Production of Myco-Nanomaterial Products from Pleurotus ostreatus (Agaricomycetes) Mushroom via Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Oyster Mushroom-Based Carbon Nanodots and Experimental Design
- (1)
- Molecular weight (kDa), fluorescence intensity (A.u.), and OMP-based CND (OMP-CND) concentration (mg/kg) were determined using high-performance liquid chromatography. For this analysis, pyrolyzed dry powders (processed at 150 °C to 240 °C) were diluted 100-fold in distilled water, vortexed (15 s), and filtered through a 0.45 µm hydrophilic PTFE syringe filter (Labex Ltd., New Delhi, India).
- (2)
- To evaluate the carbon and nitrogen (C/N) ratio and conduct UV–visible spectrophotometry, pyrolyzed dry powders were diluted ten-fold in distilled water, vortexed for 15 s, and exposed to ultrasonic cleaner (Olympus, Tokyo, Japan) treatment (30 kHz) for 20 min. The processed samples were filtered twice with filter paper (100 µm dairy filter paper). The water-soluble fractions were pre-frozen and freeze-dried at 40 °C for approximately 24 ± 2 h. These final samples (oyster mushroom powder with enhanced carbon nanodots content from the freeze-dried water-soluble fraction: OMP-CND-FL) were analyzed for their C/N ratio and fluorescence spectra.
- (3)
- To investigate the final product, Fourier Transform Infrared Spectroscopy (FTIR) and antibacterial activity assays were used, including evaluating antimicrobial effects against Escherichia coli and Staphylococcus epidermidis. FTIR investigations were performed on pyrolyzed (with the highest fluorescence intensity and CND concentration) and non-pyrolyzed freeze-dried OMPs samples.
2.2. Yield of Freeze-Dried Water-Soluble Myco-Nanomaterial Products
2.3. Characterization of Mushroom-Based Carbon Nanodots
2.4. Measurement of Carbon and Nitrogen (C/N) Content
2.5. Investigations of the Antibacterial Activity of the Final Product
2.6. Results for Fourier Transform Infrared Spectroscopy Analysis
2.7. Statistical Analysis
3. Results
3.1. Yield of Water-Soluble OMP-CNDs at Different Temperatures
3.2. Changes in Molecular Weight of CND Affected by Pyrolitic Heating Processes
3.3. Changes in Fluorescence Intensity and CND Concentration Affected by Pyrolitic Heating Processes
3.4. Evaluation of Size Distributions After Calculation of Polydispersity Index
3.5. Effect of Carbon and Carbon/Nitrogen Ratio on the Fluorescence Properties of Oyster Mushroom Powder After Pyrolysis
3.6. Antibacterial Activity of P. ostreatus-Based CNDs
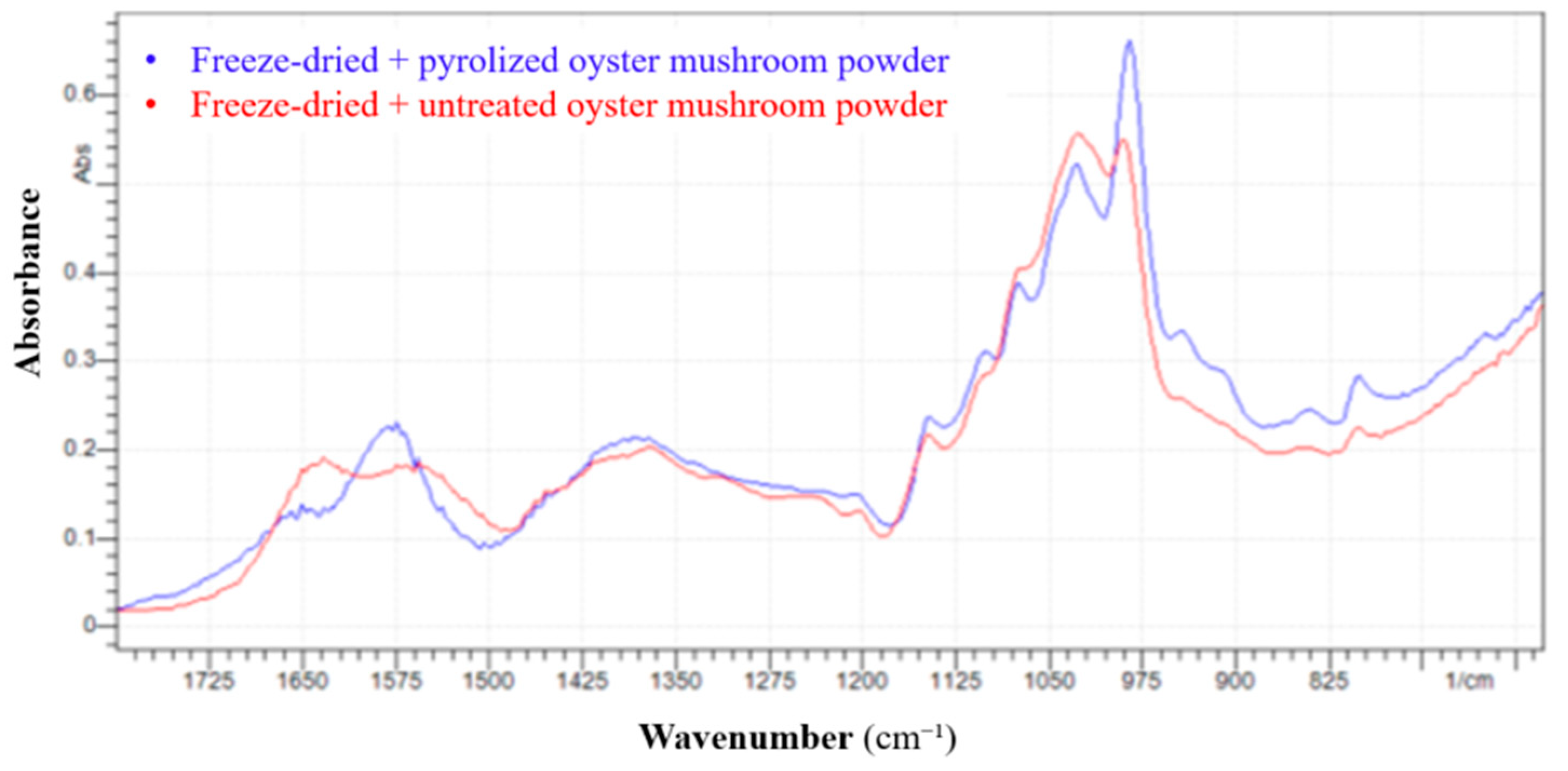
3.7. Results of FTIR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Y.; Hu, C.; Li, Z.; Wu, C.; Zeng, Y.; Peng, C. Multifunctional Carbon Nanomaterials for Diagnostic Applications in Infectious Diseases and Tumors. Mater. Today Bio 2022, 14, 100231. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, A.A.Z.; Abu-Elghait, M.; Salem, S.S. Recent Insights into Nanotechnology in Colorectal Cancer. Appl. Biochem. Biotechnol. 2024, 196, 4457–4471. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, B.G.; Pandey, V.; Sharma, S.; Patel, S.; Shah, D.P.; Kapoor, D.U. Carbon Nanodots: An Illuminating Paradigm in Production, Characterization, and Oncological Targeting Methodologies—A Review. BioNanoScience 2024, 14, 4322–4341. [Google Scholar] [CrossRef]
- Malhan, A.; Guleria, M.; Das, U.; Singh, S.; Prajapati, B.G.; Mohite, P.; Bhattacharya, S.; Chidrawar, V.R.; Puri, A.; Datta, D. Navigating the Future of Cancer Management through Carbon Nanodots: A Review. Nano-Struct. Nano-Objects 2024, 39, 101217. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Ahmed, A.; Shahadat, M.; Ul Islam, S.; Adnan, R.; Mohamad Ibrahim, M.N.; Ullah, Q. Synthesis, Characterization, and Properties of Green Carbon Nanodots. In Green Carbon Materials for Environmental Analysis: Emerging Research and Future Opportunities; Ul Islam, S., Hussain, C.M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1441, pp. 25–39. ISBN 978-0-8412-9714-2. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, K.; Zhou, R.; Shan, C. Multicolor Biomass Based Carbon Nanodots for Bacterial Imaging. Chin. Chem. Lett. 2022, 33, 798–802. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Verma, C. Carbon Nanodots: Recent Advances in Synthesis and Applications. Carbon. Lett. 2022, 32, 1603–1629. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S. Sustainable Development of Carbon Nanodots Technology: Natural Products as a Carbon Source and Applications to Food Safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Törős, G.; Peles, F.; Elramady, H.; Prokisch, J. To What Extent Can Maillard Reaction Products Influence the Probiotic and Harmful Bacteria? Egypt. J. Soil Sci. 2023, 63, 177–185. [Google Scholar] [CrossRef]
- Fricler, V.Y.; Nyashina, G.S.; Vershinina, K.Y.; Vinogrodskiy, K.V.; Shvets, A.S.; Strizhak, P.A. Microwave Pyrolysis of Agricultural Waste: Influence of Catalysts, Absorbers, Particle Size and Blending Components. J. Anal. Appl. Pyrolysis 2023, 171, 105962. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Zhang, Q.; Chai, G.; Yang, C.; Meng, Y.; Xu, H.; Chen, S. Color Characteristics and Pyrolysis Volatile Properties of Main Colored Fractions from the Maillard Reaction Models of Glucose with Three Amino Acids. LWT 2024, 192, 115739. [Google Scholar] [CrossRef]
- Lin, S.-L.; Zhang, H.; Chen, W.-H.; Wu, Y.-L.; Wu, C.-W.; Huang, S.-W. A Review on the Thermochemical Reaction Mechanisms for Distiller Pyrolysis Process. Sustain. Environ. Res. 2024, 34, 13. [Google Scholar] [CrossRef]
- Barbero, F.; Destro, E.; Bellone, A.; Di Lorenzo, L.; Brunella, V.; Perrone, G.; Damin, A.; Fenoglio, I. Hydrothermal Carbonization Synthesis of Amorphous Carbon Nanoparticles (15–150 Nm) with Fine-Tuning of the Size, Bulk Order, and the Consequent Impact on Antioxidant and Photothermal Properties. Nanoscale Adv. 2025, 7, 1391–1404. [Google Scholar] [CrossRef]
- Chen, M.; Zhai, J.; An, Y.; Li, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Liu, C.; Lin, X. Solvent-Free Pyrolysis Strategy for the Preparation of Biomass Carbon Dots for the Selective Detection of Fe3+ Ions. Front. Chem. 2022, 10, 940398. [Google Scholar] [CrossRef] [PubMed]
- Lad, U.M.; Chunekar, N.P.; Dave, D.J.; Desai, B.N.; Suthar, D.H.; Modi, C.K. Luminous Insights: Harnessing Carbon Nanodots from Black Seed Powder via Pyrolysis for Bioimaging and Antifungal Investigations. J. Fluoresc. 2024, 34, 2895–2906. [Google Scholar] [CrossRef]
- Tijani, N.A.; Hokello, J.; Awojobi, K.O.; Marnadu, R.; Shkir, M.; Ahmad, Z.; Afolabi, A.O.; Adewinbi, S.A.; Adebayo, I.A. Recent Advances in Mushroom-Mediated Nanoparticles: A Critical Review of Mushroom Biology, Nanoparticles Synthesis, Types, Characteristics and Applications. J. Drug Deliv. Sci. Technol. 2024, 96, 105695. [Google Scholar] [CrossRef]
- Acay, H.; Yildirim, A.; Erdem Güzel, E.; Kaya, N.; Baran, M.F. Evaluation and Characterization of Pleurotus eryngii Extract-Loaded Chitosan Nanoparticles as Antimicrobial Agents against Some Human Pathogens. Prep. Biochem. Biotechnol. 2020, 50, 897–906. [Google Scholar] [CrossRef]
- Zhou, H.; McClements, D.J. Recent Advances in the Gastrointestinal Fate of Organic and Inorganic Nanoparticles in Foods. Nanomaterials 2022, 12, 1099. [Google Scholar] [CrossRef]
- Cheung, M.K.; Yue, G.G.L.; Chiu, P.W.Y.; Lau, C.B.S. A Review of the Effects of Natural Compounds, Medicinal Plants, and Mushrooms on the Gut Microbiota in Colitis and Cancer. Front. Pharmacol. 2020, 11, 744. [Google Scholar] [CrossRef]
- Srinivash, M.; Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B.; Bharathakumar, S.; Gurushankar, K.; Dhanapal, K.; Karuppa Samy, K.; Babu Perumal, A. Nanomedicine for Drug Resistant Pathogens and COVID-19 Using Mushroom Nanocomposite Inspired with Bacteriocin—A Review. Inorg. Chem. Commun. 2023, 152, 110682. [Google Scholar] [CrossRef]
- De Kraker, M.E.A.; Davey, P.G.; Grundmann, H.; the BURDEN Study Group. Mortality and Hospital Stay Associated with Resistant Staphylococcus aureus and Escherichia coli Bacteremia: Estimating the Burden of Antibiotic Resistance in Europe. PLoS Med. 2011, 8, e1001104. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, V.; Kuznietsova, H.; Dziubenko, N.; Byelinska, I.; Zaderko, A.; Lysenko, T.; Skryshevsky, V. Application of Carbon Dots as Antibacterial Agents: A Mini Review. BioNanoScience 2024, 14, 1819–1831. [Google Scholar] [CrossRef]
- Li, J.; Pu, T.; Wang, Z.; Liu, T. Thermal Behavior and Pyrolysis Kinetics of Mushroom Residue with the Introduction of Waste Plastics. Polymers 2023, 15, 3824. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liao, W.; Li, Z.; Sun, L.; Chen, C. Easy Conversion of Protein-Rich Enoki Mushroom Biomass to a Nitrogen-Doped Carbon Nanomaterial as a Promising Metal-Free Catalyst for Oxygen Reduction Reaction. Nanoscale 2015, 7, 15990–15998. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yang, H.; Wu, Z.; Li, D.; Wang, Z.; Wang, D.; Wang, H.; Li, H.; Li, J. Co-Pyrolysis of Mushroom Residue Blended with Pine Sawdust/Wheat Straw for Sustainable Utilization of Biomass Wastes: Thermal Characteristics, Kinetic/Thermodynamic Analysis, and Structure Evolution of Co-Pyrolytic Char. Sustainability 2024, 16, 6677. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the Nutritional Quality of Pleurotus ostreatus (Oyster Mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; Muthu, A.; El-Ramady, H.; Daróczi, L.; Nagy, L.; Kéki, S.; Béni, Á.; Csarnovics, I.; Prokisch, J. Optimization of Extraction Conditions to Synthesize Green Carbon Nanodots Using the Maillard Reaction. Mater. Adv. 2024, 5, 3499–3505. [Google Scholar] [CrossRef]
- Törős, G.; Béni, Á.; Peles, F.; Gulyás, G.; Prokisch, J. Comparative Analysis of Freeze-Dried Pleurotus ostreatus Mushroom Powders on Probiotic and Harmful Bacteria and Its Bioactive Compounds. J. Fungi 2024, 11, 1. [Google Scholar] [CrossRef]
- Gromotka, L.; Uttinger, M.J.; Schlumberger, C.; Thommes, M.; Peukert, W. Classification and Characterization of Multimodal Nanoparticle Size Distributions by Size-Exclusion Chromatography. Nanoscale 2022, 14, 17354–17364. [Google Scholar] [CrossRef]
- Saint-Denis, T.; Goupy, J. Optimization of a Nitrogen Analyser Based on the Dumas Method. Anal. Chim. Acta 2004, 515, 191–198. [Google Scholar] [CrossRef]
- Yildiz, S.; Yilmaz, A.; Can, Z. In Vitro Bioactive Properties of Some Wild Mushrooms Collected from Kastamonu Province. Kastamonu Üniversitesi Orman. Fakültesi Derg. 2017, 17, 523–530. [Google Scholar] [CrossRef][Green Version]
- Baudot, C.; Tan, C.M.; Kong, J.C. FTIR Spectroscopy as a Tool for Nano-Material Characterization. Infrared Phys. Technol. 2010, 53, 434–438. [Google Scholar] [CrossRef]
- Balakumaran, M.D.; Ramachandran, R.; Balashanmugam, P.; Mukeshkumar, D.J.; Kalaichelvan, P.T. Mycosynthesis of Silver and Gold Nanoparticles: Optimization, Characterization and Antimicrobial Activity against Human Pathogens. Microbiol. Res. 2016, 182, 8–20. [Google Scholar] [CrossRef]
- Alvandi, H.; Hatamian-Zarmi, A.; Webster, T.J. Bioactivity and Applications of Mushroom and Polysaccharide-Derived Nanotherapeutics. In Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 415–452. [Google Scholar]
- Deng, G.; Li, J.; Liu, H.; Wang, Y. Volatile Compounds and Aroma Characteristics of Mushrooms: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 13175–13192. [Google Scholar] [CrossRef]
- Annepu, S.K.; Sharma, V.P.; Barh, A.; Kamal, S.; Shirur, M.; Kumar, S.; Bairwa, R.K.; Gupta, S.; Gupta, M.; Dutta, U. Influence of Heat Treatment and Solid-State Fermentation on the Lignocellulosic Fractions of Substrates Supporting Lentinula edodes (Berk.) Pegler Cultivation: Implications for Commercial Production. Fermentation 2023, 9, 130. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chen, J.; Xie, W.; Kuo, J.; Lu, X.; Chang, K.; Wen, S.; Sun, G.; Cai, H.; et al. Combustion Behaviors of Spent Mushroom Substrate Using TG-MS and TG-FTIR: Thermal Conversion, Kinetic, Thermodynamic and Emission Analyses. Bioresour. Technol. 2018, 266, 389–397. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, M.; Jin, Z.; Wang, G.; Li, R.; Zhang, X.; Liu, X.; Qu, J.; Wang, H. Characterization of Biochars Derived from Various Spent Mushroom Substrates and Evaluation of Their Adsorption Performance of Cu(II) Ions from Aqueous Solution. Environ. Res. 2021, 196, 110323. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bassart, Z.; Bäuerl, C.; Fabra, M.J.; Martínez-Abad, A.; Collado, M.C.; López-Rubio, A. Composition, Structural Properties and Immunomodulatory Activity of Several Aqueous Pleurotus β-Glucan-Rich Extracts. Int. J. Biol. Macromol. 2023, 253, 127255. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Engelbrecht, L.; Olla, C.; Cappai, A.; Melis, C.; Stagi, L.; Laaksonen, A.; Mocci, F. Graphene Quantum Dots and Carbon Nanodots: Modeling of Zero-Dimensional Carbon Nanomaterials. In Zero-Dimensional Carbon Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2024; pp. 411–482. ISBN 978-0-323-99535-1. [Google Scholar]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of Excitation Dependent Fluorescence in Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Pn, N.; Alexander, K.; Zavabeti, A.; Sherrell, P.C.; Ivanova, E.P.; Adhikari, B.; Naebe, M.; Bhargava, S.K. Fluorescent Nanocarbons: From Synthesis and Structure to Cancer Imaging and Therapy. Adv. Mater. 2024, 36, 2312474. [Google Scholar] [CrossRef]
- Bai, M.; Shao, X.; Wang, C.; Wang, J.; Wang, X.; Guan, P.; Hu, X. Application of Carbon-Based Nanomaterials in Alzheimer’s Disease. Mater. Horiz. 2025, 12, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Arcudi, F.; Đorđević, L.; Prato, M. Design, Synthesis, and Functionalization Strategies of Tailored Carbon Nanodots. Acc. Chem. Res. 2019, 52, 2070–2079. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, H. Novel Properties and Applications of Carbon Nanodots. Nanoscale Horiz. 2018, 3, 565–597. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, I.Y.; Sapelkin, A.V.; Sukhorukov, G.B. Carbon Nanodots: Mechanisms of Photoluminescence and Principles of Application. TrAC Trends Anal. Chem. 2017, 90, 27–37. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence Tuning in Carbon Dots: Surface Passivation or/and Functionalization, Heteroatom Doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, X.; Fatima, A.; Zhang, L.; Yuan, G.; Lian, F.; Wang, Y. Antibacterial Activity and Mechanisms of Plant Flavonoids against Gram-Negative Bacteria Based on the Antibacterial Statistical Model. Pharmaceuticals 2024, 17, 292. [Google Scholar] [CrossRef]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-Derived Carbon Dots for Toxic Metal Ion Detection and as Antibacterial and Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Prokisch, J.; Törős, G.; Nguyen, D.H.H.; Neji, C.; Ferroudj, A.; Sári, D.; Muthu, A.; Brevik, E.C.; El-Ramady, H. Nano-Food Farming: Toward Sustainable Applications of Proteins, Mushrooms, Nano-Nutrients, and Nanofibers. Agronomy 2024, 14, 606. [Google Scholar] [CrossRef]
- Sarkar, J.; Naskar, A.; Nath, A.; Gangopadhyay, B.; Tarafdar, E.; Das, D.; Chakraborty, S.; Chattopadhyay, D.; Acharya, K. Innovative Utilization of Harvested Mushroom Substrate for Green Synthesis of Silver Nanoparticles: A Multi–Response Optimization Approach. Environ. Res. 2024, 248, 118297. [Google Scholar] [CrossRef]
- Irshad, A.; Jawad, R.; Sharif, S.; Joly, N.; Ishtiaq, U.; Martin, P.; Mushtaq, Q. Bioengineering of Glucan Coated Silver Nanoparticles as Dynamic Biomedical Compound; in Vitro and in Vivo Studies. Microb. Pathog. 2024, 197, 107005. [Google Scholar] [CrossRef] [PubMed]


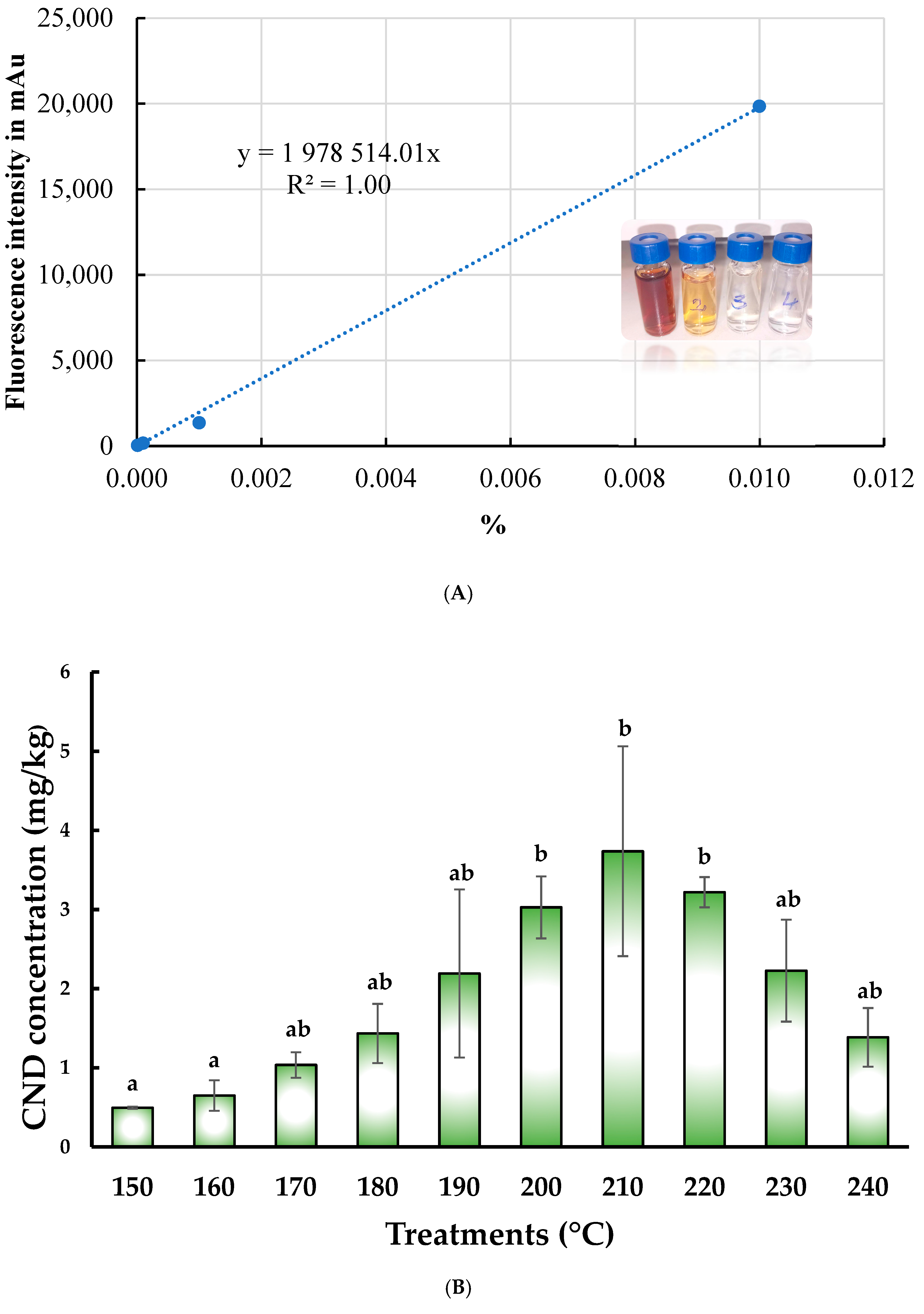
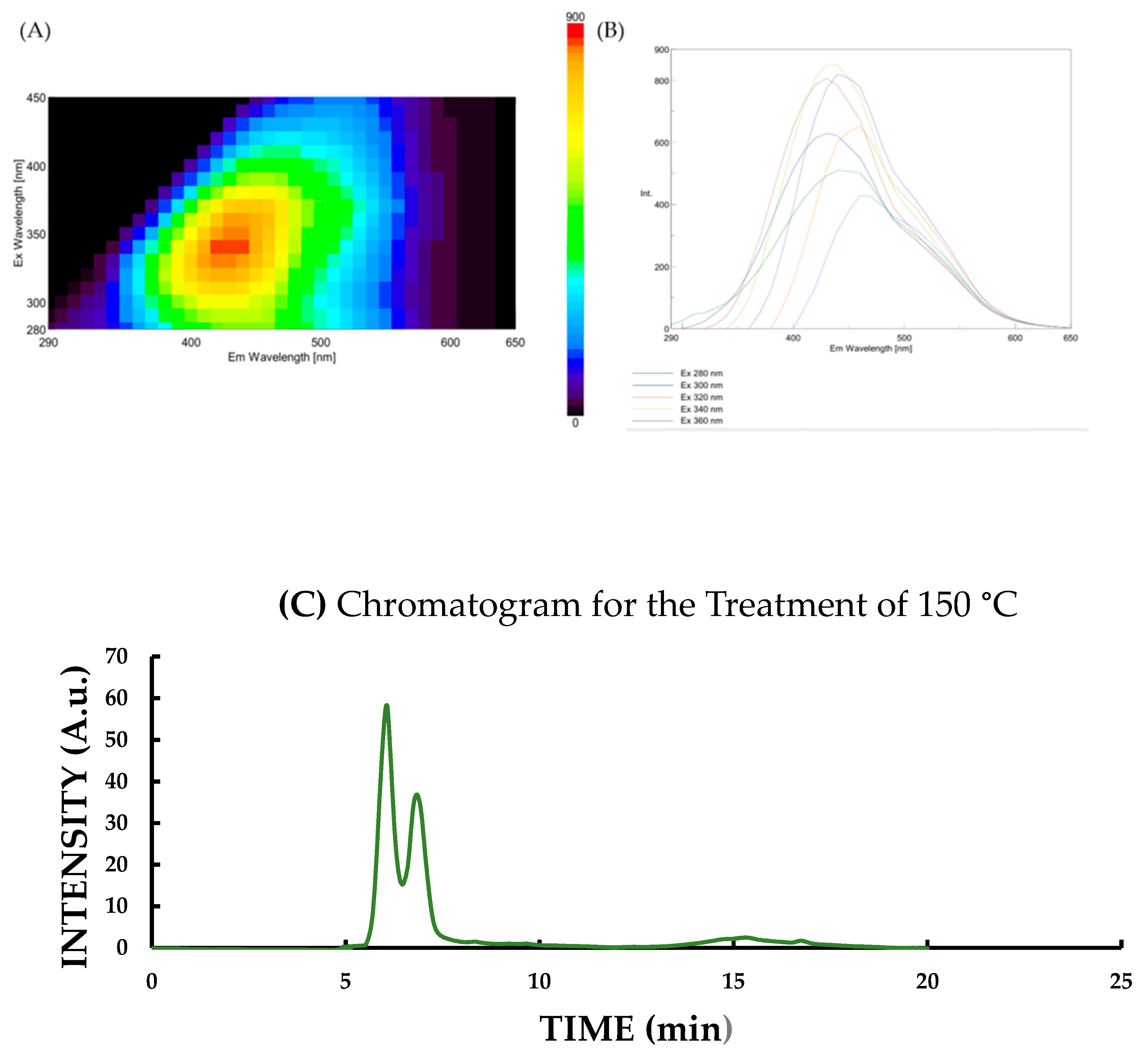
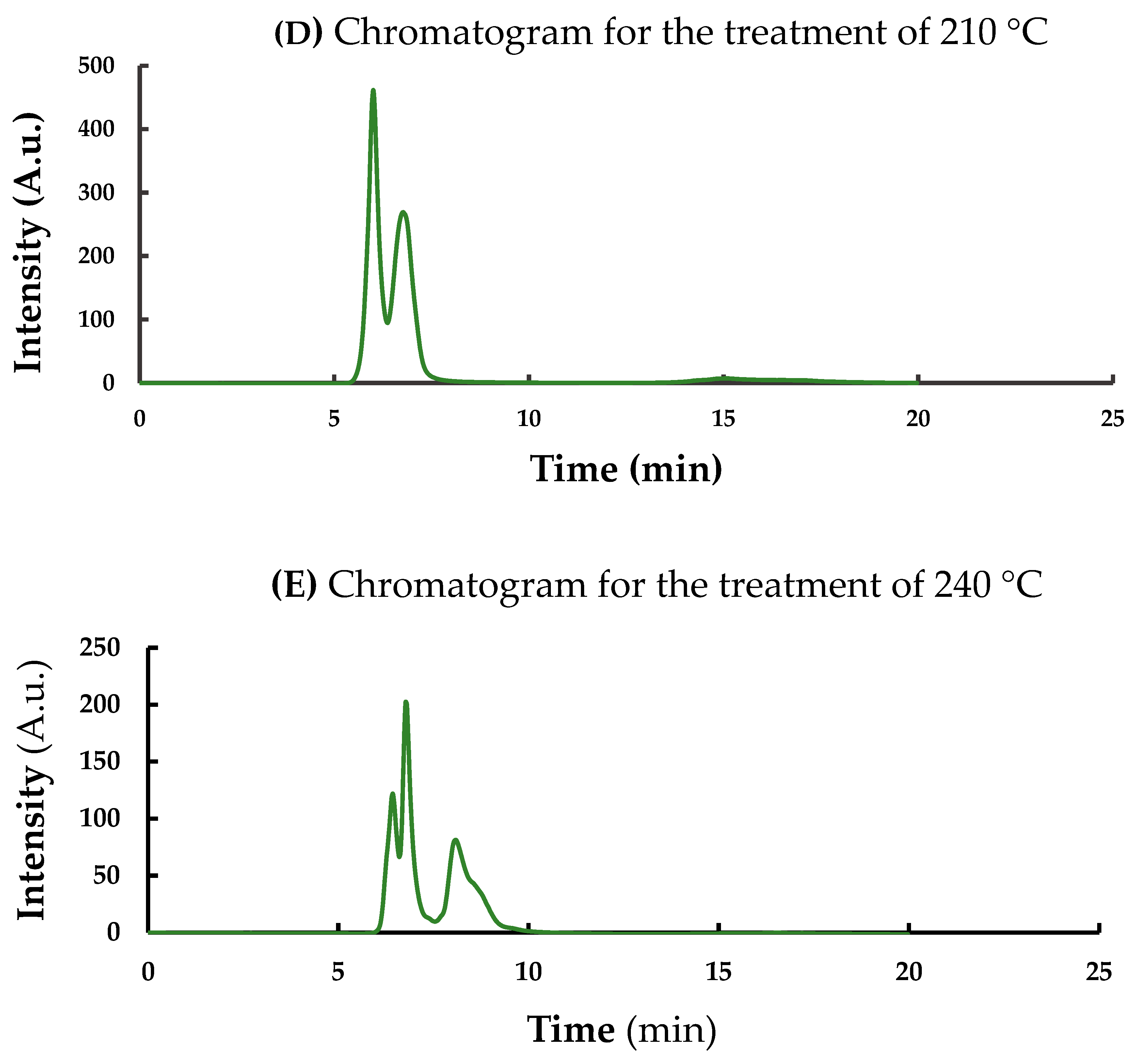

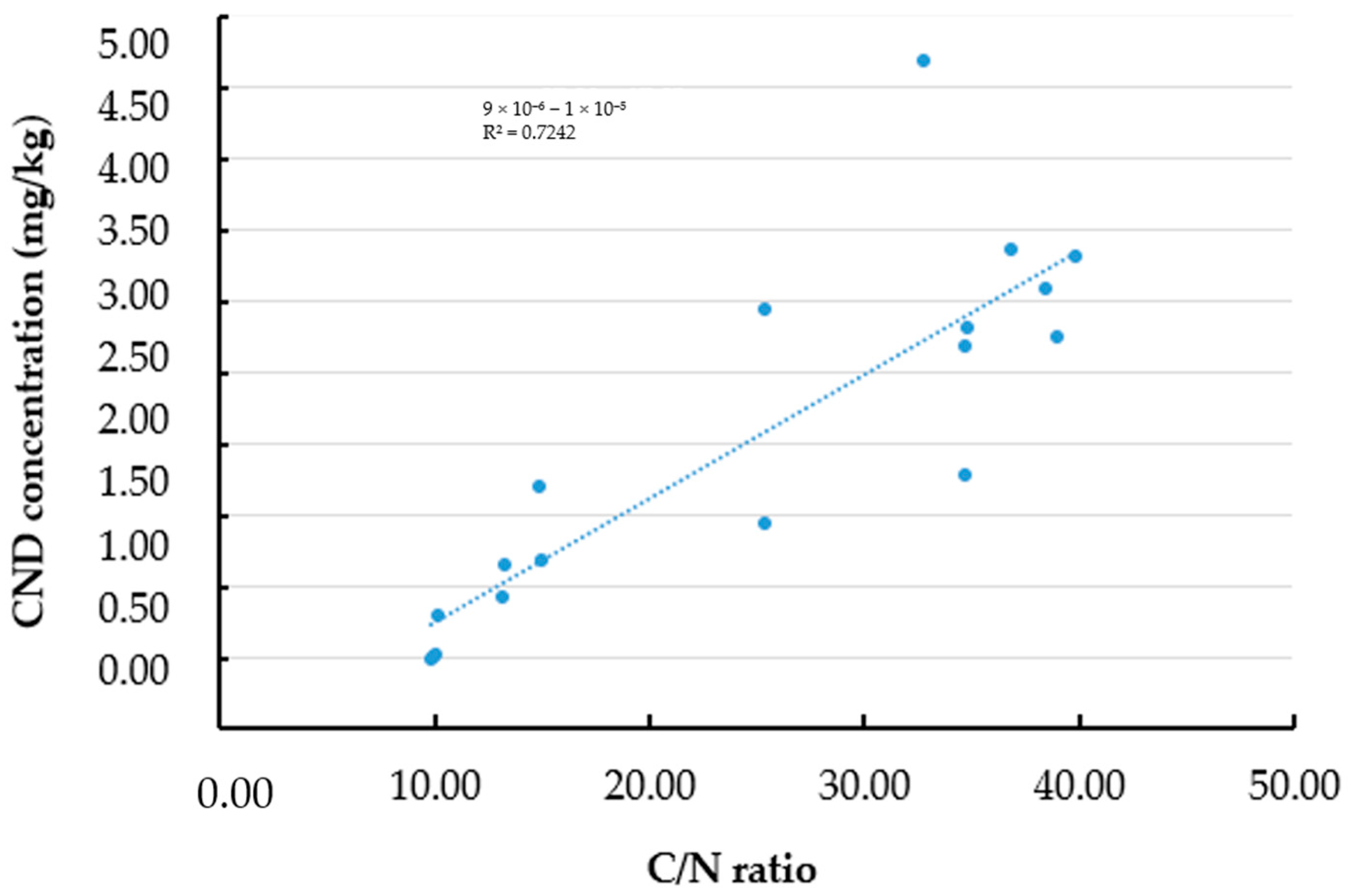
| Parameter | Description |
|---|---|
| Mobile Phase | Acetonitrile: Water (20:80, v/v), unbuffered |
| Flow Rate | 0.7 mL/min |
| Injection Volume | 5 μL |
| Excitation Wavelength | 370 nm |
| Emission Wavelength | 460 nm |
| Scan Speed | Medium (600 nm/min) |
| Integration Time | 1 s |
| Emission Scanning | Not performed (fixed excitation/emission) |
| Column Temperature | 30 °C |
| Detector Temperature | Ambient (~25 °C) |
| Sample Preparation | 100× dilution in distilled water, 0.45 μm hydrophilic PTFE filter (Labex Ltd.) |
| Mobile Phase pH | ~6.0 (unbuffered) |
| Ionic Strength | Low (no salts or buffers added) |
| Parameter | Description |
|---|---|
| Calibration Purpose | Molecular weight identification and quantification of CNDs |
| Standard Mix 1 | Bio-Rad Gel Filtration Standard (MW range: 1350–670,000 Da) |
| Components (Standard 1) | Thyroglobulin, γ-globulin, ovalbumin, myoglobin, vitamin B12 |
| Standard Mix 2 | Merck Peptide Standards |
| Components (Standard 2) | Gly-Tyr (238.2 Da), Val-Tyr-Val (379.5 Da), Met-enkephalin (573.7 Da), Leu-enkephalin (555.6 Da), Angiotensin II (1046.2 Da) |
| CND Reference Standard | Synthesized from glycine and dextrose (Nguyen et al., 2024 [28]) |
| Calibration Series | 4-point series with concentrations: 10, 100, 1000, and 10,000 g/mL |
| Solvent for Calibration | Distilled water |
| Output | Calibration curve to quantify CNDs in OMP (mushroom) samples |
| Under 200 °C | Above 200 °C | ||
|---|---|---|---|
| Temperature (°C) | Yield (%) ± SD | Temperature (°C) | Yield (%) ± SD |
| 150 | 13.20 ± 0.41 b | 200 | 6.80 ± 0.26 ab |
| 160 | 7.00 ± 0.21 ab | 210 | 7.00 ± 0.22 ab |
| 170 | 7.60 ± 0.22 b | 220 | 8.00 ± 0.27 b |
| 180 | 6.60 ± 0.24 ab | 230 | 0.80 ± 0.03 a |
| 190 | 5.60 ± 0.19 a | 240 | 0.80 ± 0.02 a |
| Under 200 °C | Above 200 °C | ||
|---|---|---|---|
| Temperature (°C) | Molecular Weight (kDa) | Temperature (°C) | Molecular Weight (kDa) |
| 150 | 602.20 ± 1.20 a | 200 | 623.20 ± 1.00 b |
| 160 | 599.20 ± 5.40 a | 210 | 618.40 ± 1.80 abcd |
| 170 | 611.80 ± 1.20 abcd | 220 | 608.50 ± 7.90 abcd |
| 180 | 614.60 ± 6.20 b | 230 | 540.10 ± 5.20 d |
| 190 | 620.60 ± 1.20 bc | 240 | 411.20 ± 6.50 d |
| Tested Samples | Escherichia coli (Million CFU/g) | Staphylococcus epidermidis (Million CFU/g) |
|---|---|---|
| Control | 1075.0 ± 16.1 a | 13.5 ± 2.1 a |
| 1 w/v% of OMP-CND-FL-210 | 605.0 ± 7.1 b | 11.5 ± 0.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törős, G.; Béni, Á.; Balláné, A.K.; Semsey, D.; Ferroudj, A.; Prokisch, J. Production of Myco-Nanomaterial Products from Pleurotus ostreatus (Agaricomycetes) Mushroom via Pyrolysis. Pharmaceutics 2025, 17, 591. https://doi.org/10.3390/pharmaceutics17050591
Törős G, Béni Á, Balláné AK, Semsey D, Ferroudj A, Prokisch J. Production of Myco-Nanomaterial Products from Pleurotus ostreatus (Agaricomycetes) Mushroom via Pyrolysis. Pharmaceutics. 2025; 17(5):591. https://doi.org/10.3390/pharmaceutics17050591
Chicago/Turabian StyleTörős, Gréta, Áron Béni, Andrea Kovács Balláné, Dávid Semsey, Aya Ferroudj, and József Prokisch. 2025. "Production of Myco-Nanomaterial Products from Pleurotus ostreatus (Agaricomycetes) Mushroom via Pyrolysis" Pharmaceutics 17, no. 5: 591. https://doi.org/10.3390/pharmaceutics17050591
APA StyleTörős, G., Béni, Á., Balláné, A. K., Semsey, D., Ferroudj, A., & Prokisch, J. (2025). Production of Myco-Nanomaterial Products from Pleurotus ostreatus (Agaricomycetes) Mushroom via Pyrolysis. Pharmaceutics, 17(5), 591. https://doi.org/10.3390/pharmaceutics17050591









