Hydrogel–Nanolipid Formulations for the Complex Anti-Inflammatory and Antimicrobial Therapy of Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preliminary Study
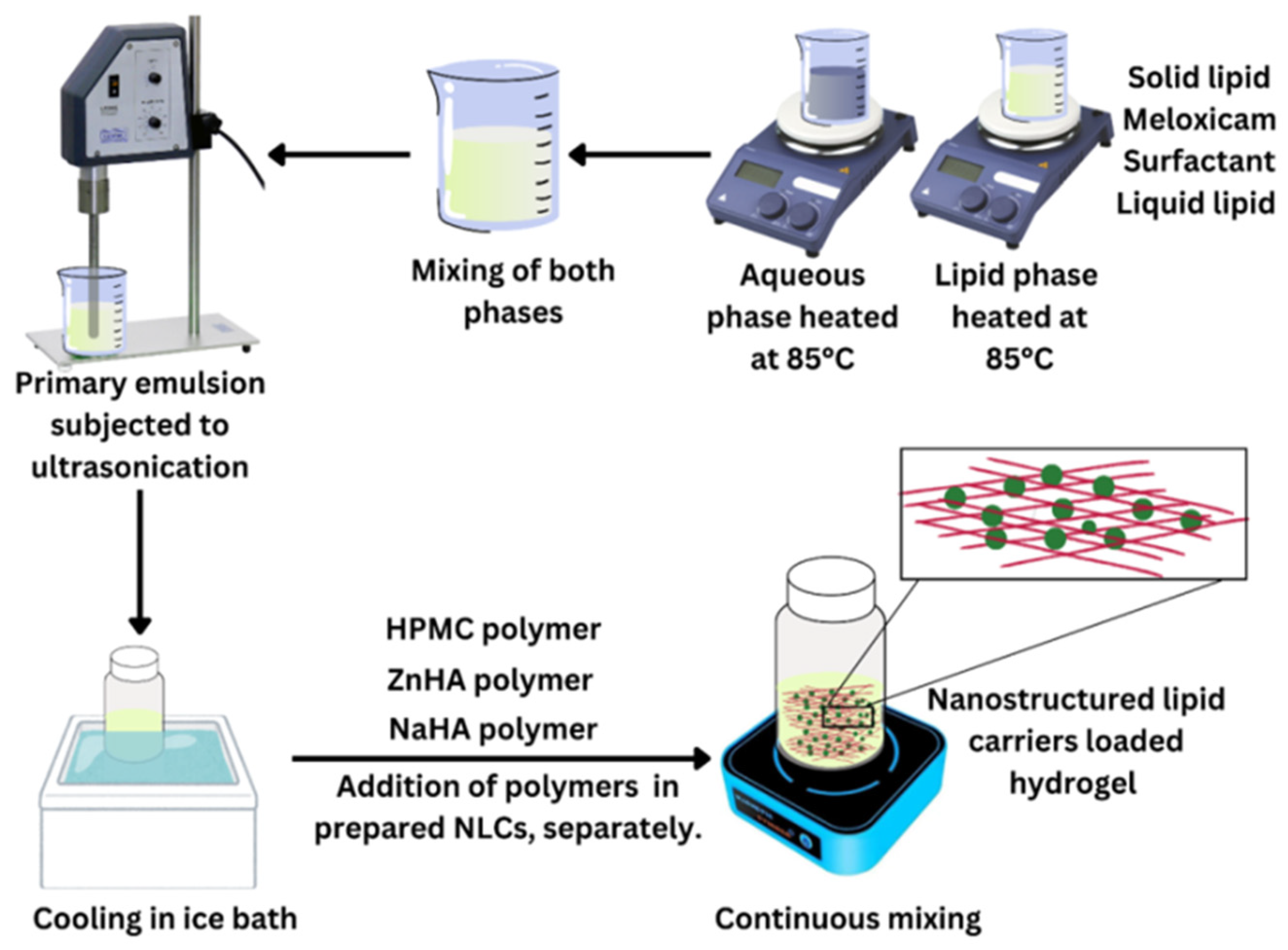
2.2.2. NLC Preparation
2.2.3. Dynamic Light Scattering Measurements of NLC–Melox
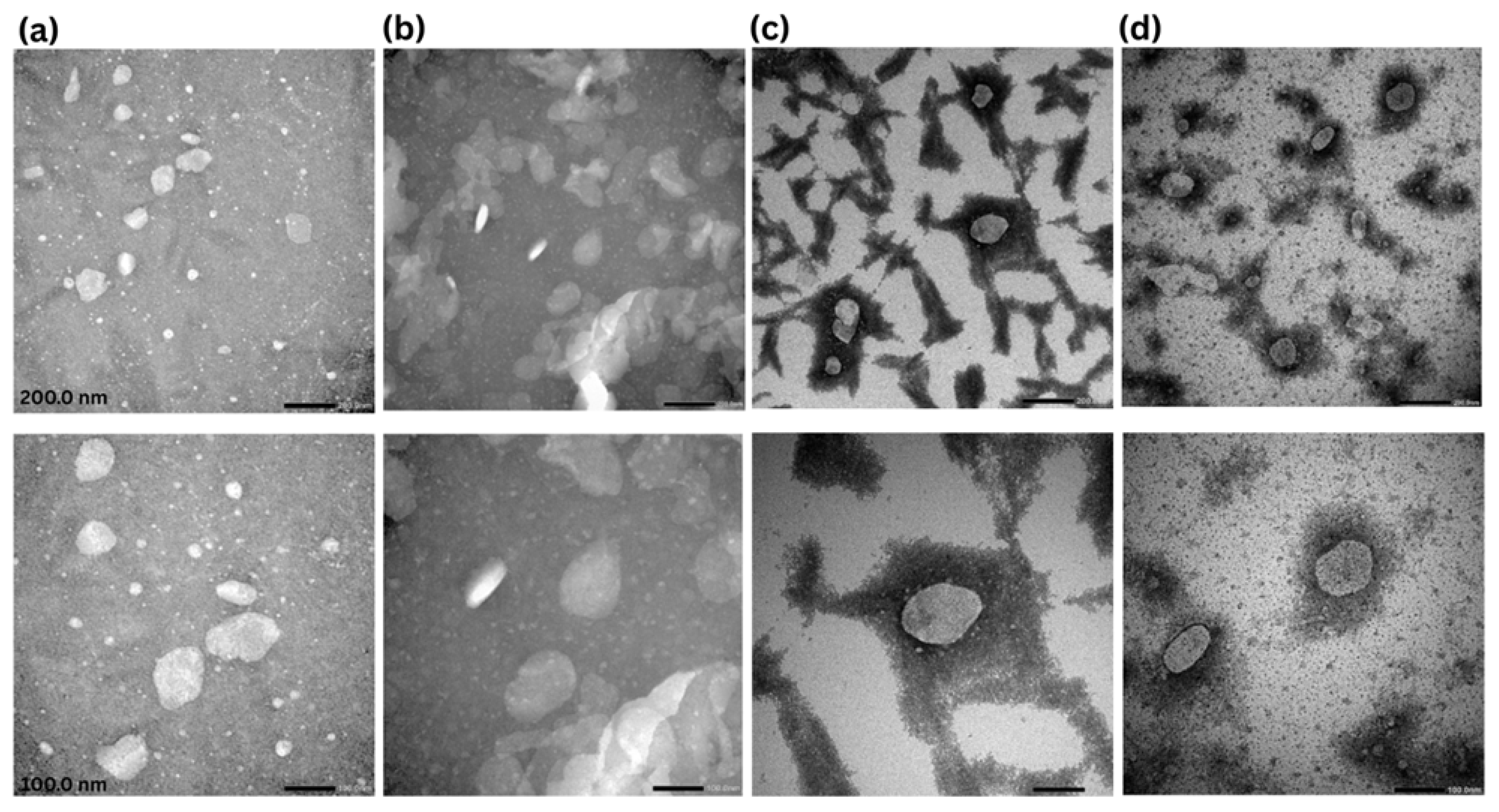
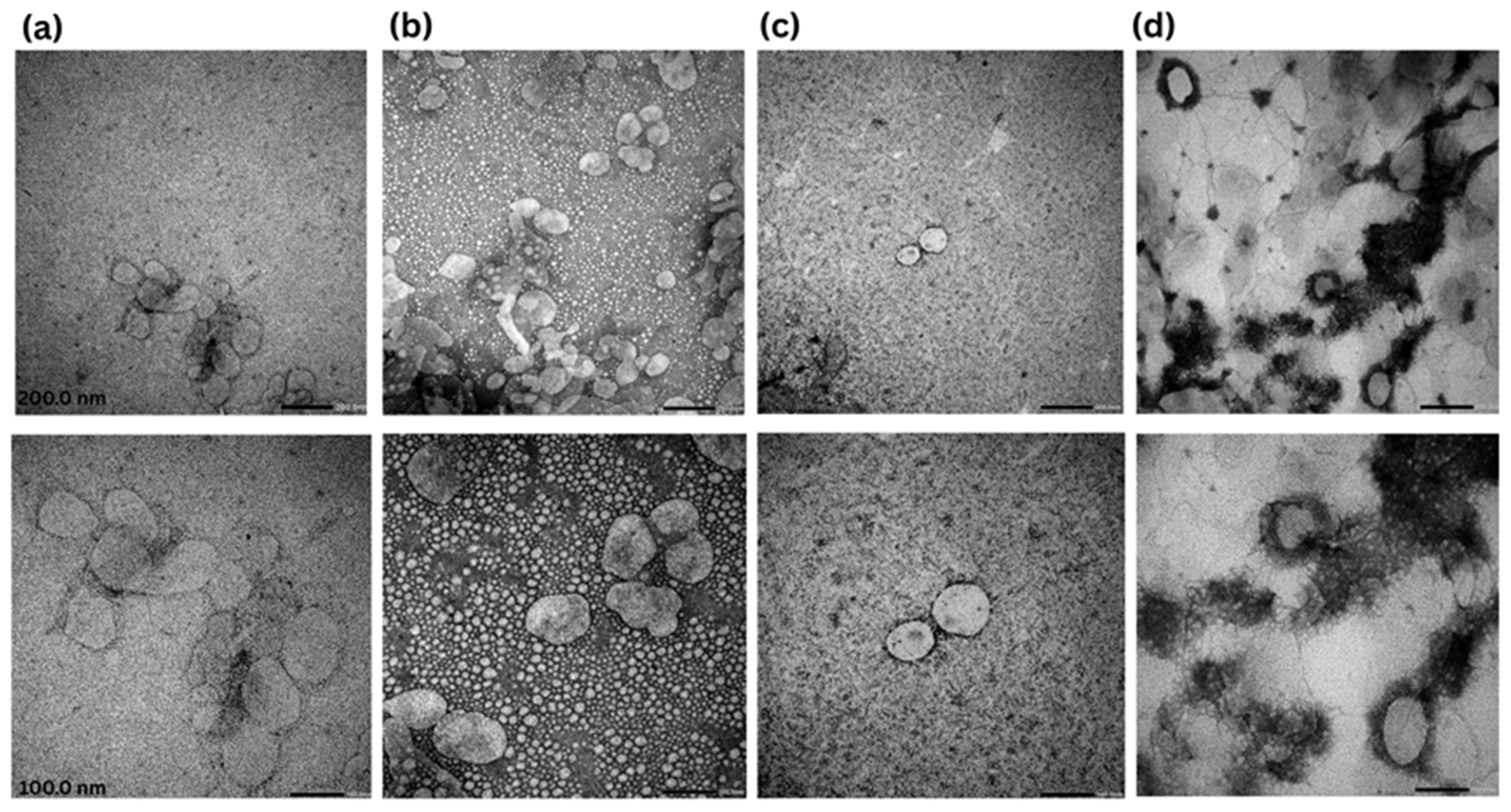
2.2.4. Morphological Analysis (Transmission Electron Microscopy (TEM))
2.2.5. Entrapment Efficiency (EE%) and Drug Loading (DL%) of Melox
2.2.6. Thermal Analysis (Differential Scanning Calorimetry)
2.2.7. Crystallographic Analysis (X-Ray Diffraction)
2.2.8. Raman Spectroscopy
2.2.9. Mucoadhesive Study
2.2.10. Rheological Analysis
2.2.11. In Vitro Release Study
2.2.12. Assessment of In Vitro Anti-Inflammatory Activity
2.2.13. In Vitro Antibacterial Study (Bacterial Cell Culture)
Agar Well Diffusion Method
Minimum Inhibitory Concentration (MIC)
Minimum Bactericidal Concentration (MBC)
2.2.14. Stability Study
2.2.15. Statistical Analysis
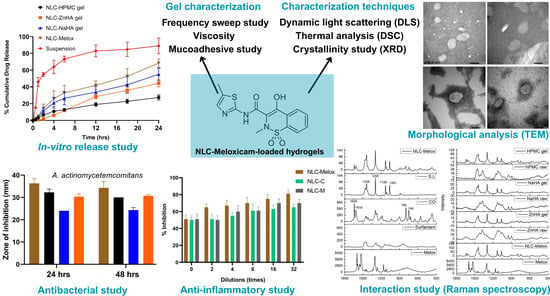
3. Results and Discussion
3.1. Dynamic Light Scattering
3.2. Transmission Electron Microscopy
3.3. Drug Loading and Entrapment Efficiency
3.4. Differential Scanning Calorimetry
3.5. X-Ray Diffraction
3.6. Raman Spectroscopy
3.7. Mucoadhesive Study
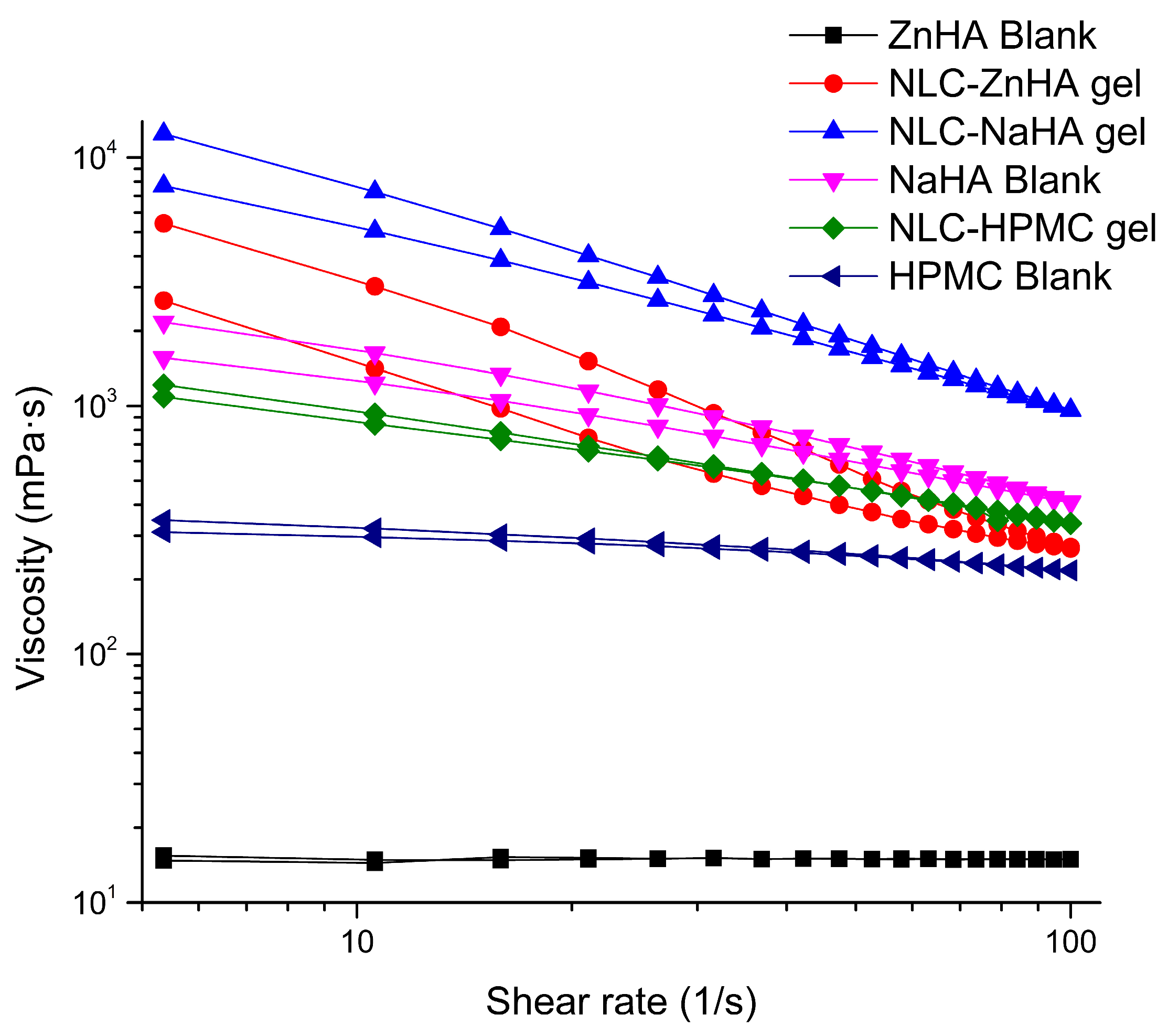
3.8. Rheological Analysis
3.9. In Vitro Release Study
3.10. In Vitro Anti-Inflammatory Study
3.11. Antibacterial Study
3.12. Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashfaq, R.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Smart biomaterial gels for periodontal therapy: A novel approach. Biomed. Pharmacother. 2025, 183, 117836. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Sisa, B.; Kovács, A.; Berkó, S.; Szécsényi, M.; Burián, K.; Vályi, P.; Budai-Szűcs, M. Factorial design of in situ gelling two-compartment systems containing chlorhexidine for the treatment of periodontitis. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 191, 106607. [Google Scholar] [CrossRef] [PubMed]
- Suriyaamporn, P.; Sahatsapan, N.; Patrojanasophon, P.; Opanasopit, P.; Kumpugdee-Vollrath, M.; Ngawhirunpat, T. Optimization of In Situ Gel-Forming Chlorhexidine-Encapsulated Polymeric Nanoparticles Using Design of Experiment for Periodontitis. AAPS PharmSciTech 2023, 24, 161. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lv, X.; Zhang, H.; Zhuang, S.; Zheng, K.; Zhou, T.; Cen, L. An injectable gel based on photo-cross-linkable hyaluronic acid and mesoporous bioactive glass nanoparticles for periodontitis treatment. Int. J. Biol. Macromol. 2024, 257, 128596. [Google Scholar] [CrossRef]
- Neduri, K.; Ailuno, G.; Zuccari, G.; Bassi, A.M.; Vernazza, S.; Schito, A.M.; Caviglioli, G.; Baldassari, S. Development of a Multilayer Film Including the Soluble Eggshell Membrane Fraction for the Treatment of Oral Mucosa Lesions. Pharmaceutics 2024, 16, 1342. [Google Scholar] [CrossRef]
- Gopalakrishna, P.K.; Jayaramu, R.A.; Boregowda, S.S.; Eshwar, S.; Suresh, N.V.; Abu Lila, A.S.; Moin, A.; Alotaibi, H.F.; Obaidullah, A.J.; Khafagy, E.-S. Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis. Gels 2023, 9, 577. [Google Scholar] [CrossRef]
- Toker, H.; Marakoglu, I.; Poyraz, O. Effect of meloxicam on gingival crevicular fluid IL-1beta and IL1 receptor antagonist levels in subjects with chronic periodontitis, and its effects on clinical parameters. Clin. Oral Investig. 2006, 10, 305–310. [Google Scholar] [CrossRef]
- Cyclooxygenase inhibitors: Any reservations? Intern. Med. J. 2001, 31, 37–41. Available online: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1445-5994.2001.00002.x (accessed on 14 February 2025). [CrossRef]
- Taha, N.F.; Mahmoud, K.M.; Soliman, A.A.F.; Emara, L.H. Anti-inflammatory and cytoprotective potentials of Meloxicam solid dispersions prepared by different techniques on lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Drug Deliv. Sci. Technol. 2021, 63, 102507. [Google Scholar] [CrossRef]
- Banerjee, K.; Madhyastha, H.; Sandur, V.R.; Manikandanath, N.T.; Thiagarajan, N.; Thiagarajan, P. Anti-inflammatory and wound healing potential of a clove oil emulsion. Colloids Surf. B Biointerfaces 2020, 193, 111102. [Google Scholar] [CrossRef]
- Mahadlek, J.; Charoenteeraboon, J.; Phaechamud, T. Zinc Oxide Gels for Periodontitis Treatment. Available online: https://www.researchgate.net/publication/266892554_Zinc_Oxide_Gels_for_Periodontitis_Treatment (accessed on 14 February 2025).
- Asghar, S.; Khan, I.U.; Salman, S.; Khalid, S.H.; Ashfaq, R.; Vandamme, T.F. Plant-derived nanotherapeutic systems to counter the overgrowing threat of resistant microbes and biofilms. Adv. Drug Deliv. Rev. 2021, 179, 114019. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Bedi, P.M.S.; Jain, N.K. Development of nanostructured lipid carriers (NLC) for controlled delivery of meloxicam. Int. J. Biomed. Nanosci. Nanotechnol. 2010, 1, 247. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, N.K.; Bedi, P.M.S. Nanostructured lipid carriers based nanogel for meloxicam delivery: Mechanistic, in-vivo and stability evaluation. Drug Dev. Ind. Pharm. 2015, 41, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Satrialdi; Husna, N.N.; Rihad, R.Q.; Utami, R.A. The Incorporation of Clove Essential Oil into Nanostructured Lipid Carrier for Improvement of the Delivery and Antioxidant Effects on the Fibroblast Cells. Pharm. Nanotechnol. 2025, 13, 372–382. [Google Scholar] [CrossRef]
- de Meneses, A.C.; Marques, E.B.P.; Leimann, F.V.; Gonçalves, O.H.; Ineu, R.P.; de Araújo, P.H.H.; de Oliveira, D.; Sayer, C. Encapsulation of clove oil in nanostructured lipid carriers from natural waxes: Preparation, characterization and in vitro evaluation of the cholinesterase enzymes. Colloids Surf. Physicochem. Eng. Asp. 2019, 583, 123879. [Google Scholar] [CrossRef]
- Pagano, C.; Giovagnoli, S.; Perioli, L.; Tiralti, M.C.; Ricci, M. Development and characterization of mucoadhesive-thermoresponsive gels for the treatment of oral mucosa diseases. Eur. J. Pharm. Sci. 2020, 142, 105125. [Google Scholar] [CrossRef]
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, J.; Pan, R.; Xu, Z.; Li, M.; Zang, R. Structures, Properties, and Bioengineering Applications of Alginates and Hyaluronic Acid. Polymers 2023, 15, 2149. [Google Scholar] [CrossRef]
- Ganguly, K.; Chaturvedi, K.; More, U.A.; Nadagouda, M.N.; Aminabhavi, T.M. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J. Control. Release Off. J. Control. Release Soc. 2014, 193, 162–173. [Google Scholar] [CrossRef]
- Ponedel’kina, I.Y.; Khaybrakhmanova, E.A.; Rakhimova, L.Y. In vitro evaluation of the mucoadhesive properties of zinc hyaluronate. Nat. Prod. Res. 2023, 0, 1–5. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Eriani, J.; Serroni, I.; Liani, G.; Borelli, V.; Castronovo, G.; Di Lenarda, R. Effectiveness of adjunctive subgingival administration of amino acids and sodium hyaluronate gel on clinical and immunological parameters in the treatment of chronic periodontitis. Ann. Stomatol. 2012, 3, 75–81. [Google Scholar]
- Favia, G.; Mariggio, M.A.; Maiorano, F.; Cassano, A.; Capodiferro, S.; Ribatti, D. Accelerated wound healing of oral soft tissues and angiogenic effect induced by a pool of aminoacids combined to sodium hyaluronate (AMINOGAM). J. Biol. Regul. Homeost. Agents 2008, 22, 109–116. [Google Scholar] [PubMed]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A Boon in Periodontal Therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Griauzdyte, V.; Jagelaviciene, E. Antimicrobial Activity of Zinc against Periodontal Pathogens: A Systematic Review of In Vitro Studies. Medicina 2023, 59, 2088. [Google Scholar] [CrossRef]
- Correia, A.C.; Costa, I.; Silva, R.; Sampaio, P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Design of experiment (DoE) of mucoadhesive valproic acid-loaded nanostructured lipid carriers (NLC) for potential nose-to-brain application. Int. J. Pharm. 2024, 664, 124631. [Google Scholar] [CrossRef]
- Noolkar, S.B.; Jadhav, N.R.; Bhende, S.A.; Killedar, S.G. Solid-State Characterization and Dissolution Properties of Meloxicam–Moringa Coagulant–PVP Ternary Solid Dispersions. AAPS PharmSciTech 2013, 14, 569–577. [Google Scholar] [CrossRef]
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by Design Based Formulation Study of Meloxicam-Loaded Polymeric Micelles for Intranasal Administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef]
- Divakar, A.; Varghese, R.M.; Kumar, S.A.; Shanmugam, R. Comparative Anti-inflammatory Activity of a Nanocomposite-Based Herbal Oral Rinse and a Commercial Oral Rinse. Cureus 2024, 16, e61548. [Google Scholar] [CrossRef]
- Varghese, R.M.; Kumar, S.A.; Shanmugam, R. Comparative Anti-inflammatory Activity of Silver and Zinc Oxide Nanoparticles Synthesized Using Ocimum tenuiflorum and Ocimum gratissimum Herbal Formulations. Cureus 2024, 16, e52995. [Google Scholar] [CrossRef]
- Eucast: MIC Determination. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 20 September 2024).
- Akkaoui, S.; Johansson, A.; Yagoubi, M.; Haubek, D.; El hamidi, A.; Rida, S.; Claesson, R.; Ennibi, O. Chemical Composition, Antimicrobial activity, In Vitro Cytotoxicity and Leukotoxin Neutralization of Essential Oil from Origanum vulgare against Aggregatibacter actinomycetemcomitans. Pathogens 2020, 9, 192. [Google Scholar] [CrossRef]
- Danaei, M.; Kalantari, M.; Raji, M.; Samareh Fekri, H.; Saber, R.; Asnani, G.P.; Mortazavi, S.M.; Mozafari, M.R.; Rasti, B.; Taheriazam, A. Probing nanoliposomes using single particle analytical techniques: Effect of excipients, solvents, phase transition and zeta potential. Heliyon 2018, 4, e01088. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.C.; Yañez, O.; Salas-Huenuleo, E.; Morales, J.O. Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics 2021, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef] [PubMed]
- Redhead, H.M.; Davis, S.S.; Illum, L. Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: In vitro characterisation and in vivo evaluation. J. Controlled Release 2001, 70, 353–363. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Kharat, M.; McClements, D.J. Fabrication and characterization of nanostructured lipid carriers (NLC) using a plant-based emulsifier: Quillaja saponin. Food Res. Int. 2019, 126, 108601. [Google Scholar] [CrossRef]
- Youssef, A.; Dudhipala, N.; Majumdar, S. Ciprofloxacin Loaded Nanostructured Lipid Carriers Incorporated into In-Situ Gels to Improve Management of Bacterial Endophthalmitis. Pharmaceutics 2020, 12, 572. [Google Scholar] [CrossRef]
- Alladi, S.; Shastri, N.R. Semi solid matrix formulations of meloxicam and tenoxicam: An in vitro and in vivo evaluation. Arch. Pharm. Res. 2015, 38, 801–812. [Google Scholar] [CrossRef]
- Ansari, M.A. Modelling of size-dependent thermodynamic properties of metallic nanocrystals based on modified Gibbs–Thomson equation. Appl. Phys. A 2021, 127, 385. [Google Scholar] [CrossRef]
- Radomska-Soukharev, A. Stability of lipid excipients in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Westesen, K.; Siekmann, B.; Koch, M.H.J. Investigations on the physical state of lipid nanoparticles by synchrotron radiation X-ray diffraction. Int. J. Pharm. 1993, 93, 189–199. [Google Scholar] [CrossRef]
- Rai, A.; Sharma, R.; Kumar Dewangan, H. Formulation and evaluation of polymeric transdermal patch of Meloxicam. Mater. Today Proc. 2022, 68, 803–808. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: Formulation, characterization, and in vitro evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Rodrigues, L.B.O.; Lima, F.A.; Alves, C.P.B.; Martins-Santos, E.; Aguiar, M.M.G.; Oliveira, C.A.; Oréfice, R.L.; Ferreira, L.A.M.; Goulart, G.A.C. Ion Pair Strategy in Solid Lipid Nanoparticles: A Targeted Approach to Improve Epidermal Targeting with Controlled Adapalene Release, Resulting Reduced Skin Irritation. Pharm. Res. 2020, 37, 148. [Google Scholar] [CrossRef]
- Asif, M.; Fatima, K.; Imam, S.S.; Alshehri, S.; Mahdi, W.A. Formulation and Evaluation of Meloxicam Hybrid nano Particles. AAPS PharmSciTech 2024, 25, 172. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M. Chapter 9—Nanocontainers in food preservation: Techniques and uses. In Smart Nanocontainers; Nguyen-Tri, P., Do, T.-O., Nguyen, T.A., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–155. ISBN 978-0-12-816770-0. [Google Scholar]
- Perez, N.; Altube, M.J.; Barbosa, L.R.S.; Romero, E.L.; Perez, A.P. Thymus vulgaris essential oil + tobramycin within nanostructured archaeolipid carriers: A new approach against Pseudomonas aeruginosa biofilms. Phytomedicine 2022, 102, 154179. [Google Scholar] [CrossRef]
- Alvarez-Figueroa, M.J.; Narváez-Araya, D.; Armijo-Escalona, N.; Carrasco-Flores, E.A.; González-Aramundiz, J.V. Design of Chitosan Nanocapsules with Compritol 888 ATO® for Imiquimod Transdermal Administration. Evaluation of Their Skin Absorption by Raman Microscopy. Pharm. Res. 2020, 37, 195. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Gualpa, F.; Ramos, L.A.; Ciobotă, V. Adulteration of clove essential oil: Detection using a handheld Raman spectrometer. Flavour Fragr. J. 2018, 33, 184–190. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. (Eds.) APPENDIX 3—A Summary of Characteristic Raman and Infrared Frequencies. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: San Diego, CA, USA, 1991; pp. 477–490. ISBN 978-0-12-451160-6. [Google Scholar]
- Larkin, P.J. Chapter 7—General Outline for IR and Raman Spectral Interpretation. In Infrared and Raman Spectroscopy, 2nd ed.; Larkin, P.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–151. ISBN 978-0-12-804162-8. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. CHAPTER 2—Alkanes. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 9–28. ISBN 978-0-12-451160-6. [Google Scholar]
- Macasoi, C.; Meltzer, V.; Stanculescu, I.; Romanitan, C.; Pincu, E. Binary Mixtures of Meloxicam and L-Tartaric Acid for Oral Bioavailability Modulation of Pharmaceutical Dosage Forms. J. Funct. Biomater. 2024, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.; Lai, J.; Yin, Z.; Wu, W. Physical characterization of meloxicam-β-cyclodextrin inclusion complex pellets prepared by a fluid-bed coating method. Particuology 2009, 7, 1–8. [Google Scholar] [CrossRef]
- Synytsya, A.; Synytsya, A.; Alexa, P.; Wagner, R.; Davídková, M.; Volka, K. Raman spectroscopic study on sodium hyaluronate: An effect of proton and γ irradiation. J. Raman Spectrosc. 2011, 42, 544–550. [Google Scholar] [CrossRef]
- Shi, S.-C.; Wu, J.-Y.; Huang, T.-F. Raman, FTIR, and XRD study of MoS2 enhanced hydroxypropyl methylcellulose green lubricant. Opt. Quantum Electron. 2016, 48, 474. [Google Scholar] [CrossRef]
- Ahuja, A.; Khar, R.K.; Ali, J. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1997, 23, 489–515. [Google Scholar] [CrossRef]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89. [Google Scholar] [CrossRef]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.-L.; Marian, E. Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Koza, J.; Straubing, S.; Schwerdtle, T.; Haase, H. In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins. Int. J. Mol. Sci. 2018, 19, 2662. [Google Scholar] [CrossRef]
- Tabernero, A.; Guastaferro, M.; González-Garcinuño, Á.; Misol, A.; Baldino, L.; Cardea, S.; del Valle, E.M.; Reverchon, E. The viscoelastic behavior of the precursor hydrogels can modify aerogel properties. J. Supercrit. Fluids 2022, 184, 105563. [Google Scholar] [CrossRef]
- Bingöl, A.Ö.; Lohmann, D.; Püschel, K.; Kulicke, W.-M. Characterization and comparison of shear and extensional flow of sodium hyaluronate and human synovial fluid. Biorheology 2010, 47, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Lapčík, L.; Lapčík, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Controlled Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Ahmed, L.; Atif, R.; Salah Eldeen, T.; Ibtihag, Y.; Omara, A.; Eltayeb, M. Study the Using of Nanoparticles as Drug Delivery System Based on Mathematical Models for Controlled Release. Available online: https://www.researchgate.net/publication/342902985_Study_the_Using_of_Nanoparticles_as_Drug_Delivery_System_Based_on_Mathematical_Models_for_Controlled_Release (accessed on 12 March 2025).
- Zhu, W.; Long, J.; Shi, M. Release Kinetics Model Fitting of Drugs with Different Structures from Viscose Fabric. Materials 2023, 16, 3282. [Google Scholar] [CrossRef]
- Guerra-Ponce, W.L.; Gracia-Vásquez, S.L.; González-Barranco, P.; Camacho-Mora, I.A.; Gracia-Vásquez, Y.A.; Orozco-Beltrán, E.; Felton, L.A. In vitro evaluation of sustained released matrix tablets containing ibuprofen: A model poorly water-soluble drug. Braz. J. Pharm. Sci. 2016, 52, 751–759. [Google Scholar] [CrossRef]
- Ameena, M.; Meignana, A.I.; Karthikeyan, R.; Rajeshkumar, S. Evaluation of the Anti-inflammatory, Antimicrobial, Antioxidant, and Cytotoxic Effects of Chitosan Thiocolchicoside-Lauric Acid Nanogel. Cureus 2023, 15, e46003. [Google Scholar] [CrossRef]
- Almira, D.; Subaidah, W.A.; Ananto, A.D.; Deccati, R.F.; Muliasari, H. In vitro concentration optimization of ethanol extract from Makasar fruit seeds (Brucea javanica L. Merr) as an anti-inflammatory agent. J. Pijar Mipa 2021, 16, 595–599. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Mahmood, U.; Masood, R.; Afzal, M.A.; Raza, Z.A.; Abid, S.; Zahir, A.; Hussain, T.; Nazir, A. Development of zinc, silver, and hyaluronic acid mediated wet spun alginate fibers for potential wound care applications. J. Ind. Text. 2022, 51, 1916S–1930S. [Google Scholar] [CrossRef]
- Barma, M.D.; Muthupandiyan, I.; Samuel, S.R.; Amaechi, B.T. Inhibition of Streptococcus mutans, antioxidant property and cytotoxicity of novel nano-zinc oxide varnish. Arch. Oral Biol. 2021, 126, 105132. [Google Scholar] [CrossRef]
- Gupta, C.; Kumari, A.; Garg, A.P.; Catanzaro, R.; Marotta, F. Comparative study of cinnamon oil and clove oil on some oral microbiota. Acta Bio-Medica Atenei Parm. 2011, 82, 197–199. [Google Scholar]
- Dillen, K.; Bridts, C.; Van der Veken, P.; Cos, P.; Vandervoort, J.; Augustyns, K.; Stevens, W.; Ludwig, A. Adhesion of PLGA or Eudragit®/PLGA nanoparticles to Staphylococcus and Pseudomonas. Int. J. Pharm. 2008, 349, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kashi, T.S.J.; Eskandarion, S.; Esfandyari-Manesh, M.; Marashi, S.M.A.; Samadi, N.; Fatemi, S.M.; Atyabi, F.; Eshraghi, S.; Dinarvand, R. Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int. J. Nanomed. 2012, 7, 221–234. [Google Scholar] [CrossRef]
- Mohammadi, G.; Nokhodchi, A.; Barzegar-Jalali, M.; Lotfipour, F.; Adibkia, K.; Ehyaei, N.; Valizadeh, H. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf. B Biointerfaces 2011, 88, 39–44. [Google Scholar] [CrossRef]
- Makade, C.S.; Shenoi, P.R.; Bhongade, B.A.; Shingane, S.A.; Ambulkar, P.C.; Shewale, A.M. Estimation of MBC: MIC Ratio of Herbal Extracts against Common Endodontic Pathogens. J. Pharm. Bioallied Sci. 2024, 16, S1414–S1416. [Google Scholar] [CrossRef]
- Xu, J.-S.; Li, Y.; Cao, X.; Cui, Y. The effect of eugenol on the cariogenic properties of Streptococcus mutans and dental caries development in rats. Exp. Ther. Med. 2013, 5, 1667–1670. [Google Scholar] [CrossRef]
- Adil, M.; Singh, K.; Verma, P.K.; Khan, A.U. Eugenol-induced suppression of biofilm-forming genes in Streptococcus mutans: An approach to inhibit biofilms. J. Glob. Antimicrob. Resist. 2014, 2, 286–292. [Google Scholar] [CrossRef]

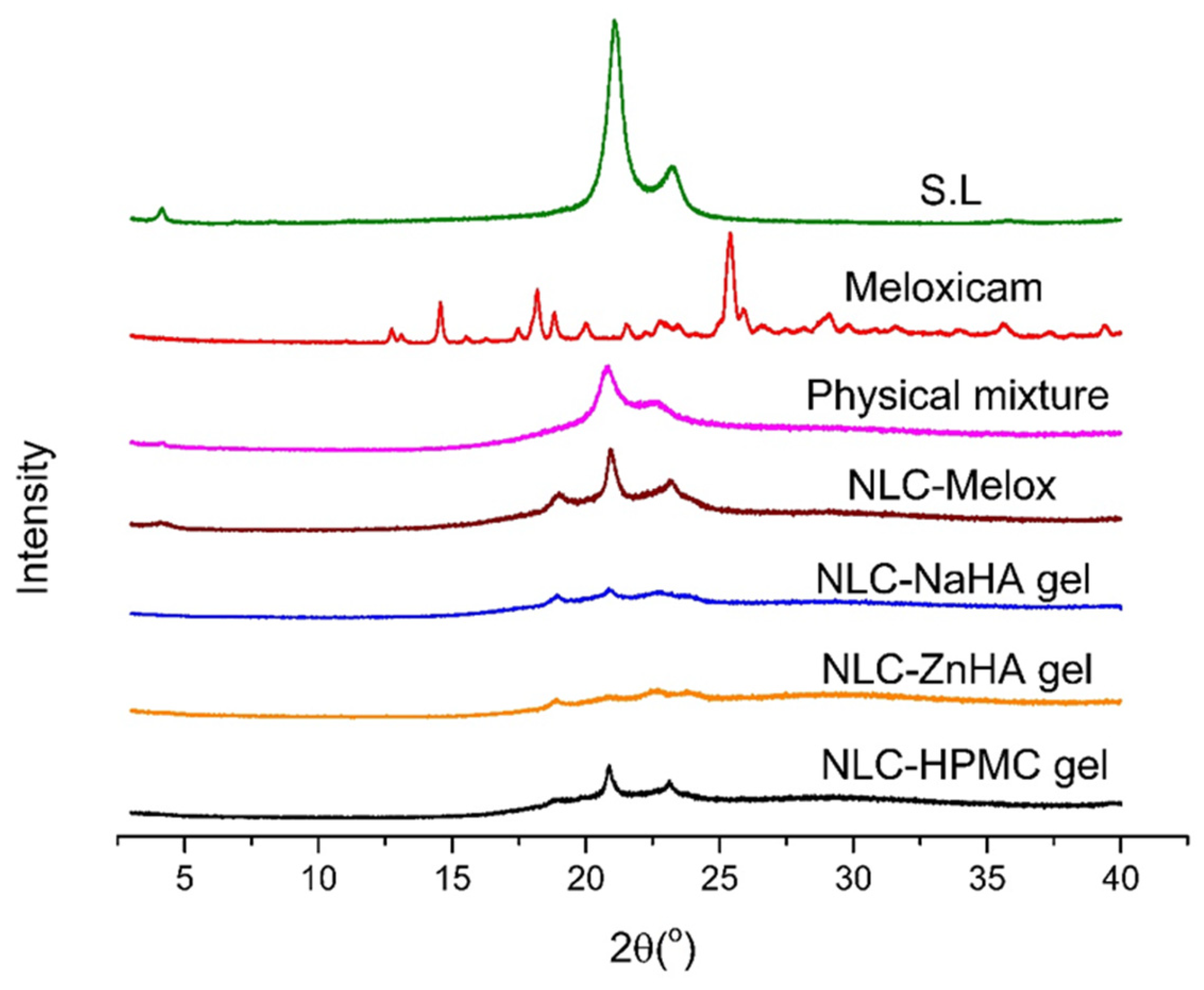
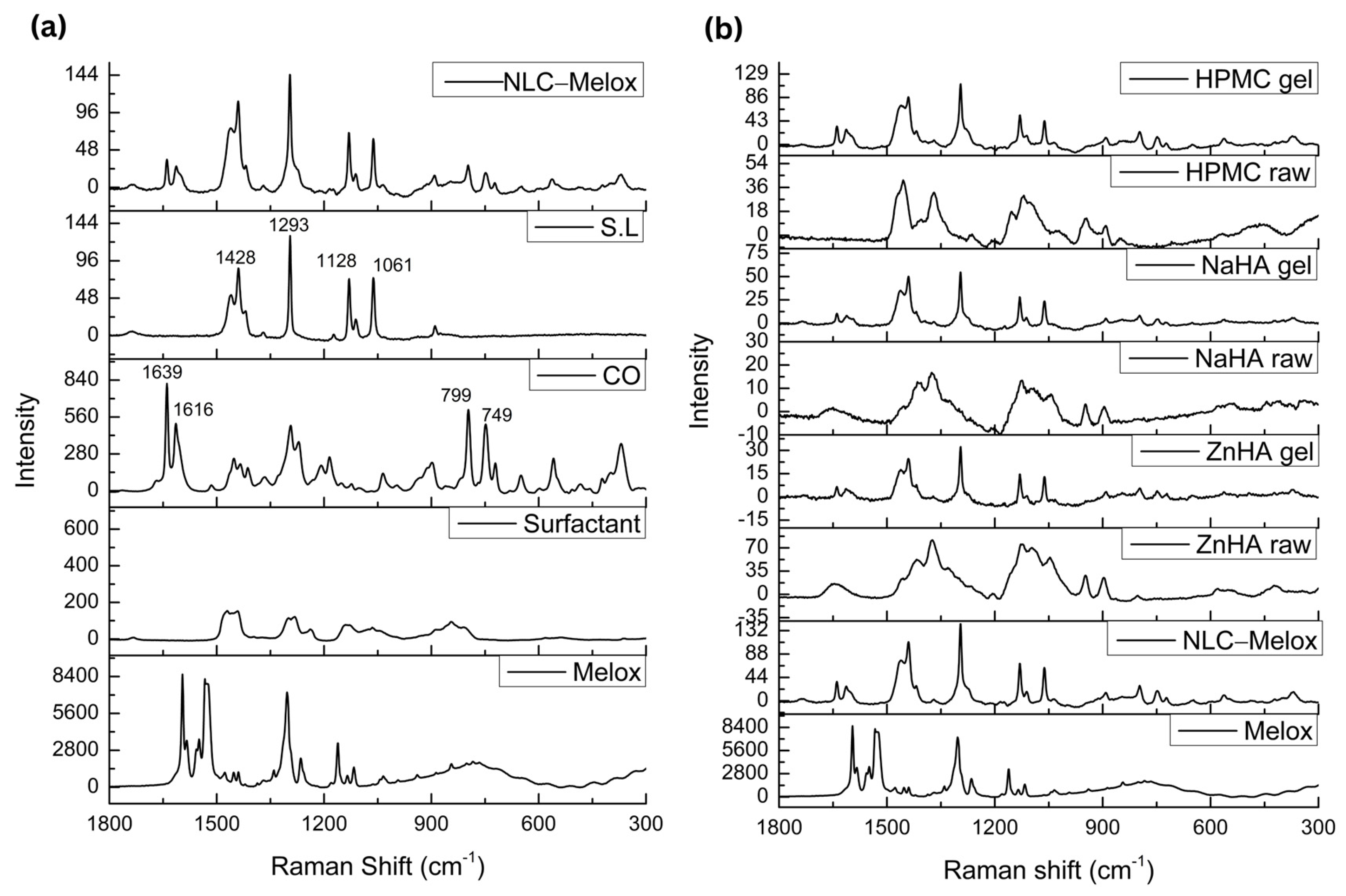
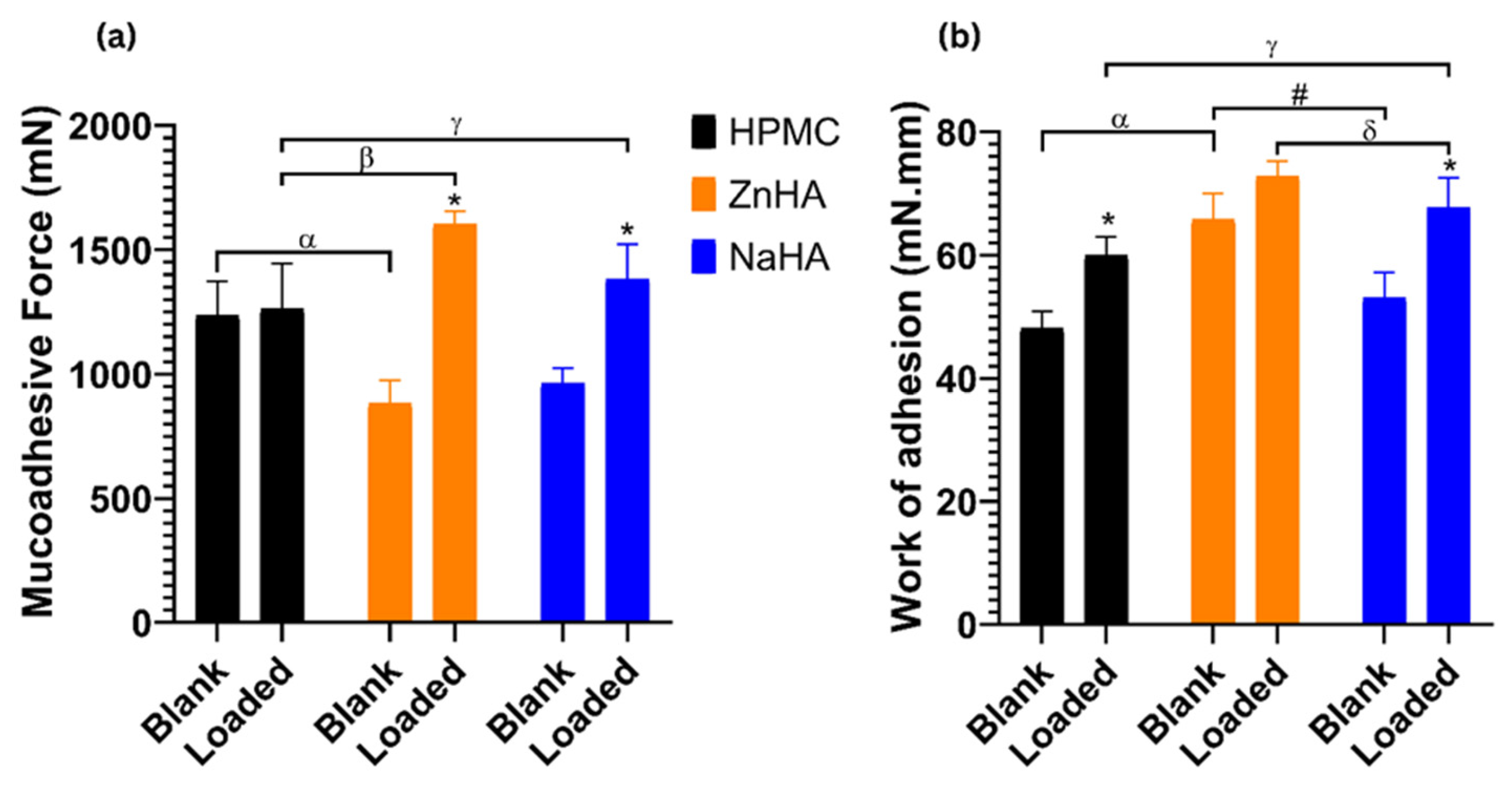
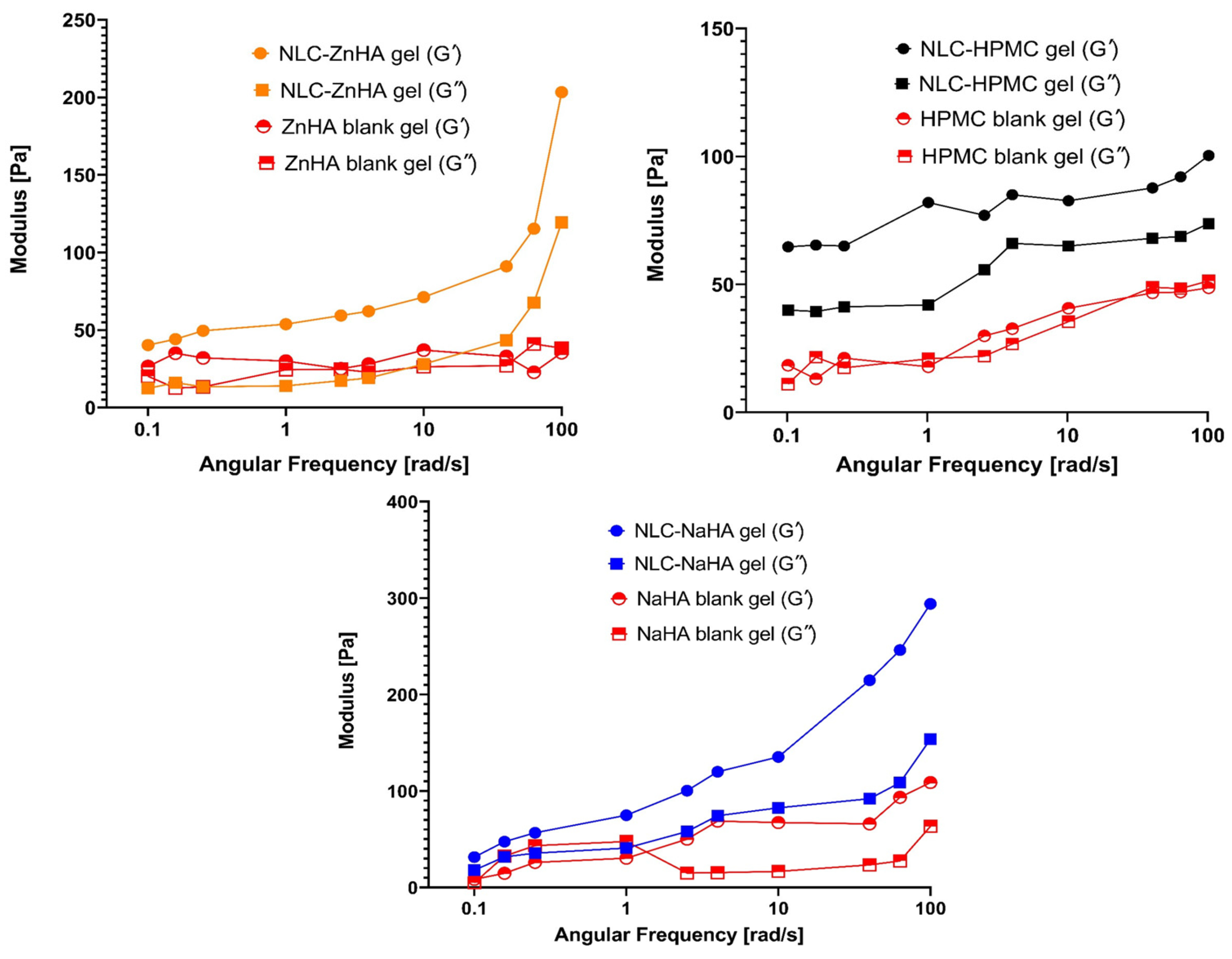


| Formulations | S.L (%) | CO (%) | Surfactant (%) | Melox (μg/mL) | HPMC (%) | ZnHA (%) | NaHA (%) |
|---|---|---|---|---|---|---|---|
| NLC–Melox | 3 | 2 | 2.5 | 250 | - | - | - |
| NLC-HPMC gel | 3 | 2 | 2.5 | 250 | 1 | - | - |
| NLC-ZnHA gel | 3 | 2 | 2.5 | 250 | - | 1 | - |
| NLC-NaHA gel | 3 | 2 | 2.5 | 250 | - | - | 1 |
| Formulations | Enthalpy Change (J/g) | Onset Temperature(°C) | Peak Point (°C) | Endset Temperature (°C) | CI% |
|---|---|---|---|---|---|
| NLC-NaHA gel | 67.51 | 61.12 | 68.03 | 72.39 | 22.4 |
| NLC-ZnHA gel | 61.05 | 62.95 | 68.99 | 73.36 | 20.2 |
| NLC-HPMC gel | 55.63 | 60.96 | 68.98 | 73.35 | 18.4 |
| NLC–Melox | 49.11 | 65.57 | 69.15 | 70.42 | 16.3 |
| S.L | 100.3 | 71.49 | 73.05 | 74.97 | - |
| Kinetic Release Models | NLC–Melox | NLC-HPMC Gel | NLC-ZnHA Gel | NLC-NaHA Gel |
|---|---|---|---|---|
| Zero order | 0.88 | 0.86 | 0.97 | 0.88 |
| First order | 0.95 | 0.90 | 0.97 | 0.94 |
| Higuchi | 0.96 | 0.97 | 0.85 | 0.96 |
| Korsmeyer–Peppas | 0.98 | 0.99 | 0.98 | 0.98 |
| (n) | 0.61 | 0.58 | 0.95 | 0.60 |
| Formulations | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| A. actinomycetemcomitans | S. mutans | |||
| 24 h | 48 h | 24 h | 48 h | |
| NLC-ZnHA gel | 30.3 ± 1.3 | 30.7 ± 0.6 | N.A. | N.A |
| NLC-NaHA gel | 24.0 ± 0.0 | 24.3 ± 1.2 | N.A. | N.A. |
| NLC-HPMC gel | 32.3 ± 1.5 | 30.0 ± 0.0 | N.A. | N.A |
| ZnHA blank gel | 25.0 ± 1.0 | 24.7 ± 1.2 | 14.5 ± 0.0 | 14.2 ± 0.3 |
| NaHA blank gel | N.A. | N.A. | N.A. | N.A. |
| HPMC blank gel | N.A. | N.A. | N.A. | N.A. |
| NLC–Melox | 36.3 ± 2.1 | 34.3 ± 2.9 | 14.7 ± 1.0 | 14.4 ± 1.1 |
| NLC-C | 33.3 ± 2.3 | 33.3 ± 1.2 | N.A. | N.A. |
| Free CO | 28.3 ± 2.1 | 26.3 ± 1.5 | 17.7 ± 0.6 | 17.5 ± 0.5 |
| NLC-M | N.A. | N.A. | N.A. | N.A. |
| Free Melox | N.A. | N.A. | N.A. | N.A. |
| Formulations | A. actinomycetemcomitans | S. mutans | ||
|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| NLC–Melox | 312.5 * | 312.5 * | 625 * | 5000 * |
| NLC-C | 156.2 * | 625 * | 625 * | 1250 * |
| CO | 312.5 * | 1250 * | 1250 * | 5000 * |
| NLC-M | >250 ** | >250 ** | >250 ** | >250 ** |
| Melox | >250 ** | >250 ** | >250 ** | >250 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashfaq, R.; Tóth, N.; Kovács, A.; Berkó, S.; Katona, G.; Ambrus, R.; Polgár, T.F.; Szécsényi, M.; Burián, K.; Budai-Szűcs, M. Hydrogel–Nanolipid Formulations for the Complex Anti-Inflammatory and Antimicrobial Therapy of Periodontitis. Pharmaceutics 2025, 17, 620. https://doi.org/10.3390/pharmaceutics17050620
Ashfaq R, Tóth N, Kovács A, Berkó S, Katona G, Ambrus R, Polgár TF, Szécsényi M, Burián K, Budai-Szűcs M. Hydrogel–Nanolipid Formulations for the Complex Anti-Inflammatory and Antimicrobial Therapy of Periodontitis. Pharmaceutics. 2025; 17(5):620. https://doi.org/10.3390/pharmaceutics17050620
Chicago/Turabian StyleAshfaq, Rabia, Nóra Tóth, Anita Kovács, Szilvia Berkó, Gábor Katona, Rita Ambrus, Tamás Ferenc Polgár, Mária Szécsényi, Katalin Burián, and Mária Budai-Szűcs. 2025. "Hydrogel–Nanolipid Formulations for the Complex Anti-Inflammatory and Antimicrobial Therapy of Periodontitis" Pharmaceutics 17, no. 5: 620. https://doi.org/10.3390/pharmaceutics17050620
APA StyleAshfaq, R., Tóth, N., Kovács, A., Berkó, S., Katona, G., Ambrus, R., Polgár, T. F., Szécsényi, M., Burián, K., & Budai-Szűcs, M. (2025). Hydrogel–Nanolipid Formulations for the Complex Anti-Inflammatory and Antimicrobial Therapy of Periodontitis. Pharmaceutics, 17(5), 620. https://doi.org/10.3390/pharmaceutics17050620