days 6 and 14 | Cmin: 10–20 | Day 6: Cmin: 8.7 ± 1.6 Cmax: 13.12 ± 3.1 Day 14: Cmin: 15 ± 4.4 Cmax: 26.25 ± 8.5 Week 4: Cmin: 13.5 ± 5 | AUC0–24h: Day 6: 235.67 ± 47.9 Day 14: 423.86 ± 86 | Tmax: Day 6: 2 h (1–4.1) Day 14: 3.5 h (0–16.1) |
| [21] | PRT: 0.15 mg/kg/day 1st dose: Within the 1st 24 h post-LT | Days 2, 7, 15, and 30 | Cmin: 8–10 | Cmin: Day 2: 8.9 (5.3–11.6) Day 7: 7.5 (5.4–9.7) Day 15: 8.78 (6.9–10.65) Day 30: 9.7 (8.17–11.9) | No data | No data |
| [22] | PRT: 0.2 mg/kg/day 1st dose: Within 12 h post-LT | Days 1, 3, 7, and 14 (SS conditions) | Cmin: 10–20 | Day 1: Cmin: 9.97 ± 6.7 Cmax: 21.29 ± 10.53 Day 3: Cmin: 14.06 ± 7.11 Cmax: 27.82 ± 11.72 Day 7: Cmin: 11.06 ± 5.63 Cmax: 23.2 ± 9.83 Day 14: Cmin: 10.47 ± 4.14 Cmax: 24.85 ± 7.24 | AUC0–24h: Day 1: 320.44 ± 186.93; Day 3: 452.06 ± 213.19; Day 7: 358.6 ± 146.62; Day 14: 353.42 ± 109.42 | Tmax: 3–4 h Day 1: 3 h (1–14) Day 3: 4 h (1–13) Day 7: 3 h (1–8) Day 14: 3 h (1–6) |
| [23] | Initial iv TAC and switch to PRT (Day 5) (iv:PRT 1:6) | Days 6 and 21 (SS conditions) | Cmin: 10–15 | Day 6: Cmin: 8.68 ± 3.35 Cmax: 16.3 Day 21: Cmin: 8.83 ± 3.61 Cmax: 25.1 | AUC0–24h: Day 6: 257.3 Day 21: 308.2 | Tmax (h): Day 6: 3.5 Day 21: 2.8 |
| [24] | PRT: 0.15 ± 0.05 mg/kg/day 1st dose: In the morning on the day post-LT | Daily until 3 weeks after LT | Cmin: 10–15 (PODs 1–7), 8–12 (PODs 8–14), 6–10 (after POD 15) | Week 3: Cmin: 9 ± 4.5 Cmax: 22.3 ± 8.6 | AUC0–24h: Week 3: 330 ± 103 | Week 3: Tmax: 4 h (0–12) Cl/F: 0.319 ± 0.205 L/h/kg |
| [25] | PRT: 5.26 ± 1.91 mg/day | Days 3, 5, 7, 15, and 30 | No data | Cmin: Day 3: 2.42 ± 2.75 Day 5: 4.17 ± 2.05 Day 7: 6.06 ± 3.03 Day 15: 6.69 ± 2.71 Day 30: 7.96 ± 4.16 | No data | No data |
| [26] | Initial IRT and switch to PRT (day 7) (IRT:PRT 1:1) | Cmin: days 5, 7, and 30; PK profile: day 14 | Cmin: 6–10 | Cmin: 8.5 (5.4–10.2) | AUC0–24h: Non compartmental 251.3 (95%, CI 108.5–460.7) Model-predicted: 235.6 (95% CI, 139.6–598.7) | Cl: 5.11 (95% CI, 4.36–5.93) L/h Vc: 86.9 (95% CI, 41.1–126) L Vp: 142 (95% CI, 88.4–196) L |
| [9] | PRT via nasogastric 0.2 mg/kg/day or 0.15– 0.175 mg/kg/day (if TAC + BAS) 1st dose: Immediately post-LT | Days 1 and 3 | Cmin: 5–15 | Day 1: Cmin: 5.27 Cmax: 15.1 Day 3 Cmin: 8.77 Cmax:19.1 | AUC0–24h: Day 1: 193 Day 3: 301 | Tmax (h): Day 1: 2 (2–24) Day 3: 4.5 (0.5– 24) |
| [2] | PRT: 0.1 mg/kg/day 1st dose: within 6–12 h post-LT | Day 7 | AUC0–24h: 240–300 | 8.6 (0.8–55) | AUC0–24h: 316.5 (34.5–775.2) | Cl/F: 48.7 (18.9–91.3) L/h Vd/F: 589 (335–2857) L |
| [28] | PRT: 1 mg | Weeks 1, 2, and 3 and month 1 | Cmin: 5 | Cmin: Week 1: 2.36 Week 2: 2.9 Week 3: 2.6 Month 1: 2.91 | No data | No data |
| [29] | Initial IRT and switch to PRT (day 3 to 5) (IRT:PRT 1:1) | Weeks 1, 2, and 4 | Cmin: 6–12 | Cmin: Week 1: 5.8 ± 2.4 Week 2: 8.2 ± 3 Week 4: 7.8 ± 3.3 | No data | No data |
| LCPT pharmacokinetic studies | ||||||
| [25] | LCPT: 5.19 ± 1.72 mg/day | Days 3, 5, 7, 15, and 30 | No data | Cmin: Day 3: 5.05 ± 3.58 Day 5: 7.35 ± 5.12 Day 7: 8.03 ± 5.44 Day 15: 8.62 ± 7.86 Day 30: 9.1 ± 5.78 | No data | No data |
| [27] | LCPT: 0.07 to 0.11 mg/kg/day except if black: 0.09–0.13 mg/kg/day 1st dose: Between 1 and 3 days post-LT | Days 1, 7, and 14 | Cmin: 5–20 | Day 1: Cmin: 3.22 ± 2.39 Cmax: 5.95 ± 3.46 Day 7: Cmin: 7.33 ± 3.54 Cmax: 17.15 ± 7.9 Day 14: Cmin: 7.41 ± 4.17 Cmax: 21.3 ± 9.93 | AUC0–24h: Day 1: 68.18 ± 37.4 Day 7: 251.29 ± 102.6 Day 14: 279.59 ± 139.86 | Tmax (h): Day 1: 12 (1.48–24.2) Day 7: 4 (0–12) Day 14: 4 (1–16) |
| [29] | Initial IRT and switch to LCPT (day 3 to 5) (IRT:PRT 1:1) | Weeks 1, 2, and 4 | Cmin: 6–12 | Cmin: Week 1: 6.6 ± 3.4 Week 2: 8 ± 3.9 Week 4: 7.7 ± 4.4 | No data | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barriga-Rodríguez, P.; Falcón-Cubillo, M.; Mejías-Trueba, M.; Ciudad-Gutiérrez, P.; Guisado-Gil, A.B.; Gómez-Bravo, M.Á.; Porras-López, M.; Gil-Navarro, M.V.; Herrera-Hidalgo, L. Pharmacokinetics of Different Tacrolimus Formulations in the Early Post-Liver Transplant Period: A Scoping Review. Pharmaceutics 2025, 17, 619. https://doi.org/10.3390/pharmaceutics17050619
Barriga-Rodríguez P, Falcón-Cubillo M, Mejías-Trueba M, Ciudad-Gutiérrez P, Guisado-Gil AB, Gómez-Bravo MÁ, Porras-López M, Gil-Navarro MV, Herrera-Hidalgo L. Pharmacokinetics of Different Tacrolimus Formulations in the Early Post-Liver Transplant Period: A Scoping Review. Pharmaceutics. 2025; 17(5):619. https://doi.org/10.3390/pharmaceutics17050619
Chicago/Turabian StyleBarriga-Rodríguez, Paloma, Marta Falcón-Cubillo, Marta Mejías-Trueba, Pablo Ciudad-Gutiérrez, Ana Belén Guisado-Gil, Miguel Ángel Gómez-Bravo, Manuel Porras-López, María Victoria Gil-Navarro, and Laura Herrera-Hidalgo. 2025. "Pharmacokinetics of Different Tacrolimus Formulations in the Early Post-Liver Transplant Period: A Scoping Review" Pharmaceutics 17, no. 5: 619. https://doi.org/10.3390/pharmaceutics17050619
APA StyleBarriga-Rodríguez, P., Falcón-Cubillo, M., Mejías-Trueba, M., Ciudad-Gutiérrez, P., Guisado-Gil, A. B., Gómez-Bravo, M. Á., Porras-López, M., Gil-Navarro, M. V., & Herrera-Hidalgo, L. (2025). Pharmacokinetics of Different Tacrolimus Formulations in the Early Post-Liver Transplant Period: A Scoping Review. Pharmaceutics, 17(5), 619. https://doi.org/10.3390/pharmaceutics17050619






